Single-Step Engineered Gelatin-Based Hydrogel for Integrated Prevention of Postoperative Adhesion and Promotion of Wound Healing
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of GPP
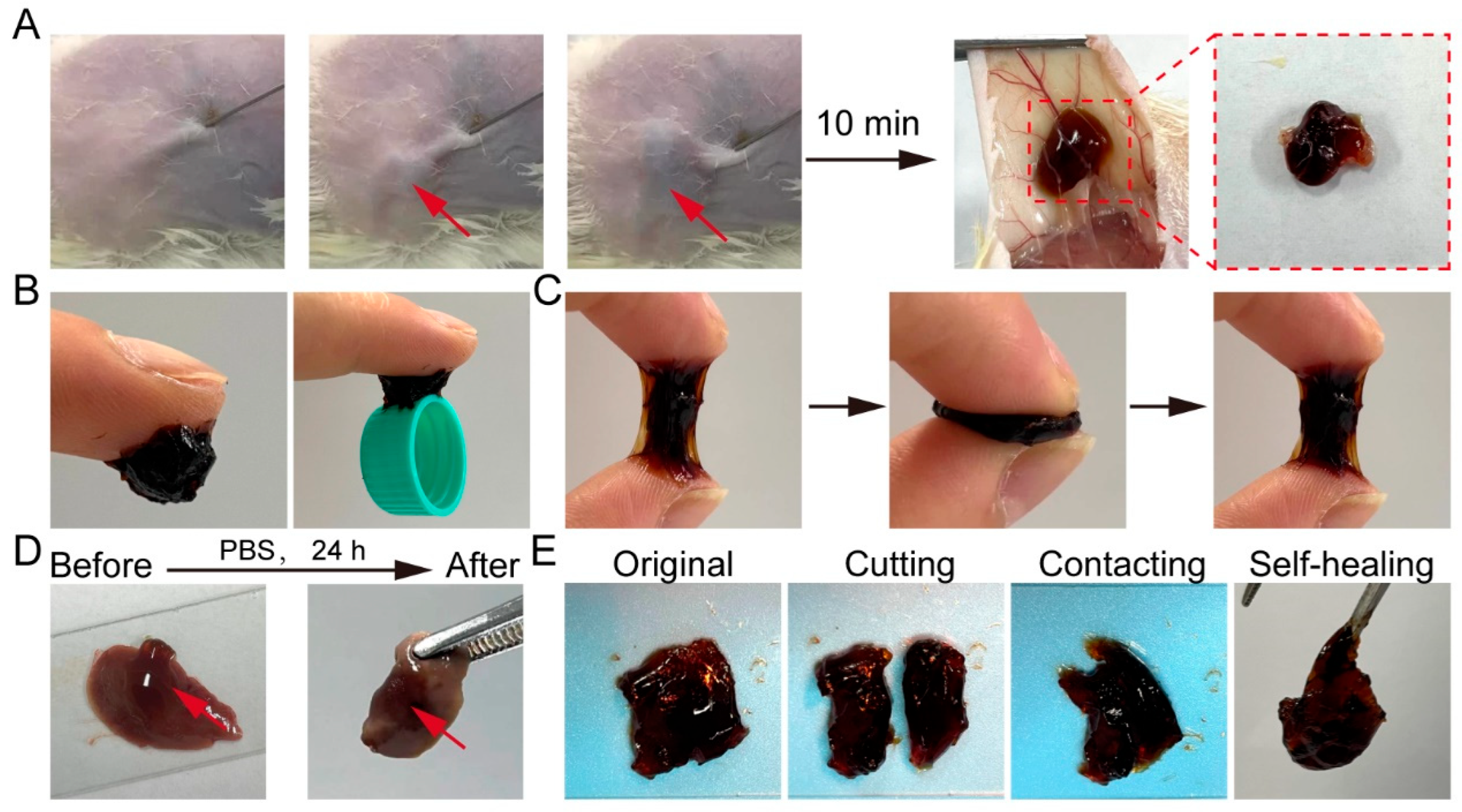
2.2. Injectability, Tissue Adhesive, and Self-Healing Properties of GPP
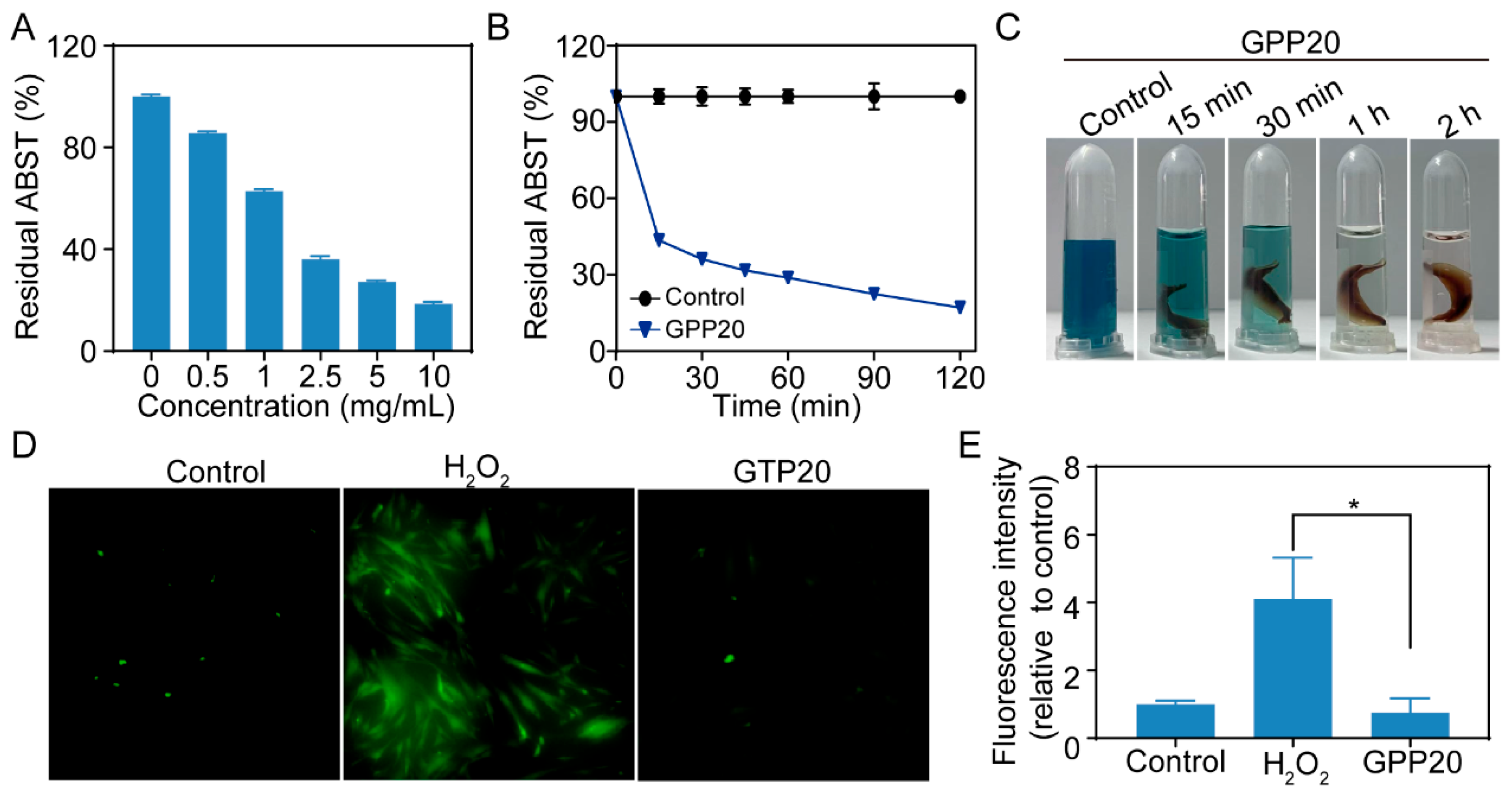
2.3. Antioxidatant Activity of GPP
2.4. Biocompatibility of GPP
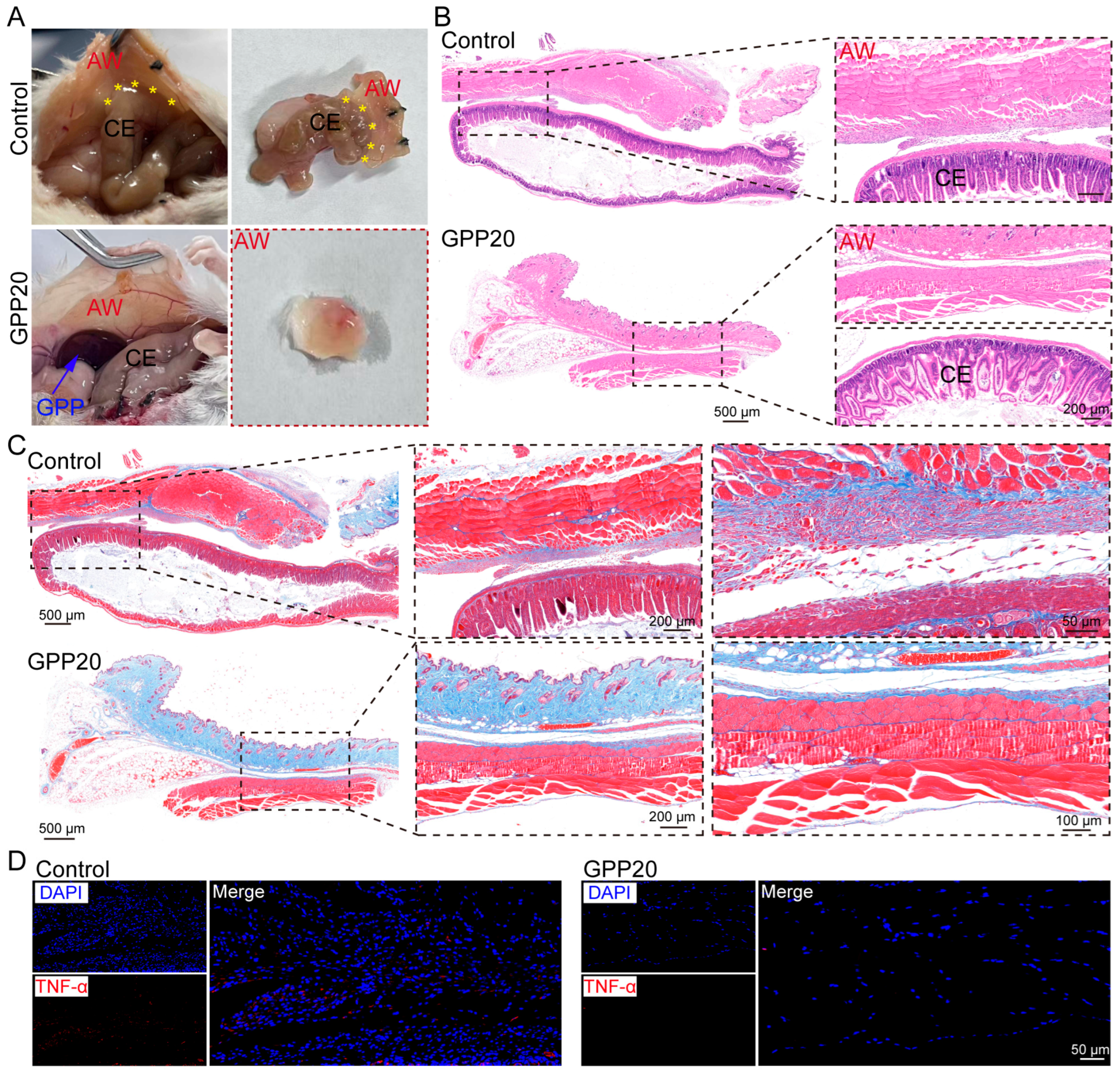
2.5. Postoperative Anti-Adhesion Efficacy of GPP
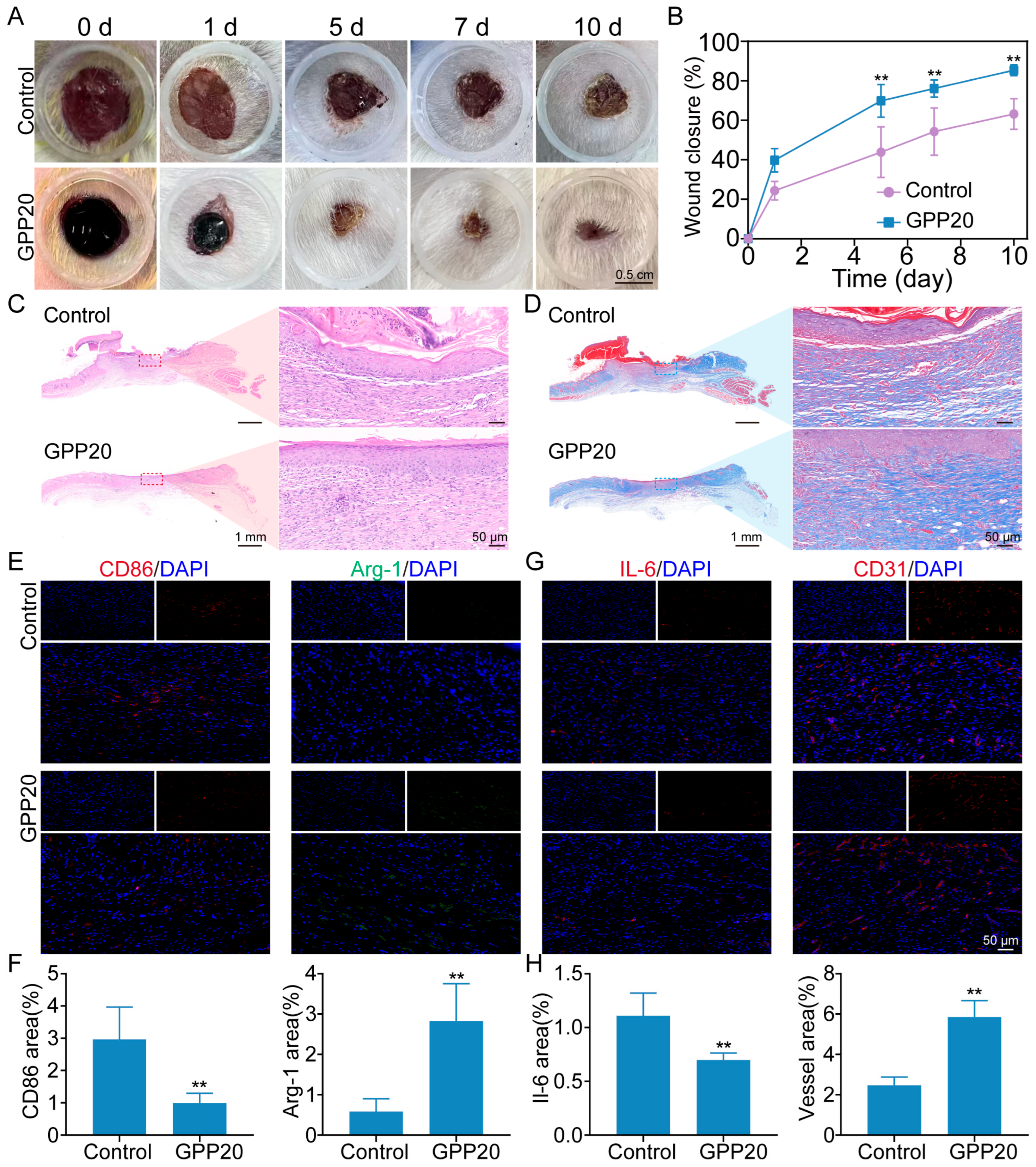
2.6. Wound Healing Performance of GPP
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| PBS | Phosphate-buffered saline |
References
- Liao, J.; Li, X.; Fan, Y. Prevention strategies of postoperative adhesion in soft tissues by applying biomaterials: Based on the mechanisms of occurrence and development of adhesions. Bioact. Mater. 2023, 26, 387–412. [Google Scholar] [CrossRef]
- Hassanabad, A.F.; Zarzycki, A.N.; Jeon, K.; Dundas, J.A.; Vasanthan, V.; Deniset, J.F.; Fedak, P.W.M. Prevention of post-operative adhesions: A comprehensive review of present and emerging strategies. Biomolecules 2021, 11, 1027. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of biomaterial-based postoperative adhesion barriers. Macromol. Biosci. 2021, 21, 2000395. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Ebrahimnia, M.; Niaki, D.S.; Solhi, M.; Rabbani, S.; Haeri, A. Recent advances in the preventative strategies for postoperative adhesions using biomaterial-based membranes and micro/nano-drug delivery systems. J. Drug Deliv. Sci. Tec. 2023, 85, 104539. [Google Scholar] [CrossRef]
- Anvari-Yazdi, A.F.; Badea, I.; Chen, X. Biomaterials in postoperative adhesion barriers and uterine tissue engineering. Gels 2025, 11, 441. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Lai, Y.; Wang, Y.; Liu, P.; Shen, J. An adhesive/anti-adhesive janus tissue patch for efficient closure of bleeding tissue with inhibited postoperative adhesion. Adv. Sci. 2023, 10, e2301427. [Google Scholar] [CrossRef]
- Lv, Y.; Cai, F.; Zhao, X.; Zhu, X.; Wei, F.; Zheng, Y.; Shi, X.; Yang, J. Bioinspired microstructured janus bioadhesive for the prevention of abdominal and intrauterine adhesions. Adv. Funct. Mater. 2024, 34, 2314402. [Google Scholar] [CrossRef]
- Li, X.; Zou, B.; Zhao, N.; Wang, C.; Du, Y.; Mei, L.; Wang, Y.; Ma, S.; Tian, X.; He, J.; et al. Potent anti-adhesion barrier combined biodegradable hydrogel with multifunctional turkish galls extract. ACS Appl. Mater. Interfaces 2018, 10, 24469–24479. [Google Scholar] [CrossRef]
- Hassanabad, A.F.; Zarzycki, A.N.; Jeon, K.; Deniset, J.F.; Fedak, P.W.M. Post-operative adhesions: A comprehensive review of mechanisms. Biomedicines 2021, 9, 867. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xia, X.; Kang, X.; Song, P.; Liu, Z.; Wang, M.; Lu, X.; Guan, W.; Liu, S. A review of physiological and cellular mechanisms underlying fibrotic postoperative adhesion. Int. J. Biol. Sci. 2021, 17, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, C.; Zhang, X.; Lin, Y.; Yang, H.; Zhang, Y.S.; Ding, J. An oxidative stress-responsive electrospun polyester membrane capable of releasing anti-bacterial and anti-inflammatory agents for postoperative anti-adhesion. J. Control. Release 2021, 335, 359–368. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, Y.; Pan, Q.; Fan, Z.; Li, G.; Pei, M.; Zhang, J.; Wang, T.; Zhou, G.; Wang, X.; et al. Multifunctional calcium polyphenol networks reverse the hostile microenvironment of trauma for preventing postoperative peritoneal adhesions. Biomater. Sci. 2023, 11, 6848–6861. [Google Scholar] [CrossRef]
- Abueva, C.D.; Ryu, H.S.; Park, S.Y.; Lee, H.; Padalhin, A.R.; Min, J.W.; Chung, P.S.; Woo, S.H. Trimethyl chitosan postoperative irrigation solution modulates inflammatory cytokines related to adhesion formation. Carbohyd. Polym. 2022, 288, 119380. [Google Scholar] [CrossRef]
- Yuan, L.; Wei, H.; Pan, Z.; Deng, X.; Yang, L.; Wang, Y.; Lu, D.; Li, Z.; Luo, F.; Li, J.; et al. A bioinspired injectable antioxidant hydrogel for prevention of postoperative adhesion. J. Mater. Chem. B 2024, 12, 6968–6980. [Google Scholar] [CrossRef]
- Wan, Z.; He, J.; Yang, Y.; Chong, T.; Wang, J.; Guo, B.; Xue, L. Injectable adhesive self-healing biocompatible hydrogel for haemostasis, wound healing, and postoperative tissue adhesion prevention in nephron-sparing surgery. Acta Biomater. 2022, 152, 157–170. [Google Scholar] [CrossRef]
- Popescu, I.; Pelin, I.M.; Rosca, I.; Constantin, M. One-pot synthesis of gelatin/gum arabic hydrogels embedding silver nanoparticles as antibacterial materials. Gels 2025, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, C.; Zi, Y.; Gong, H.; Chen, L.; Kan, G.; Wang, X.; Zhong, J. Effects of polyphenol and gelatin types on the physicochemical properties and emulsion stabilization of polyphenol-crosslinked gelatin conjugates. Food Chem. X 2024, 22, 101250. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, A.; Lin, L.; El-Sohaimy, S.A.; Li, Y.; Wu, L.; Zhang, H. Schiff base cross-linked dialdehyde cellulose/gelatin composite aerogels as porous structure templates for oleogels preparation. Int. J. Biol. Macromol. 2023, 224, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Yang, D.; Xiao, Y.; Zhu, Y.; Chen, H.; Ni, S.; Zheng, S.; Zhu, L.; Shen, J.; Zhang, X. Facile general injectable gelatin/metal/tea polyphenol double nanonetworks remodel wound microenvironment and accelerate healing. Adv. Sci. 2023, 11, 2305405. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Tech. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Chen, J.M.; An, X.Y.; Xu, L.; Gao, Y.; Zhou, M.Q.; Liu, Z.G. Adhesive nanoparticle-in-microgel system with ros scavenging capability and hemostatic activity for postoperative adhesion prevention. Small 2024, 20, 2306598. [Google Scholar] [CrossRef]
- Chen, J.M.; Pan, C.; Gao, Y.; Chen, Q.H.; An, X.Y.; Liu, Z.G. Reactive oxygen species scavenging injectable hydrogel potentiates the therapeutic potential of mesenchymal stem cells in skin flap regeneration. ACS Appl. Mater. Interfaces 2024, 16, 17120–17128. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Gu, Z.; Zhou, L.; Liu, S.; Li, C.; Zhang, M.; Xu, Y.; Zhang, P.; Wen, Y. Janus mucosal dressing with a tough and adhesive hydrogel based on synergistic effects of gelatin, polydopamine, and nano-clay. Acta Biomater. 2022, 149, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Kong, Y.; Alimi, O.A.; Kuss, M.A.; Tu, H.; Hu, W.; Rafay, A.; Vikas, K.; Shi, W.; Lerner, M.; et al. Multifunctional microgel-based cream hydrogels for postoperative abdominal adhesion prevention. ACS Nano 2023, 17, 3847–3864. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Zhu, L.; Wen, Y.; Zhang, T.; Ren, P.; Wang, F.; Ji, Z. Combination of polypropylene mesh and in situ injectable mussel-inspired hydrogel in laparoscopic hernia repair for preventing post-surgical adhesions in the piglet model. ACS Biomater. Sci. Eng. 2020, 6, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Guan, P.; Xiao, C.; Liu, C.; Guan, Y.; Lin, Y.; Tian, Y.; Ren, K.; Huang, Y.; Fu, R.; et al. Injectable intrinsic photothermal hydrogel bioadhesive with on-demand removability for wound closure and MRSA-infected wound healing. Adv. Healthc. Mater. 2023, 12, 2203306. [Google Scholar]
- Zhao, X.; Zhang, M.; Guo, B.; Ma, P.X. Mussel-inspired injectable supramolecular and covalent bond crosslinked hydrogels with rapid self-healing and recovery properties via a facile approach under metal-free conditions. J. Mater. Chem. B 2016, 4, 6644–6651. [Google Scholar]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable self-healing adhesive chitosan hydrogel with antioxidative, antibacterial, and hemostatic activities for rapid hemostasis and skin wound healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, S.; Li, F.; Wang, Z.; Li, J.; Xia, C.; Yu, Y.; Cai, L.; Huang, Z. Self-healable and biodegradable soy protein-based protective functional film with low cytotoxicity and high mechanical strength. Chem. Eng. J. 2021, 404, 126505. [Google Scholar] [CrossRef]
- Wu, K.; Li, Y.Z.; Wang, H.; Xiao, J.; Ma, W.Y.; Li, L. High-strength and antioxidant gelatin/(oxidized) olive polyphenol films by melt extrusion method. Food Hydrocolloid 2025, 168, 111483. [Google Scholar] [CrossRef]
- Wang, M.; Lin, S.; Liu, M.; Jiao, J.; Mi, H.; Sun, J.; Liu, Y.; Guo, R.; Liu, S.; Fu, H.; et al. An injectable and rapidly degraded carboxymethyl chitosan/polyethylene glycol hydrogel for postoperative antiadhesion. Chem. Eng. J. 2023, 463, 142283. [Google Scholar] [CrossRef]
- Feng, W.; Wang, Z. Tailoring the swelling-shrinkable behavior of hydrogels for biomedical applications. Adv. Sci. 2023, 10, 2303326. [Google Scholar] [CrossRef]
- Hwang, U.; Moon, H.; Park, J.; Jung, H.W. Crosslinking and swelling properties of ph-responsive poly(ethylene glycol)/poly(acrylic acid) interpenetrating polymer network hydrogels. Polymers 2024, 16, 2149. [Google Scholar] [CrossRef]
- Wang, H.; Yi, X.; Liu, T.; Liu, J.; Wu, Q.; Ding, Y.; Liu, Z.; Wang, Q. An integrally formed janus hydrogel for robust wet-tissue adhesive and anti-postoperative adhesion. Adv. Mater. 2023, 35, e2300394. [Google Scholar] [CrossRef]
- Zhou, M.Q.; An, X.Y.; Liu, Z.G.; Chen, J.M. Biosafe polydopamine-decorated MnO2 nanoparticles with hemostasis and antioxidative properties for postoperative adhesion prevention. ACS Biomater. Sci. Eng. 2024, 10, 1031–1039. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Ouyang, X.; Yang, Y.; Chen, Y.; Luo, Q.; Zhang, Y.; Zhu, D.; Yu, X.; Li, L. Biomimetic hybrid hydrogel for hemostasis, adhesion prevention and promoting regeneration after partial liver resection. Bioact. Mater. 2022, 11, 41–51. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, J.; Zhang, J.; Yang, L.; Guo, X.; Tian, R.; Wu, H.; Li, Y.; Gu, Z. In situ fabrication of robust polyphenolic hydrogels for skin protection and repair. Chem. Mater. 2023, 35, 2191–2201. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, N.; Wen, S.; Wang, X.; Huang, X.; Xia, A. The mechanism study of enhanced antioxidant capacity: Intermolecular hydrogen bonds between epigallocatechin gallate and theanine in tea. LWT 2023, 189, 115523. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Fareed, M.S.; He, Y.; Lu, Y.; Yang, C.; Wang, Z.; Su, J.; Wang, P.; Yan, W.; et al. An injectable peptide hydrogel constructed of natural antimicrobial peptide J-1 and ADP shows anti-infection, hemostasis, and antiadhesion efficacy. ACS Nano 2022, 16, 7636–7650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-zno composite hydrogel for accelerating wound healing. Carbohyd. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.Y.; Wan, X.Z.; Shi, L.X.; Ye, M.S.; Zhang, Y.K.; Wang, S.T. A viscous-biofluid self-pumping organohydrogel dressing to accelerate diabetic wound healing. Adv. Mater. 2024, 36, 2401539. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, F.; Liao, Y.; Li, H.; Gao, K.; Liang, X.; Jiang, H.; Chen, F.; Wu, J.; Wang, Q.; et al. Biomimetic nanostructural materials based on placental amniotic membrane-derived nanofibers for self-healing and anti-adhesion during cesarean section. Biomaterials 2025, 317, 123081. [Google Scholar] [CrossRef]
- Lu, W.; Wang, X.; Kong, C.; Chen, S.; Hu, C.; Zhang, J. Hemoadhican-based bioabsorbable hydrogel for preventing postoperative adhesions. ACS Appl. Mater. Interfaces 2024, 16, 17267–17284. [Google Scholar] [CrossRef]
- Farhadpour, M.; Liu, G.; Zhao, Q.; You, Q.; Pan, M.; Bagheri, R.; Pircheraghi, G.; Shao, M. Crosslinked polyfluorene-based membranes with well-balanced properties for anion exchange membrane fuel cells. Chem. Eng. J. 2025, 509, 161203. [Google Scholar] [CrossRef]
- Jing, Y.; Deng, Z.; Yang, X.; Li, L.; Gao, Y.; Li, W. Ultrathin two-dimensional polydopamine nanosheets for multiple free radical scavenging and wound healing. Chem. Commun. 2020, 56, 10875–10878. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Sun, L.; Chen, J.; Su, M.; Liu, Z. Single-Step Engineered Gelatin-Based Hydrogel for Integrated Prevention of Postoperative Adhesion and Promotion of Wound Healing. Gels 2025, 11, 797. https://doi.org/10.3390/gels11100797
Wu X, Sun L, Chen J, Su M, Liu Z. Single-Step Engineered Gelatin-Based Hydrogel for Integrated Prevention of Postoperative Adhesion and Promotion of Wound Healing. Gels. 2025; 11(10):797. https://doi.org/10.3390/gels11100797
Chicago/Turabian StyleWu, Xinyu, Lei Sun, Jianmei Chen, Meiling Su, and Zongguang Liu. 2025. "Single-Step Engineered Gelatin-Based Hydrogel for Integrated Prevention of Postoperative Adhesion and Promotion of Wound Healing" Gels 11, no. 10: 797. https://doi.org/10.3390/gels11100797
APA StyleWu, X., Sun, L., Chen, J., Su, M., & Liu, Z. (2025). Single-Step Engineered Gelatin-Based Hydrogel for Integrated Prevention of Postoperative Adhesion and Promotion of Wound Healing. Gels, 11(10), 797. https://doi.org/10.3390/gels11100797







