Cannabidiol Encapsulation in Polymeric Hydrogels and Its Controlled Release: A Review
Abstract
1. Introduction
2. Physicochemical Properties of CBD and Its Derivatives
2.1. Molecular Structure, Solubility, and Stability Issues
2.2. Interaction of CBD with Polymeric Materials
3. Polymeric Matrix Systems: Fundamentals and Classification
3.1. Natural vs. Synthetic Polymers
3.2. Biodegradable and Biocompatible Matrices
4. Encapsulation Techniques and Technologies
4.1. Encapsulation by Emulsion Solvent Evaporation
4.2. Encapsulation by Electrospinning Process
4.3. Supercritical Fluid Processing
5. CBD–Polymer Interactions: Mechanistic Insights
5.1. Chemical Bonding vs. Physical Entrapment
5.2. Controlled Release Behavior and Kinetics
5.3. Influence of Polymer Characteristics on Drug Loading and Release
6. Material Performance and Characterization Approaches
6.1. Morphology and Encapsulation Efficiency
6.2. Thermal Properties
6.3. Swelling Behavior, Degradation, and Release Profile
7. Challenges, Gaps, and Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faiz, M.B.; Naeem, F.; Irfan, M.; Aslam, M.A.; Estevinho, L.M.; Ateşşahin, D.A.; Alshahrani, A.M.; Calina, D.; Khan, K.; Sharifi-Rad, J. Exploring the therapeutic potential of cannabinoids in cancer by modulating signaling pathways and addressing clinical challenges. Discov. Oncol. 2024, 15, 490. [Google Scholar] [CrossRef]
- Shah, S.A.; Gupta, A.S.; Kumar, P. Emerging role of cannabinoids and synthetic cannabinoid receptor 1/cannabinoid receptor 2 receptor agonists in cancer treatment and chemotherapy-associated cancer management. J. Cancer Res. Ther. 2021, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Challa, S.K.R.; Misra, N.N.; Martynenko, A. Drying of cannabis—State of the practices and future needs. Dry. Technol. 2021, 39, 2055–2064. [Google Scholar] [CrossRef]
- Kuzumi, A.; Yoshizaki-Ogawa, A.; Fukasawa, T.; Sato, S.; Yoshizaki, A. The Potential Role of Cannabidiol in Cosmetic Dermatology: A Literature Review. Am. J. Clin. Dermatol. 2024, 25, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Nawfer, N.; Dharmawansa, K.S.; Redha, A.A.; Rupasinghe, H.V. Recent advances in cannabidiol (CBD) extraction: A review of potential eco-friendly solvents and advanced technologies. Green Anal. Chem. 2025, 13, 100270. [Google Scholar] [CrossRef]
- Pillai, S.K.; Kera, N.H.; Kleyi, P.; de Beer, M.; Magwaza, M.; Ray, S.S. Stability, biofunctional, and antimicrobial characteristics of cannabidiol isolate for the design of topical formulations. Soft Matter 2024, 20, 2348–2360. [Google Scholar] [CrossRef]
- Samara, E.; Bialer, M.; Mechoulam, R. Pharmacokinetics of cannabidiol in dogs. Drug Metab. Dispos. 1988, 16, 469–472. [Google Scholar] [CrossRef]
- Freire, N.F.; Cordani, M.; Aparicio-Blanco, J.; Sanchez, A.I.F.; Dutra, L.; Pinto, M.C.; Zarrabi, A.; Pinto, J.C.; Velasco, G.; Fialho, R. Preparation and characterization of PBS (Polybutylene Succinate) nanoparticles containing cannabidiol (CBD) for anticancer application. J. Drug Deliv. Sci. Technol. 2024, 97, 105833. [Google Scholar] [CrossRef]
- Ortiz-Romero, N.; Ochoa-Martínez, L.A.; González-Herrera, S.M.; Rutiaga-Quiñones, O.M.; Gallegos-Infante, J.A. Avances en las investigaciones sobre la encapsulación mediante gelación iónica: Una revisión sistemática. TecnoLógicas 2021, 24, e1962. [Google Scholar] [CrossRef]
- Barbosa-Nuñez, J.A.; Espinosa-Andrews, H.; Cardona, A.A.V.; Haro-González, J.N. Polymer-based encapsulation in food products: A comprehensive review of applications and advancements. J. Futur. Foods 2025, 5, 36–49. [Google Scholar] [CrossRef]
- Jose, M.S.; Sumathi, S. A review of electrospun polymeric fibers as potential drug delivery systems for tunable release kinetics. J. Sci. Adv. Mater. Devices 2025, 10, 100933. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Eagleston, L.R.M.; Kalani Yazd, N.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24, 24. [Google Scholar] [CrossRef]
- Brighenti, V.; Protti, M.; Anceschi, L.; Zanardi, C.; Mercolini, L.; Pellati, F. Emerging challenges in the extraction, analysis and bioanalysis of cannabidiol and related compounds. J. Pharm. Biomed. Anal. 2021, 192, 113633. [Google Scholar] [CrossRef]
- Tihăuan, B.–.M.; Onisei, T.; Slootweg, W.; Gună, D.; Iliescu, C.; Chifiriuc, M.C. Cannabidiol—A friend or a foe? Eur. J. Pharm. Sci. 2025, 208, 107036. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 1150, 122188. [Google Scholar] [CrossRef] [PubMed]
- Koryťáková, A.; Chatziadi, A.; Rohlíček, J.; Zmeškalová, E.; Beránek, J.; Šoóš, M. Stability study and structural insights into cannabidiol cocrystals. CrystEngComm 2025, 27, 2154–2165. [Google Scholar] [CrossRef]
- Kosović, E.; Sýkora, D.; Kuchař, M. Stability Study of Cannabidiol in the Form of Solid Powder and Sunflower Oil Solution. Pharmaceutics 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Bini, A.; Salerno, S.; Protti, S.; Pollastro, F.; Profumo, A.; Morini, L.; Merli, D. Photodegradation of cannabidiol (CBD) and Δ9-THC in cannabis plant material. Photochem. Photobiol. Sci. 2024, 23, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Jaidee, W.; Siridechakorn, I.; Nessopa, S.; Wisuitiprot, V.; Chaiwangrach, N.; Ingkaninan, K.; Waranuch, N. Kinetics of CBD, Δ9-THC Degradation and Cannabinol Formation in Cannabis Resin at Various Temperature and pH Conditions. Cannabis Cannabinoid Res. 2022, 7, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Buerhop, C.; Stroyuk, O.; Mashkov, O.; Barabash, A.; Hauch, J.; Peters, I. Polymer encapsulation impact on potential-induced degradation in PV modules revealed by a multi-modal field study. Sol. Energy Mater. Sol. Cells 2024, 277, 113111. [Google Scholar] [CrossRef]
- Yi, L.; Shi, L.; Móczó, J.; Pukánszky, B. Encapsulation of a drug into electrospun fibers spun from water soluble polymers to control solubility and release. Heliyon 2024, 10, e38935. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Sarnelli, S.; Palazzo, I.; Scognamiglio, M.; Reverchon, E. Production of cannabidiol nanoparticles loaded in polyvinylpyrrolidone microparticles by supercritical CO2 assisted atomization and dissolution enhancement effect. Adv. Powder Technol. 2025, 36, 104749. [Google Scholar] [CrossRef]
- Bhatia, S. Natural Polymers vs. Synthetic Polymer. In Natural Polymer Drug Delivery Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 95–118. [Google Scholar] [CrossRef]
- Rezazadeh, A.; Bazardeh, M.E.; Ghasempour, Z.; Kia, E.M. Gelatin/pectin complex coacervation for encapsulation of microwave-assisted extraction of bioactive compounds from red onion skin. Int. J. Biol. Macromol. 2025, 319, 145416. [Google Scholar] [CrossRef] [PubMed]
- Tripty, M.R.; Nupur, A.H.; Jany, J.F.; Toma, M.A.; Mazumder, A.R. Encapsulation of mango peel bioactive compounds in milk, gum acacia, and maltodextrin improves its stability. NFS J. 2025, 39, 100227. [Google Scholar] [CrossRef]
- Afra, S.; Koch, M.; Żur-Pińska, J.; Dolatshahi, M.; Bahrami, A.R.; Sayed, J.E.; Moradi, A.; Matin, M.M.; Włodarczyk-Biegun, M.K. Chitosan/Nanohydroxyapatite/Hydroxyethyl-cellulose-based printable formulations for local alendronate drug delivery in osteoporosis treatment. Carbohydr. Polym. Technol. Appl. 2024, 7, 100418. [Google Scholar] [CrossRef]
- Secerli, J.; Adatepe, Ş.; Altuntas, S.; Topal, G.R.; Erdem, O.; Bacanlı, M. In vitro toxicity of naringin and berberine alone, and encapsulated within PMMA nanoparticles. Toxicol. Vitr. 2023, 89, 105580. [Google Scholar] [CrossRef] [PubMed]
- Rezagholizade-Shirvan, A.; Soltani, M.; Shokri, S.; Radfar, R.; Arab, M.; Shamloo, E. Bioactive compound encapsulation: Characteristics, applications in food systems, and implications for human health. Food Chem. X 2024, 24, 101953. [Google Scholar] [CrossRef]
- Machtakova, M.; Thérien-Aubin, H.; Landfester, K. Polymer nano-systems for the encapsulation and delivery of active biomacromolecular therapeutic agents. Chem. Soc. Rev. 2021, 51, 128–152. [Google Scholar] [CrossRef]
- Rahul, P.B.; Tiwari, R.K.; Dash, K.K.; Sharma, M. Recent advances in encapsulation of pomegranate peel extract and combination of wall materials: A review of encapsulation technologies, characterization and applications in the food industry. Sustain. Food Technol. 2025, 3, 123–144. [Google Scholar] [CrossRef]
- Yuan, M.; Hu, M.; Dai, F.; Fan, Y.; Deng, Z.; Deng, H.; Cheng, Y. Application of synthetic and natural polymers in surgical mesh for pelvic floor reconstruction. Mater. Des. 2021, 209, 109984. [Google Scholar] [CrossRef]
- Suhas, P.; Mahesh, B.; Divakara, S.; Nanjundaswamy, G.; Prasad, C.M.; Sionkowska, A.; Popat, K.C.; Gowda, D.C. Synergistic approaches in natural and synthetic polymer blends for biomedical applications-A review. Eur. Polym. J. 2025, 236, 114161. [Google Scholar] [CrossRef]
- Zwicker, P.; Hornschuh, M.; Schmidt, T.; Schäfer, J.; Becker-Willinger, C.; Jochum, M.; Kramer, A.; Müller, G. A biocompatible polylactide-ε-caprolactone polymer coated with poly(hexamethylene biguanide) displays antibacterial properties against slime-producing S. epidermidis. Mater. Adv. 2025, 6, 2423–2434. [Google Scholar] [CrossRef]
- Moutinho, L.G.; Soares, E.; Oliveira, M. Biodegradability assessment of cork polymer composites for sustainable packaging applications. Next Mater. 2025, 8, 100904. [Google Scholar] [CrossRef]
- Gu, X.; Ding, F.; Yang, Y.; Liu, J. Tissue Engineering in Peripheral Nerve Regeneration. In Neural Regeneration; Academic Press: Cambridge, MA, USA, 2015; pp. 73–99. [Google Scholar] [CrossRef]
- Dai, L.; Zhan, X.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Composite zein—Propylene glycol alginate particles prepared using solvent evaporation: Characterization and application as Pickering emulsion stabilizers. Food Hydrocoll. 2018, 85, 281–290. [Google Scholar] [CrossRef]
- Kim, B.K.; Hwang, S.J.; Park, J.B.; Park, H.J. Preparation and characterization of drug-loaded polymethacrylate microspheres by an emulsion solvent evaporation method. J. Microencapsul. 2002, 19, 811–822. [Google Scholar] [CrossRef]
- Safari, H.; Felder, M.L.; Kaczorowski, N.; Eniola-Adefeso, O. Effect of the Emulsion Solvent Evaporation Technique Cosolvent Choice on the Loading Efficiency and Release Profile of Anti-CD47 from PLGA Nanospheres. J. Pharm. Sci. 2022, 111, 2525–2530. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Wang, S.; Wang, Y.; Liu, H.; Huo, X.; Ma, H.; Ma, Z.; Xiong, H. Microencapsulation of oxalic acid via oil-in-oil (O/O) emulsion solvent evaporation. Powder Technol. 2017, 320, 405–411. [Google Scholar] [CrossRef]
- David, C.; de Souza, J.F.; Silva, A.F.; Grazioli, G.; Barboza, A.S.; Lund, R.G.; Fajardo, A.R.; Moraes, R.R. Cannabidiol-loaded microparticles embedded in a porous hydrogel matrix for biomedical applications. J. Mater. Sci. Mater. Med. 2024, 35, 14. [Google Scholar] [CrossRef] [PubMed]
- Villate, A.; Barreto, G.P.; Nicolás, M.S.; Aizpurua-Olaizola, O.; Olivares, M.; Usobiaga, A. Development, Characterization and In Vitro Gastrointestinal Release of PLGA Nanoparticles Loaded with Full-Spectrum Cannabis Extracts. AAPS PharmSciTech 2024, 25, 120. [Google Scholar] [CrossRef]
- de la Ossa, D.H.P.; Ligresti, A.; Gil-Alegre, M.; Aberturas, M.; Molpeceres, J.; Di Marzo, V.; Suárez, A.T. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: Development, characterization and in vitro evaluation of their antitumoral efficacy. J. Control. Release 2012, 161, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.B.; dos Santos, F.N.; Costa, I.H.d.L.; Kringel, D.H.; Zavareze, E.d.R.; Dias, A.R.G. Essential oil encapsulation by electrospinning and electrospraying using food proteins: A review. Food Res. Int. 2023, 170, 112970. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.B.; Fonseca, L.M.; Siebeneichler, T.J.; Crizel, R.L.; dos Santos, F.N.; Hackbart, H.C.d.S.; Kringel, D.H.; Meinhart, A.D.; Zavareze, E.d.R.; Dias, A.R.G. Curcumin encapsulation in capsules and fibers of potato starch by electrospraying and electrospinning: Thermal resistance and antioxidant activity. Food Res. Int. 2022, 162, 112111. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Encapsulation in food industry with emerging electrohydrodynamic techniques: Electrospinning and electrospraying—A review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef]
- Reksamunandar, R.P.; Edikresnha, D.; Munir, M.M.; Damayanti, S. Khairurrijal Encapsulation of β-carotene in poly(vinylpyrrolidone) (PVP) by Electrospinning Technique. Procedia Eng. 2017, 170, 19–23. [Google Scholar] [CrossRef]
- Alfonso, I.; Calvo-Correas, T.; Eceiza, A.; Claver, A.; Torresi, S.; García, J.A.; Zalakain, I. Recycling bovine ear tags for phase change material encapsulation via electrospinning. Sustain. Mater. Technol. 2025, 45, e01449. [Google Scholar] [CrossRef]
- Andriotis, E.G.; Chachlioutaki, K.; Monou, P.K.; Bouropoulos, N.; Tzetzis, D.; Barmpalexis, P.; Chang, M.-W.; Ahmad, Z.; Fatouros, D.G. Development of Water-Soluble Electrospun Fibers for the Oral Delivery of Cannabinoids. AAPS PharmSciTech 2021, 22, 23. [Google Scholar] [CrossRef]
- Cruz, O.B.; Lou, L.; Mohammed, S.M.A.K.; Murickan, R.T.; Benedetti, L.; Lin, Y.-M.; Dolmetsch, T.; Agarwal, A. Wearable, Ultralow Power, and Needleless Electrospinning Equipment for Cannabidiol-Loaded Patch Fabrication. ACS Appl. Mater. Interfaces 2025, 17, 48145–48159. [Google Scholar] [CrossRef]
- Wenzel, J.; Samaniego, C.S.; Wang, L.; Burrows, L.; Tucker, E.; Dwarshuis, N.; Ammerman, M.; Zand, A. Antioxidant potential of Juglans nigra, black walnut, husks extracted using supercritical carbon dioxide with an ethanol modifier. Food Sci. Nutr. 2017, 5, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.A.; Vazifedoost, M.; Hajirostamloo, B.; Didar, Z.; Nematshahi, M.M. Supercritical fluid extraction and encapsulation of Rivas (Rheum ribes) flower: Principal component analysis (PCA). Heliyon 2025, 11, e41746. [Google Scholar] [CrossRef]
- Naziruddin, M.; Jawaid, M.; Elais, R.; Sanny, M.; Fouad, H.; Yusof, N.; Abdul-Mutalib, N. Supercritical fluid extraction of torch ginger: Encapsulation, metabolite profiling, and antioxidant activity. J. King Saud Univ. Sci. 2023, 35, 102700. [Google Scholar] [CrossRef]
- Cerro, D.; Rojas, A.; Torres, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Nanoencapsulation of food-grade bioactive compounds using a supercritical fluid extraction of emulsions process: Effect of operational variables on the properties of nanocapsules and new perspectives. LWT 2023, 184, 115115. [Google Scholar] [CrossRef]
- Fuentes-Ríos, D.; Moya-Utrera, F.; Moreno, J.; Mesas, C.; Doña-Flores, M.; Sarabia, F.; López-Romero, J.M.; Melguizo, C.; Prados, J. Synthesis, characterization and antitumor activity of a poly-4-Vinyl pyridine-co-cannabidiol polymer. Eur. Polym. J. 2024, 219, 113328. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Rodrigues, P.C.; Beyer, U.; Schumacher, P.; Roth, T.; Fiebig, H.H.; Unger, C.; Messori, L.; Orioli, P.; Paper, D.H.; Mülhaupt, R.; et al. Acid-sensitive polyethylene glycol conjugates of doxorubicin: Preparation, in vitro efficacy and intracellular distribution. Bioorganic Med. Chem. 1999, 7, 2517–2524. [Google Scholar] [CrossRef]
- Ma, Z.; Bitter, J.H.; Boom, R.M.; Nikiforidis, C.V. Encapsulation of cannabidiol in hemp seed oleosomes. Food Res. Int. 2024, 195, 114948. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Zhao, R.; Freeman, K.; McHenry, M.A.; Wang, C.; Guo, M. Impact of carrier oil on interfacial properties, CBD partition and stability of emulsions formulated by whey protein or whey protein-maltodextrin conjugate. LWT 2022, 168, 113933. [Google Scholar] [CrossRef]
- Korzhikov-Vlakh, V.; Sinitsyna, E.; Stepanova, M.; Korzhikova-Vlakh, E.; Tennikova, T. Comparison of Different Aliphatic Polyester-Based Microparticles as Protein Delivery Systems. Polymers 2025, 17, 2676. [Google Scholar] [CrossRef]
- Lakshani, N.; Wijerathne, H.S.; Sandaruwan, C.; Kottegoda, N.; Karunarathne, V. Release Kinetic Models and Release Mechanisms of Controlled-Release and Slow-Release Fertilizers. ACS Agric. Sci. Technol. 2023, 3, 939–956. [Google Scholar] [CrossRef]
- Martín-Camacho, U.d.J.; Rodríguez-Barajas, N.; Sánchez-Burgos, J.A.; Pérez-Larios, A. Weibull β value for the discernment of drug release mechanism of PLGA particles. Int. J. Pharm. 2023, 640, 123017. [Google Scholar] [CrossRef]
- Lozza, I.; Martín-Sabroso, C.; Torres-Suárez, A.I.; Fraguas-Sánchez, A.I. In situ forming PLA and PLGA implants for the parenteral administration of Cannabidiol. Int. J. Pharm. 2024, 661, 124468. [Google Scholar] [CrossRef]
- Morakul, B.; Junyaprasert, V.B.; Sakchaisri, K.; Teeranachaideekul, V. Cannabidiol-Loaded Nanostructured Lipid Carriers (NLCs) for Dermal Delivery: Enhancement of Photostability, Cell Viability, and Anti-Inflammatory Activity. Pharmaceutics 2023, 15, 537. [Google Scholar] [CrossRef] [PubMed]
- Toncheva-Moncheva, N.; Dimitrov, E.; Grancharov, G.; Momekova, D.; Petrov, P.; Rangelov, S. Cinnamyl-Modified Polyglycidol/Poly(ε-Caprolactone) Block Copolymer Nanocarriers for Enhanced Encapsulation and Prolonged Release of Cannabidiol. Pharmaceutics 2023, 15, 2128. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Alkurdi, M.; Al Bawab, A.Q.; Hammad, A.M.; Tayyem, R.; Abu Obeed, A.; Abufara, M. Encapsulation of morin in lipid core/PLGA shell nanoparticles significantly enhances its anti-inflammatory activity and oral bioavailability. Saudi Pharm. J. 2023, 31, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Chu, C.; Xue, F.; Li, J.; Bai, J. Structure-function integrated biodegradable Mg/polymer composites: Design, manufacturing, properties, and biomedical applications. Bioact. Mater. 2024, 39, 74–105. [Google Scholar] [CrossRef]
- Rojas, K.; Verdugo-Molinares, M.G.; Vallejo-Cardona, A.A. Use of encapsulating polymers of active compounds in the pharmaceutical and food industry. Food Chem. Adv. 2024, 4, 100619. [Google Scholar] [CrossRef]
- Zhang, S.; Li, L.; Kumar, A. Materials Characterization Techniques; Taylor & Francis: London, UK, 2008. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Dernaika, F.; Halawy, L.; Zeaiter, J.; Kawrani, S.; Mroue, D.; Lteif, A.; Kourani, S.; Mehanna, M.; Abboud, C.; Mroueh, M.; et al. Development and characterization of a zeolite based drug delivery system: Application to cannabidiol oral delivery. Heliyon 2024, 10, e37373. [Google Scholar] [CrossRef]
- Ansari, M.J.; Alshahrani, S.M. Nano-encapsulation and characterization of baricitinib using poly-lactic-glycolic acid co-polymer. Saudi Pharm. J. 2019, 27, 491–501. [Google Scholar] [CrossRef]
- Saiyasombat, W.; Yimpetch, C.; Chathiran, W.; Chimasangkanan, J.; Srichamnong, W. Effect of microencapsulation using β-cyclodextrin and β-glucan as coating agents on physicochemical properties and phytocannabinoids retention of cannabis flower oil extract. NFS J. 2025, 38, 100209. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.; Jithendra, T.; Madhavi, C.; Rao, K.C. Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J. Pharm. Anal. 2021, 11, 191–199. [Google Scholar] [CrossRef]
- Muhamad, H.; Ward, A.; Patel, K.; Williamson, J.; Blunt, L.; Conway, B.; Østergaard, J.; Asare-Addo, K. Investigation into the swelling and dissolution behaviour of Polymer-Excipient blends of PEO Utilising dissolution imaging. Int. J. Pharm. 2024, 666, 124850. [Google Scholar] [CrossRef]
- Lohani, A.; Saxena, R.; Duarte, J.G.; Khan, S.; Figueiras, A.; Mascarenhas-Melo, F. Tailored polymeric hydrogels for regenerative medicine and drug delivery: From material design to clinical applications. Int. J. Pharm. 2025, 681, 125818. [Google Scholar] [CrossRef] [PubMed]
- Carton, F.; Rizzi, M.; Canciani, E.; Sieve, G.; Di Francesco, D.; Casarella, S.; Di Nunno, L.; Boccafoschi, F. Use of Hydrogels in Regenerative Medicine: Focus on Mechanical Properties. Int. J. Mol. Sci. 2024, 25, 11426. [Google Scholar] [CrossRef]
- Hu, X.; Liang, R.; Li, J.; Liu, Z.; Sun, G. Mechanically strong hydrogels achieved by designing homogeneous network structure. Mater. Des. 2019, 163, 107547. [Google Scholar] [CrossRef]
- Ovando-Medina, V.M.; Reyes-Palacios, G.A.; García-Montejano, L.A.; Antonio-Carmona, I.D.; Martínez-Gutiérrez, H. Electroactive polyacrylamide/chitosan/polypyrrole hydrogel for captopril release controlled by electricity. J. Vinyl Addit. Technol. 2021, 27, 679–690. [Google Scholar] [CrossRef]
- Funk, N.L.; Fantaus, S.; Beck, R.C.R. Immediate release 3D printed oral dosage forms: How different polymers have been explored to reach suitable drug release behaviour. Int. J. Pharm. 2022, 625, 122066. [Google Scholar] [CrossRef] [PubMed]
- Korelc, K.; Tzanova, M.M.; Larsson, A.; Grassi, M.; Di Cagno, M.P.; Tho, I. A simplified method to interpret the mechanism of drug release from thin polymeric films by drug diffusivity measurements. Int. J. Pharm. 2025, 675, 125491. [Google Scholar] [CrossRef]
- Cui, S.; Bahraminia, M.; Rouabhia, M.; Semlali, A.; Béland, F.; Zhang, Z. Design, characterization, and release profile of a cannabidiol (CBD)-rich polyvinyl alcohol hydrogel. Mater. Adv. 2024, 5, 7244–7255. [Google Scholar] [CrossRef]
- Zhang, H.; Lan, L.-M.; Hu, H.-J.; Chen, Y.; Hu, T.; Cheng, H.; Hu, X.; Tang, S.; Liao, X.-P.; Jiang, G.-B. Cannabidiol-loaded hydrogel contact lenses for on-demand pH regulation and enhanced corneal alkali burn repair. J. Control. Release 2025, 383, 113859. [Google Scholar] [CrossRef]
- Wang, C.; Cui, B.; Sun, Y.; Wang, C.; Guo, M. Preparation, stability, antioxidative property and in vitro release of cannabidiol (CBD) in zein-whey protein composite nanoparticles. LWT 2022, 162, 113466. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, F.; Rodrigues, A. Pickering emulsions stabilized with chitosan/gum Arabic particles: Effect of chitosan degree of deacetylation on the physicochemical properties and cannabidiol (CBD) topical delivery. J. Mol. Liq. 2022, 355, 118993. [Google Scholar] [CrossRef]
- Lozza, I.; Martín-Sabroso, C.; Hurtado-Marcos, C.; Montejo-Rubio, C.; Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Cannabidiol-loaded-injectable depot formulation for the treatment of triple-negative breast cancer: Design, development, in-vitro and in-ovo evaluation of its anticancer activity. Int. J. Pharm. 2025, 678, 125710. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Sun, Y.; Wang, C.; Guo, M. Fabrication and characterization of a cannabidiol-loaded emulsion stabilized by a whey protein-maltodextrin conjugate and rosmarinic acid complex. J. Dairy Sci. 2022, 105, 6431–6446. [Google Scholar] [CrossRef]

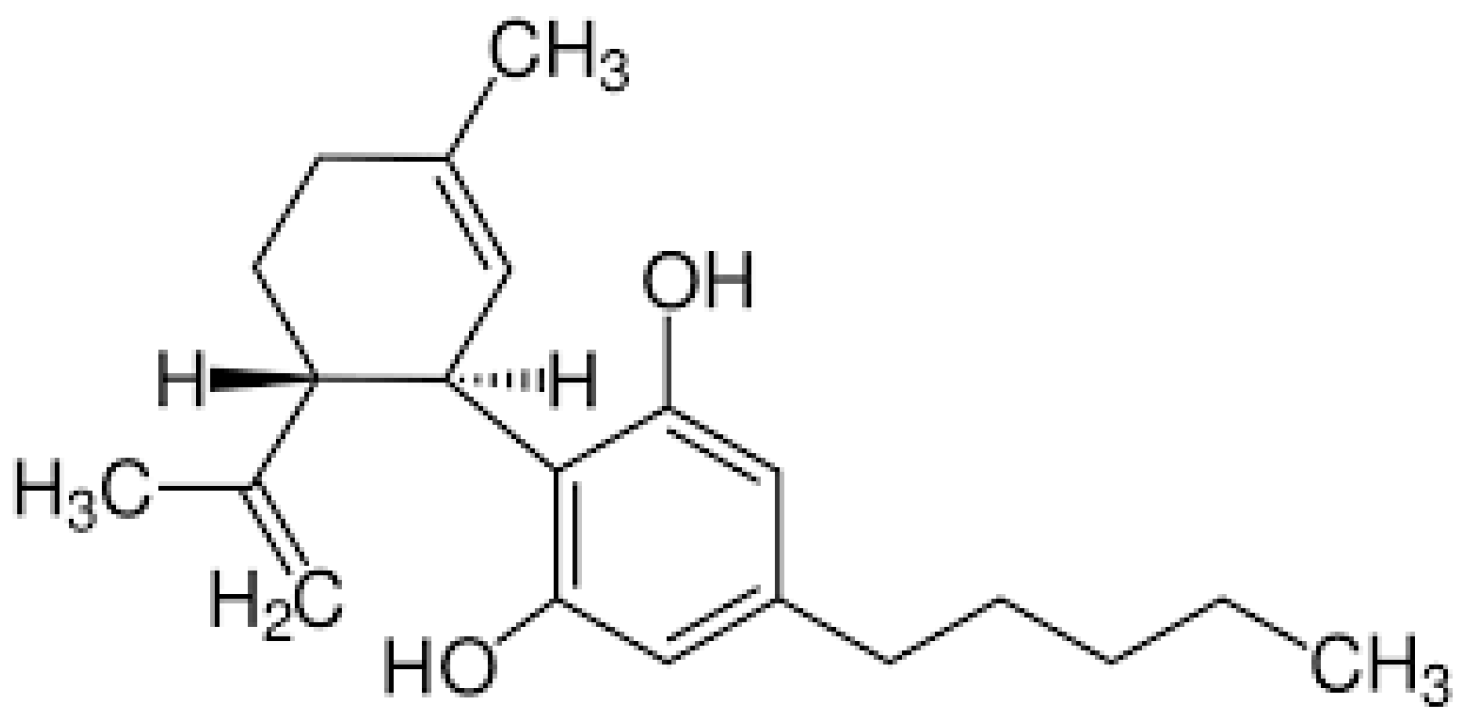
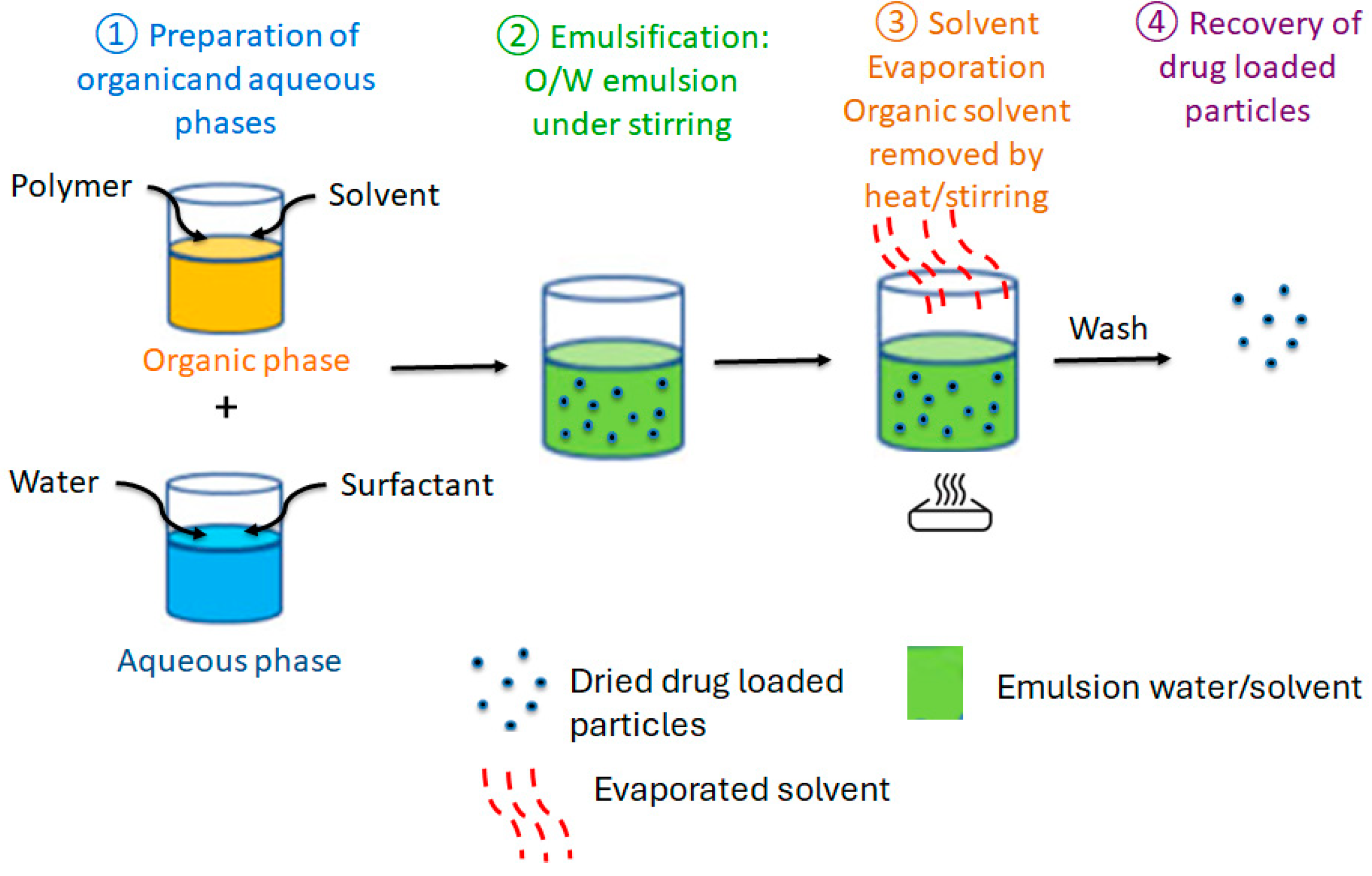

| Encapsulation Technique | Polymeric System Used | Applications | Main Results Achieved |
|---|---|---|---|
| Double emulsion/solvent evaporation technique [8] | Poly (butylene succinate) (PBS) | Anticancer application | Controlled release of CBD in the first 3–5 h at approximately 50% and slow release after the first hours 75% in 72 h. |
| Drop-by-drop anti-solvent precipitation method [84] | Zeina and Zein-whey protein (WP) | Protection of compounds in food processing | Controlled release of CBD in vitro in simulated gastric and intestinal fluid, with release around 75% in CBD/Zein and 92% in CBD/Zein-WP. |
| Oil-water emulsion [59] | Whey protein (WP) and WP-maltodextrin | Emulsion-based delivery systems | Using WP-MD as a stabilizer with a 50:50 medium-chain triglyceride (MCT) and long-chain triglyceride (LCT) ratio produced more stable emulsions, suitable for long-term CBD preservation in 16 days at 55 °C. |
| Pickering emulsión (PEs) [85] | High and low DDA chitosan/gum Arabic | Topical delivery of CBD | CH/GA particles containing high-DDA chitosan showed good affinity and adherence to skin cells. Both formulations achieved over 95% CBD recovery, regarded as optimal under OECD ex vivo skin absorption guidelines. The skin absorbed roughly 2.9% and 4.3% of the total CBD for high- and low-DDA chitosan systems, respectively. |
| Supercritical CO2 atomization [23] | Polivinylpyrrolidone (PVP) | Oral controlled-release system | CBD release powder fully dissolved in around 240 min, whereas the 55 nm CBD nanoparticle with PVP was completely released in just 20 min |
| Solvent exchange process [86] | Polycaprolactone (PCL) | Evaluation of anticancer activity | One of the formulations suppressed the proliferation and migration of MDA-MB-231 and 4T1 cells and demonstrated an antiangiogenic effect in in ovo models. |
| Oil-water emulsion [87] | Whey protein (WP) Whey protein-maltodextrin (WP-MD) WP-MD-Rosmarinic acid (RA) | Design emulsion systems that protect active substances from environmental conditions. | Results showed that WP-MD-RA was an efficient emulsifier, producing fine droplets and enhancing pH and salt stability. It provided the greatest CBD protection against UV and heat degradation and maintained a small particle size during storage at 4 °C. |
| Freeze–thaw method [82] | Conjugated systems of Poly(vinyl alcohol) (PVA), propylene glycol (PG) and vegetable glycerine (VG) | Controlled release into the system | CBD release was evaluated after 24 h. The PVA system released 60% of the CBD, while the PVA-PG and PVA-PG-VG systems released 65%, and the PVA-PG system released 75% of the encapsulated CBD. |
| Injectable Solid-in-Oil or In Situ Forming Implants [63] | PLA-202/203 and PLGA 502H/503 | Production of in situ forming implants (ISFIs) for cancer application | PLGA 502 implants prepared with DMSO as the solvent and a CBD/polymer ratio of 5:100 (w/w) exhibited an initial release below 25% and nearly constant release over one month, a crucial period for cancer therapy applications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovando-Medina, V.M.; García-Martínez, C.A.; Farias-Cepeda, L.; Antonio-Carmona, I.D.; Dector, A.; Olivares-Ramírez, J.M.; Ortiz-Verdin, A.A.; Martínez-Gutiérrez, H.; Rivas Martínez, E.N. Cannabidiol Encapsulation in Polymeric Hydrogels and Its Controlled Release: A Review. Gels 2025, 11, 815. https://doi.org/10.3390/gels11100815
Ovando-Medina VM, García-Martínez CA, Farias-Cepeda L, Antonio-Carmona ID, Dector A, Olivares-Ramírez JM, Ortiz-Verdin AA, Martínez-Gutiérrez H, Rivas Martínez EN. Cannabidiol Encapsulation in Polymeric Hydrogels and Its Controlled Release: A Review. Gels. 2025; 11(10):815. https://doi.org/10.3390/gels11100815
Chicago/Turabian StyleOvando-Medina, Víctor M., Carlos A. García-Martínez, Lorena Farias-Cepeda, Iveth D. Antonio-Carmona, Andrés Dector, Juan M. Olivares-Ramírez, Alondra Anahí Ortiz-Verdin, Hugo Martínez-Gutiérrez, and Erika Nohemi Rivas Martínez. 2025. "Cannabidiol Encapsulation in Polymeric Hydrogels and Its Controlled Release: A Review" Gels 11, no. 10: 815. https://doi.org/10.3390/gels11100815
APA StyleOvando-Medina, V. M., García-Martínez, C. A., Farias-Cepeda, L., Antonio-Carmona, I. D., Dector, A., Olivares-Ramírez, J. M., Ortiz-Verdin, A. A., Martínez-Gutiérrez, H., & Rivas Martínez, E. N. (2025). Cannabidiol Encapsulation in Polymeric Hydrogels and Its Controlled Release: A Review. Gels, 11(10), 815. https://doi.org/10.3390/gels11100815







