Structure–Function Relationships in High-Moisture Meat Analogues: Effects of Soybean Residue (Okara) on Plant Protein–Starch Gels
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition
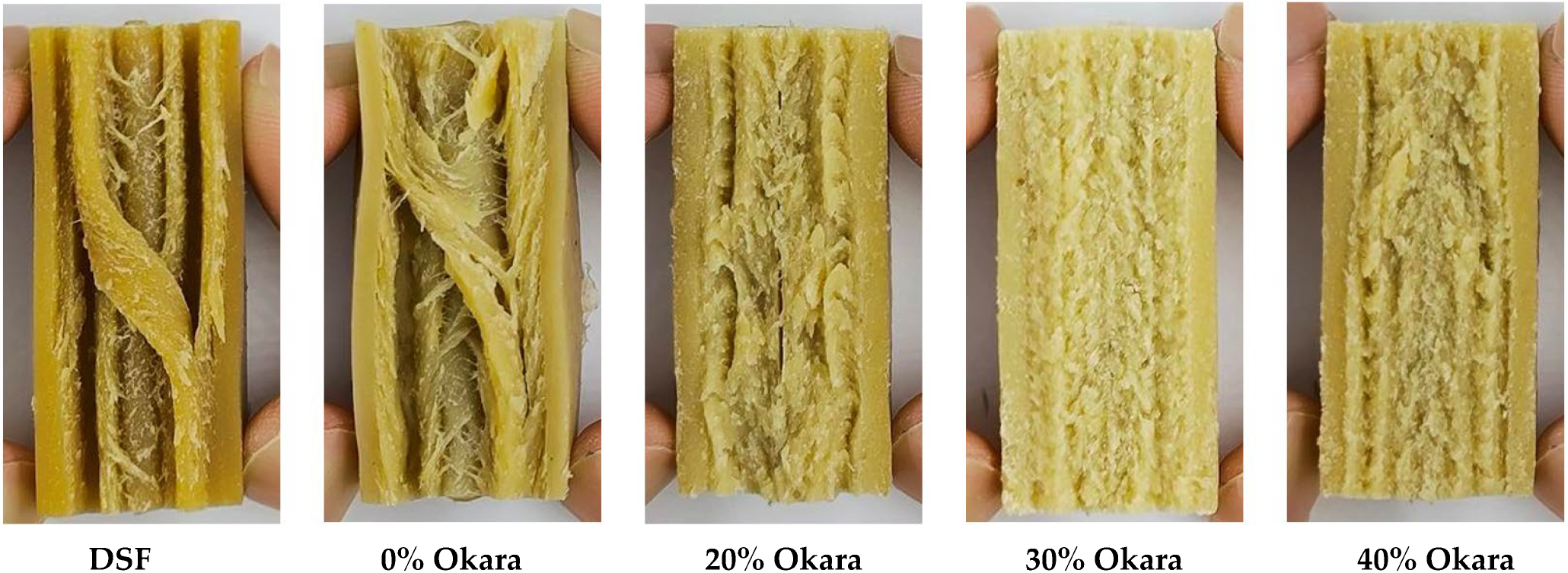
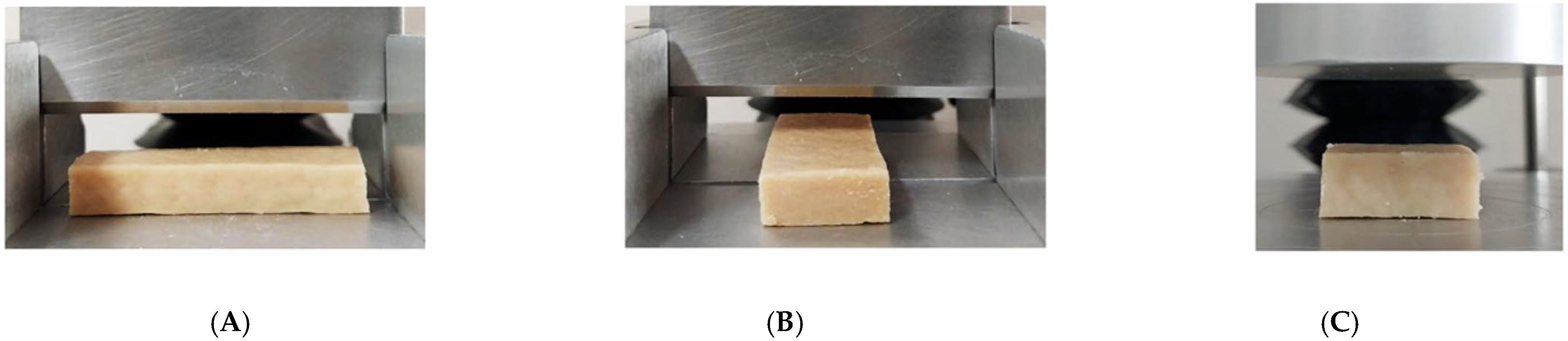
2.2. Macrostructure and Fibrous Integrity
2.3. Instrumental Texture Characterization Using Multivariate Analysis
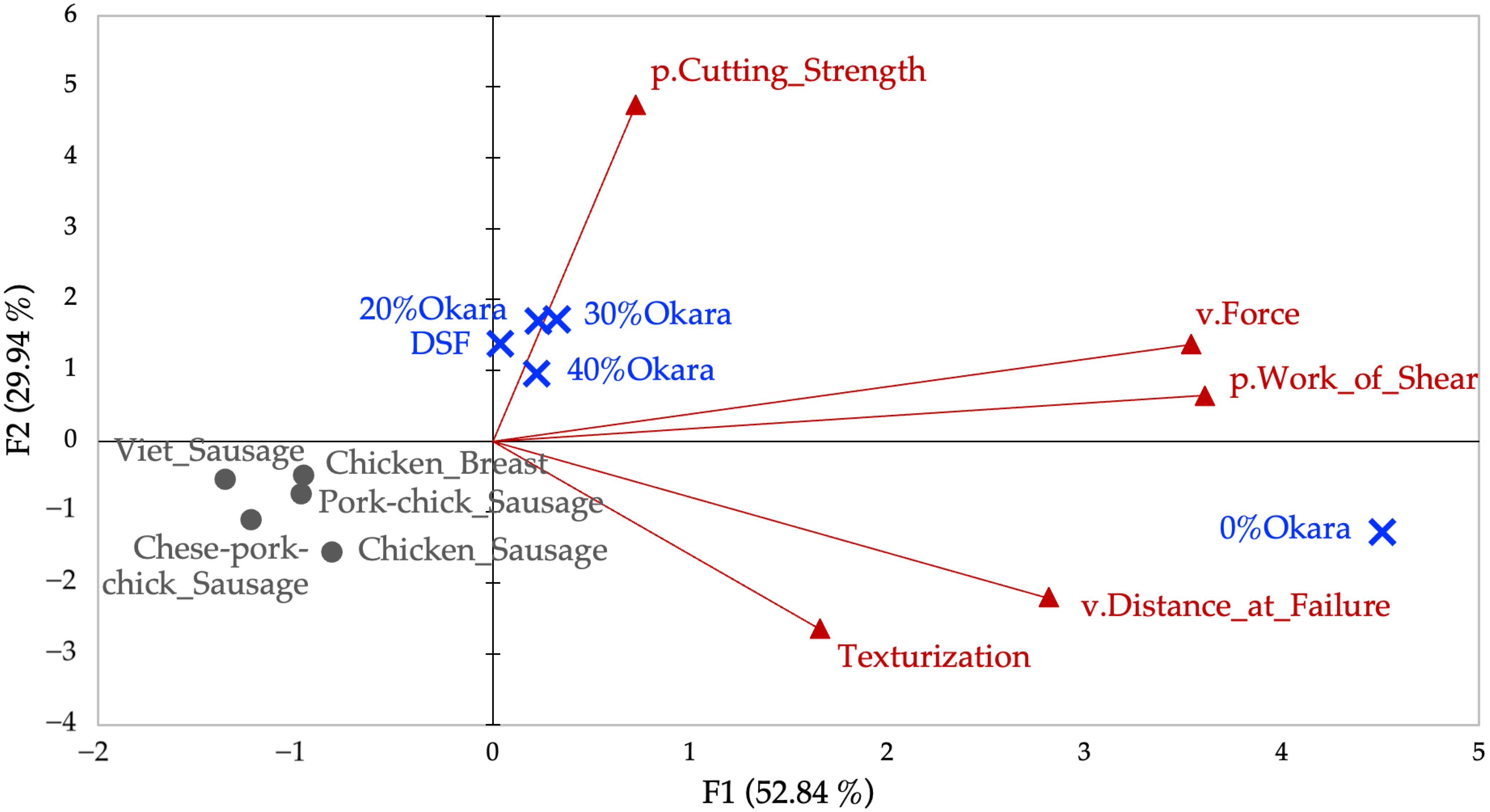
2.3.1. PCA of Cutting Test Variables
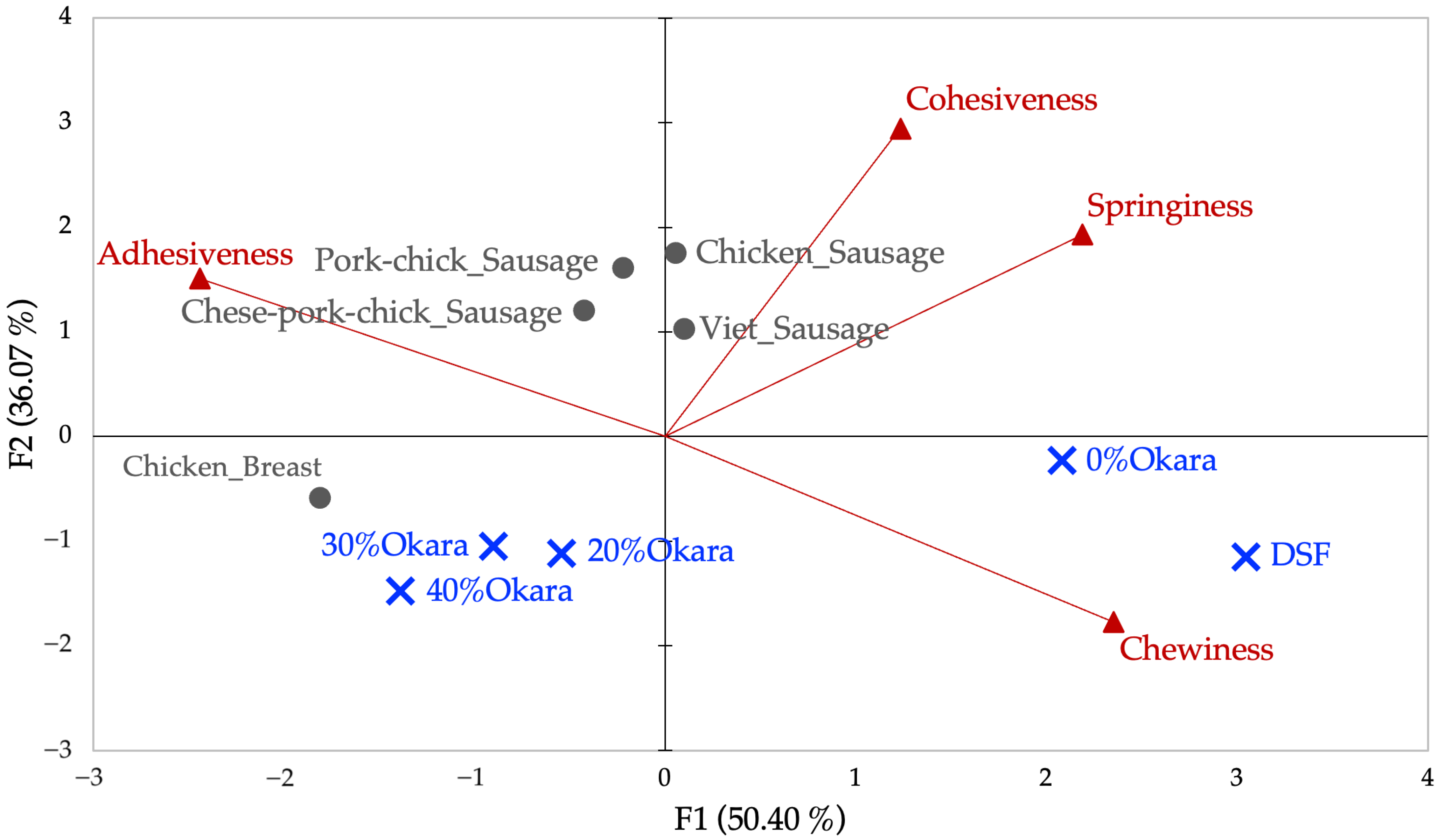
2.3.2. PCA of TPA Variables
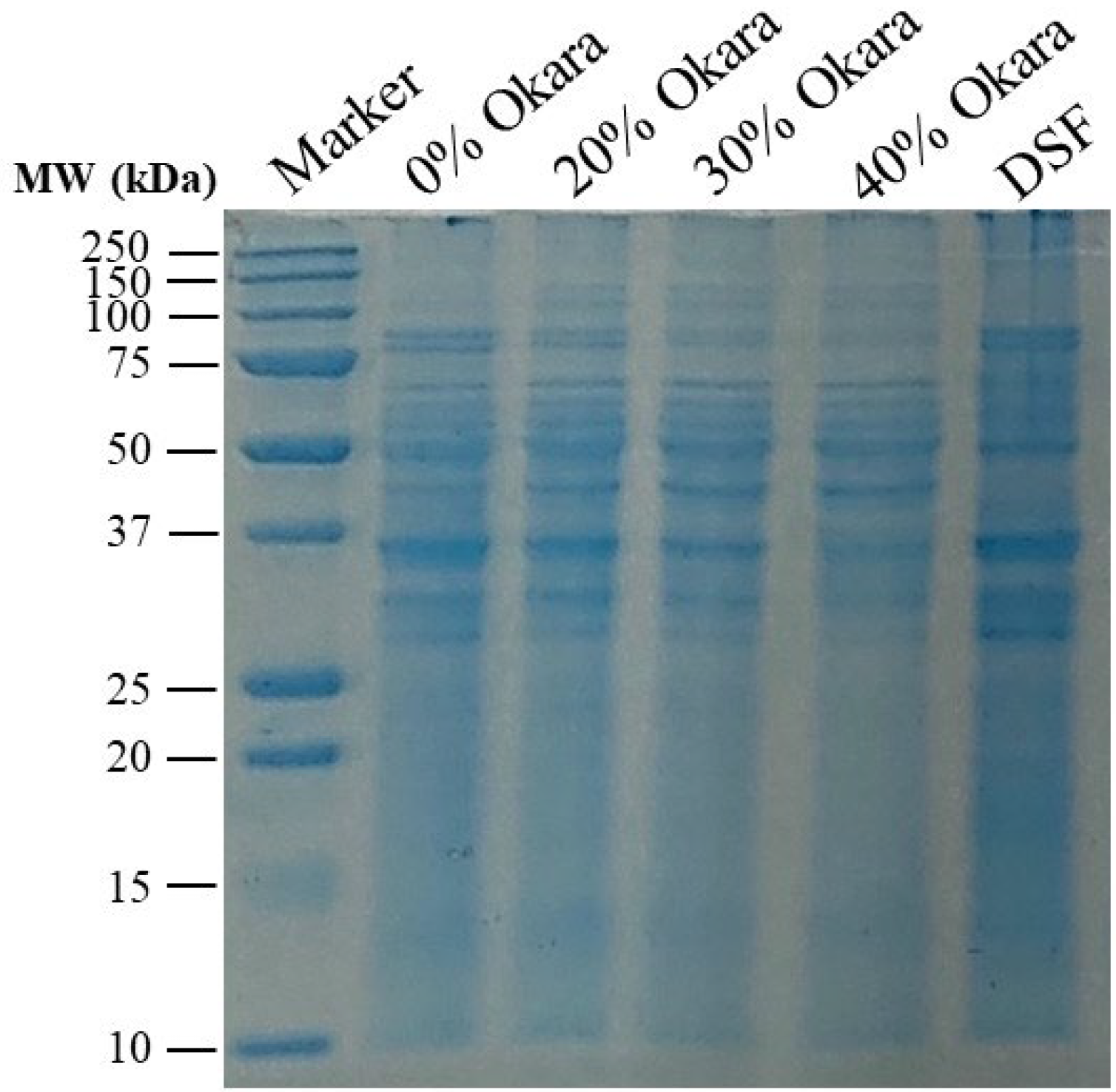
2.4. Protein-Level Characterization Using SDS-PAGE
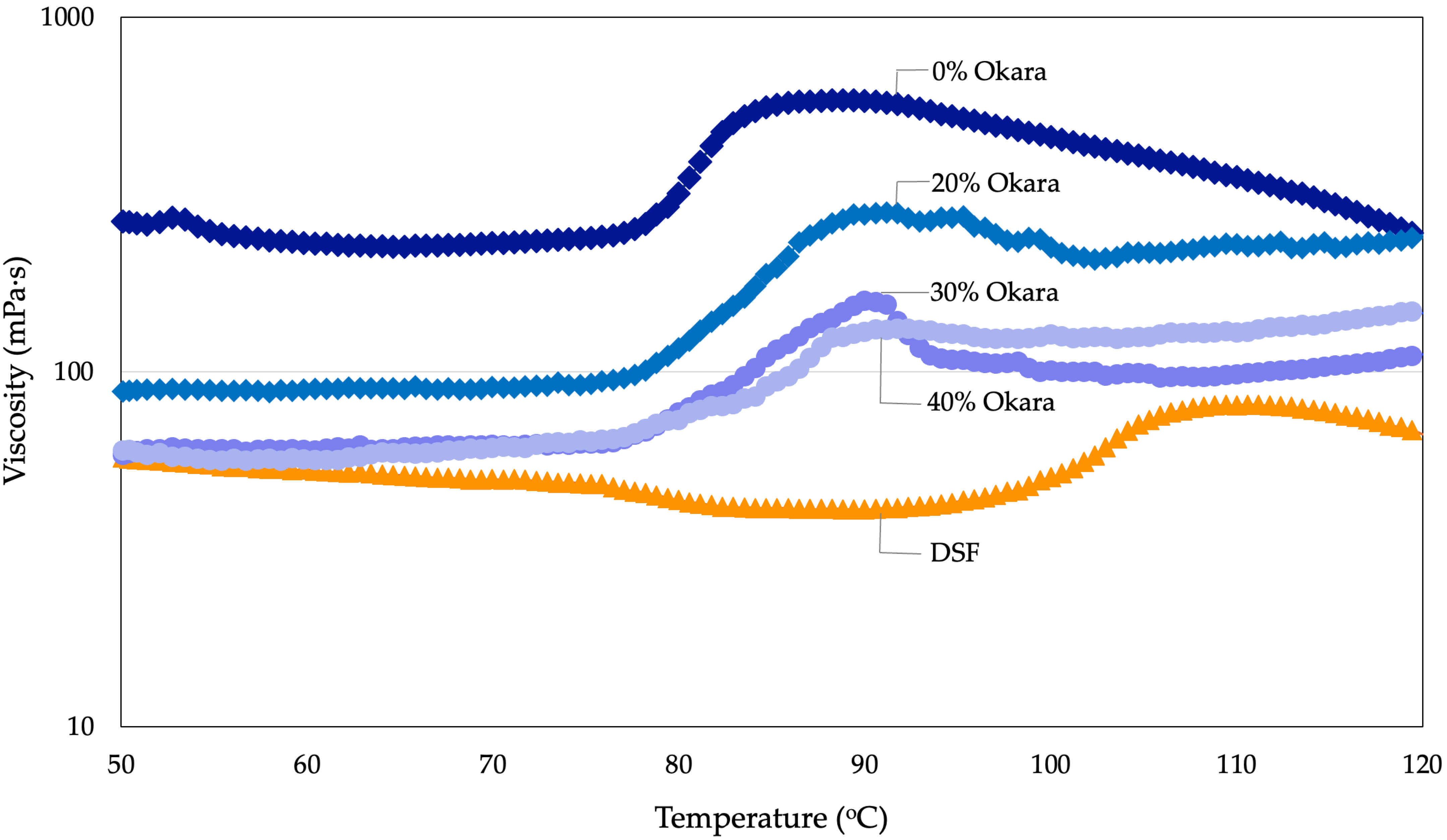
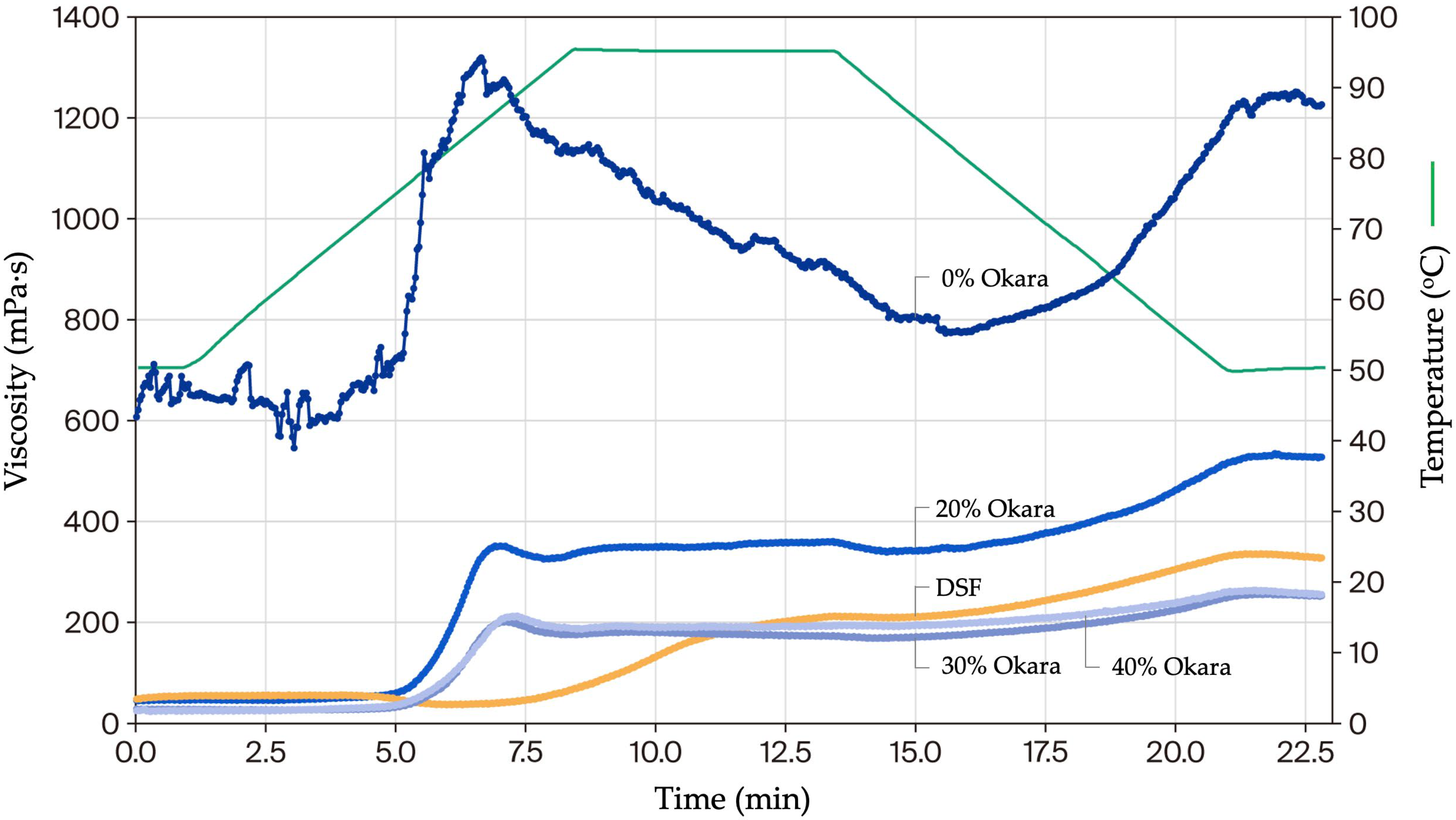
2.5. Pasting Properties of HMMA Flour Mixtures
2.5.1. Gelling Behavior Under Pressure
2.5.2. Pasting Properties Under Atmospheric Conditions
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of High-Moisture Meat Analogue (HMMA)
4.3. Proximate Composition and Nutritional Value
4.4. Functional Properties of HMMA Samples
4.4.1. Sample Preparation
4.4.2. Cholesterol Adsorption Capacity (CAC)
4.4.3. Glucose Adsorption Capacity (GAC)
4.5. SDS PAGE
4.6. Rheometer-Based Pasting Properties of HMMA Flour Mixtures
4.6.1. Determination of Gelling Behavior Under Pressure
4.6.2. Determination of Pasting Properties (RVA-like Starch Cell Test)
4.7. Texture Profile Analysis and Cutting Strengt
4.8. Data Processing and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation/Variable | Definition |
| DSF | Defatted soy flour |
| GAC | Glucose adsorption capacity |
| HMMA | High-moisture meat analogue |
| HME | High-moisture extrusion |
| PBMA | Plant-based meat analogue |
| PCA | Principal component analysis |
| RVA | Rapid Visco Analyzer |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SPI | Soy protein isolate |
| TDF | Total dietary fiber |
| TPA | Texture profile analysis |
| p.Force | Maximum cutting force |
| p.Work_of_Shear | Work of shear (energy required to cut through sample) |
| p.Cutting_Strength | Cutting strength (force normalized by cross-sectional area) |
| p.Distance_at_Failure | Blade displacement at fracture |
| v.Force | Maximum cutting force |
| v.Work_of_Shear | Work of shear (energy required to cut through sample) |
| v.Cutting_Strength | Cutting strength (force normalized by cross-sectional area) |
| v.Distance_at_Failure | Blade displacement at fracture |
| Note: “p.” and “v.” denote directions relative to the extrusion flow, as defined in Section 4.7 (Materials and Methods). | |
Appendix A
| Variable | Loading | Squared Cosine | Contribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| Force | 0.978 | −0.176 | 0.003 | 0.957 | 0.031 | 0.000 | 20.504 | 1.229 | 0.002 |
| Hardness | 0.968 | −0.220 | 0.021 | 0.938 | 0.049 | 0.000 | 20.092 | 1.921 | 0.107 |
| Gumminess | 0.990 | −0.046 | −0.049 | 0.980 | 0.002 | 0.002 | 20.998 | 0.082 | 0.584 |
| Chewiness | 0.992 | 0.025 | −0.051 | 0.984 | 0.001 | 0.003 | 21.077 | 0.024 | 0.649 |
| Cohesiveness | −0.115 | 0.974 | −0.175 | 0.013 | 0.948 | 0.031 | 0.283 | 37.505 | 7.605 |
| Resilience | 0.193 | 0.937 | −0.256 | 0.037 | 0.878 | 0.066 | 0.794 | 34.709 | 16.212 |
| Springiness | 0.311 | 0.779 | 0.544 | 0.097 | 0.606 | 0.296 | 2.076 | 23.982 | 73.308 |
| Adhesiveness | −0.813 | −0.118 | 0.079 | 0.662 | 0.014 | 0.006 | 14.174 | 0.547 | 1.533 |
| Variable Name | Loading | Squared Cosine | Contribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | PC1 | PC2 | PC3 | |
| p.Force | 0.932 | 0.323 | −0.016 | 0.869 | 0.104 | 0.000 | 17.628 | 3.602 | 0.032 |
| p.Work_of_Shear | 0.946 | −0.214 | −0.073 | 0.894 | 0.046 | 0.005 | 18.147 | 1.585 | 0.670 |
| p.Cutting_Strength | 0.517 | 0.838 | 0.051 | 0.267 | 0.703 | 0.003 | 5.416 | 24.330 | 0.322 |
| p.Distance_at_Failure | 0.488 | −0.768 | −0.089 | 0.238 | 0.590 | 0.008 | 4.834 | 20.420 | 0.996 |
| v.Force | 0.986 | −0.043 | 0.082 | 0.973 | 0.002 | 0.007 | 19.744 | 0.065 | 0.849 |
| v.Work_of_Shear | 0.983 | −0.038 | −0.009 | 0.967 | 0.001 | 0.000 | 19.613 | 0.050 | 0.011 |
| v.Cutting_Strength | 0.594 | 0.779 | 0.066 | 0.353 | 0.607 | 0.004 | 7.166 | 21.009 | 0.540 |
| v.Distance_at_Failure | 0.553 | −0.728 | −0.372 | 0.306 | 0.529 | 0.138 | 6.214 | 18.322 | 17.374 |
| Texturization | 0.247 | −0.554 | 0.794 | 0.061 | 0.307 | 0.630 | 1.239 | 10.616 | 79.206 |
References
- Dekkers, B.L.; Boom, R.M.; van der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- McClements, D.J.; Grossmann, L. A brief review of the science behind the design of healthy and sustainable plant-based foods. npj Sci. Food 2021, 5, 17. [Google Scholar] [CrossRef]
- Aghagholizadeh, R.; Rigi, A.A. High-Moisture Extrusion in Plant-Based Meat: Challenges and Emerging Trends. J. Food Process Eng. 2025, 48, e70107. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, A.; Zhang, Z.; Zhang, T.; Jiang, L.; Zhou, W.; Zhang, Y.; Sui, X. Advancing molecular understanding in high moisture extrusion for plant-based meat analogs: Challenges and perspectives. Food Chem. 2024, 460, 140458. [Google Scholar] [CrossRef]
- Dinali, M.; Liyanage, R.; Silva, M.; Newman, L.; Adhikari, B.; Wijesekara, I.; Chandrapala, J. Fibrous Structure in Plant-Based Meat: High-Moisture Extrusion Factors and Sensory Attributes in Production and Storage. Food Rev. Int. 2024, 40, 2940–2968. [Google Scholar] [CrossRef]
- Oppen, D.; Grossmann, L.; Weiss, J. Insights into characterizing and producing anisotropic food structures. Crit. Rev. Food Sci. Nutr. 2024, 64, 1158–1176. [Google Scholar] [CrossRef]
- Schmid, E.-M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High moisture extrusion cooking of meat analogs: A review of mechanisms of protein texturization. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.F.; Bouché, O.; Schlangen, M.; der Goot, A.J.v.; Corredig, M. Relationship between rheological parameters and structure formation in high moisture extrusion of plant protein biopolymers. Food Hydrocoll. 2025, 160, 110843. [Google Scholar] [CrossRef]
- Gräfenhahn, M.; Beyrer, M. Influence of temperature and shear rate during cooling on the rheological and textural properties of pea protein-based meat analogues. J. Food Eng. 2025, 399, 112625. [Google Scholar] [CrossRef]
- Shrestha, S.; van ’t Hag, L.; Haritos, V.; Dhital, S. Fate of pulse globulin proteins molecular Structure and composition on high moisture extrusion. Food Hydrocoll. 2024, 149, 109512. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Zhang, J.; Liu, S.; Tian, Y.; Cheng, T.; Guo, Z.; Wang, Z. Improve the fiber structure and texture properties of plant-based meat analogues by adjusting the ratio of soy protein isolate (SPI) to wheat gluten (WG). Food Chem. X 2024, 24, 101962. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, D.W. Advancements in plant based meat analogs enhancing sensory and nutritional attributes. npj Sci. Food 2024, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT—Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Fei, C.; Li, L.; Zhao, R.; Wang, X.; Fan, B.; Liu, L.; Wang, F.; Huang, Y. Texture and structure of high-moisture extrudates produced from soybean protein isolates with different 7S/11S globulin ratios. Food Hydrocoll. 2026, 171, 111771. [Google Scholar] [CrossRef]
- Wu, C.; Hua, Y.; Chen, Y.; Zhang, C.; Kong, X. Effect of 7S/11S Ratio on the Network Structure of Heat-induced Soy Protein Gels: A Study of Probe Release. RSC Adv. 2016, 6, 101981–101987. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, J.; Xie, Y.; Luo, W.; Zhang, Y.; He, X.; Liu, D.; Han, J. Effect of Extrusion Temperature on the Structure of High-moisture Extruded Mung Bean Protein. Sci. Technol. Food Ind. 2022, 43, 130–136. [Google Scholar]
- Tian, T.; Ren, K.; Cao, X.; Peng, X.; Zheng, L.; Dai, S.; Tong, X.; Zeng, Q.; Qiu, S.; Wang, H.; et al. High moisture extrusion of soybean-wheat co-precipitation protein: Mechanism of fibrosis based on different extrusion energy regulation. Food Hydrocoll. 2023, 144, 108950. [Google Scholar] [CrossRef]
- Huang, Z.; Qu, Y.; Hua, X.; Wang, F.; Jia, X.; Yin, L. Recent advances in soybean protein processing technologies: A review of preparation, alterations in the conformational and functional properties. Int. J. Biol. Macromol. 2023, 248, 125862. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, D.; Li, M.; Wen, X.; Ni, Y. The Interactions of Soy Protein and Wheat Gluten for the Development of Meat-like Fibrous Structure. Molecules 2023, 28, 7431. [Google Scholar] [CrossRef]
- Gasparre, N.; van den Berg, M.; Oosterlinck, F.; Sein, A. High-Moisture Shear Processes: Molecular Changes of Wheat Gluten and Potential Plant-Based Proteins for Its Replacement. Molecules 2022, 27, 5855. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Fan, Y.; Annamalai, P.K.; Bhandari, B.; Prakash, S. Characteristics of faba bean protein-based high-moisture meat analogues incorporating brewers’ spent grain through extrusion. Innov. Food Sci. Emerg. Technol. 2025, 100, 103919. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhao, X.; Sun, P.; Zhao, D.; Jiang, L.; Xiaonan, S. The texture of plant protein-based meat analogs by high moisture extrusion: A review. J. Texture Stud. 2022, 54, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Krintiras, G.; Gobel, J.; Bouwman, W.; Goot, A.J.; Stefanidis, G. On characterization of anisotropic plant protein structures. Food Funct. 2014, 5, 3233–3240. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Annamalai, P.K.; Wang, J.; Gu, B.; Chen, X.D.; Bhandari, B.; Prakash, S. Influence of extrusion processing conditions on fibrous structure, texture, and digestibility of plant-based meat analogues incorporating faba bean protein and brewers’ spent grain. Food Hydrocoll. 2026, 170, 111706. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Boonbumrung, S.; Pantoa, T. Valorization of Soybean Residue (Okara) by Supercritical Carbon Dioxide Extraction: Compositional, Physicochemical, and Functional Properties of Oil and Defatted Powder. Foods 2023, 12, 2698. [Google Scholar] [CrossRef]
- Kantrong, H.; Aussanasuwannakul, A.; Rodkwan, N.; Chitisankul, W.T. Effect of mechanical treatment from extrusion process on physicochemical properties of okara cellulose powder. Sci. Rep. 2024, 14, 22193. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Puntaburt, K.; Pantoa, T. Enhancing Gluten-Free Crispy Waffles with Soybean Residue (Okara) Flour: Rheological, Nutritional, and Sensory Impacts. Foods 2024, 13, 2951. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Teangpook, C.; Treesuwan, W.; Puntaburt, K.; Butsuwan, P. Effect of the Addition of Soybean Residue (Okara) on the Physicochemical, Tribological, Instrumental, and Sensory Texture Properties of Extruded Snacks. Foods 2022, 11, 2967. [Google Scholar] [CrossRef]
- Porcel, M.V.O.; Campderros, M.E.; Rinaldoni, A.N. Effect of Okara flour addition on the physical and sensory quality of wheat bread. MOJ Food Process. Technol. 2017, 4, 184–190. [Google Scholar] [CrossRef]
- Wan Ibadullah, W.Z.; Mokhtar, F.; Abedin, N.H.; Nor-Khaizura, M.A.R.; Ibrahim, N.; Mustapha, N.A. Okara-Fortified Wheat Bread: Effect on Nutritional, Physicochemical and Sensory Properties. J. Biochem. Microbiol. Biotechnol. 2024, 12, 110–113. [Google Scholar] [CrossRef]
- Lu, F.; Cui, Z.; Liu, Y.; Li, B. The Effect of Okara on the Qualities of Noodle and Steamed Bread. Adv. J. Food Sci. Technol. 2013, 5, 960–968. [Google Scholar] [CrossRef]
- Cui, L.; Cui, S.; Ma, P.; Zhang, L.; Zhang, H. Effect of soybean dregs powder on sensory evaluation of Chinese steamed bread (CSB) and textural properties of wheat dough and CSB. Food Sci. Nutr. 2014, 35, 85–88. [Google Scholar]
- Maeda, H.; Nakamura, A. 24—Soluble Soybean Polysaccharide. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2009; pp. 693–709. [Google Scholar]
- Salarbashi, D.; Bazeli, J.; Tafaghodi, M. Environment-friendly green composites based on soluble soybean polysaccharide: A review. Int. J. Biol. Macromol. 2019, 122, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, S.; Shi, Z.; Zhai, L.; Fu, J.; Zhao, J. Purification, characterization, and antioxidant activity of polysaccharides from Okara. J. Food Process. Preserv. 2022, 46, e16411. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, L.; Sun, J.; Korma, S.A.; Al-Asmari, F.; Xie, M.; Yu, M. Soluble Soybean Polysaccharide Improves Quality and Shelf Life of Peanut Butter. Foods 2025, 14, 2180. [Google Scholar] [CrossRef]
- Tan, N.K.; Devnani, B.; Sanders, J.; Rosenfeld, S.; Liu, H.; Moraca, F.; Grossmann, L. Hydrophobic plant protein-polysaccharide composite enables high moisture extrusion of anisotropic textures at low temperature. Food Hydrocoll. 2026, 170, 111711. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Ye, Y.; Zhu, Z.; Geng, S.; Ning, D.; Sun, J.; Sheng, L.; Ji, J.; Zhang, Y.; Sun, X. High-moisture extrusion processing of walnut meal waste: Mechanism of fibrous formation and enhancement of characteristics. Food Hydrocoll. 2025, 166, 111334. [Google Scholar] [CrossRef]
- Chen, Q.; Guan, J.; Wang, Z.; Wang, Y.; Wang, X.; Chen, Z. Improving the Gelation Properties of Pea Protein Isolates Using Psyllium Husk Powder: Insight into the Underlying Mechanism. Foods 2024, 13, 3413. [Google Scholar] [CrossRef]
- Plattner, B.J.; Hong, S.; Li, Y.; Talavera, M.J.; Dogan, H.; Plattner, B.S.; Alavi, S. Use of Pea Proteins in High-Moisture Meat Analogs: Physicochemical Properties of Raw Formulations and Their Texturization Using Extrusion. Foods 2024, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Fei, L. Okara dietary fiber and hypoglycemic effect of okara foods. Bioact. Carbohydr. Diet. Fibre 2013, 2, 126–132. [Google Scholar]
- Tsubaki, S.; Nakauchi, M.; Ozaki, Y.; Azuma, J.-i. Microwave Heating for Solubilization of Polysaccharide and Polyphenol from Soybean Residue (Okara). Food Sci. Technol. Res. 2009, 15, 307–314. [Google Scholar] [CrossRef]
- Zhang, G.; Hao, M.; He, Y.; Ahmad, I.; Ding, Y.; Lyu, F. Structural, physicochemical, and functional properties of insoluble dietary fiber derived from okara by ViscozymeL. J. Food Sci. 2023, 88, 1994–2008. [Google Scholar] [CrossRef]
- Nakamura, A. Development of Soybean Soluble Polysaccharide Derived from “Okara”, and Application as a Functional Food Ingredient. Nippon Shokuhin Kagaku Kogaku Kaishi 2011, 58, 559–566. [Google Scholar] [CrossRef]
- Pérez-López, E.; Cela, D.; Costabile, A.; Mateos-Aparicio, I.; Rupérez, P. In vitro fermentability and prebiotic potential of soyabean Okara by human faecal microbiota. Br. J. Nutr. 2016, 116, 1116–1124. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Singkammo, S. Multiscale Characterization of Rice Starch Gelation and Retrogradation Modified by Soybean Residue (Okara) and Extracted Dietary Fiber Using Rheology, Synchrotron Wide-Angle X-Ray Scattering (WAXS), and Fourier Transform Infrared (FTIR) Spectroscopy. Foods 2025, 14, 1862. [Google Scholar] [CrossRef]
- Aussanasuwannakul, A.; Butsuwan, P. Evaluating Microbiological Safety, Sensory Quality, and Packaging for Online Market Success of Roasted Pickled Fish Powder. Foods 2024, 13, 861. [Google Scholar] [CrossRef]
- McCleary, B.V. Measurement of Dietary Fiber: Which AOAC Official Method of AnalysisSM to Use. J. AOAC Int. 2023, 106, 917–930. [Google Scholar] [CrossRef]
- Lumivero. XLSTAT Statistical and Data Analysis Solution; Lumivero, L.L.C.: New York, NY, USA, 2025. [Google Scholar]


| Composition/Functional Property | 0% Okara | 20% Okara | 30% Okara | 40% Okara | DSF |
|---|---|---|---|---|---|
| Moisture (%) | 53.28 ± 0.44 a | 55.11 ± 0.26 b | 58.73 ± 0.29 c | 52.53 ± 0.47 a | 57.30 ± 0.43 b |
| Protein (%) | 35.06 ± 0.08 d | 27.25 ± 0.25 c | 24.56 ± 0.49 a | 25.75 ± 0.09 b | 24.63 ± 0.33 a |
| Fat (%) | 1.63 ± 0.02 b | 3.21 ± 0.05 c | 3.71 ± 0.05 d | 5.14 ± 0.05 e | 0.62 ± 0.10 a |
| Ash (%) | 1.42 ± 0.02 d | 1.03 ± 0.01 b | 1.01 ± 0.02 a | 1.15 ± 0.01 c | 3.03 ± 0.04 e |
| Carbohydrate (%) | 8.61 ± 0.51 a | 13.40 ± 0.56 c | 11.98 ± 0.37 b | 15.44 ± 0.47 e | 14.42 ± 0.68 d |
| Total dietary fiber (g/100 g) | 0.00 ± 0.00 a | 3.58 ± 0.02 c | 4.94 ± 0.04 d | 7.59 ± 0.08 e | 1.58 ± 0.02 b |
| Total energy (kcal/100 g) | 189.66 ± 2.09 c | 191.51 ± 0.84 d | 179.61 ± 1.20 b | 210.98 ± 2.00 e | 161.57 ± 1.69 a |
| Energy from fat (kcal/100 g) | 14.89 ± 0.19 b | 29.11 ± 0.20 c | 33.28 ± 0.48 d | 46.33 ± 0.58 e | 5.56 ± 0.51 a |
| CAC (mg/g, pH 2) | 4.64 ± 0.00 a | 4.66 ± 0.01 b | 4.77 ± 0.00 d | 4.80 ± 0.00 e | 4.75 ± 0.00 c |
| CAC (mg/g, pH 7) | 4.63 ± 0.00 a | 4.65 ± 0.00 b | 4.68 ± 0.00 c | 4.77 ± 0.00 e | 4.71 ± 0.00 d |
| GAC (mM/g) | 39.49 ± 2.25 b | 42.07 ± 1.31 c | 50.95 ± 1.05 d | 54.35 ± 2.16 e | 31.93 ± 1.44 a |
| Condition | Property | 0% Okara | 20% Okara | 30% Okara | 40% Okara | DSF |
|---|---|---|---|---|---|---|
| Pressurized | Pasting temperature (°C) 1 | 78.3 ± 0.7 b | 76.4 ± 0.5 a | 77.5 ± 0.6 a,b | 77.3 ± 0.8 a | 97.3 ± 0.7 c |
| (50–120 °C, 30 bar) | Peak viscosity (mPa·s) 2 | 583.8 ± 0.9 e | 270.8 ± 10.9 d | 158.5 ± 1.3 c | 132.5 ± 1.2 b | 80.7 ± 1.0 a |
| Final viscosity (mPa·s) 3 | 228.5 ± 1.1 e | 201.3 ± 0.8 c | 104.5 ± 1.2 b | 210.6 ± 1.2 d | 50.4 ± 1.0 a | |
| Atmospheric | Pasting temperature (°C) | 67.5 ± 4.5 b | 71.1 ± 1.8 c | 70.4 ± 3.6 c | 69.2 ± 1.9 c | 53.2 ± 1.5 a |
| (50–95 °C, 1 bar) | Peak viscosity (mPa·s) | 1318.3 ± 7.0 d | 360.7 ± 1.2 c | 201.5 ± 0.7 a | 212.5 ± 0.7 b | 211.4 ± 0.7 b |
| Final viscosity (mPa·s) | 1209.7 ± 16.5 d | 525.9 ± 13.3 c | 253.3 ± 8.9 a | 257.1 ± 7.8 a | 327.6 ± 2.2 b |
| HMMA Sample | * Ratio of Flour (%) | ||||
|---|---|---|---|---|---|
| Okara Flour | Soy Protein Isolate | Wheat Gluten | Corn Starch | Defatted Soy Flour | |
| 0% Okara | 0 | 50 | 40 | 10 | 0 |
| 20% Okara | 20 | 30 | 40 | 10 | 0 |
| 30% Okara | 30 | 20 | 40 | 10 | 0 |
| 40% Okara | 40 | 10 | 40 | 10 | 0 |
| DSF | 0 | 0 | 0 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aussanasuwannakul, A.; Pantoa, T.; Pengpinit, W. Structure–Function Relationships in High-Moisture Meat Analogues: Effects of Soybean Residue (Okara) on Plant Protein–Starch Gels. Gels 2025, 11, 805. https://doi.org/10.3390/gels11100805
Aussanasuwannakul A, Pantoa T, Pengpinit W. Structure–Function Relationships in High-Moisture Meat Analogues: Effects of Soybean Residue (Okara) on Plant Protein–Starch Gels. Gels. 2025; 11(10):805. https://doi.org/10.3390/gels11100805
Chicago/Turabian StyleAussanasuwannakul, Aunchalee, Thidarat Pantoa, and Worapol Pengpinit. 2025. "Structure–Function Relationships in High-Moisture Meat Analogues: Effects of Soybean Residue (Okara) on Plant Protein–Starch Gels" Gels 11, no. 10: 805. https://doi.org/10.3390/gels11100805
APA StyleAussanasuwannakul, A., Pantoa, T., & Pengpinit, W. (2025). Structure–Function Relationships in High-Moisture Meat Analogues: Effects of Soybean Residue (Okara) on Plant Protein–Starch Gels. Gels, 11(10), 805. https://doi.org/10.3390/gels11100805







