Physicochemical Characterization of Soluble and Insoluble Fibers from Berry Pomaces
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Characterization of SDF and IDF from Berry Pomace
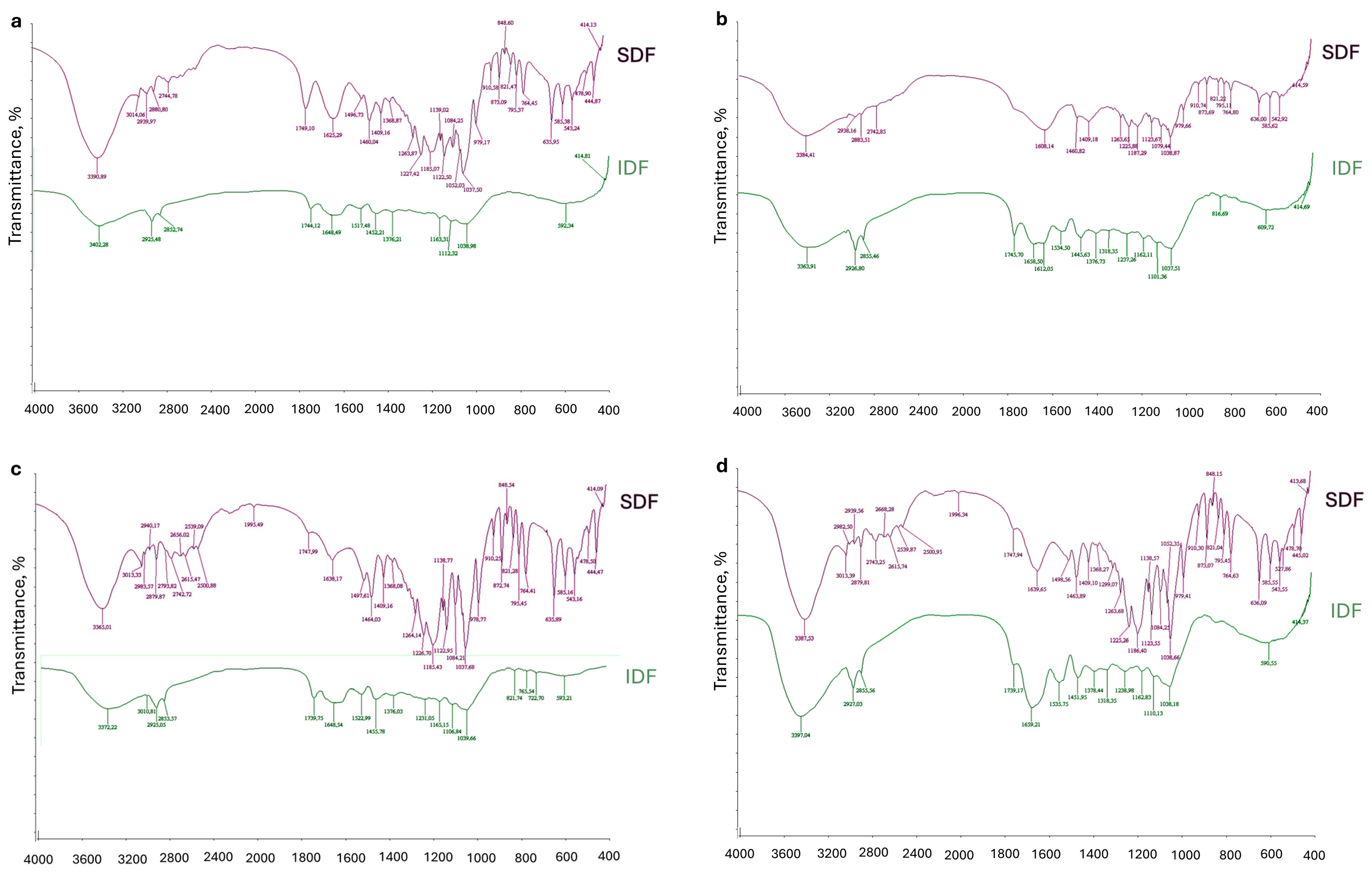
2.2. FT-IR of SDF and IDF Fractions
2.3. Technological Properties of SDF and IDF Fractions
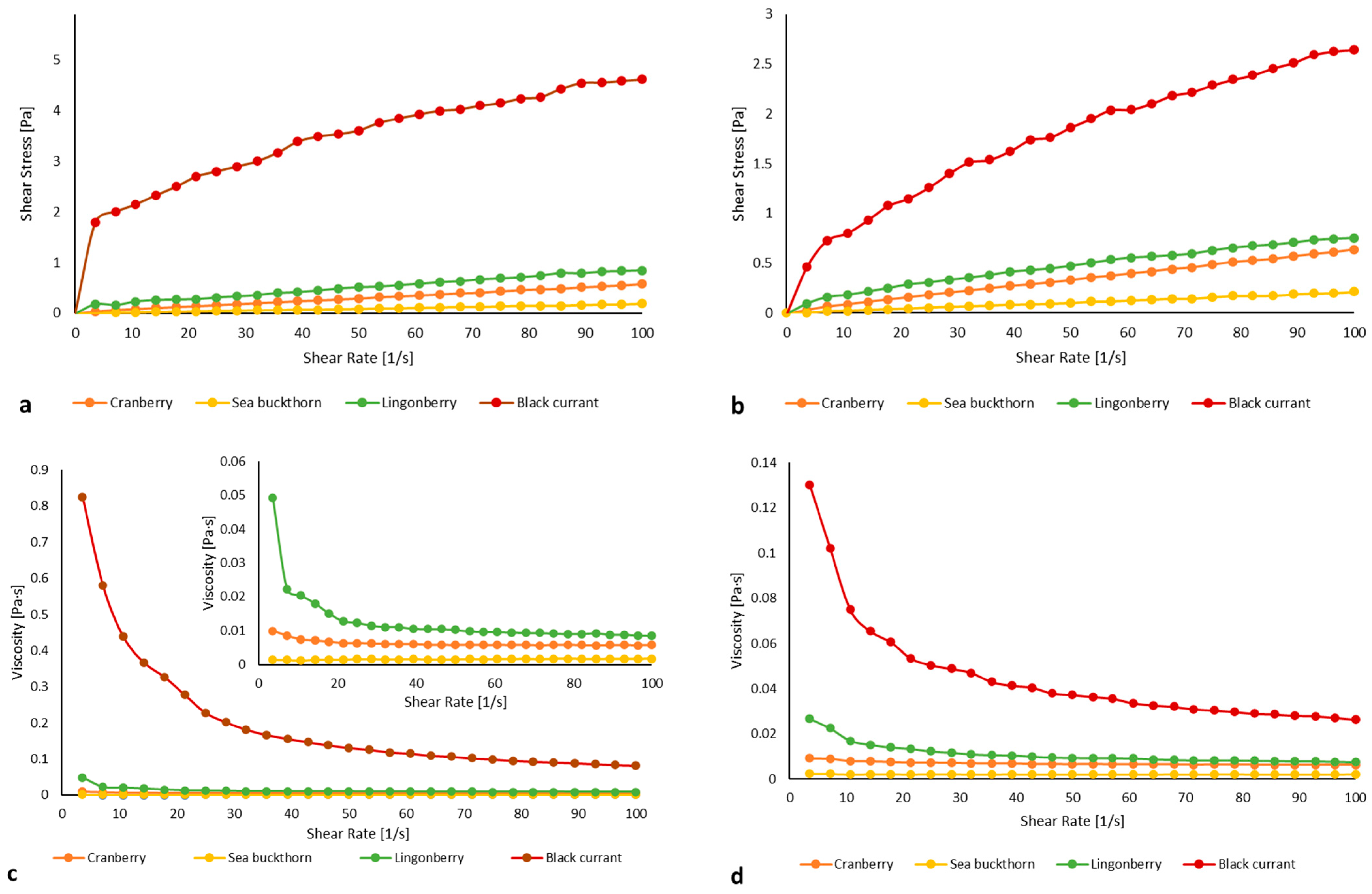
2.4. Static Rheological Properties of SDF Fraction
3. Conclusions
4. Materials and Methods
4.1. Berry Pomaces
4.2. Preparation of IDF and SDF Fractions
4.3. Determination of Chemical Composition
4.4. Technological Properties
4.5. FT-IR
4.6. Determination of Static Rheological Properties
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BD | Bulk density |
| DF | Dietary fiber |
| FT-IR | Fourier transform infrared |
| IDF | Insoluble dietary fiber |
| ORC | Oil retention capacity |
| K | Consistency index |
| SDF | Soluble dietary fiber |
| WRC | Water retention capacity |
| WSC | Water swelling capacity |
| WSI | Solubility index |
| n | flow behavior index |
| ηap | apparent viscosity |
References
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Coleman, C.M.; Ferreira, D. Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity. Molecules 2020, 25, 881. [Google Scholar] [CrossRef]
- FDA. Authors Health Claims: Fruits, Vegetables, and Grain Products That Contain Fiber, Particularly Soluble Fiber, and Risk of Coronary Heart Disease. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-E/section-101.77 (accessed on 28 May 2022).
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Use of Berry Pomace to Design Functional Foods. Food Rev. Int. 2023, 39, 3204–3224. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-Aligned Dietary Fiber Definitions Help to Bridge the “Fiber Gap”. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- Al Faruq, A.; Farahnaky, A.; Torley, P.J.; Buckow, R.; Eri, R.; Majzoobi, M. Sustainable Approaches to Boost Soluble Dietary Fibre in Foods: A Path to Healthier Foods. Food Hydrocoll. 2025, 162, 110880. [Google Scholar] [CrossRef]
- Alba, K.; MacNaughtan, W.; Laws, A.P.; Foster, T.J.; Campbell, G.M.; Kontogiorgos, V. Fractionation and Characterisation of Dietary Fibre from Blackcurrant Pomace. Food Hydrocoll. 2018, 81, 398–408. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine Grape Pomace as Antioxidant Dietary Fibre for Enhancing Nutritional Value and Improving Storability of Yogurt and Salad Dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, Textural and Rheological Properties of Probiotic Yogurt Fortified with Fibre-Rich Pineapple Peel Powder during Refrigerated Storage. LWT 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Islam, M.R.; Hassan, Y.I.; Das, Q.; Lepp, D.; Hernandez, M.; Godfrey, D.V.; Orban, S.; Ross, K.; Delaquis, P.; Diarra, M.S. Dietary Organic Cranberry Pomace Influences Multiple Blood Biochemical Parameters and Cecal Microbiota in Pasture-Raised Broiler Chickens. J. Funct. Foods 2020, 72, 104053. [Google Scholar] [CrossRef]
- Reißner, A.-M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and Physicochemical Properties of Dried Berry Pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, C.; Fu, X.; Huang, Q.; Chen, Q. Physicochemical, Functional and Biological Properties of Soluble Dietary Fibers Obtained from Rosa Roxburghii Tratt Pomace Using Different Extraction Methods. Process Biochem. 2023, 128, 40–48. [Google Scholar] [CrossRef]
- Zou, X.; Xu, X.; Chao, Z.; Jiang, X.; Zheng, L.; Jiang, B. Properties of Plant-Derived Soluble Dietary Fibers for Fiber-Enriched Foods: A Comparative Evaluation. Int. J. Biol. Macromol. 2022, 223, 1196–1207. [Google Scholar] [CrossRef]
- Mada, T.; Duraisamy, R.; Guesh, F. Optimization and Characterization of Pectin Extracted from Banana and Papaya Mixed Peels Using Response Surface Methodology. Food Sci. Nutr. 2022, 10, 1222–1238. [Google Scholar] [CrossRef]
- Bayar, N.; Kriaa, M.; Kammoun, R. Extraction and Characterization of Three Polysaccharides Extracted from Opuntia Ficus Indica Cladodes. Int. J. Biol. Macromol. 2016, 92, 441–450. [Google Scholar] [CrossRef]
- Roman, L.; Guo, M.; Terekhov, A.; Grossutti, M.; Vidal, N.P.; Reuhs, B.L.; Martinez, M.M. Extraction and Isolation of Pectin Rich in Homogalacturonan Domains from Two Cultivars of Hawthorn Berry (Crataegus pinnatifida). Food Hydrocoll. 2021, 113, 106476. [Google Scholar] [CrossRef]
- Jarrín-Chacón, J.P.; Núñez-Pérez, J.; Espín-Valladares, R.d.C.; Manosalvas-Quiroz, L.A.; Rodríguez-Cabrera, H.M.; Pais-Chanfrau, J.M. Pectin Extraction from Residues of the Cocoa Fruit (Theobroma cacao L.) by Different Organic Acids: A Comparative Study. Foods 2023, 12, 590. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Andersson, A.A.M.; Andersson, R.; Kamal-Eldin, A. Lignin Is the Main Determinant of Total Dietary Fiber Differences between Date Fruit (Phoenix dactylifera L.) Varieties. NFS J. 2020, 21, 16–21. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, Y.; Hu, J.; Ding, J.; Yang, P.; Liu, Y.; Lu, Y.; Jin, Y.; Tang, H.; Liu, Y.; et al. A Novel Polysaccharide from Lonicerae Japonicae Caulis: Characterization and Effects on the Function of Fibroblast-like Synoviocytes. Carbohydr. Polym. 2022, 292, 119674. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and Characterization of Nanofibers from Agricultural Residues—Wheat Straw and Soy Hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR Spectroscopy, a Reliable Method for Routine Analysis of the Degree of Methylesterification of Pectin in Different Fruit- and Vegetable-Based Matrices. Food Chem. 2015, 176, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, M.; Dell’Anna, R.; Amato, A.; Balestrini, R.; Dal Santo, S.; Monti, F.; Zenoni, S. Active Rearrangements in the Cell Wall Follow Polymer Concentration during Postharvest Withering in the Berry Skin of Vitis Vinifera Cv. Corvina. Plant Physiol. Biochem. 2019, 135, 411–422. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Lv, Y.; He, Q. Extraction and Functional Properties of Water-Soluble Dietary Fiber from Apple Pomace. J. Food Process Eng. 2014, 37, 293–298. [Google Scholar] [CrossRef]
- Deng, M.; Lin, Y.; Dong, L.; Jia, X.; Shen, Y.; Liu, L.; Chi, J.; Huang, F.; Zhang, M.; Zhang, R. Physicochemical and Functional Properties of Dietary Fiber from Pummelo (Citrus grandis L. Osbeck) and Grapefruit (Citrus paradisi Mcfad) Cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical Characterization of Five Types of Citrus Dietary Fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Wu, A.; Song, Y.; Li, Q.; Liao, X.; Zhao, J. Towards Understanding Pectin-Protein Interaction and the Role of Pectin in Plant-Based Meat Analogs Constructing. LWT 2024, 202, 116325. [Google Scholar] [CrossRef]
- Caparino, O.A.; Tang, J.; Nindo, C.I.; Sablani, S.S.; Powers, J.R.; Fellman, J.K. Effect of Drying Methods on the Physical Properties and Microstructures of Mango (Philippine ‘Carabao’ Var.) Powder. J. Food Eng. 2012, 111, 135–148. [Google Scholar] [CrossRef]
- Alba, K.; Campbell, G.M.; Kontogiorgos, V. Dietary Fibre from Berry-Processing Waste and Its Impact on Bread Structure: A Review. J. Sci. Food Agric. 2019, 99, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Wittmüss, M.; Amft, J.; Heyn, T.R.; Schwarz, K. Oil Binding Capacity and Related Physicochemical Properties of Commercial Plant Protein Products. Food Biosci. 2024, 59, 103823. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical Profile, Functional and Antioxidant Properties of Tomato Peel Fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Mahloko, L.M.; Silungwe, H.; Mashau, M.E.; Kgatla, T.E. Bioactive Compounds, Antioxidant Activity and Physical Characteristics of Wheat-Prickly Pear and Banana Biscuits. Heliyon 2019, 5, e02479. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional Properties, Bioactive Compounds, and in Vitro Gastrointestinal Digestion Study of Dried Fruit Pomace Powders as Functional Food Ingredients. LWT 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I. Peanut Protein Concentrate: Production and Functional Properties as Affected by Processing. Food Chem. 2007, 103, 121–129. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The Role of Dietary Fiber in the Bioaccessibility and Bioavailability of Fruit and Vegetable Antioxidants. J. Food Sci. 2011, 76, R6. [Google Scholar] [CrossRef]
- Xu, G.Y.; Liao, A.M.; Huang, J.H.; Zhang, J.G.; Thakur, K.; Wei, Z.J. The Rheological Properties of Differentially Extracted Polysaccharides from Potatoes Peels. Int. J. Biol. Macromol. 2019, 137, 1–7. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-Related Gelling of Pectins and Linking with Other Natural Compounds: A Review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Sommer, S.; Hoffmann, J.L.; Fraatz, M.A.; Zorn, H. Upcycling of Black Currant Pomace for the Production of a Fermented Beverage with Wolfiporia cocos. J. Food Sci. Technol. 2023, 60, 1313–1322. [Google Scholar] [CrossRef]
- Lau, A.T.Y.; Arvaj, L.; Strange, P.; Goodwin, M.; Barbut, S.; Balamurugan, S. Effect of Cranberry Pomace on the Physicochemical Properties and Inactivation of Salmonella during the Manufacture of Dry Fermented Sausages. Curr. Res. Food Sci. 2021, 4, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Jaśniewska, A.; Diowksz, A. Wide Spectrum of Active Compounds in Sea Buckthorn (Hippophae Rhamnoides) for Disease Prevention and Food Production. Antioxidants 2021, 10, 1279. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C.; Li, J.; Hussain, S.; Yan, S.; Wang, Q. Effects of Extrusion on Structural and Physicochemical Properties of Soluble Dietary Fiber from Nodes of Lotus Root. LWT 2018, 93, 204–211. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, C.; Qiu, W.Y.; Chen, T.T.; Yang, Y.; Wang, W.H.; Zhang, H.N. Ultrasonic Treatment at Different PH Values Affects the Macromolecular, Structural, and Rheological Characteristics of Citrus Pectin. Food Chem. 2021, 341, 128216. [Google Scholar] [CrossRef]
- Moreira, H.R.; Munarin, F.; Gentilini, R.; Visai, L.; Granja, P.L.; Tanzi, M.C.; Petrini, P. Injectable Pectin Hydrogels Produced by Internal Gelation: PH Dependence of Gelling and Rheological Properties. Carbohydr. Polym. 2014, 103, 339–347. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kastner, H.; Urbisch, A.; Kroh, L.W.; Drusch, S. Thermal Degradation of Citrus Pectin in Low-Moisture Environment—Influence of Acidic and Alkaline Pre-Treatment. Food Hydrocoll. 2019, 86, 104–115. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Li, L.; Liu, H. Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Aguayo-Mendoza, M.G.; Ketel, E.C.; van der Linden, E.; Forde, C.G.; Piqueras-Fiszman, B.; Stieger, M. Oral Processing Behavior of Drinkable, Spoonable and Chewable Foods Is Primarily Determined by Rheological and Mechanical Food Properties. Food Qual. Prefer. 2019, 71, 87–95. [Google Scholar] [CrossRef]
- Xie, J.; Peng, G.; Hu, X.; Xie, J.; Chen, Y.; Dong, R.; Si, J.; Yang, C.; Yu, Q. Physicochemical Characteristics of Soluble Dietary Fiber Obtained from Grapefruit Peel Insoluble Dietary Fiber and Its Effects on Blueberry Jam. Foods 2022, 11, 3735. [Google Scholar] [CrossRef] [PubMed]
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, Enzymatic-Ultrasonic and Alkaline Extraction of Soluble Dietary Fibre from Flaxseed—A Physicochemical Approach. Food Hydrocoll. 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Chokeberry Pomace Valorization into Food Ingredients by Enzyme-Assisted Extraction: Process Optimization and Product Characterization. Food Bioprod. Process. 2017, 105, 36–50. [Google Scholar] [CrossRef]
- Megazyme. Total Dietary Fiber Assay Procedure; Megazyme: Bray, Ireland, 2017. [Google Scholar]
- Helrich, K. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; AOAC International: Arlington, VA, USA, 1990. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of Carrot (Daucus carota Linn. Var. Sativa Hoffm.) Pomace Insoluble Dietary Fiber with Complex Enzyme Method, Ultrafine Comminution, and High Hydrostatic Pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Jagelavičiutė, J.; Čižeikienė, D.; Bašinskienė, L. Enzymatic Modification of Apple Pomace and Its Application in Conjunction with Probiotics for Jelly Candy Production. Appl. Sci. 2025, 15, 599. [Google Scholar] [CrossRef]
| DF Source | Yield, % | Moisture, % | Protein, g/100 g d·m. | Reducing Sugars, g/100 g d·m. | |
|---|---|---|---|---|---|
| Cranberry | SDF | 20.71 ± 0.91 c | 8.19 ± 0.22 c | 24.17 ± 0.2 f | 9.15 ± 0.2 f |
| IDF | 66.12 ± 2.94 e | 4.55 ± 0.21 a | 3.92 ± 0.03 b | 2.0 ± 0.03 a | |
| Black currant | SDF | 13.12 ± 0.60 b | 9.42 ± 0.57 d | 29.09 ± 0.2 g | 12.57 ± 0.4 g |
| IDF | 46.96 ± 1.82 d | 5.71 ± 0.76 b | 8.67 ± 0.5 e | 6.58 ± 0.1 c | |
| Lingonberry | SDF | 15.80 ± 0.64 b | 8.42 ± 0.26 cd | 39.67 ± 0.2 h | 8.52 ± 0.2 e |
| IDF | 70.60 ± 2.36 e | 4.71 ± 0.23 ab | 4.08 ± 0.4 c | 3.81 ± 0.04 b | |
| Sea buckthorn | SDF | 7.40 ± 0.29 a | 9.12 ± 0.32 cd | 5.62 ± 0.03 d | 11.91 ± 0.3 g |
| IDF | 68.00 ± 2.45 e | 5.21 ± 0.21 b | 2.84 ± 0.02 a | 7.97 ± 0.1 d | |
| Dietary Fiber Source | WRC, g/g d·m. | WSC, mL/g d·m. | ORC, g/g d·m. | WSI, % | BD, g/mL d·m. | |
|---|---|---|---|---|---|---|
| Cranberry | SDF | 8.95 ± 0.10 d | 1.10 ± 0.0 b | 2.64 ± 0.12 b | 71.8 ± 0.6 g | 0.32 ± 0.01 c |
| IDF | 10.69 ± 0.36 e | 2.52 ± 0.06 e | 7.24 ± 0.32 f | 4.8 ± 0.3 a | 0.13 ± 0.01 a | |
| Black currant | SDF | 13.04 ± 0.1 f | 2.20 ± 0.0 d | 5.48 ± 0.2 d | 64.1 ± 0.1 e | 0.32 ±0.0 c |
| IDF | 9.60 ± 0.6 de | 3.15 ± 0.0 f | 3.34 ± 0.2 c | 4.5 ± 0.1 a | 0.17 ± 0.0 b | |
| Lingonberry | SDF | 15.36 ± 0.08 g | 0.55 ± 0.0 a | 15.36 ± 0.08 g | 69.42 ± 0.2 f | 0.36 ± 0.0 d |
| IDF | 6.53 ± 0.03 c | 1.57 ± 0.01 c | 6.53 ± 0.03 e | 7.85 ± 0.0 b | 0.19 ± 0.0 b | |
| Sea buckthorn | SDF | 4.10 ± 0.05 a | 2.81 ± 0.21 e | 1.62 ± 0.03 a | 20.7 ± 0.1 d | 0.31 ± 0.1 c |
| IDF | 4.68 ± 0.21 b | 3.82 ± 0.16 g | 1.70 ± 0.07 a | 12.1 ± 0.3 c | 0.34 ± 0.1 cd | |
| Dietary Fiber Source | L* | a* | b* | |
|---|---|---|---|---|
| Cranberry | SDF | 58.63 ± 0.41 f | 12.78 ± 0.07 g | 15.20 ± 0.04 e |
| IDF | 46.37 ± 0.21 d | 14.28 ± 0.05 h | 11.68 ± 0.02 c | |
| Black currant | SDF | 23.64 ± 0.20 a | 8.14 ± 0.08 e | 2.80 ± 0.05 a |
| IDF | 37.36 ± 0.02 b | 8.62 ± 0.01 f | 3.93 ± 0.01 b | |
| Lingonberry | SDF | 56.36 ± 0.22 e | 0.91 ± 0.0 a | 19.12 ± 0.04 g |
| IDF | 37.61 ± 0.13 c | 1.22 ± 0.0 b | 18.71 ± 0.02 f | |
| Sea buckthorn | SDF | 65.64 ± 0.12 g | 5.59 ± 0.01 d | 12.76 ± 0.04 d |
| IDF | 56.66 ± 0.02 e | 5.20 ± 0.00 c | 21.85 ± 0.01 h | |
| DF Source | pH | K (Pa sn) | n | ηap (Pa s) | R2 |
|---|---|---|---|---|---|
| Cranberry | 4.70 ± 0.01 b | 0.0099 ± 0.0004 c | 0.528 ± 0.02 b | 0.0058 ± 0.0004 c | 0.9990 |
| 7.00 ± 0.01 d | 0.0053 ± 0.0002 b | 0.718 ± 0.01 e | 0.0063 ± 0.0001 d | 0.9990 | |
| Black currant | 5.14 ± 0.02 c | 1.0520 ± 0.083 g | 0.317 ± 0.015 a | 0.0729 ± 0.0090 g | 0.9810 |
| 7.00 ± 0.03 d | 0.2389 ± 0.0052 f | 0.524 ± 0.005 b | 0.0371 ± 0.0003 f | 0.9970 | |
| Lingonberry | 4.73 ± 0.01 b | 0.0579 ± 0.0047 e | 0.563 ± 0.018 c | 0.0103 ± 0.0007 e | 0.9463 |
| 7.00 ± 0.02 d | 0.0428 ± 0.0023 d | 0.621 ± 0.007 d | 0.0097 ± 0.0005 e | 0.9970 | |
| Sea buckthorn | 4.57 ± 0.02 a | 0.0022 ± 0.0001 a | 0.925 ± 0.034 f | 0.0016 ± 0.0001 a | 0.9920 |
| 7.00 ± 0.03 d | 0.0022 ± 0.00001 a | 0.992 ± 0.009 g | 0.0021 ± 0.0002 b | 0.9980 |
| Pomace | SDF, g/100 d·m. | IDF, g/100 g d·m. | Proteins, g/100 g d·m. | Lipids, g/100 g d·m. | Ash, g/100 g d·m. |
|---|---|---|---|---|---|
| Cranberry | 12.24 ± 0.54 | 61.78 ± 0.22 | 7.60 ± 0.09 | 7.13 ± 0.39 | 1.00 ± 0.01 |
| Black currant | 8.85 ± 0.35 | 42.20± 0.90 | 9.74 ± 0.46 | 8.67 ± 0.10 | 4.02 ± 0.03 |
| Lingonberry | 8.94 ± 0.15 | 68.26± 1.47 | 9.03 ± 0.01 | 7.60 ± 0.04 | 1.24 ± 0.01 |
| Sea buckthorn | 6.72 ± 0.44 | 63.93± 0.68 | 25.45 ± 0.41 | 1.80 ± 0.20 | 1.53 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagelavičiūtė, J.; Šimkutė, S.; Kairė, A.; Kaminskytė, G.; Bašinskienė, L.; Čižeikienė, D. Physicochemical Characterization of Soluble and Insoluble Fibers from Berry Pomaces. Gels 2025, 11, 796. https://doi.org/10.3390/gels11100796
Jagelavičiūtė J, Šimkutė S, Kairė A, Kaminskytė G, Bašinskienė L, Čižeikienė D. Physicochemical Characterization of Soluble and Insoluble Fibers from Berry Pomaces. Gels. 2025; 11(10):796. https://doi.org/10.3390/gels11100796
Chicago/Turabian StyleJagelavičiūtė, Jolita, Simona Šimkutė, Aurelija Kairė, Gabrielė Kaminskytė, Loreta Bašinskienė, and Dalia Čižeikienė. 2025. "Physicochemical Characterization of Soluble and Insoluble Fibers from Berry Pomaces" Gels 11, no. 10: 796. https://doi.org/10.3390/gels11100796
APA StyleJagelavičiūtė, J., Šimkutė, S., Kairė, A., Kaminskytė, G., Bašinskienė, L., & Čižeikienė, D. (2025). Physicochemical Characterization of Soluble and Insoluble Fibers from Berry Pomaces. Gels, 11(10), 796. https://doi.org/10.3390/gels11100796






