Synthesis of a CCNC–Silica–Graphene Oxide Porous Monolith for Efficient Copper Ion Removal
Abstract
1. Introduction
2. Results and Discussion
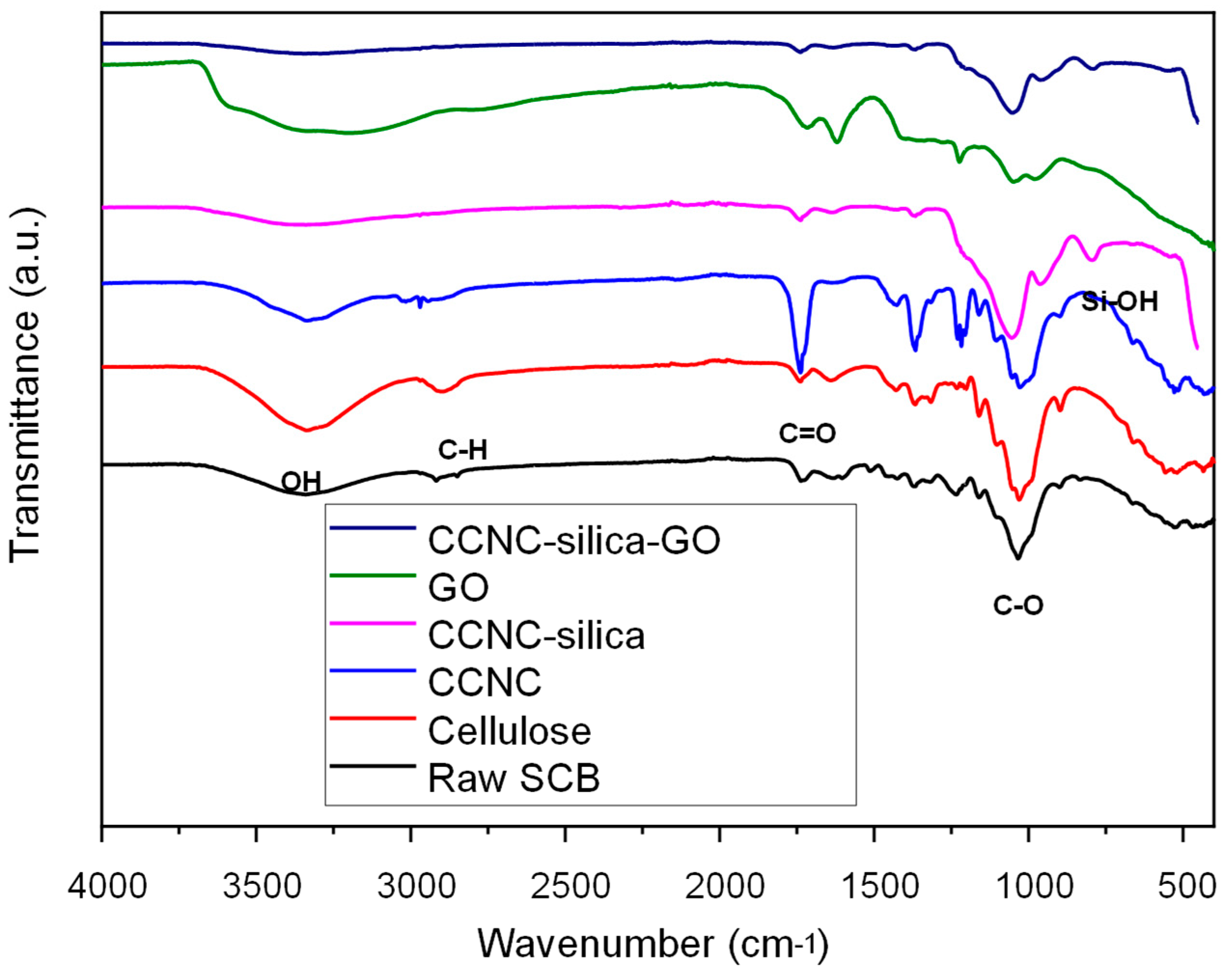
2.1. FTIR Spectroscopy Analysis
2.2. X-Ray Diffraction Analysis
2.3. SEM Analysis
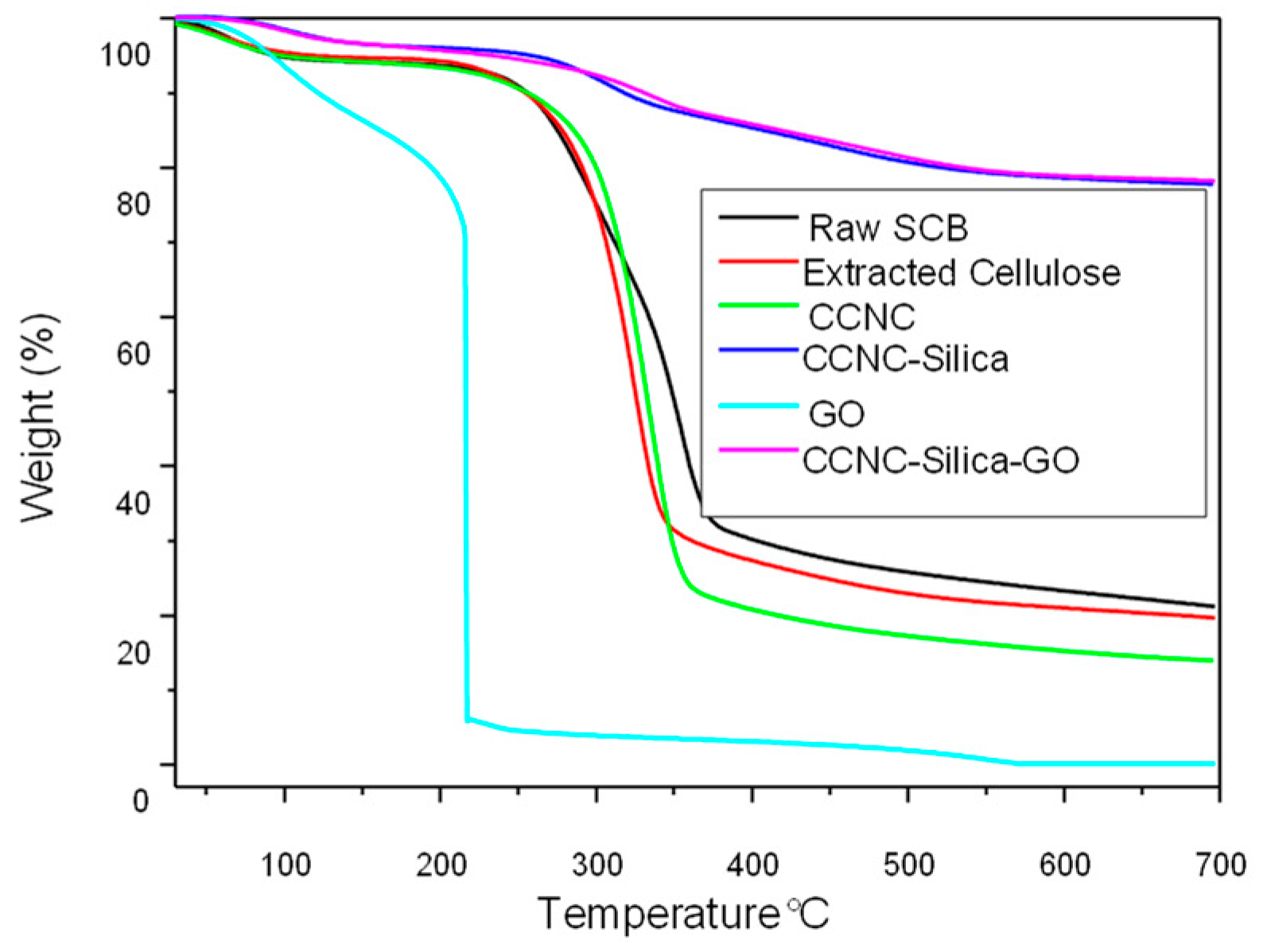
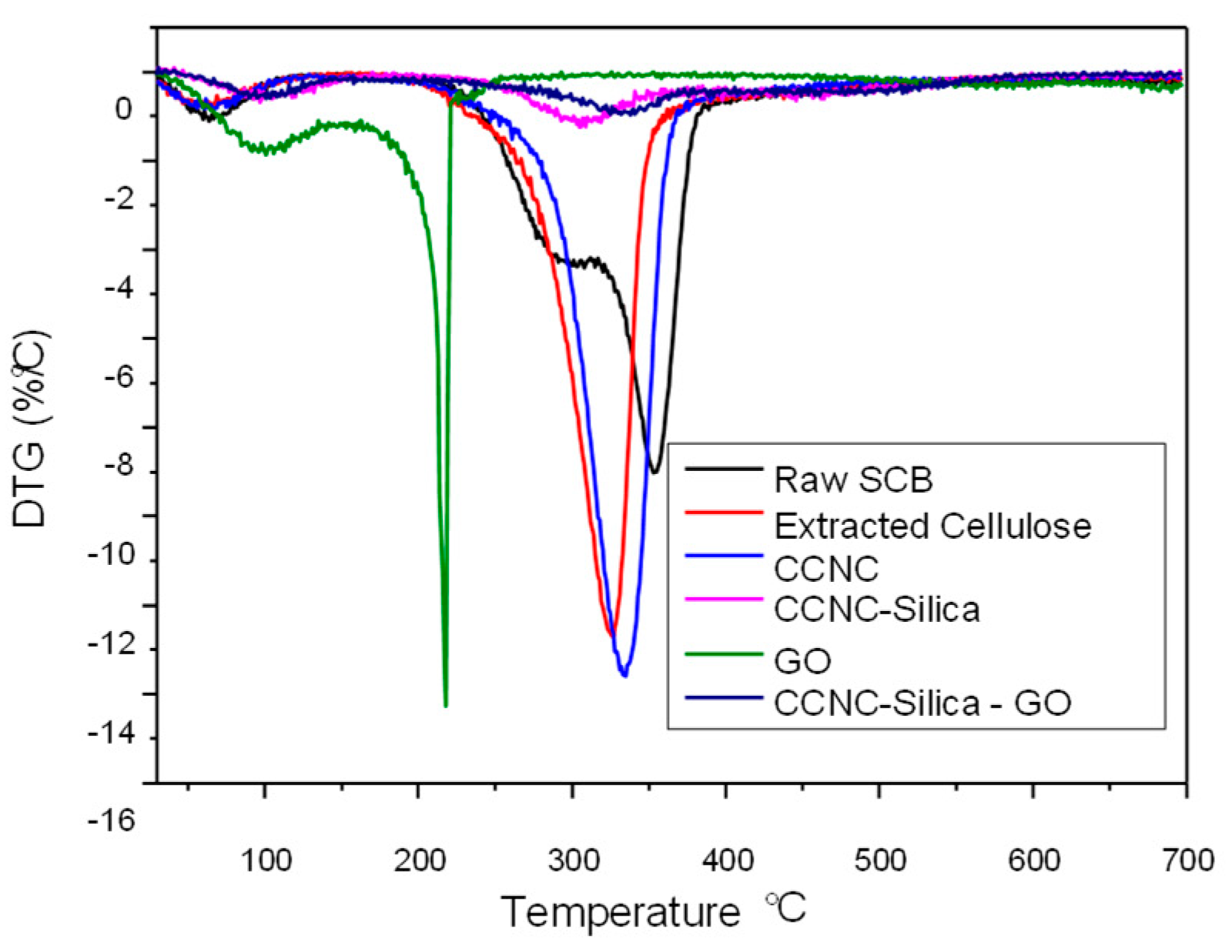
2.4. TGA
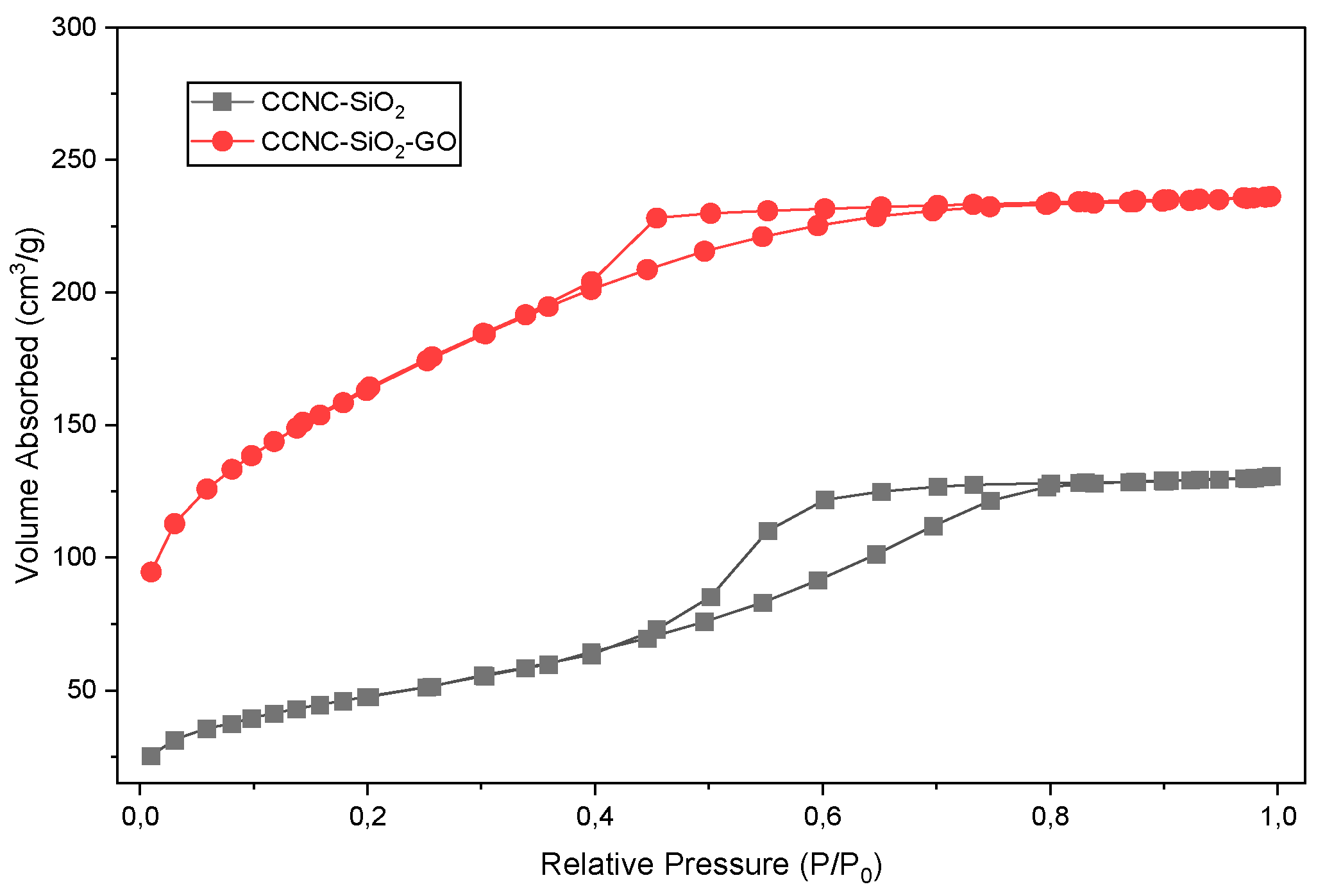
2.5. BET Analysis
2.6. Factors Affecting Copper Adsorption
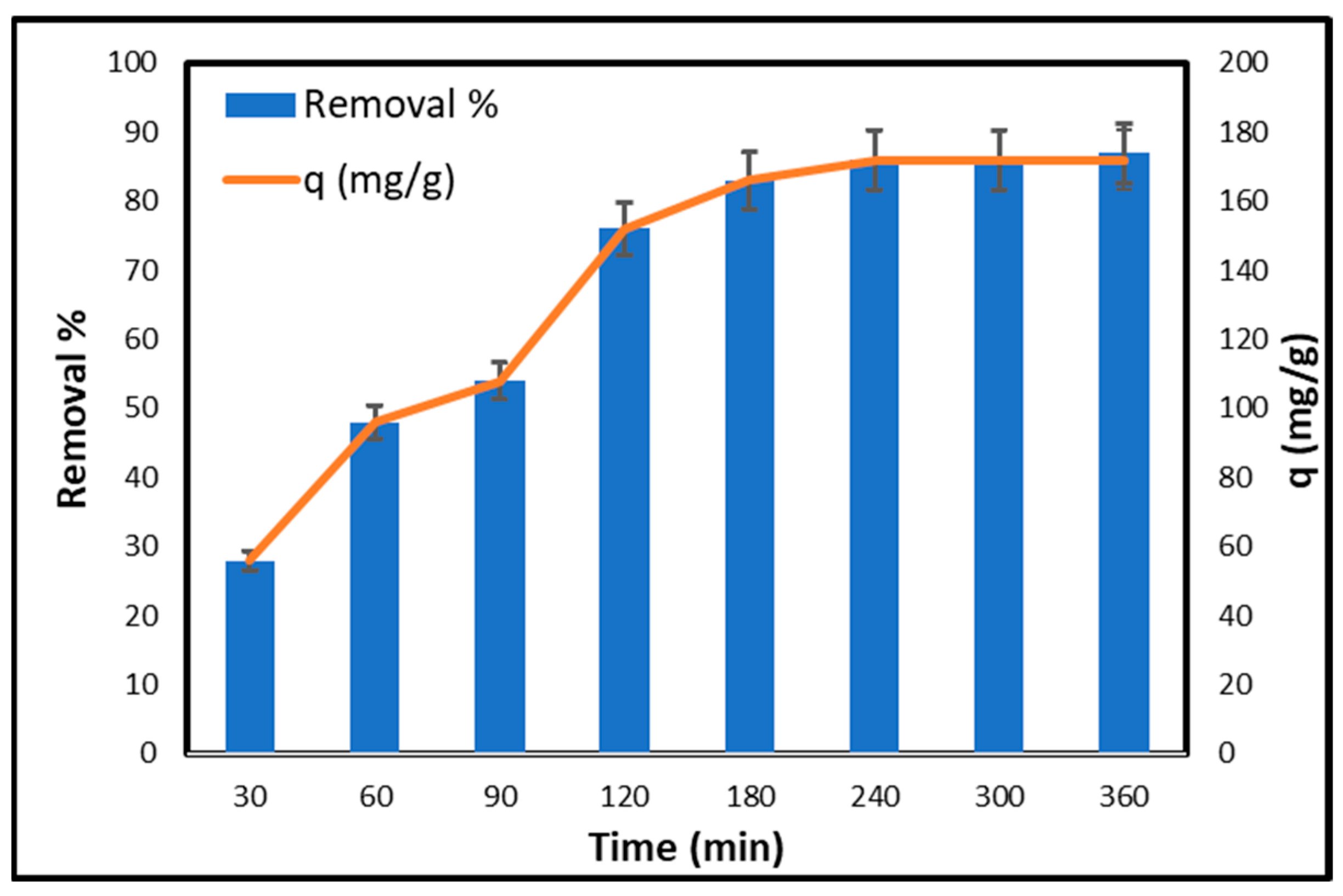
2.6.1. Effect of Contact Time
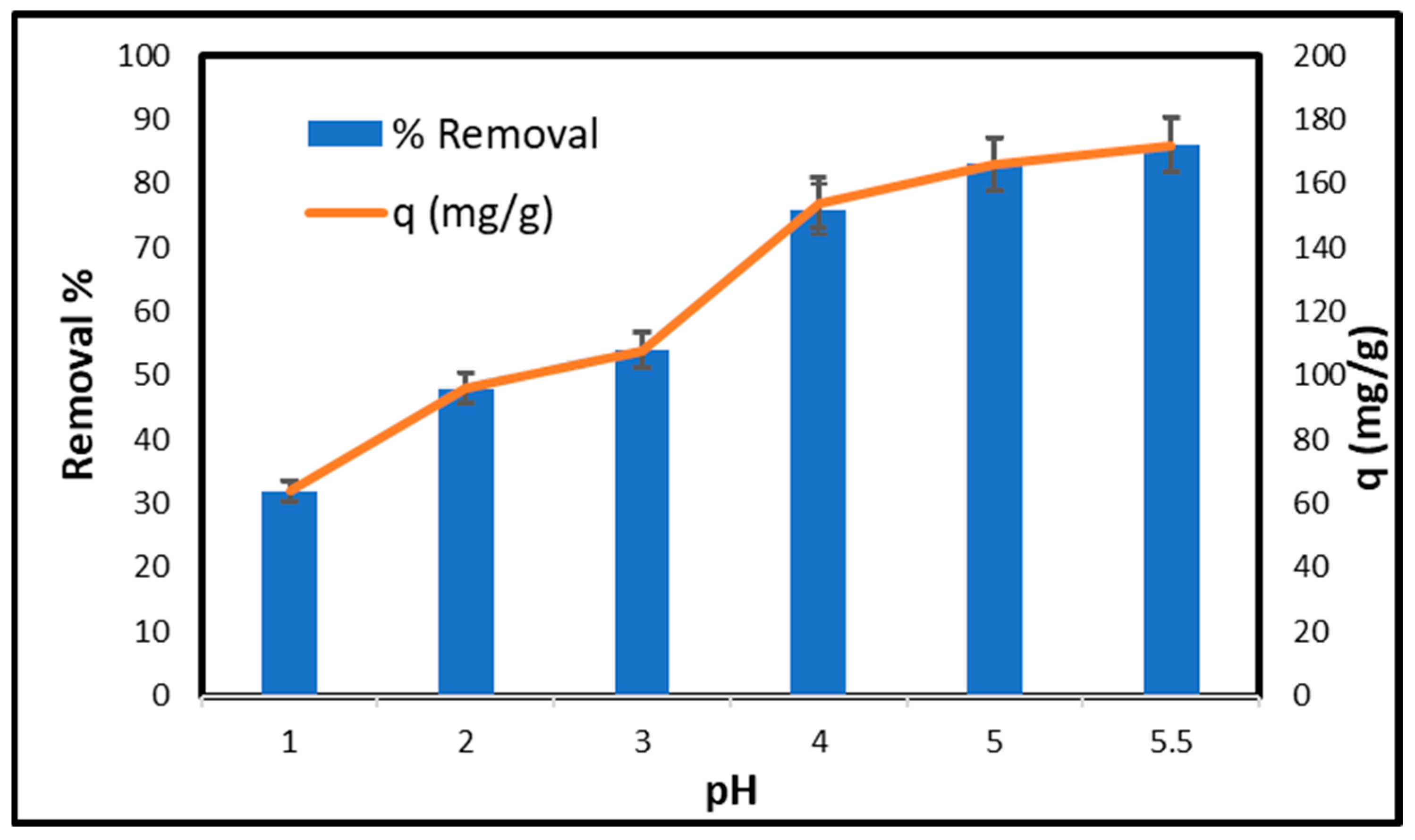
2.6.2. Effect of pH
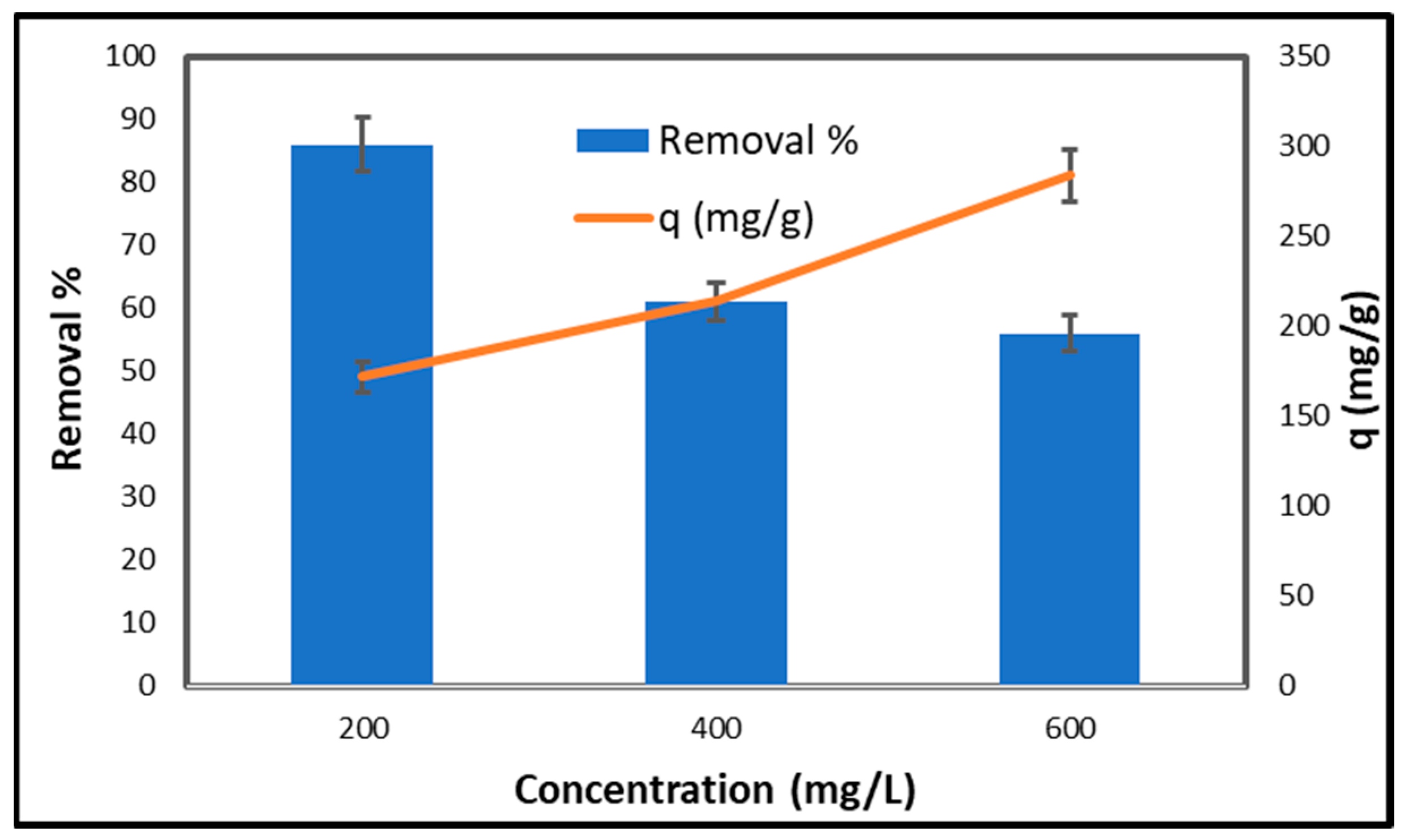
2.6.3. Effect of Initial Copper Concentration
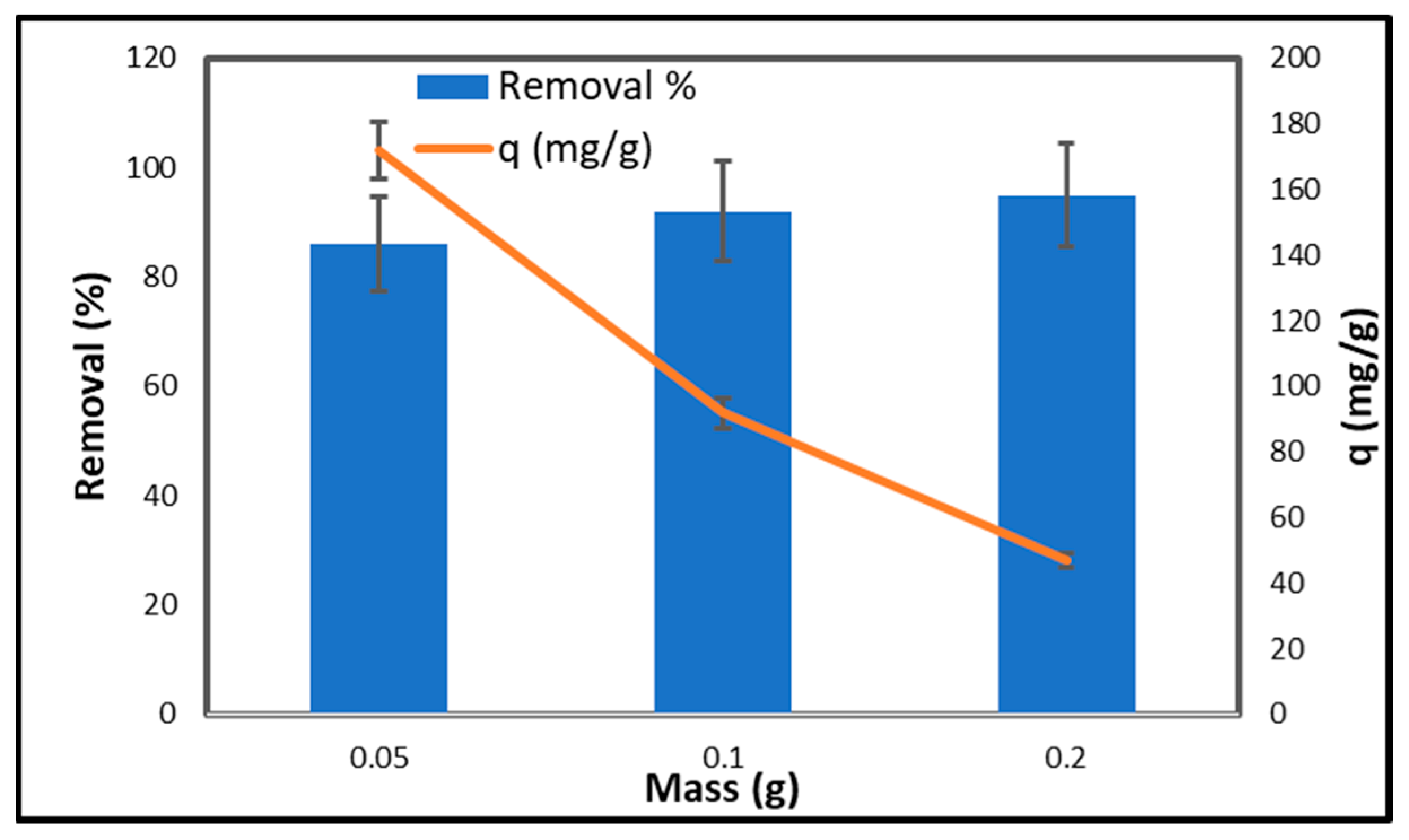
2.6.4. Effect of Adsorbent Dose
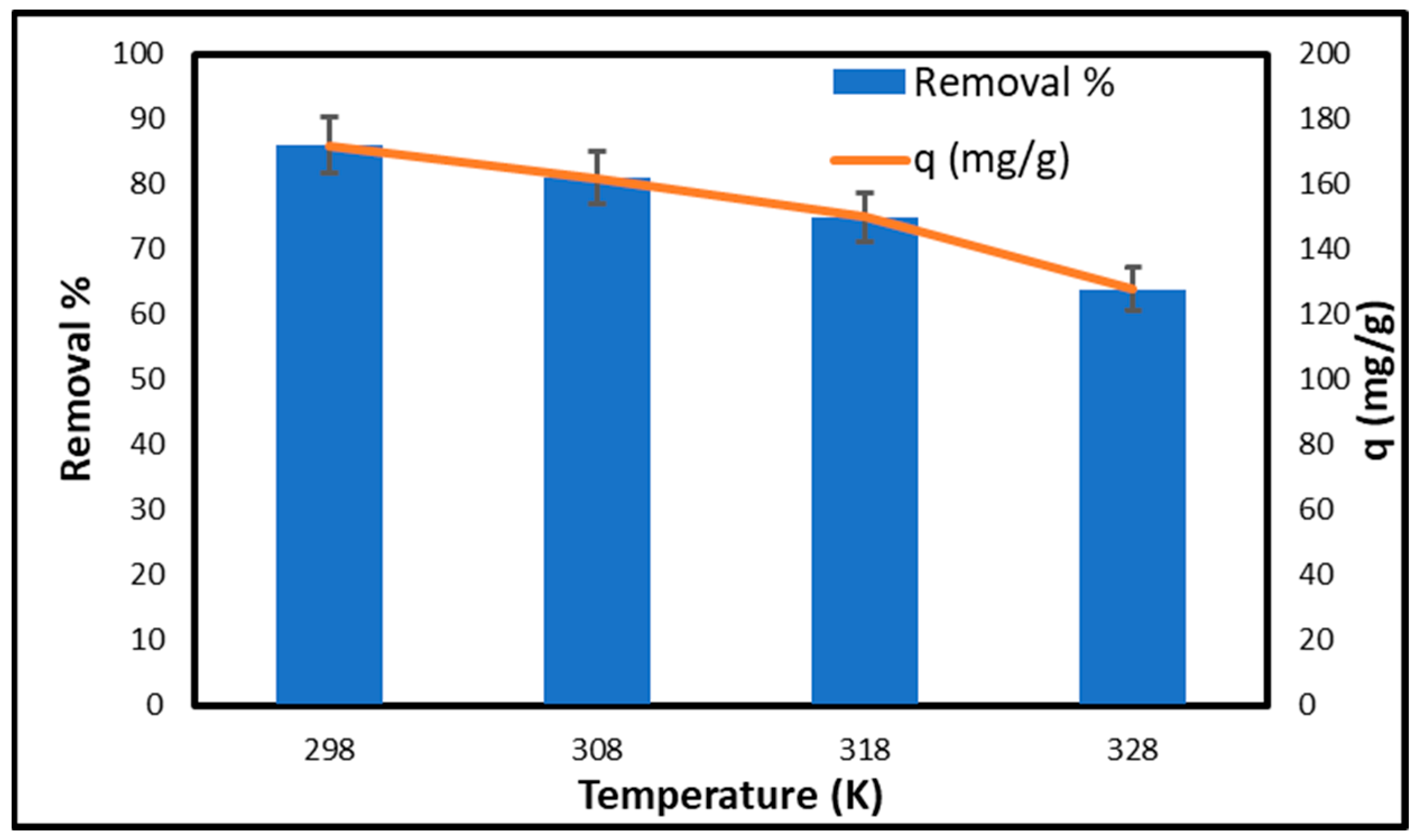
2.6.5. Effect of Temperature
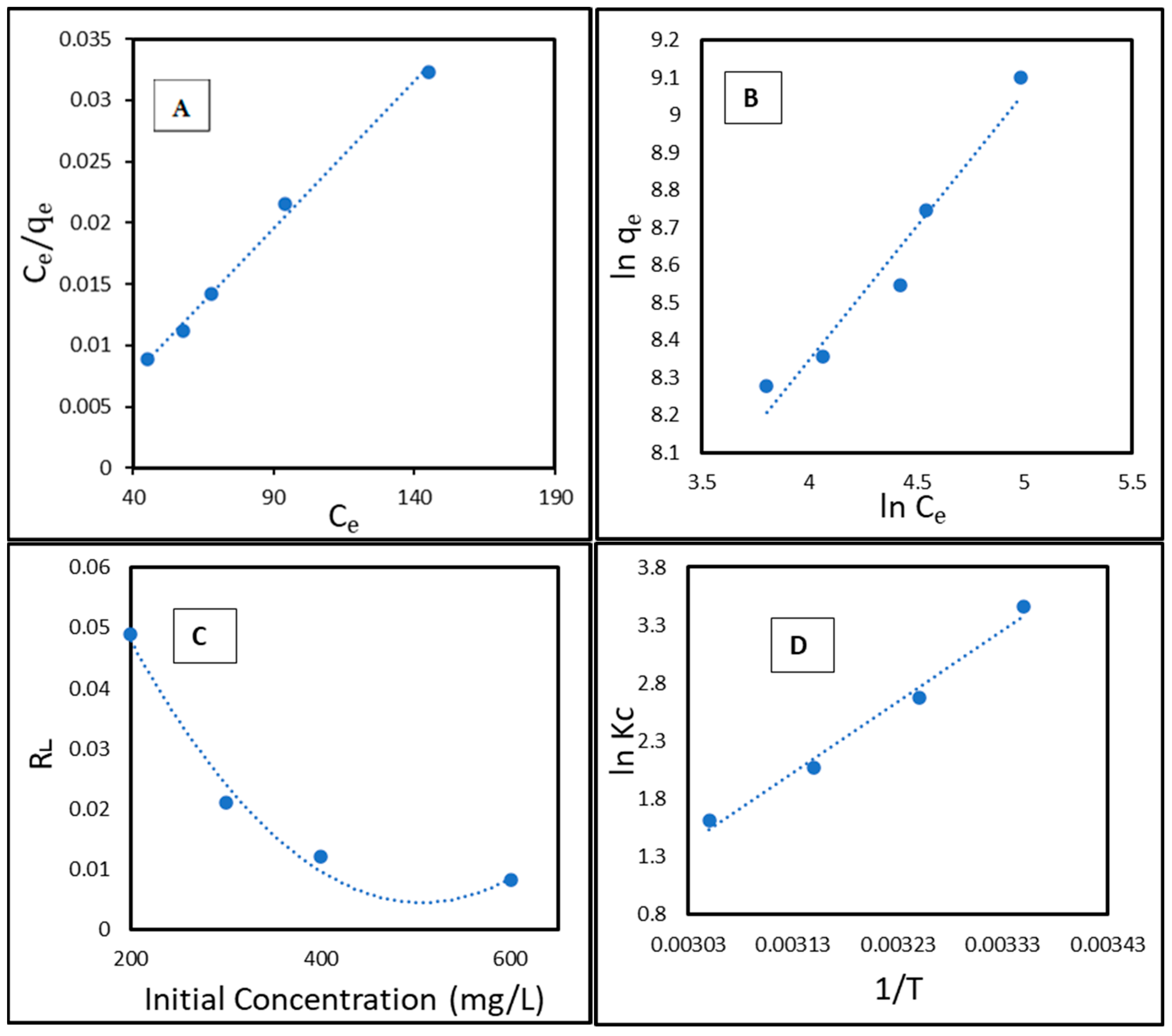
2.7. Adsorption Isotherm
2.8. Thermodynamic Studies
2.9. Kinetic Studies
2.10. Elucidation of the Cu(II) Sorption Mechanism
2.11. Cellulose-Based Silica Composite Porous Monolith Reusability
2.12. Comparison of Adsorption Capacity and Reusability with Other Reported Materials
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of Cellulose
4.3. Preparation of Carboxylate Cellulose Nanocrystals
4.4. Preparation of CCNC–Silica Hydrogel
4.5. Preparation of Graphene Oxide
4.6. Preparation of CCNC–Silica–GO Hybrid Composite Porous Monolith
4.7. Characterization Methods
4.7.1. Fourier-Transform Infra-Red Spectroscopy
4.7.2. X-Ray Diffraction
4.7.3. Scanning Electron Microscopy
4.7.4. Thermogravimetric Analysis
4.8. Adsorption Experiments
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Qin, G.; Zhang, Y.; Sun, J.; Wang, S.; Jiang, L. Heavy metal removal from aqueous solutions by calcium silicate powder from waste coal fly-ash. J. Clean. Prod. 2018, 182, 776–782. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Wu, Y.; Hu, S.; Zhang, Y. Dynamic characteristics of heavy metal accumulation in the farmland soil over Xiaoqinling gold-mining region, Shaanxi, China. Environ. Earth Sci. 2019, 78, 25. [Google Scholar] [CrossRef]
- Laus, R.; De Favere, V.T. Competitive adsorption of Cu (II) and Cd (II) ions by chitosan crosslinked with epichlorohydrin–triphosphate. Bioresour. Technol. 2011, 102, 8769–8776. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu (II), Cd (II), Pb (II), and Hg (II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef]
- Khumalo, N.L.; Mohomane, S.; Basson, A.K.; Motaung, T.E. Functional cellulose-based gels used in wastewater remediation, with a focus on heavy metals and dye removal: A critical review. Cellul. Chem. Technol. 2025, 59, 665–682. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Petersková, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Extraction of valuable metal ions (Cs, Rb, Li, U) from reverse osmosis concentrate using selective sorbents. Desalination 2012, 286, 316–323. [Google Scholar] [CrossRef]
- Verma, V.K.; Tewari, S.; Rai, J.P.N. Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 2008, 99, 1932–1938. [Google Scholar] [CrossRef]
- Kong, A.; Ji, Y.; Ma, H.; Song, Y.; He, B.; Li, J. A novel route for the removal of Cu (II) and Ni (II) ions via homogeneous adsorption by chitosan solution. J. Clean. Prod. 2018, 192, 801–808. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Diao, K.; Huang, W.; Wang, J.; Deng, W.; Lei, F.; Goodman, B.A. Direct identification of Cu (II) species adsorbed on rosin-derived resins using electron paramagnetic resonance (EPR) spectroscopy. Chemosphere 2018, 210, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Qiao, H.; Zhou, Y.; Yu, F.; Wang, E.; Min, Y.; Huang, Q.; Pang, L.; Ma, T. Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 2015, 141, 297–303. [Google Scholar] [CrossRef]
- Yu, H.Y.; Zhang, D.Z.; Lu, F.F.; Yao, J. New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain. Chem. Eng. 2016, 4, 2632–2643. [Google Scholar] [CrossRef]
- Lam, E.; Hemraz, U.D. Preparation and surface functionalization of carboxylated cellulose nanocrystals. Nanomaterials 2021, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Ahmad, I.; Siddiqui, W.A.; Ahmad, T. Synthesis, characterization of silica nanoparticles and adsorption removal of Cu2+ ions in aqueous solution. Int. J. Emerg. Technol. Adv. Eng. 2017, 7, 439–445. [Google Scholar]
- Tripathi, A.; Ranjan, M.R. Heavy metal removal from wastewater using low cost adsorbents. J. Bioremed. Biodeg. 2015, 6, 315. [Google Scholar] [CrossRef]
- Rahman, M.S.; Sathasivam, K.V. Heavy metal adsorption onto Kappaphycus sp. from aqueous solutions: The use of error functions for validation of isotherm and kinetics models. BioMed Res. Int. 2015, 2015, 126298. [Google Scholar] [CrossRef]
- Gao, Z.Z.; Qi, N.; Chen, W.J.; Zhao, H. Construction of hydroxyethyl cellulose/silica/graphitic carbon nitride solid foam for adsorption and photocatalytic degradation of dyes. Arab. J. Chem. 2022, 15, 104105. [Google Scholar] [CrossRef]
- Khumalo, N.L.; Mohomane, S.M.; Elumalai, V.; Motaung, T.E. Fabrication of a Carboxylate Cellulose Nanocrysal-Silica-TiO2 Aerogel for Enhanced Photocatalytic Degradation of Methylene Blue. Materials 2025, 18, 4702. [Google Scholar] [CrossRef]
- Khumalo, N.L.; Mohomane, S.M.; Motaung, T.E. Effect of Acetylation on the Morphology and Thermal Properties of Maize Stalk Cellulose Nanocrystals: A Comparative Study of Green-Extracted CNC vs. Acid Hydrolysed Followed by Acetylation. Crystals 2024, 14, 636. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Abu Elgoud, E.M.; Aly, H.F. Graphene oxide modified with carboxymethyl cellulose for high adsorption capacities towards Nd (III) and Ce (III) from aqueous solutions. Cellulose 2022, 29, 9831–9846. [Google Scholar] [CrossRef]
- Mazibuko, M.T.; Onwubu, S.C.; Mokhothu, T.H.; Paul, V.; Mdluli, P.S. Unlocking Heavy Metal Remediation Potential: A Review of Cellulose–Silica Composites. Sustainability 2024, 16, 3265. [Google Scholar] [CrossRef]
- Khumalo, N.L.; Mohomane, S.M.; Malevu, T.D.; Motloung, S.V.; Koao, L.F.; Motaung, T.E. Effect of Acid Concentration on Structural, Thermal, and Morphological Properties of Cellulose Nanocrystals from Sugarcane Bagasse and Their Reinforcement in Poly (Furfuryl) Alcohol Composites. Crystals 2025, 15, 403. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, X.; Chen, M. Cellulose nanowhiskers isolation and properties from acid hydrolysis combined with high pressure homogenization. BioResources 2013, 8, 933–943. [Google Scholar] [CrossRef]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet explosion: A universal and efficient pretreatment process for lignocellulosic biorefineries. BioEnergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Sun, R.C.; Fowler, P.; Baird, M.S. Inhomogeneities in the chemical structure of sugarcane bagasse lignin. J. Agric. Food Chem. 2003, 51, 6719–6725. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Curling, S.F.; Hale, M.D. On the effect of heat on the chemical composition and dimensions of thermally-modified wood. Polym. Degrad. Stab. 2009, 94, 2184–2193. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, X.F.; Sun, R.C.; Su, Y.Q. Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohydr. Polym. 2004, 56, 195–204. [Google Scholar] [CrossRef]
- Habibi, Y.; Vignon, M.R. Optimization of cellouronic acid synthesis by TEMPO-mediated oxidation of cellulose III from sugar beet pulp. Cellulose 2008, 15, 177–185. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Shange, M.G.; Khumalo, N.L.; Mohomane, S.M.; Motaung, T.E. Factors affecting silica/cellulose nanocomposite prepared via the sol–gel technique: A review. Materials 2024, 17, 1937. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Design, synthesis, and performance of adsorbents for heavy metal removal from wastewater: A review. J. Mater. Chem. A 2022, 10, 1047–1085. [Google Scholar] [CrossRef]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar] [CrossRef]
- Alammar, A. Sustainable Materials for Liquid-Phase Separations in Harsh Environments. Ph.D. Thesis, The University of Manchester, Manchester, UK, 2022. [Google Scholar]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Luo, J.; Wang, H. Preparation, thermal insulation and flame retardance of cellulose nanocrystal aerogel modified by TiO2. Int. J. Heat Technol. 2018, 36, 614–618. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, Z.; Cao, B.; Chen, X.; Song, H. Enhancements of thermal insulation and mechanical property of silica aerogel monoliths by mixing graphene oxide. Mater. Chem. Phys. 2017, 187, 183–190. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef]
- Morita, Y.; Koizumi, G.; Sakamoto, H.; Suye, S.I. Evaluation of protein adsorption onto a polyurethane nanofiber surface having different segment distributions. Mater. Chem. Phys. 2017, 187, 1–4. [Google Scholar] [CrossRef]
- Yan, M.; Huang, W.; Li, Z. Chitosan cross-linked graphene oxide/lignosulfonate composite aerogel for enhanced adsorption of methylene blue in water. Int. J. Biol. Macromol. 2019, 136, 927–935. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Y.; Li, Q.; Chen, X.; Xu, X. Synthesis of high-performance sodium carboxymethyl cellulose-based adsorbent for effective removal of methylene blue and Pb (II). Int. J. Biol. Macromol. 2019, 126, 107–117. [Google Scholar] [CrossRef]
- Ren, F.; Li, Z.; Tan, W.Z.; Liu, X.H.; Sun, Z.F.; Ren, P.G.; Yan, D.X. Facile preparation of 3D regenerated cellulose/graphene oxide composite aerogel with high-efficiency adsorption towards methylene blue. J. Colloid Interface Sci. 2018, 532, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Q.; Liu, T.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; Xia, L. Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr. Polym. 2013, 95, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Synthesis and characterization of multi-amino-functionalized cellulose for arsenic adsorption. Carbohydr. Polym. 2013, 92, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of graphene oxide using modified hummers method: Solvent influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
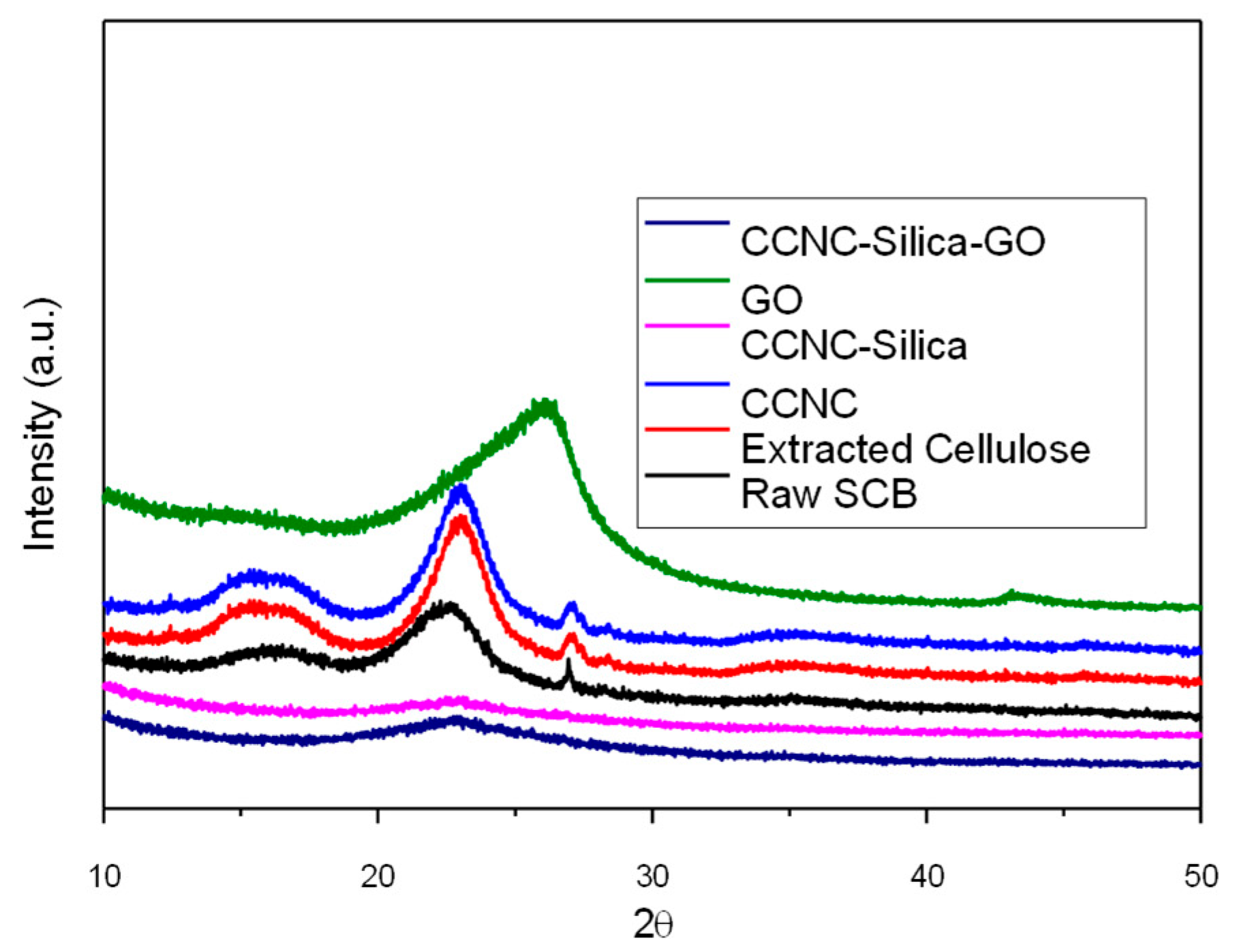



| Fiber Type | CI (%) (Segal) | CI (%) (Deconvolution) |
|---|---|---|
| Raw SCB | 29 | 27 |
| Extracted cellulose | 66 | 63 |
| CCNC | 58 | 54 |
| CCNC–silica porous monolith | - | 42 |
| CCNC–silica–GO porous monolith | - | 21 |
| Sample | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| CCNC-SiO2 | 312 | 0.12 | 1.15 |
| CCNC-SiO2-GO | 512 | 0.37 | 4.24 |
| Cellulose-Based Silica Porous Monoliths | Removal (%) | Adsorption Capacity (mg/g) |
|---|---|---|
| CCNC silica | 22 | 32 |
| CCNC–silica–GO | 86 | 172 |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| Material | qmax (mg/g) | KL (L/mg) | R2 | KF (mg1−1/ⁿ L1/ⁿ g−1) | n | R2 |
| CCNC–silica–GO | 172 | 0.27 | 0.993 | 57.32 | 3.57 | 0.908 |
| T (K) | ΔG° (KJ·mol−1) | ΔH° (KJ·mol−1) | ΔS° (J·mol−1·K−1) |
|---|---|---|---|
| 298 | −6.22 | −37.24 | −105.25 |
| 308 | −5.32 | ||
| 318 | −4.46 | ||
| 328 | −3.65 |
| Co (mg/L) | qe, exp (mg/g) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|---|
| qe, cal (mg/g) | k1 (min−1) | R2 | qe, cal (mg/g) | k2 (g/mg min) | R2 | ||
| 200 | 172 | 155 | 0.0158 | 0.941 | 186.24 | 0.00028 | 0.995 |
| 400 | 214 | 182 | 0.0154 | 0.932 | 130.46 | 0.00025 | 0.994 |
| 600 | 284 | 196 | 0.0149 | 0.921 | 124.62 | 0.00022 | 0.992 |
| Adsorbent Material | Maximum Capacity (mg/g) | Reusability (Cycles/% Retention) | Key Features/Limitations | Reference |
|---|---|---|---|---|
| CCNC-SiO2-GO Porous monolith (This work) | 172 | 5/>70% | Monolithic porous monolith, high capacity, excellent reusability, from waste biomass. | - |
| Carboxylated CNC | 149.3 | 4/~85% | High capacity from functionalization, but powdered form. | [12] |
| Mesoporous Silica (SBA-15) | 41.2 | - | High surface area, but low intrinsic capacity for Cu(II). | [16] |
| Graphene Oxide (GO) | 117.5 | - | High capacity, but prone to aggregation and difficult recovery. | [45] |
| Chitosan/GO Composite Beads | 88.7 | 5/~85% | Good reusability, but moderate capacity. | [9] |
| Cellulose/Silica Composite | 105.0 | - | Binary composite, moderate capacity. | [23] |
| Alginate/GO/Cellulose Foam | 181.8 | 5/~90% | High capacity and reusability, similar performance tier. | [50] |
| EDTA-modified CNC/Silica | 158.0 | 4/~80% | High capacity from chelating agent, requires complex modification. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumalo, N.; Mohomane, S.; Elumalai, V.; Motaung, T. Synthesis of a CCNC–Silica–Graphene Oxide Porous Monolith for Efficient Copper Ion Removal. Gels 2025, 11, 832. https://doi.org/10.3390/gels11100832
Khumalo N, Mohomane S, Elumalai V, Motaung T. Synthesis of a CCNC–Silica–Graphene Oxide Porous Monolith for Efficient Copper Ion Removal. Gels. 2025; 11(10):832. https://doi.org/10.3390/gels11100832
Chicago/Turabian StyleKhumalo, Nduduzo, Samson Mohomane, Vetrimurugan Elumalai, and Tshwafo Motaung. 2025. "Synthesis of a CCNC–Silica–Graphene Oxide Porous Monolith for Efficient Copper Ion Removal" Gels 11, no. 10: 832. https://doi.org/10.3390/gels11100832
APA StyleKhumalo, N., Mohomane, S., Elumalai, V., & Motaung, T. (2025). Synthesis of a CCNC–Silica–Graphene Oxide Porous Monolith for Efficient Copper Ion Removal. Gels, 11(10), 832. https://doi.org/10.3390/gels11100832






