Development of Efficient In-Situ Cleaning Methods for Stained Textile Relics
Abstract
1. Introduction
2. Results and Discussion
2.1. Appearance Morphology Analysis of Stained Textile Relics Before and After Cleaning
2.2. Cleaning Efficiency Analysis of Stained Textile Relics Before and After Cleaning
2.3. Mechanical Strength Analysis of Stained Textiles Before and After Cleaning
2.4. EDS Analysis of Stained Textile Relics Before and After Cleaning
2.5. Analysis of In-Situ Cleaning Mechanism
3. Conclusions
4. Material and Methods
4.1. Experimental Materials and Equipment
4.2. Experimental Process and Design
4.2.1. Preparation of Simulated Samples of Stained Textile Relics
4.2.2. Development Experiment of Efficient In-Situ Cleaning Method for Stained Textile Relics
4.3. Experimental Testing Indicators and Methods
4.3.1. Appearance Morphology
4.3.2. Washing Efficiency
4.3.3. Mechanical Strength Test
4.3.4. Energy-Dispersive Spectrometer (EDS)
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, Y.; Cao, X.; Su, Z.; Wang, G.; Ling, X.; Pan, W. Deterioration Characteristics Analysis and Grade Estimation of Textile Relics in Moist Buried Environment. Russ. J. Nondestruct. Test. 2025, 61, 70–90. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Han, J.; Huang, X.; Luo, H. Restoring Ancient Civilizations with “Herit-Materials”: Technological Advances in its Studies. Sci. China Technol. Sci. 2023, 66, 1952–1974. [Google Scholar] [CrossRef]
- Xiao, W.; Xian, Y.; Yu, C.; Wang, Y.; Sun, L.; Li, Y. Microinvasive Analysis of Textile Relics from an Ancient Silk Road Turquoise Mining Site. Sci. China Technol. Sci. 2023, 66, 2286–2296. [Google Scholar] [CrossRef]
- Guan, M.; Chen, Y.; Li, X.; Wang, Y.; Kang, X.; Hu, X.; Qu, L.; Li, G. Evaluation of Color Degradation on Unearthed Silks: A Data Analytical Strategy based on Micro-fading Spectrometry. J. Cult. Herit. 2024, 66, 501–510. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, H.; Zhang, W.; Yang, R.; Su, B.; Zhao, X.; Zhou, Y.; Dai, X. Insight of Silk Relics of Mineralized Preservation in Maoling Mausoleum using Two Enzyme-linked Immunological Methods. J. Archaeol. Sci. 2020, 115, 105089. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Shi, L.; Han, Q.; Lin, C.; Zhang, L.; Wu, M.; Zhang, W. Degradation Pathways and Mechanisms Insight of Indigo and Shikonin with Experiments and Quantum Chemical Calculations. Dye. Pigment. 2023, 218, 111455. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Z.; Hu, J.; Zhou, G.; Xu, G.; Yang, W.; Zhang, J. Insight into the Measurements for Determining the Ageing Degree of Ancient Silk. Polym. Degrad. Stab. 2022, 196, 109833. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, H.; Jia, L.; Chen, N.; Zhou, Y. Study on the Aging Degree of Historical Silk by the Surface Resistance Method. Russ. J. Nondestruct. Test. 2021, 57, 408–416. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, W.; Yang, H.; Ma, C.; Zhou, Y.; Dai, X. An Immunomagnetic Bead Enrichment Technique to Improve the Detection Efficiency for Trace Silk Protein, its’ Application. J. Cult. Herit. 2019, 38, 46–52. [Google Scholar] [CrossRef]
- Chen, R.; Hu, M.; Zheng, H.; Yang, H.; Zhou, L.; Zhou, Y.; Peng, Z.; Hu, Z.; Wang, B. Proteomics and Immunology Provide Insight into the Degradation Mechanism of Historic and Artificially Aged Silk. Anal. Chem. 2020, 92, 2435–2442. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Liu, M.; Liu, Y.; Zheng, H.; Hu, Z.; Zhou, Y.; Wang, B. Lanthanide-Labeled Immunochromatographic Strip Assay for the On-Site Identification of Ancient Silk. ACS Sens. 2017, 2, 569–575. [Google Scholar] [CrossRef]
- Wei, Y.; Ling, X.; Su, Z.; Cao, X.; Guo, J.; Wan, Z.; Liu, K.; Pan, W. Development of Suitable Washing Methods for Textile Relics Adsorbing Composite Organic/Inorganic Stains. J. Text. Inst. 2024, 116, 1788–1804. [Google Scholar] [CrossRef]
- Wei, Y.; Cao, X.; Ling, X.; Su, Z.; Wan, Z.; Liu, K.; Shemin, C.; Pan, W. Develop an Environment-friendly Detergent for Textile Relics Adsorbing Soil/rust Stains. J. Surfact. Deterg. 2024, 27, 301–315. [Google Scholar] [CrossRef]
- Miao, B.; Zhao, Z.; Guo, P.; Li, H.; Wang, Y. Cleaning Iron Rust Compounds from Cotton Textiles: Application to Qing Dynasty Armor. Herit. Sci. 2023, 11, 56. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R.; Poggi, G. Colloid and Materials Science for the Conservation of Cultural Heritage: Cleaning, Consolidation, and Deacidification. Langmuir 2013, 29, 5110–5122. [Google Scholar] [CrossRef]
- Natali, I.; Carretti, E.; Angelova, L.; Baglioni, P.; Weiss, R.G.; Dei, L. Structural and Mechanical Properties of “Peelable” Organ Aqueous Dispersions with Partially Hydrolyzed Poly (Vinyl Acetate)-Borate Networks: Applications to Cleaning Painted Surfaces. Langmuir 2011, 27, 13226–13235. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Cui, R.; Niu, L. Study on the Technological Process and Artistic Characteristics of Ancient Chinese Zhuanghua Silk Fabric. Fibres Text. East. Eur. 2021, 29, 105–111. [Google Scholar] [CrossRef]
- Gong, Y.; Qiao, C.; Zhong, B.; Zhong, J.; Gong, D. Analysis and Characterization of Materials Used in Heritage Theatrical Figurines. Herit. Sci. 2020, 8, 13. [Google Scholar] [CrossRef]
- Ahmed, H.E.; Kolisis, F.N. An Investigation into the Removal of Starch Paste Adhesives from Historical Textiles by Using the Enzyme α-amylase. J. Cult. Herit. 2011, 12, 169–179. [Google Scholar] [CrossRef]
- He, J.; Wu, Z.-y.; Zhang, S.; Zhou, Y.; Zhao, F.; Peng, Z.-q.; Hu, Z.-w. Optimization of Microwave-Assisted Extraction of Tea Saponin and Its Application on Cleaning of Historic Silks. J. Surfact. Deterg. 2014, 17, 919–928. [Google Scholar] [CrossRef]
- Meng, Q.; Li, X.; Geng, J.; Liu, C.; Ben, S. A Biological Cleaning Agent for Removing Mold Stains from Paper Artifacts. Herit. Sci. 2023, 11, 243. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, H.; Su, Z.; Tao, S.; Cao, X.; Wan, Z.; Pan, W. Develop an Optimal Daily Washing Care for Technical Jacket by Balancing Washing Efficiency and Functional Degradation. J. Eng. Fibers Fabr. 2023, 18, 1–16. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C.; Koch, K. Self-cleaning Efficiency of Artificial Superhydrophobic Surfaces. Langmuir 2009, 25, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Wang, J.; Ding, J.; Zhao, X.; Dong, W.; Lu, Z.; Li, X. Preparing a Microemulsion-Loaded Hydrogel for Cleaning Wall Paintings and Coins. Herit. Sci. 2024, 12, 149. [Google Scholar] [CrossRef]
- Furstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and Self-Cleaning Properties of Artificial Superhydrophobic Surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef]
- Heckenthaler, T.; Sadhujan, S.; Morgenstern, Y.; Natarajan, P.; Bashouti, M.; Kaufman, Y. Self-Cleaning Mechanism: Why Nanotexture and Hydrophobicity Matter. Langmuir 2019, 35, 15526–15534. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Liao, Z.; Ueda, Y.; Yoshikawa, K.; Zhang, G. Microbubbles for Effective Cleaning of Metal Surfaces Without Chemical Agents. Langmuir 2022, 38, 769–776. [Google Scholar] [CrossRef]
- Ding, S.; Liu, W.; Li, X.; Feng, Y. The Ecology of Microbiome on Cultural Relics: The Linkage of Assembly, Composition and Biodeterioration. J. Cult. Herit. 2025, 71, 412–418. [Google Scholar] [CrossRef]
- Baglioni, P.; Dei, L.; Carretti, E.; Giorgi, R. Gels for the Conservation of Cultural Heritage. Langmuir 2009, 25, 8373–8374. [Google Scholar] [CrossRef]
- Khan, M.; Das, S.; Roy, A.; Roy, S. Reusable Sugar-Based Gelator for Marine Oil-Spill Recovery and Waste Water Treatment. Langmuir 2023, 39, 899–908. [Google Scholar] [CrossRef]
- Heger, A.; Koenig, L.; Reckert, A.; Schroeder, M.; Kilic, F.; Emami, F.; Ritz-Timme, S. Further Experiments and Remarks Regarding the Possible Formation of Blood Stains on the Turin Shroud: Stains Attributed to the Nailing of the Hands. Int. J. Leg. Med. 2024, 138, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lian, X.; Yang, Z.; Zhou, Y.; Zhang, X.; Wang, Y. Self-Shaping Microemulsion Gels for Cultural Relic Cleaning. Langmuir 2021, 37, 11474–11483. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhang, L.; Tan, K.; Dong, S.; He, Y.; Ye, L.; Zhao, W.; Gu, L.; Prati, S.; Bai, J. A New Deep Eutectic Solvent-based Green Gel for the Removal of Polymeric Coating from Mural Painting. J. Cult. Herit. 2025, 71, 51–60. [Google Scholar] [CrossRef]
- Morlotti, M.; Forlani, F.; Saccani, I.; Sansonetti, A. Evaluation of Enzyme Agarose Gels for Cleaning Complex Substrates in Cultural Heritage. Gels 2024, 10, 14. [Google Scholar] [CrossRef]
- Sun, M.; Zou, J.; Zhang, H.; Zhang, B. Measurement of Reversible Rate of Conservation Materials Based on Gel Cleaning Approach. J. Cult. Herit. 2015, 16, 719–727. [Google Scholar] [CrossRef]
- GB/T 7974-2013; Paper, Board and Pulps—Measurement of Diffuse Blue Reflectance Factor—D65 Brightness (Diff/Geometry, Outdoor Daylight Conditions). General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2013.
- GB/T 3923.1-2013; Textiles—Tensile Properties of Fabrics—Part 1: Determination of Maximum Force and Elongation at Maximum Force Using the Strip Method. General Administration of Quality Supervision, Inspection and Quaran-Tine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2013.

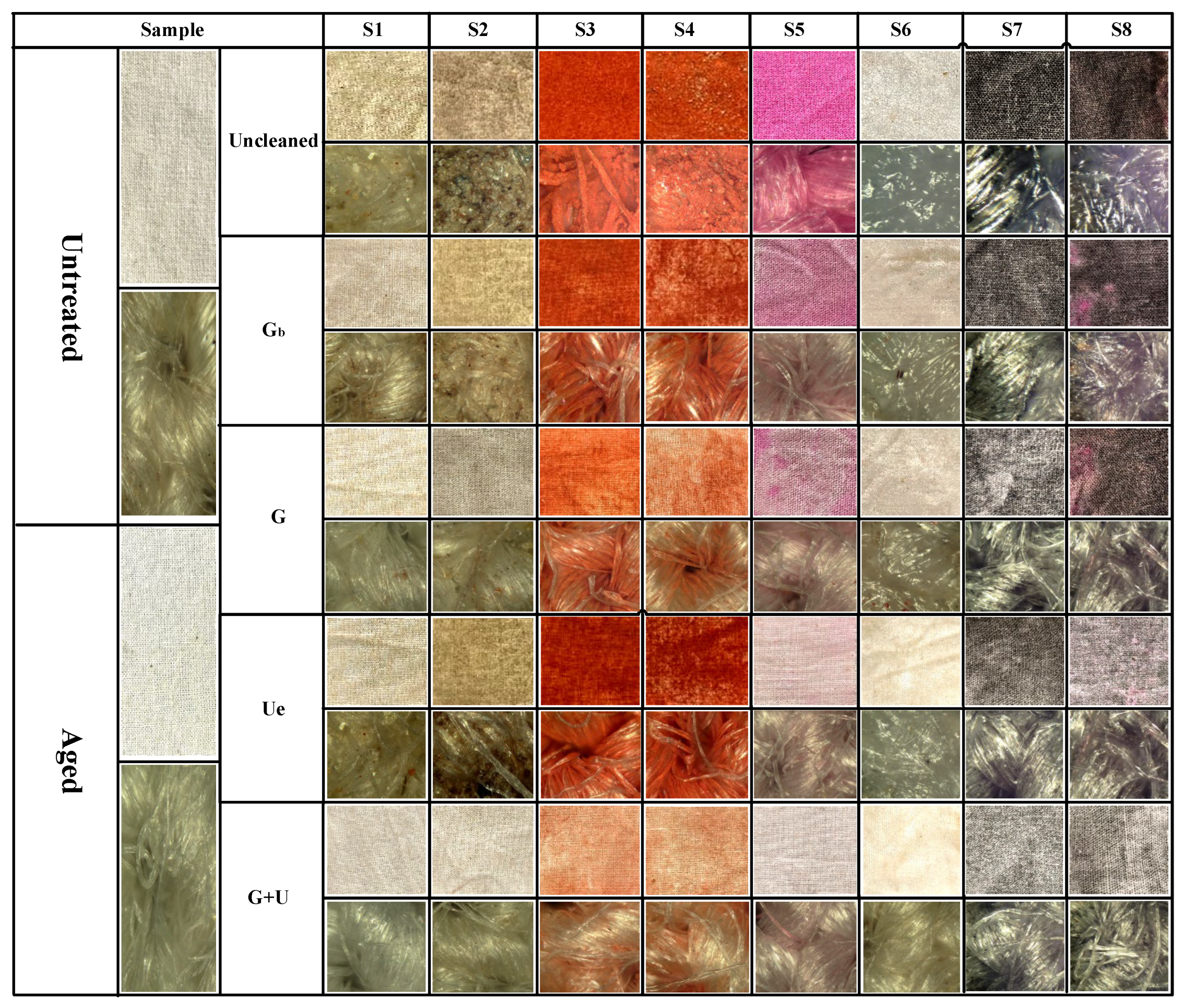
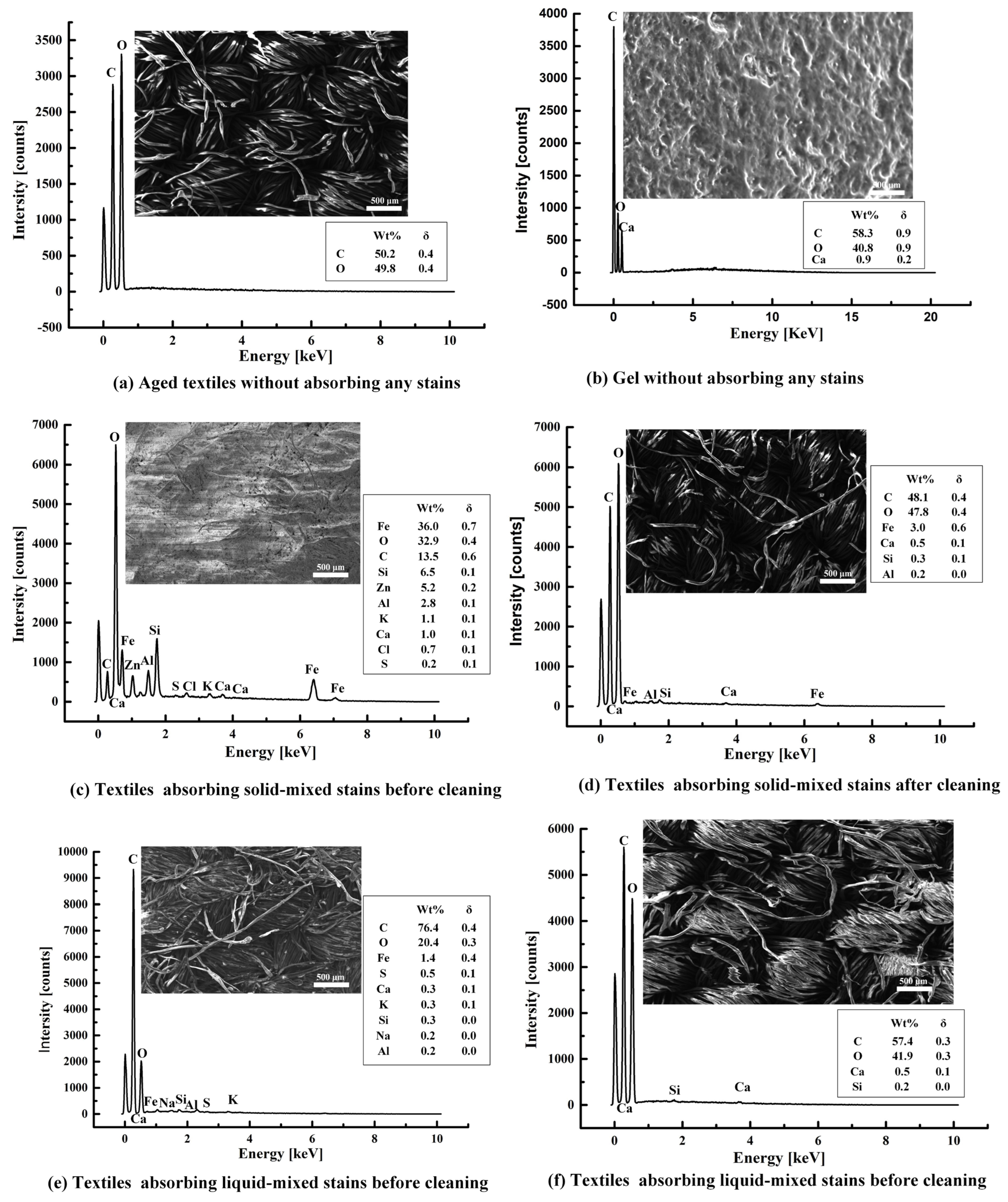
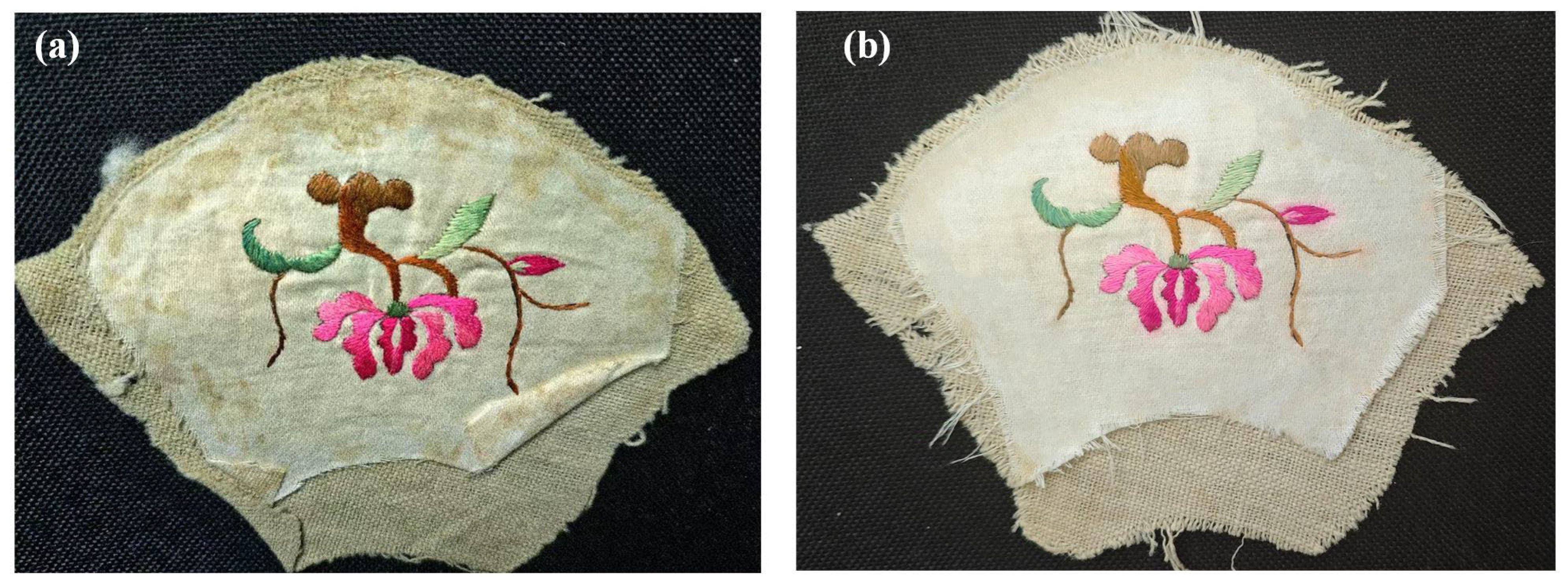
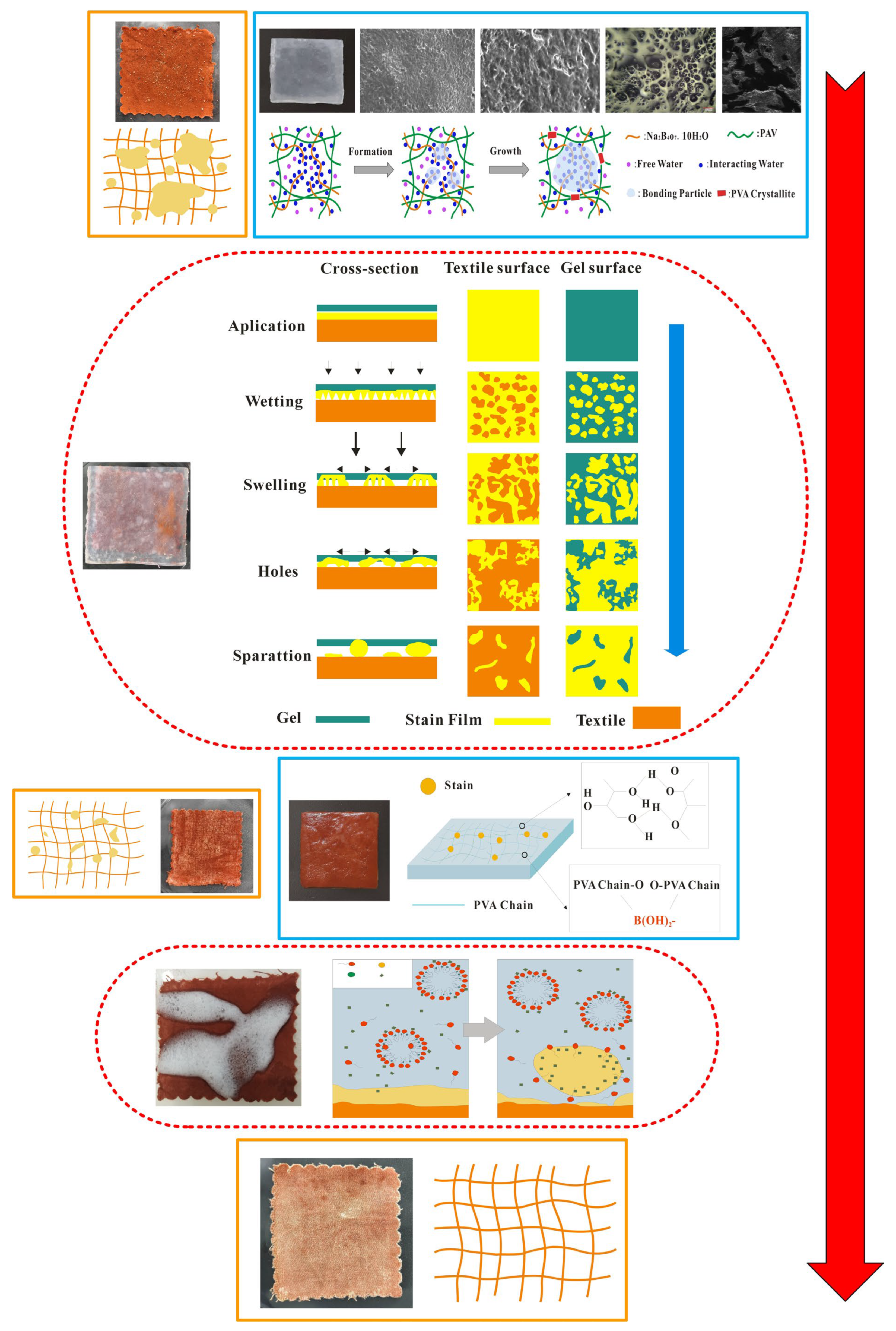
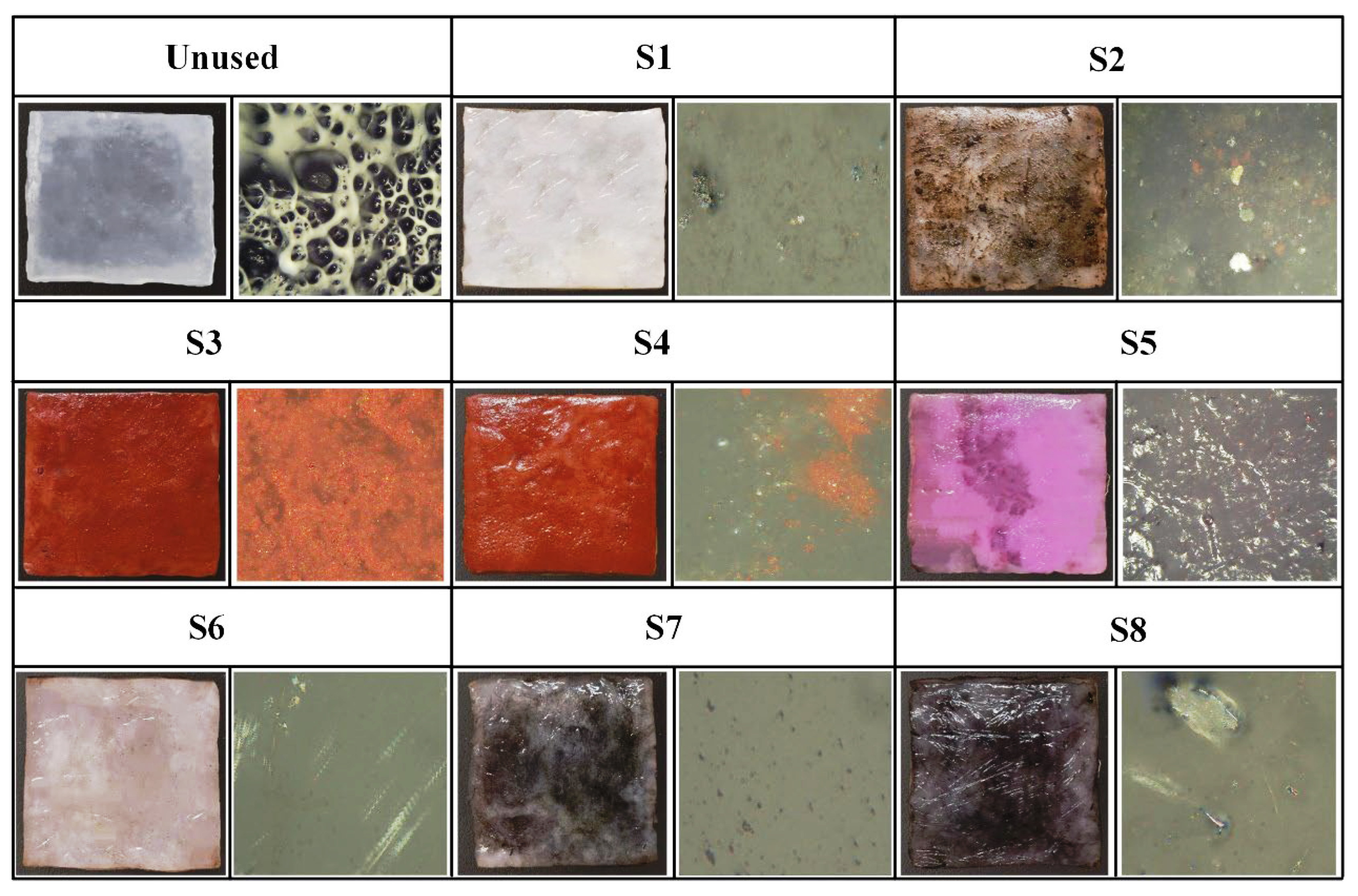
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Wv | R | 63.27 | 49.84 | 23.68 | 29.89 | 34.46 | 69.96 | 21.17 | 22.69 | |
| R1 | Gb | 68.94 | 53.13 | 25.43 | 31.64 | 41.75 | 88.76 | 23.36 | 23.68 | |
| G | 73.23 | 58.45 | 27.26 | 33.66 | 46.35 | 87.27 | 28.59 | 25.94 | ||
| Ue | 60.44 | 44.99 | 20.21 | 23.05 | 73.54 | 86.88 | 47.69 | 44.91 | ||
| G+U | 84.08 | 78.46 | 52.72 | 56.91 | 82.21 | 85.74 | 48.82 | 46.24 | ||
| CD | Gb | 5.67 | 3.29 | 1.75 | 1.77 | 7.29 | −1.2 | 2.19 | 0.99 | |
| G | 9.96 | 8.61 | 3.58 | 3.79 | 11.89 | −2.69 | 7.42 | 3.25 | ||
| Ue | −2.83 | −4.85 | −3.47 | −6.84 | 39.08 | −3.08 | 26.52 | 22.22 | ||
| G+U | 20.81 | 28.62 | 29.04 | 27.02 | 47.75 | −4.22 | 27.65 | 23.55 | ||
| CR, % | Gb | 26.06 | 9.35 | 2.85 | 3.21 | 14.42 | 24.34 | 3.43 | 1.59 | |
| G | 45.77 | 24.47 | 5.84 | 6.87 | 23.51 | 54.56 | 11.62 | 5.21 | ||
| Ue | −13.01 | −13.78 | −5.66 | −12.40 | 77.28 | 62.47 | 41.53 | 35.64 | ||
| G+U | 95.63 | 81.33 | 47.33 | 49.00 | 94.42 | 85.60 | 43.30 | 37.78 | ||
| Yarn Direction | Stain Type | Breaking Strength (N) | Retention Rate of Breaking Strength (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ta | Tb | |||||||||
| Gb | G | Ue | G+U | Gb | G | Ue | G+U | |||
| Warp | S1 | 9.31 | 9.28 | 9.19 | 9.23 | 9.24 | 99.15 | 98.18 | 98.61 | 98.72 |
| S2 | 9.27 | 9.26 | 9.27 | 9.26 | 9.25 | 98.93 | 99.04 | 98.93 | 98.82 | |
| S3 | 9.29 | 9.27 | 9.25 | 9.28 | 9.26 | 99.04 | 98.82 | 99.15 | 98.93 | |
| S4 | 9.15 | 9.16 | 9.22 | 9.21 | 9.27 | 97.86 | 98.50 | 98.40 | 99.04 | |
| S5 | 9.23 | 9.21 | 9.18 | 9.17 | 9.18 | 98.40 | 98.08 | 97.97 | 98.08 | |
| S6 | 9.28 | 9.19 | 9.18 | 9.26 | 9.16 | 98.18 | 98.08 | 98.93 | 97.86 | |
| S7 | 9.29 | 9.26 | 9.22 | 9.27 | 9.23 | 98.93 | 98.50 | 99.04 | 98.61 | |
| S8 | 9.14 | 9.17 | 9.27 | 9.21 | 9.16 | 97.97 | 99.04 | 98.40 | 97.86 | |
| Weft | S1 | 8.69 | 8.65 | 8.59 | 8.66 | 8.29 | 98.41 | 97.72 | 98.52 | 94.31 |
| S2 | 8.58 | 8.57 | 8.56 | 8.58 | 8.56 | 97.49 | 97.38 | 97.61 | 97.38 | |
| S3 | 8.82 | 8.75 | 8.79 | 8.58 | 8.76 | 99.54 | 100 | 97.61 | 99.65 | |
| S4 | 8.47 | 8.48 | 8.43 | 8.46 | 8.45 | 96.47 | 95.90 | 96.24 | 96.13 | |
| S5 | 8.76 | 8.73 | 8.74 | 8.74 | 8.73 | 99.32 | 99.43 | 99.43 | 99.32 | |
| S6 | 8.77 | 8.74 | 8.71 | 8.75 | 8.72 | 99.43 | 99.09 | 99.54 | 99.20 | |
| S7 | 8.73 | 8.69 | 8.59 | 8.75 | 8.71 | 98.86 | 97.72 | 99.54 | 99.09 | |
| S8 | 8.67 | 8.68 | 8.73 | 8.29 | 8.65 | 98.75 | 99.32 | 94.31 | 98.41 | |
| Stage | Aged Steps | Process Diagram | |
|---|---|---|---|
| Aged stage | First, fabric was cut into standard 5 cm × 5 cm swatches. These swatches were immersed in a 1 mol/L hydrochloric acid solution at a liquor ratio of 1:30 for 7 h, with agitation every hour. After 7 h, the samples were removed, rinsed with deionized water until a neutral pH (pH = 7) was achieved, and air-dried at room temperature in the shade. Then, moderately aged samples with tensile strength retention rates of 10–20% were obtained. |  | |
| Stain contamination stage | Stain type | Stain contamination steps | Process diagram |
| Sand stain (S1) | First, the artificially aged samples were laid flat on the workbench and treated with deionized water to achieve a moist state with a 20% moisture content. Secondly, sandy soil was uniformly sprinkled onto the surface of the moist sample at a mass ratio of 10:1 (sandy soil to textile). To ensure even distribution of the sandy soil, a spatula was used to gently spread and press it. Finally, the moist samples with absorbed sandy soil were air-dried at room temperature for 48 h to allow the stains to fully adhere, yielding substitute samples of textile relics with adsorbed sand stains. |  | |
| Clay stain (S2) | First, the artificially aged samples were placed flat on the workbench and treated with deionized water to achieve a moist state with a 20% moisture content. Secondly, clay (passed through a 16-mesh sieve) was uniformly sprinkled onto the surface of the moist samples at a mass ratio of 10:1 (clay to textile). To ensure even distribution, a spatula was used to gently spread and press the clay. Finally, the moist samples with absorbed clay were left to stand at room temperature for 48 h to ensure full adhesion of the stains, thus obtaining substitute samples of textile relics with adsorbed clay stains. | 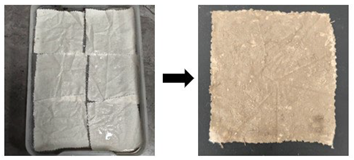 | |
| Rust stain (S3) | First, artificially aged samples were placed flat on the workbench and treated with deionized water to achieve a moist state with a 20% moisture content. Next, iron rust was uniformly sprinkled onto the surface of the moist samples at a mass ratio of 10:1 (clay to textile). To ensure even distribution of the clay, a spatula was used to gently spread and press it. Finally, the moist samples with absorbed iron rust were left at room temperature for 48 h. Deionized water was sprayed every 12 h to ensure full adhesion of the stains, thus obtaining substitute samples of textile relics with adsorbed iron rust stains. |  | |
| Mixed solid stain (S4) | First, artificially aged samples were placed flat on the workbench and treated with deionized water to achieve a moist state with a 20% moisture content. Next, the mixed solids (4 sand/4 clay/2 rust) were uniformly sprinkled onto the surface of the moist samples at a mass ratio of 10:1 (mixed solids to textile). To ensure even distribution of the mixed solids, a spatula was used to gently spread and press them. Finally, the moist samples with absorbed mixed solids were left to stand at room temperature for 48 h. Deionized water was sprayed every 12 h to ensure sufficient adhesion of the stains, thus obtaining substitute textile relic samples with adsorbed mixed solid stains. | 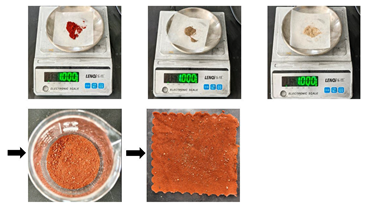 | |
| Blood stain (S5) | First, aged samples were placed flat on a horizontal workbench. Then, a rubber-tipped dropper was used to evenly apply 1 mL of the blood stain solution (diluted at a 1:1 ratio with deionized water and artificial blood) onto the sample surface. Finally, the samples were left to absorb the blood stains at room temperature for 48 h, thus obtaining a substitute sample of textile relics with adsorbed blood stains. |  | |
| Oil stain (S6) | First, aged samples were placed flat on a horizontal workbench. Then, a rubber-tipped dropper was used to evenly apply 1 mL of lard onto the sample surface. Finally, the samples were left to absorb the lard at room temperature for 48 h, thus obtaining a substitute sample of textile relics with adsorbed oil stains. |  | |
| Ink stain (S7) | First, aged samples were placed flat on a horizontal workbench. Then, a rubber-tipped dropper was used to evenly apply 1 mL of the ink-stained solution (diluted at a 5:1 ratio of deionized water to ink) onto the sample’s surface. Finally, the samples were allowed to absorb the ink at room temperature for 48 h to obtain a substitute sample of textile relics with adsorbed ink stains. |  | |
| Mixed liquid stain (S8) | First, aged samples were placed into a mixed liquid stain solution prepared at a 1:3 ratio of deionized water to a blend of blood, ink, and lard (1:1:1). The samples were submerged for 30 min, then removed and air-dried at room temperature for 48 h. This yielded substitute samples of textile relics with adsorbed mixed liquid stains. | 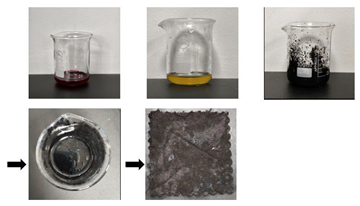 | |
| Cleaning Method | Description of Specific Cleaning Methods |
|---|---|
| Gb | Firstly, add 10% PVA powder to deionized water, stir and heat to 90 °C, and continue stirring until completely dissolved (10 min). Then, stop heating and cool to room temperature (30 min). Slowly drip 1% borax solution into PVA solution to form a precursor gel. Additionally, to ensure sufficient cross-linking and curing, place the obtained precursor gel in a freezer for 3 h, and then remove it to thaw at room temperature, thus obtaining a blank gel. Apply the obtained blank gel directly onto the surface of the contaminated textiles; then, place a 5 N weight on top of the textiles and maintain it for 10 min to ensure uniform adhesion to the stained textiles. Use tweezers to peel off the gel, repeating the pressing and peeling actions 10–15 times until the blank gel loses its adhesion to the stains. Furthermore, to enhance the gel’s adhesiveness during the cleaning process, each time gel is peeled off, use a garment steamer to spray steam for 10 s from a distance of 10 cm to soften the gel.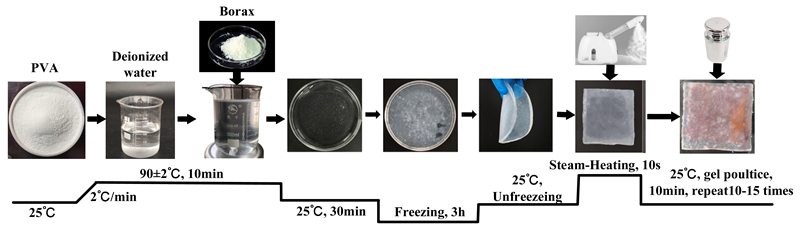 |
| G | Firstly, add 10% PVA powder to deionized water, stir and heat to 90 °C, and continue stirring until completely dissolved (10 min). Then, stop heating and cool to room temperature (30 min). Then, add the cleaning agent at a mass ratio of 1 (cleaning agent):20 (contaminated fabric), stir for 5 min, and slowly drip a 1% borax solution to form a precursor gel. Additionally, to ensure sufficient cross-linking and curing, place the obtained precursor gel in a freezer for 3 h, and then remove it to thaw at room temperature, thus obtaining a cleaning gel. Apply the obtained cleaning gel directly onto the surface of the contaminated textiles; then, place a 5 N weight on top of the textiles and maintain it for 10 min to ensure uniform adhesion to the stained textiles. Use tweezers to peel off the gel, repeating the pressing and peeling actions 10–15 times until the cleaning gel loses its adhesion to the stains. Furthermore, to enhance the gel’s adhesiveness during the cleaning process, each time gel is peeled off, use a garment steamer to spray steam for 10 s from a distance of 10 cm to soften the gel.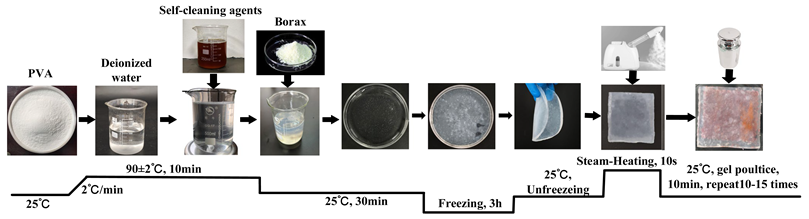 |
| Ue | Firstly, prepare a cleaning solution at a mass ratio of 1 (detergent):20 (deionized water), and add the solution to emulsification equipment via an infusion pump, forming a cleaning unit with the soiled cloth placed in the cleaning tank. Then, perform the cleaning using an ultrasonic vibrating brush. During the process, place a superabsorbent polymer pad under the stained textile to absorb wastewater, preventing backflow and recontamination. Set the ultrasonic vibration frequency to 20,000 Hz and maintain a reciprocating rate of 30 cycles/min for 10 min to complete the treatment.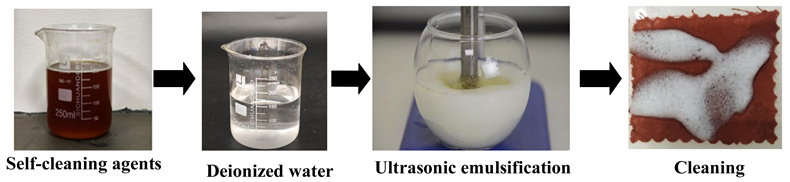 |
| G+U | First, use the cleaning gel poultice cleaning method to remove loose stains from the surface of the stained textiles. Then, perform the improved ultrasonic emulsification cleaning treatment to perform emulsification cleaning for slits, interior areas, and stubborn stains. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Guo, J.; Su, Z.; Yu, K.; Ling, X.; Zhang, Z.; Liu, K.; Pan, W. Development of Efficient In-Situ Cleaning Methods for Stained Textile Relics. Gels 2025, 11, 830. https://doi.org/10.3390/gels11100830
Wei Y, Guo J, Su Z, Yu K, Ling X, Zhang Z, Liu K, Pan W. Development of Efficient In-Situ Cleaning Methods for Stained Textile Relics. Gels. 2025; 11(10):830. https://doi.org/10.3390/gels11100830
Chicago/Turabian StyleWei, Yuhui, Jinxia Guo, Zhaowei Su, Kui Yu, Xue Ling, Zhenlin Zhang, Kaixuan Liu, and Wei Pan. 2025. "Development of Efficient In-Situ Cleaning Methods for Stained Textile Relics" Gels 11, no. 10: 830. https://doi.org/10.3390/gels11100830
APA StyleWei, Y., Guo, J., Su, Z., Yu, K., Ling, X., Zhang, Z., Liu, K., & Pan, W. (2025). Development of Efficient In-Situ Cleaning Methods for Stained Textile Relics. Gels, 11(10), 830. https://doi.org/10.3390/gels11100830










