The Potential of Dosimetry and the Visualization of Microbeam Arrays in NIPAM Gel at the PETRA III Synchrotron
Abstract
1. Introduction
2. Results and Discussion
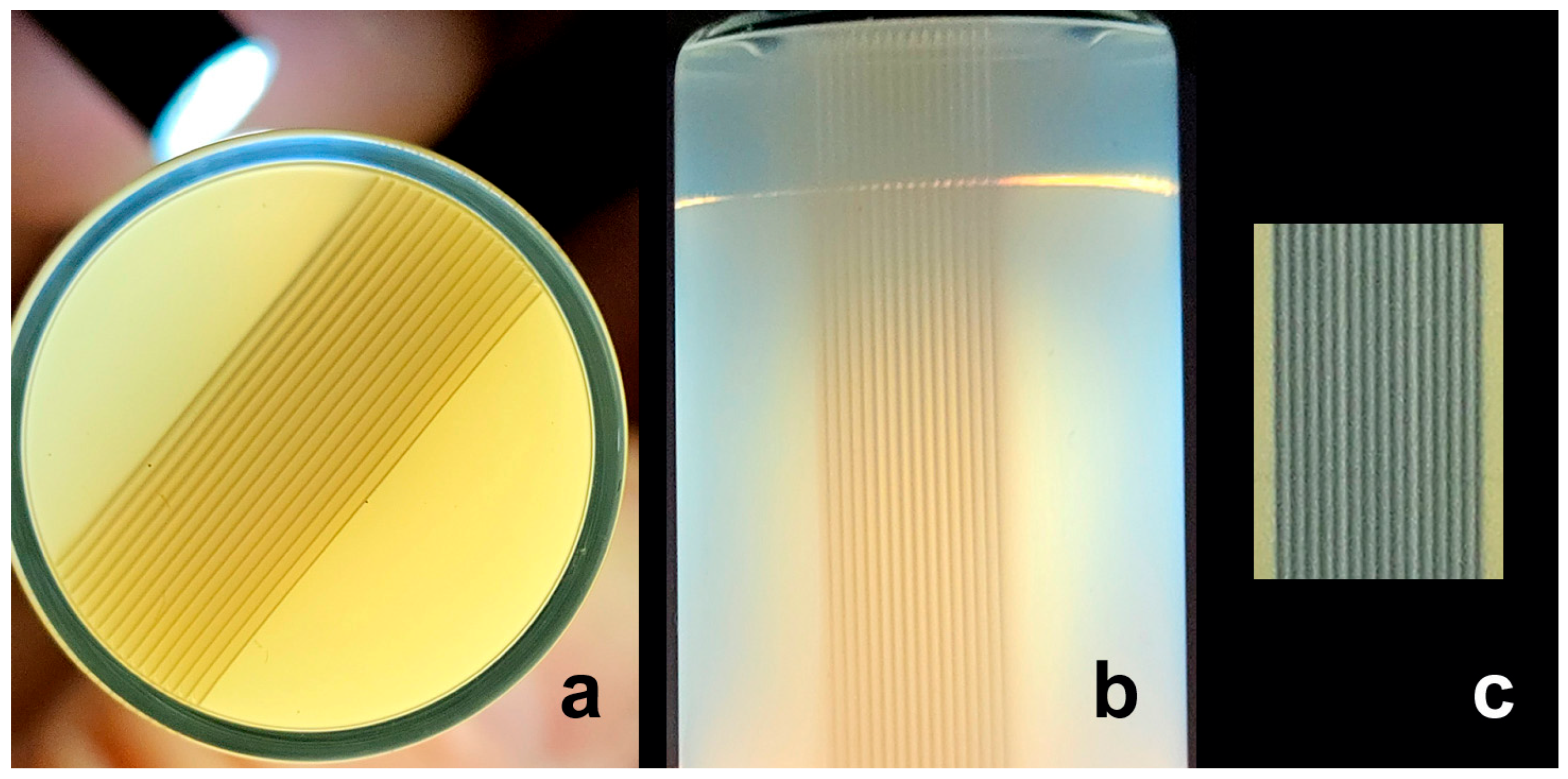
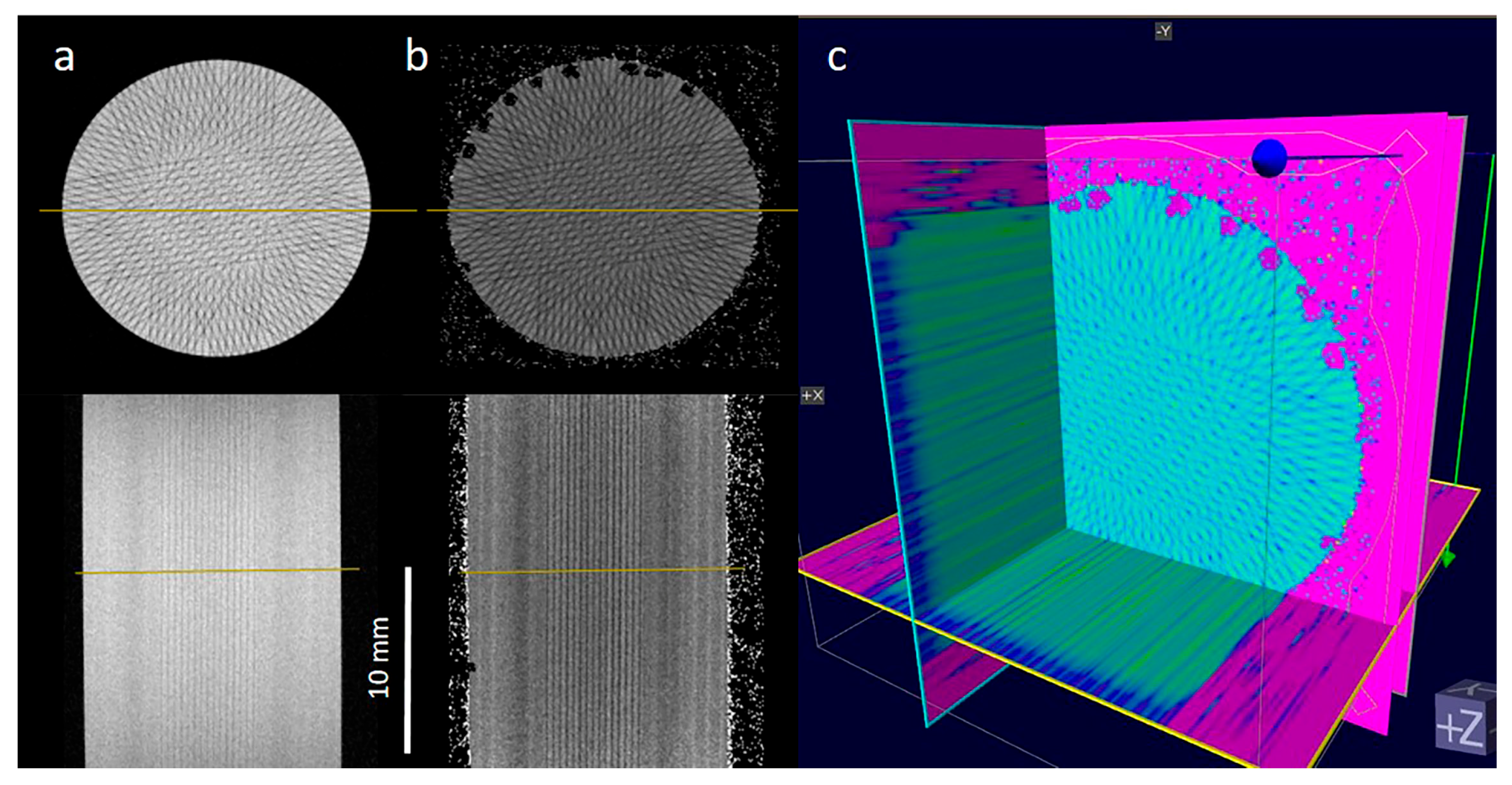
2.1. Recording the Geometry of Microbeam Irradiation Patterns in NIPAM Gel
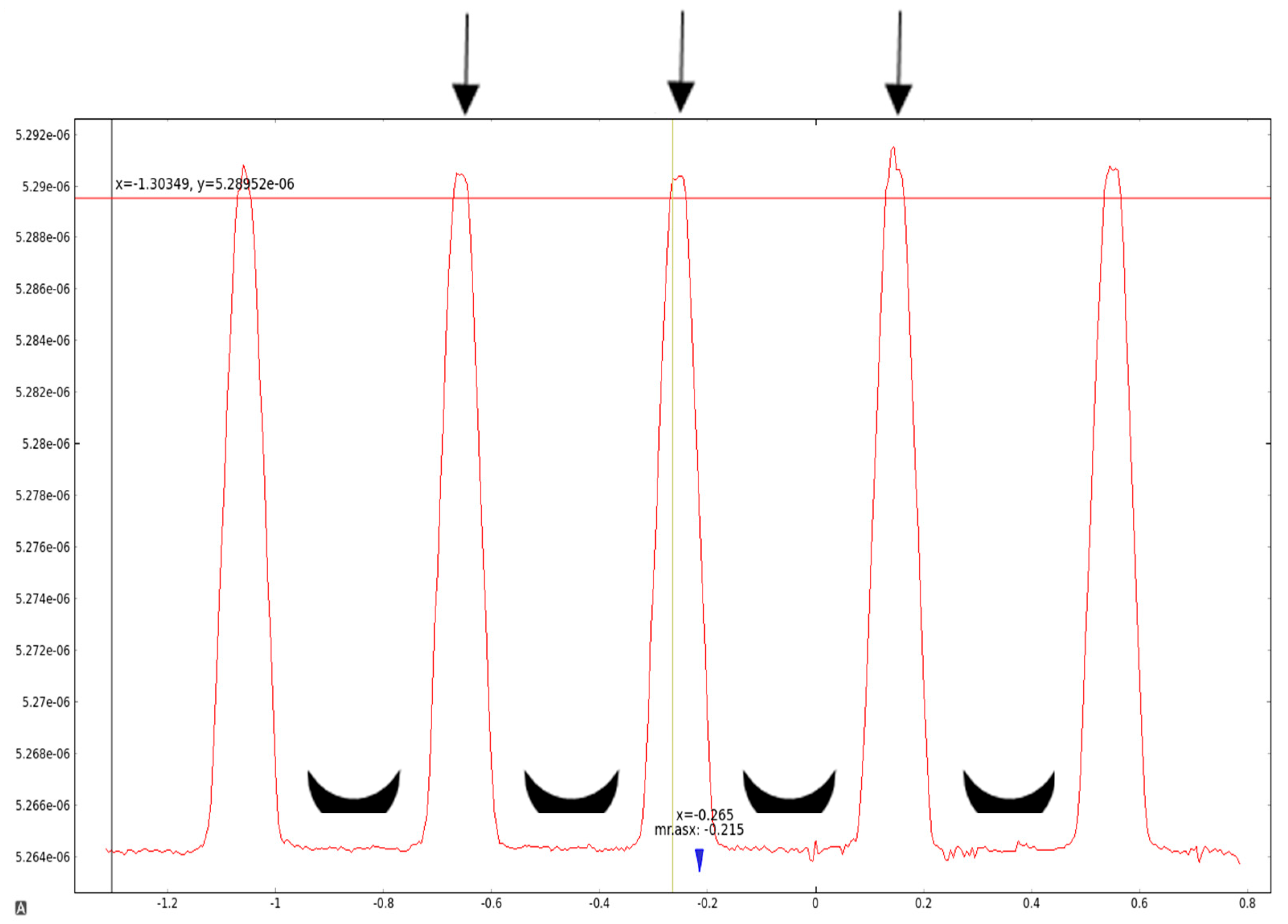
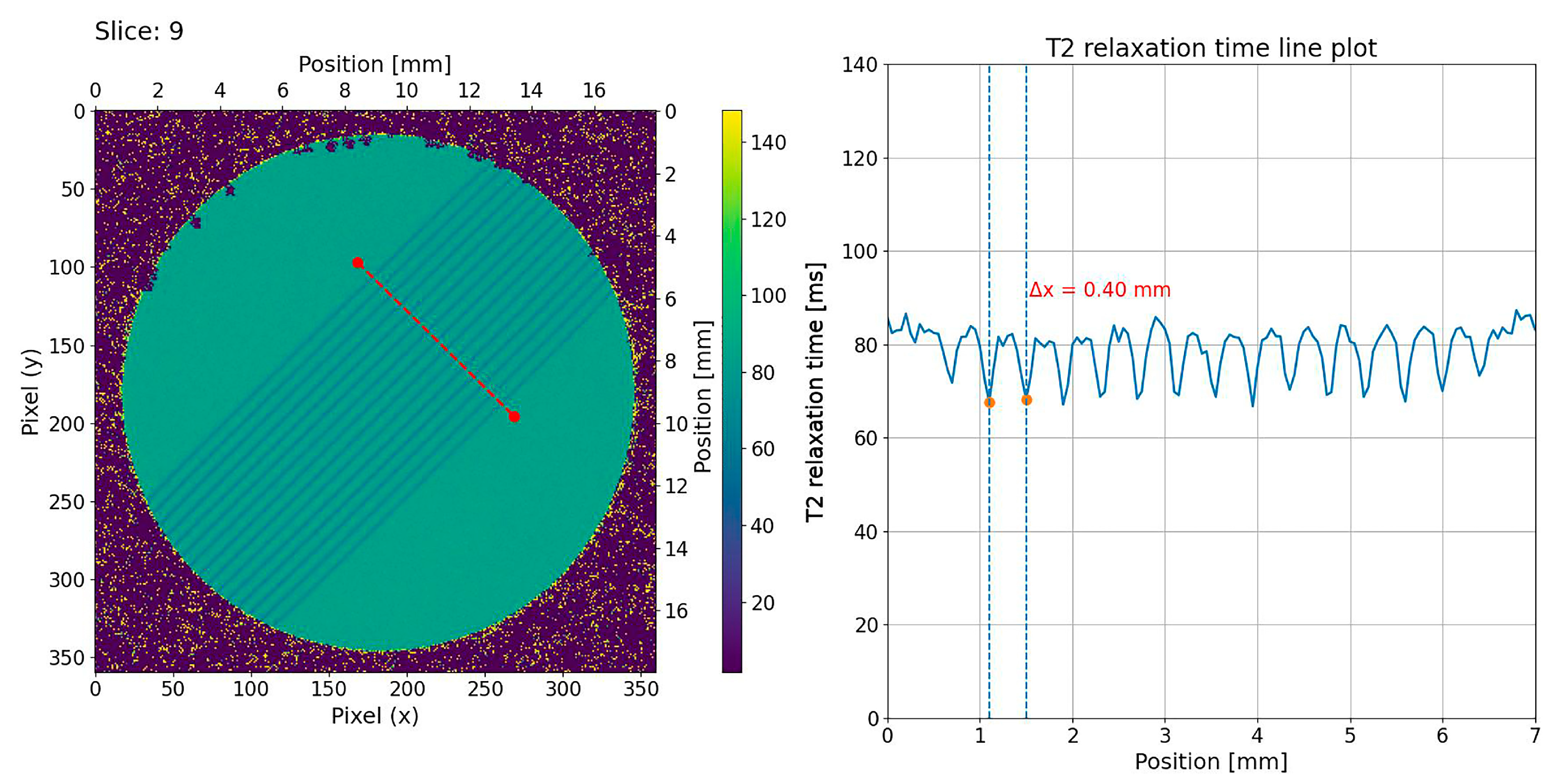
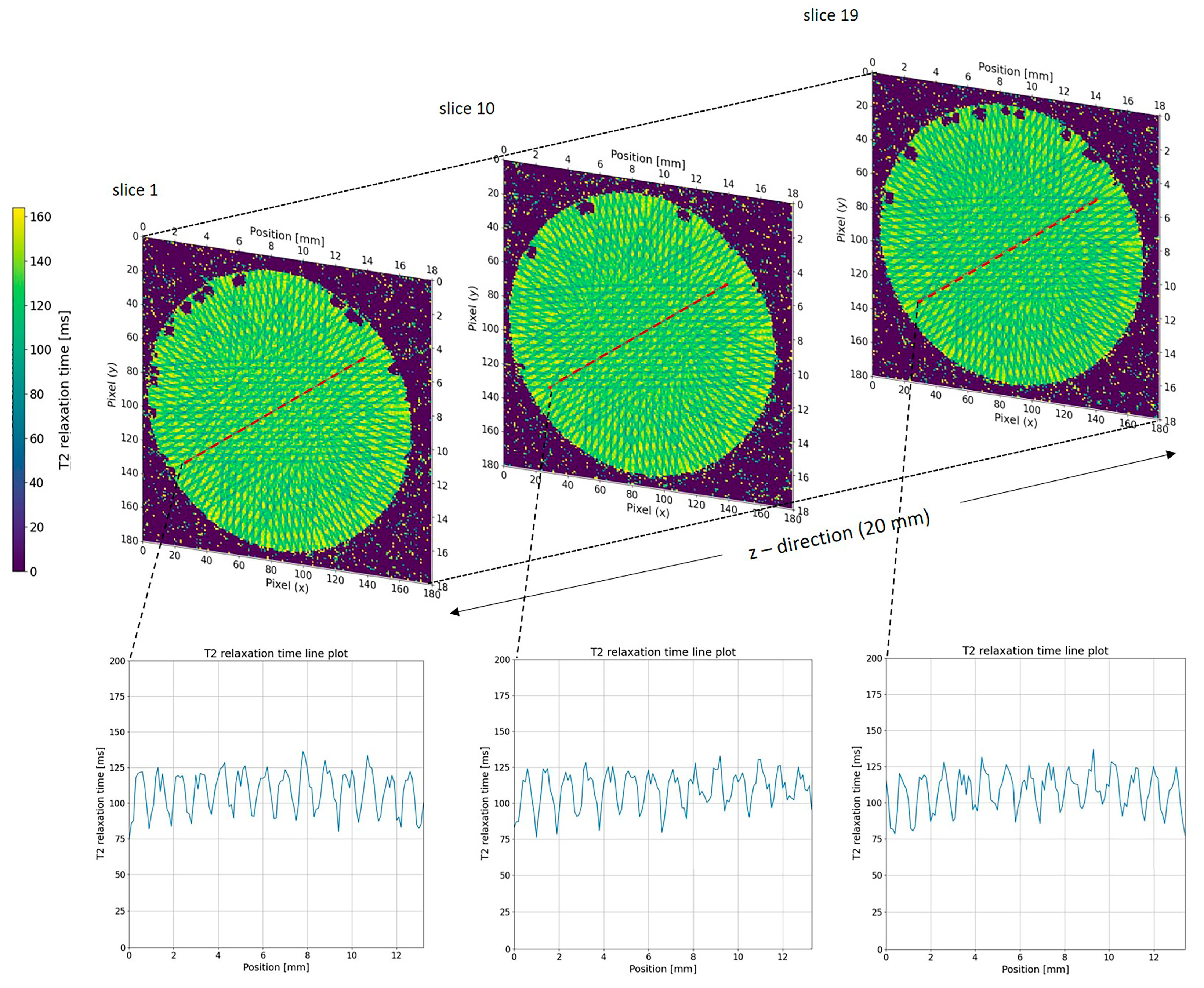
2.2. MRI Analysis of NIPAM Gel Irradiated with Microbeams
3. Conclusions
4. Materials and Methods
4.1. NIPAM Gel Production
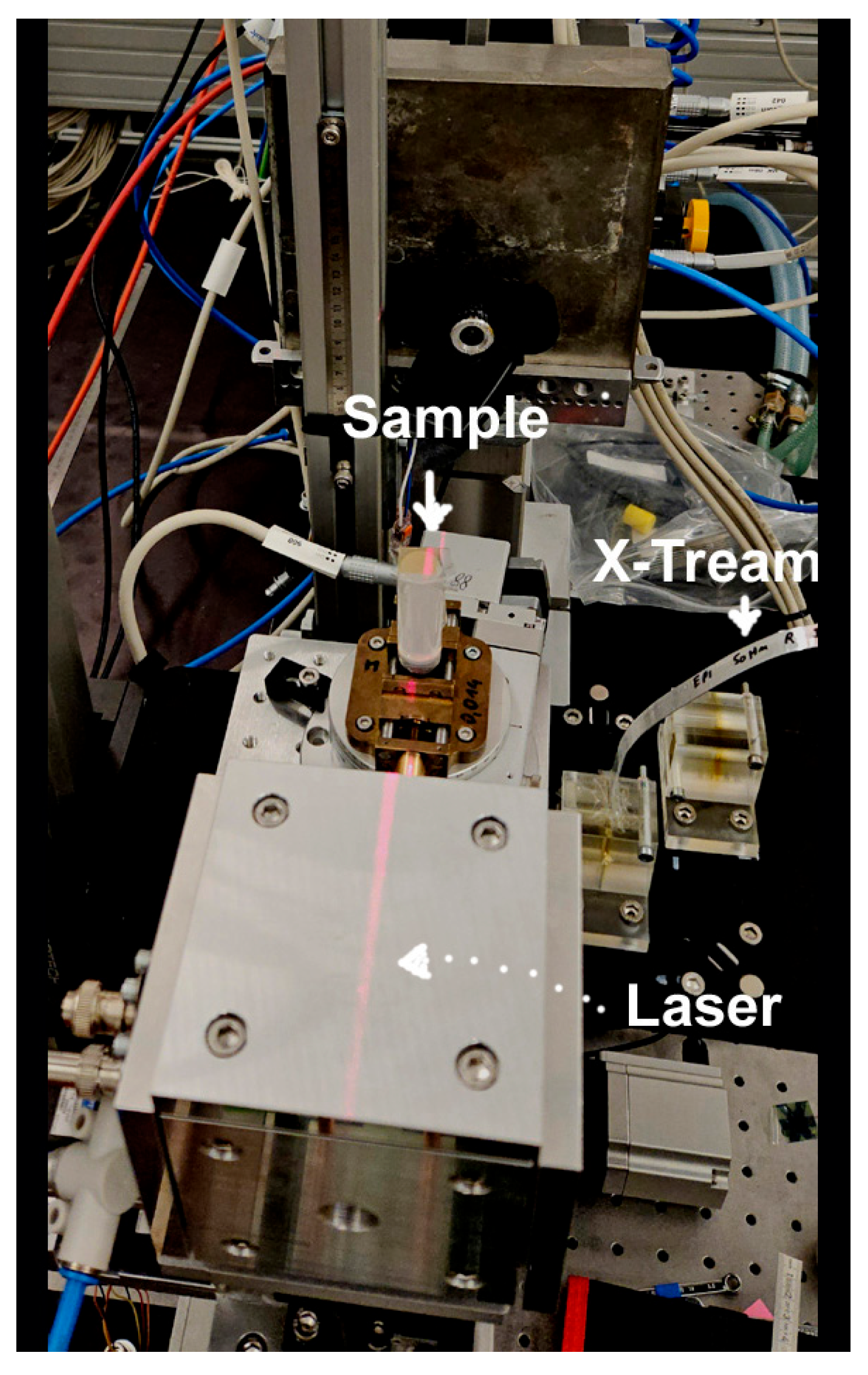
4.2. Microbeam Irradiation and Dosimetry
4.3. Magnetic Resonance Imaging of Gels After Irradiation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| MRT | Microbeam radiotherapy |
| NIPAM | N-isopropyl acrylamide |
| SFRT | Spatially fractionated radiotherapy |
| SNR | Signal-to-noise ratio |
| SRS | Stereotactic Radio Surgery |
| TE | Echo Time |
| TR | Repetition Time |
References
- Two Win Nobel for Immune Regulation Discoveries. Cancer Discov. 2018, 8, 1338–1339. [CrossRef]
- Chowdhury, P.S.; Chamoto, K.; Honjo, T. Combination therapy strategies for improving PD-1 blockade efficacy: A new era in cancer immunotherapy. J. Intern. Med. 2018, 283, 110–120. [Google Scholar] [CrossRef]
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef]
- Eling, L.; Kefs, S.; Keshmiri, S.; Balosso, J.; Calvet, S.; Chamel, G.; Drevon-Gaud, R.; Flandin, I.; Gaudin, M.; Giraud, L.; et al. Neuro-Oncologic Veterinary Trial for the Clinical Transfer of Microbeam Radiation Therapy: Acute to Subacute Radiotolerance after Brain Tumor Irradiation in Pet Dogs. Cancers 2024, 16, 2701. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.-F.; Balosso, J.; Bayat, S.; Berkvens, P.; Berruyer, G.; Bräuer-Krisch, E.; Brochard, T.; Chamel, G.; Desagneaux, A.; Drevon-Gaud, R.; et al. Toward Neuro-Oncologic Clinical Trials of High-Dose-Rate Synchrotron Microbeam Radiation Therapy: First Treatment of a Spontaneous Canine Brain Tumor. Int. J. Radiat. Oncol. 2022, 113, 967–973. [Google Scholar] [CrossRef]
- Winter, J.; Dimroth, A.; Roetzer, S.; Zhang, Y.; Krämer, K.; Petrich, C.; Matejcek, C.; Aulenbacher, K.; Zimmermann, M.; Combs, S.E.; et al. Heat management of a compact x-ray source for microbeam radiotherapy and FLASH treatments. Med. Phys. 2022, 49, 3375–3388. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Galek, M.; Matejcek, C.; Wilkens, J.J.; Aulenbacher, K.; Combs, S.E.; Bartzsch, S. Clinical microbeam radiation therapy with a compact source: Specifications of the line-focus X-ray tube. Phys. Imaging Radiat. Oncol. 2020, 14, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bartzsch, S.; Oelfke, U. Line focus X-ray tubes—A new concept to produce high brilliance X-rays. Phys. Med. Biol. 2017, 62, 8600. [Google Scholar] [CrossRef]
- Bouchet, A.; Bräuer-Krisch, E.; Prezado, Y.; El Atifi, M.; Rogalev, L.; Le Clec’h, C.; Laissue, J.A.; Pelletier, L.; Le Duc, G. Better Efficacy of Synchrotron Spatially Microfractionated Radiation Therapy Than Uniform Radiation Therapy on Glioma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 1485–1494. [Google Scholar] [CrossRef]
- Steel, H.; Brüningk, S.C.; Box, C.; Oelfke, U.; Bartzsch, S.H. Quantification of Differential Response of Tumour and Normal Cells to Microbeam Radiation in the Absence of FLASH Effects. Cancers 2021, 13, 3238. [Google Scholar] [CrossRef]
- Archer, J.; Li, E.; Davis, J.; Cameron, M.; Rosenfeld, A.; Lerch, M. High spatial resolution scintillator dosimetry of synchrotron microbeams. Sci. Rep. 2018, 9, 6873. [Google Scholar] [CrossRef]
- Davis, J.A.; Petasecca, M.; Cullen, A.; Paino, J.R.; Archer, J.; Rosenfeld, A.B.; Lerch, M.L.F. X-Tream dosimetry of synchrotron radiation with the PTW microDiamond. J. Instrum. 2019, 14, P10037. [Google Scholar] [CrossRef]
- Petasecca, M.; Cullen, A.; Fuduli, I.; Espinoza, A.; Porumb, C.; Stanton, C.; Aldosari, A.H.; Bräuer-Krisch, E.; Requardt, H.; Bravin, A.; et al. X-Tream: A novel dosimetry system for Synchrotron Microbeam Radiation Therapy. J Synchrotron Radiat. 2022, 29 Pt 1, 125–137. [Google Scholar] [CrossRef]
- Fournier, P.; Cornelius, I.; Donzelli, M.; Requardt, H.; Nemoz, C.; Petasecca, M.; Bräuer-Krisch, E.; Rosenfeld, A.; Lerch, M. X-Tream quality assurance in synchrotron X-ray microbeam radiation therapy. J. Synchrotron. Radiat. 2016, 23 Pt 5, 1180–1190. [Google Scholar] [CrossRef]
- Pellicioli, P.; Bartzsch, S.; Donzelli, M.; Krisch, M.; Bräuer-Krisch, E. High resolution radiochromic film dosimetry: Comparison of a microdensitometer and an optical microscope. Phys. Med. 2019, 65, 106–113. [Google Scholar] [CrossRef]
- Hombrink, G.; Wilkens, J.J.; Combs, S.E.; Bartzsch, S. Simulation and measurement of microbeam dose distribution in lung tissue. Phys. Med. 2020, 75, 77–82. [Google Scholar] [CrossRef]
- Bartzsch, S.; Lott, J.; Welsch, K.; Bräuer-Krisch, E.; Oelfke, U. Micrometer-resolved film dosimetry using a microscope in microbeam radiation therapy. Med Phys. 2015, 42, 4069–4079. [Google Scholar] [CrossRef]
- McErlean, C.M.; Bräuer-Krisch, E.; Adamovics, J.; Doran, S.J. Assessment of optical CT as a future QA tool for synchrotron x-ray microbeam therapy. Phys. Med. Biol. 2016, 61, 320–337. [Google Scholar] [CrossRef]
- Gagliardi, F.M.; Cornelius, I.; Blencowe, A.; Franich, R.D.; Geso, M. High resolution 3D imaging of synchrotron generated microbeams. Med. Phys. 2015, 42, 6973–6986. [Google Scholar] [CrossRef] [PubMed]
- Sagsoz, M.E.; Korkut, O.; Gallo, S. Advancements in Tissue-Equivalent Gel Dosimeters. Gels 2025, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1. [Google Scholar] [CrossRef]
- Day, M.J.; Stein, G. Chemical effects of ionizing radiation in some gels. Nature 1950, 166, 146147. [Google Scholar] [CrossRef]
- De Deene, Y. Radiation Dosimetry by Use of Radiosensitive hydrogels and polymers: Mechanisms, state-of-the-art and perspective from 3D to 4D. Gels 2022, 19, 599. [Google Scholar] [CrossRef]
- El Sayed, M.M. Production of polymer hydrogel composites and their applications. J Polym. Environ. 2023, 31, 2855–2879. [Google Scholar] [CrossRef]
- Ceberg, S.; Gagne, I.; Gustafsson, H.; Scherman, J.B.; Korreman, S.S.; Kjaer-Kristoffersen, F.; Hilts, M.; Bäck, S.Å.J. RapidArc treatment verification in 3D using polymer gel dosimetry and Monte Carlo simulation. Phys. Med. Biol. 2010, 55, 4885–4898. [Google Scholar] [CrossRef]
- Senden, R.J.; De, J.P.; McAuley, K.B.; Schreiner, L.J. Polymer gel dosimeters with reduced toxicity: A preliminary investigation of the NMR and optical dose-response using different monomers. Phys. Med. Biol. 2006, 51, 3301–3314. [Google Scholar] [CrossRef]
- Farajollahi, A.; Pak, F.; Horsfield, M.; Myabi, Z. The Basic Radiation Properties of the N-Isopropylacrylamide Based Polymer Gel Dosimeter. Int. J. Rad. Res. 2014, 12, 347–354. [Google Scholar]
- Cheng, K.Y.; Hsieh, L.L.; Shih, C.T. A Comprehensive Evaluation of NIPAM Polymer Gel Dosimeters on Three Orthogonal Planes and Temporal Stability Analysis. PLoS ONE 2016, 11, e0155797. [Google Scholar] [CrossRef] [PubMed]
- Kunkyab, T.; Lakrad, K.; Jirasek, A.; Oldham, M.; Quinn, B.; Hyde, D.; Adamson, J. Clinical applicability of Linac-integrated CBCT based NIPAM 3D dosimetry: A dual-institutional investigation. Phys. Med. Biol. 2024, 69, 155002. [Google Scholar] [CrossRef]
- Waldenberg, C.; Karlsson Hauer, A.; Gustafsson, C.; Ceberg, S. Dose integration and dose rate characteristics of a NiPAM polymer gel MRI dosimeter system. J. Phys. Conf. Ser. 2017, 847, 012063. [Google Scholar] [CrossRef]
- Pak, F.; Farajollahi, A.; Movafaghi, A.; Naseri, A. Influencing Factors on Reproducibility and Stability of MRI NIPAM Polymer Gel Dosimeter. BioImpacts BI 2013, 3, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Dale, R.G.; Finst, P.; Khaksar, S.J. Biological equivalent dose assessment of the consequences of hypofractionated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Fiedler, S.; Mewes, C.; Gargioni, E.; Klingenberg, J.; Abreu Faria, G.; Lerch, M.; Petasecca, M.; Prehn, F.; Wegner, M.; et al. The Microbeam Insert at the White Beam Beamline P61A at the Synchrotron PETRA III/DESY: A New Tool for High Dose Rate Irradiation Research. Cancers 2022, 14, 5137. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, M.; Felici, G.; Galante, F.; Gasparini, A.; Giuliano, L.; Heinrich, S.; Pacitti, M.; Prestopino, G.; Vanreusel, V.; Verellen, D.; et al. Design, realization, and characterization of a novel diamond detector prototype for FLASH radiotherapy dosimetry. Med. Phys. 2022, 49, 1902–1910. [Google Scholar] [CrossRef]
- Bräuer-Krisch, E.; Requardt, H.; Brochard, T.; Berruyer, G.; Renier, M.; Laissue, J.A.; Bravin, A. New technology enables high precision multislit collimators for microbeam radiation therapy. Rev. Sci. Instrum. 2009, 80, 74301. [Google Scholar] [CrossRef]



| Sequence | Component | Amount | Temperature |
| 1 | H2O, de-ionized | 300 mL | ambient to |
| 2 | Gelatin | 17 g | 45 °C |
| 3 | NIPAM | 10 g | 45 °C |
| 4 | BIS | 10 g | 45 °C |
| 5 | THPC | 1 mL | 38 °C |
| Sequence Name | Repetition Time (TR) | Echo Time (TE) | Field of View (FoV) | Matrix | Resolution | Slice Number/Slice Thickness | Acq. Time |
| T2-Map-MSME axial | 2635 ms | 12 Echos, spacing 7.5 ms (7.5–0 ms) | 18 × 18 mm | 180 × 180 | 100 × 100 µm | 20/1 mm | 31:37 min:s (4 Averages) |
| T2-Map-MSME axial (higher resolution) | 2812 ms | 12 Echos, spacing 10 ms (10–180 ms) | 18 × 18 mm | 360 × 360 | 50 × 50 µm | 20/1 mm | 8:26 hrs:s (30 Averages) |
| T2-Map-MSME frontal | 3375.8 ms | 12 Echos, spacing 15 ms (15–180 ms) | 40 × 20 mm | 180 × 180 | 100 × 100 µm | 17/1 mm | 31:37 min:s (4 Averages) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breslin, T.; Kügele, M.; de Rover, V.; Fiedler, S.; Lindner, T.; Klingenberg, J.; Faria, G.A.; Frerker, B.; Nuesken, F.; Ceberg, S.; et al. The Potential of Dosimetry and the Visualization of Microbeam Arrays in NIPAM Gel at the PETRA III Synchrotron. Gels 2025, 11, 814. https://doi.org/10.3390/gels11100814
Breslin T, Kügele M, de Rover V, Fiedler S, Lindner T, Klingenberg J, Faria GA, Frerker B, Nuesken F, Ceberg S, et al. The Potential of Dosimetry and the Visualization of Microbeam Arrays in NIPAM Gel at the PETRA III Synchrotron. Gels. 2025; 11(10):814. https://doi.org/10.3390/gels11100814
Chicago/Turabian StyleBreslin, Thomas, Malin Kügele, Vincent de Rover, Stefan Fiedler, Tobias Lindner, Johannes Klingenberg, Guilherme Abreu Faria, Bernd Frerker, Frank Nuesken, Sofie Ceberg, and et al. 2025. "The Potential of Dosimetry and the Visualization of Microbeam Arrays in NIPAM Gel at the PETRA III Synchrotron" Gels 11, no. 10: 814. https://doi.org/10.3390/gels11100814
APA StyleBreslin, T., Kügele, M., de Rover, V., Fiedler, S., Lindner, T., Klingenberg, J., Faria, G. A., Frerker, B., Nuesken, F., Ceberg, S., Ceberg, C., Lerch, M., Hildebrandt, G., & Schültke, E. (2025). The Potential of Dosimetry and the Visualization of Microbeam Arrays in NIPAM Gel at the PETRA III Synchrotron. Gels, 11(10), 814. https://doi.org/10.3390/gels11100814






