Plant-Based Sunscreen Emulgel: UV Boosting Effect of Bilberry and Green Tea NaDES Extracts
Abstract
1. Introduction
2. Results and Discussion
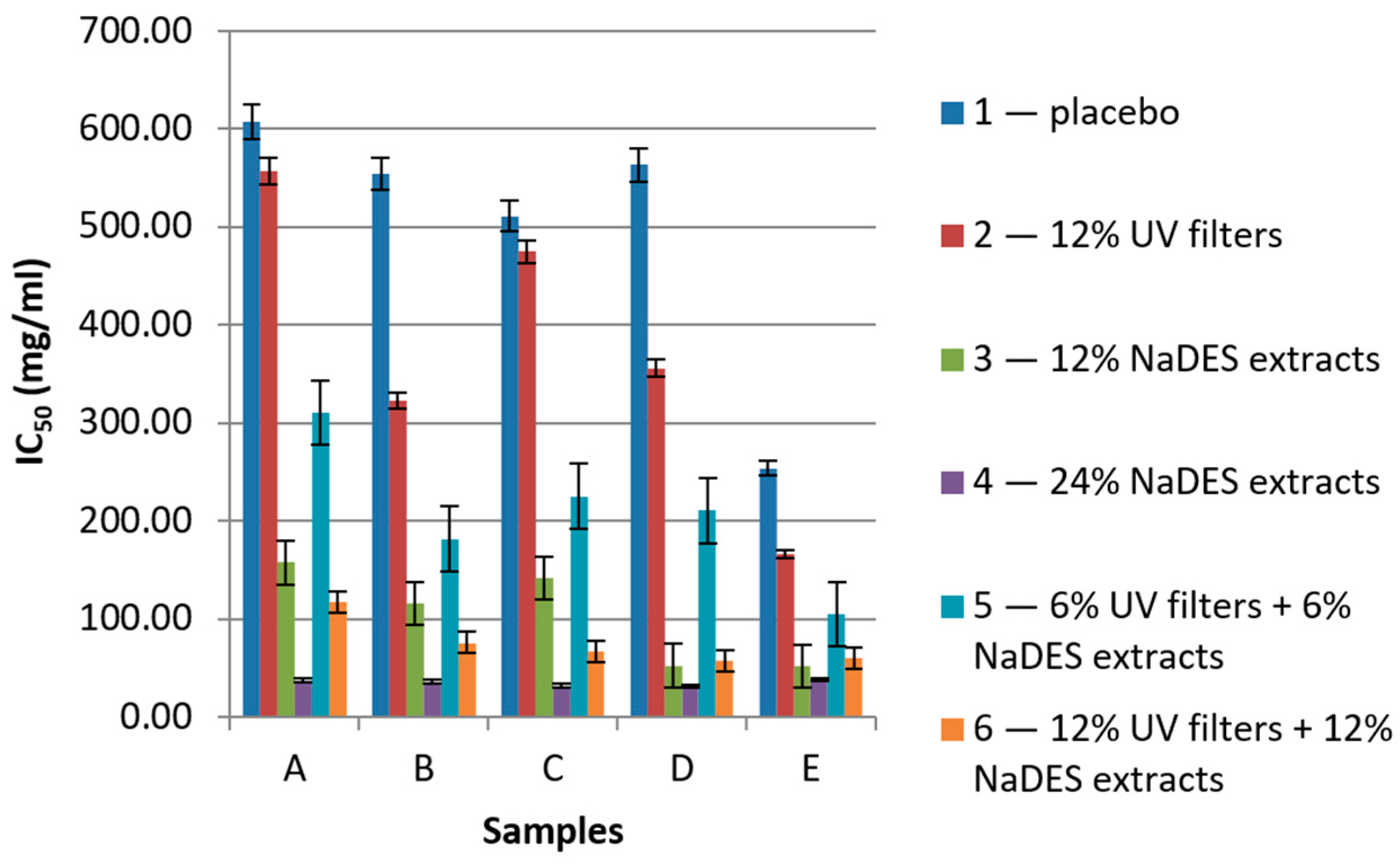
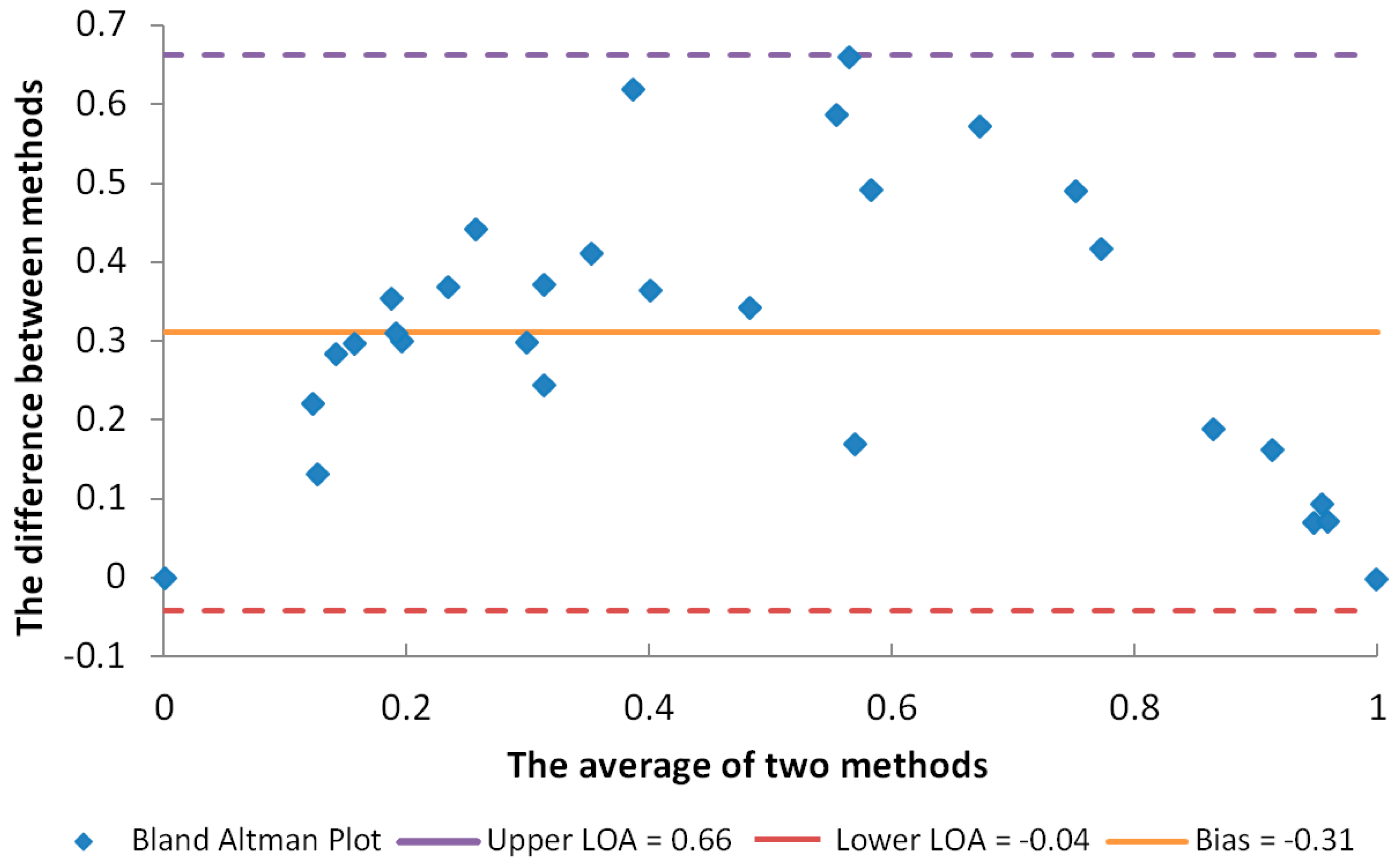
2.1. Determination of UV-Protective Activity
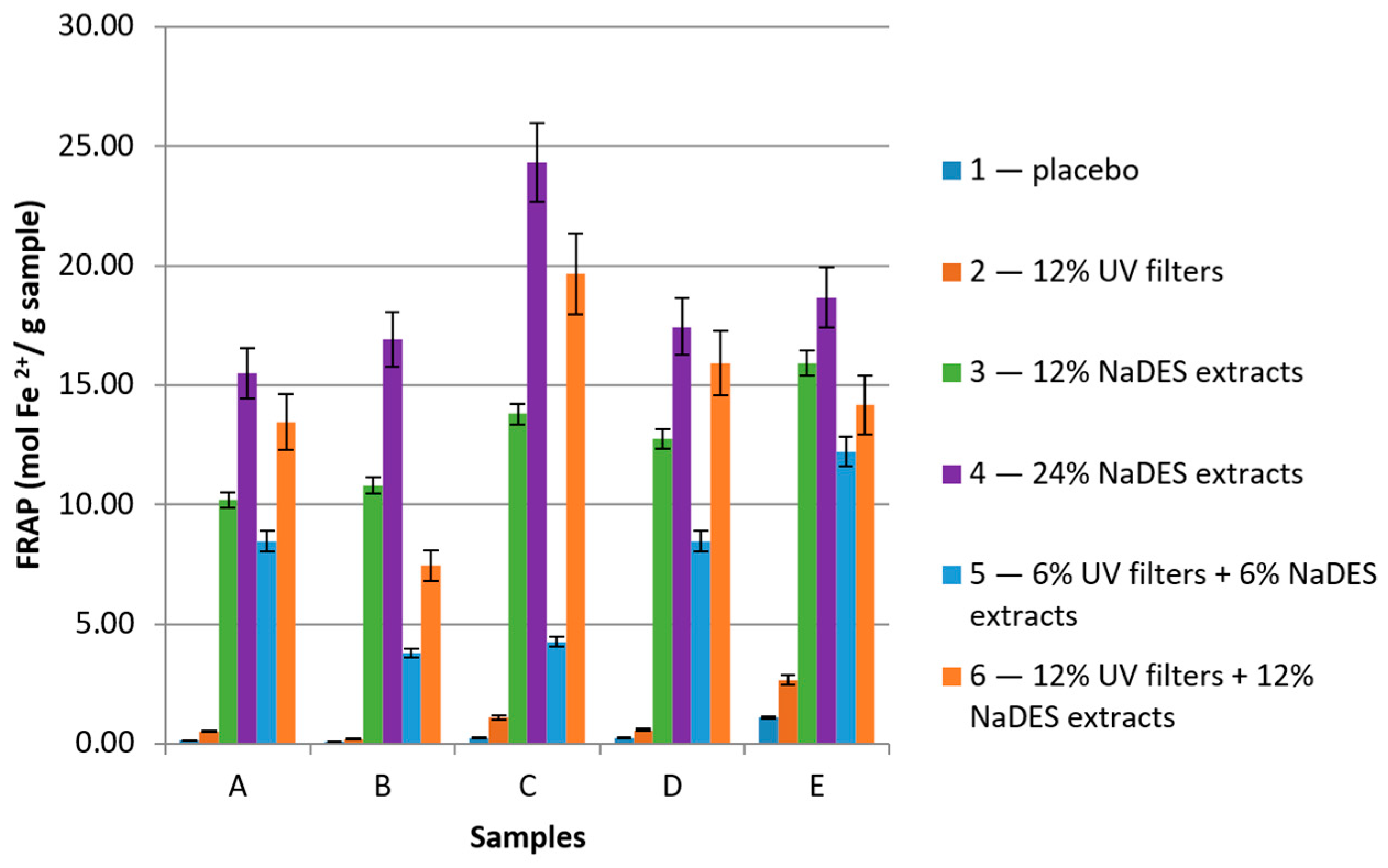
2.2. Determination of Antioxidant Activity
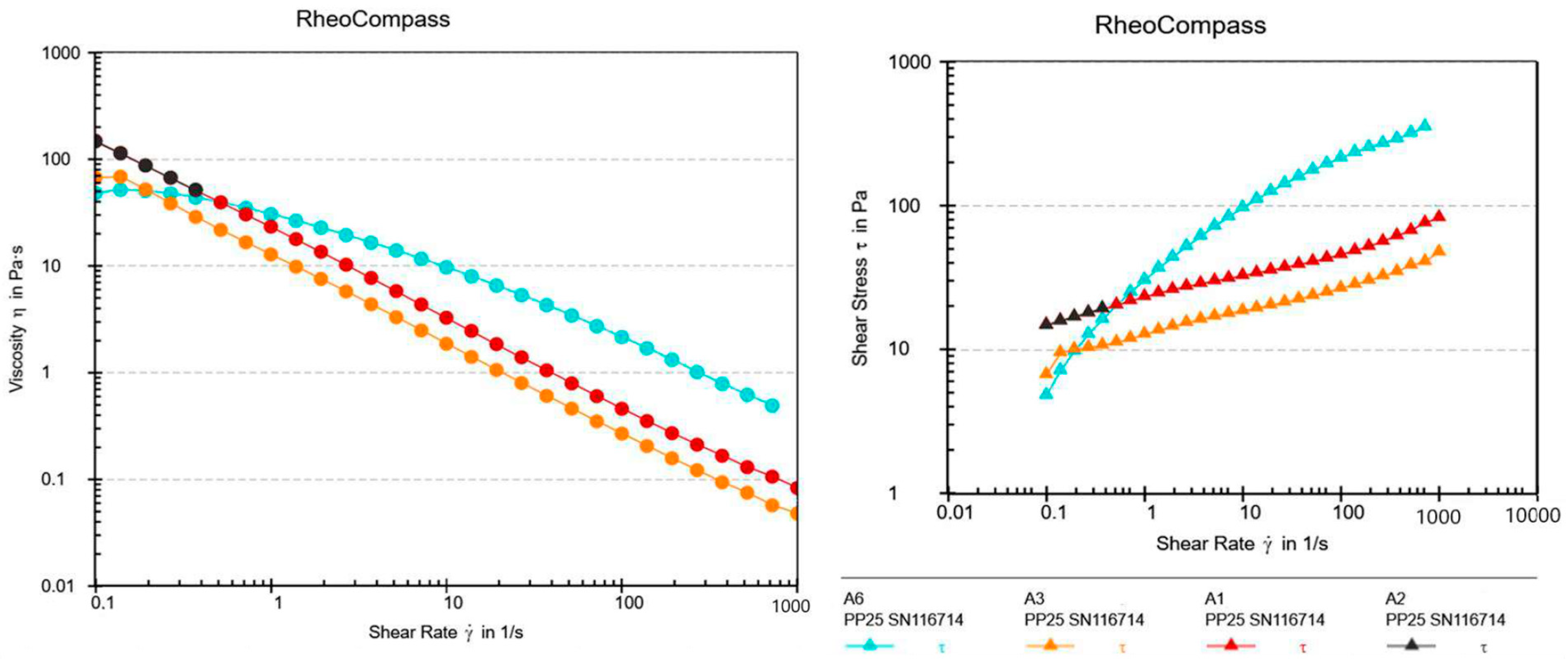
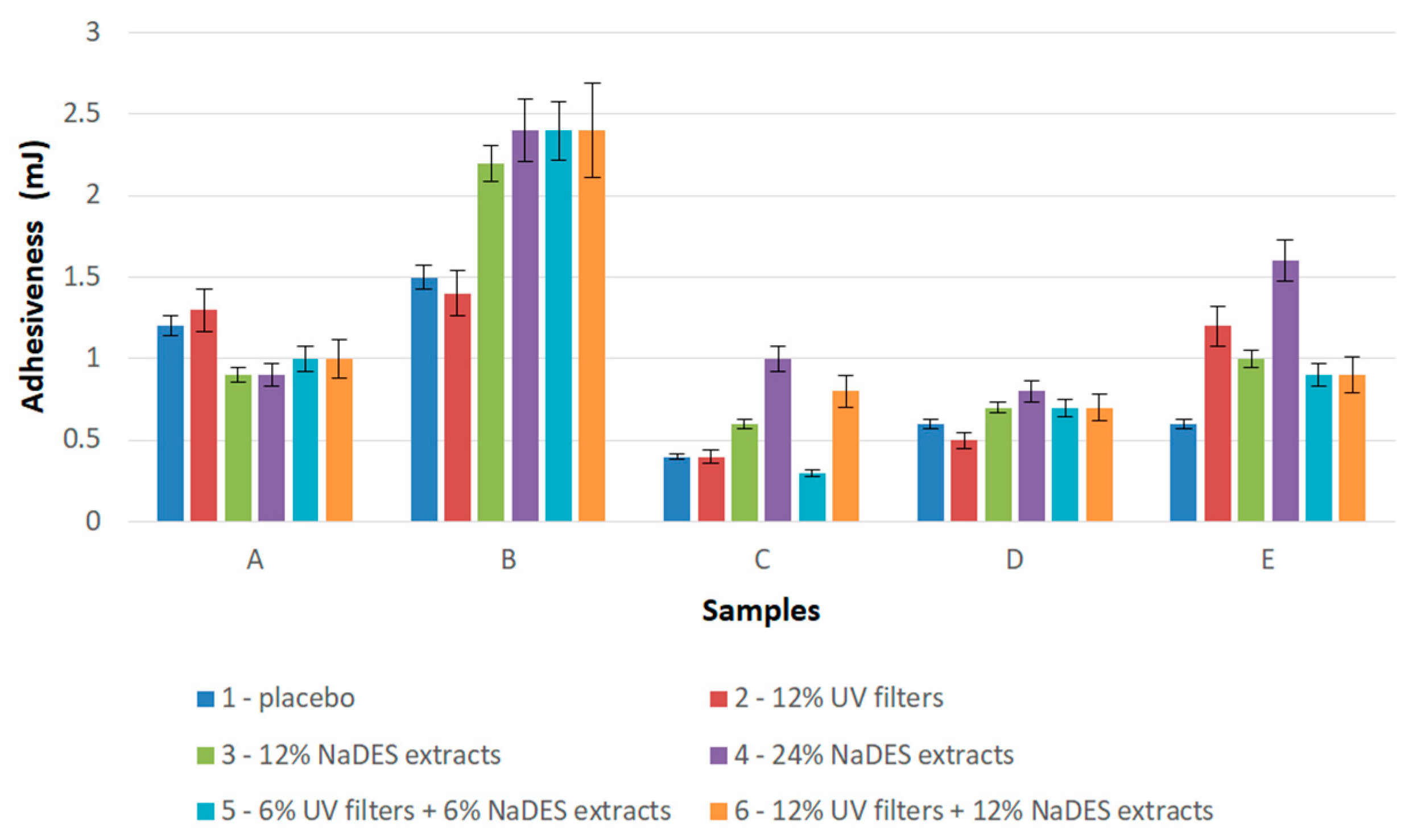
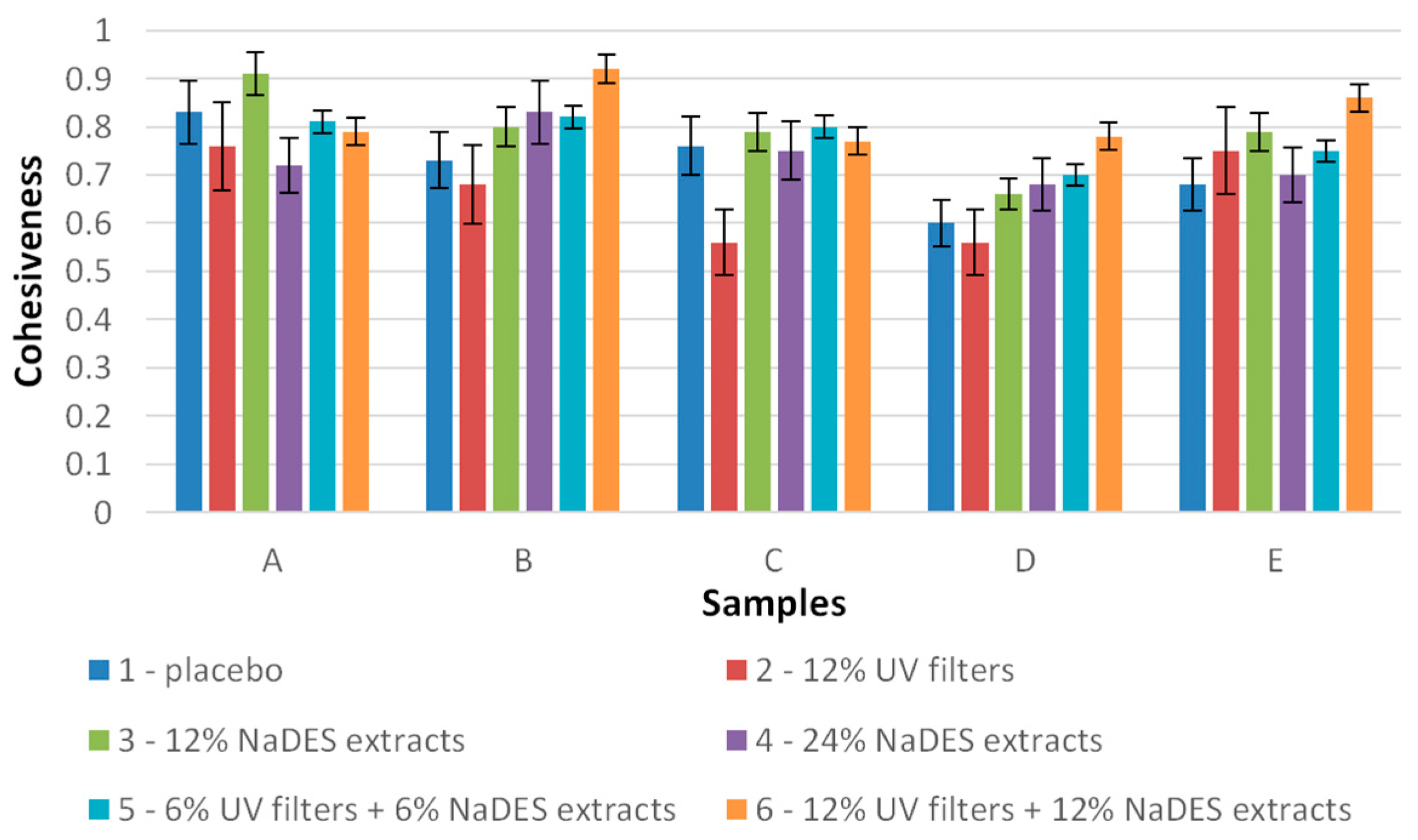
2.3. Texture Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. NaDES Extract Preparation
4.3. SPF Calculation Using Mansur Equation
4.4. Semi-Solid Samples
4.4.1. Hydrogel Preparation
4.4.2. Cream (Emulsion) Preparation
4.4.3. Emulgel Preparation
4.5. Physicochemical Characterization of the Samples
4.5.1. Physicochemical and Organoleptic Characterization
4.5.2. Stability Assays
4.5.3. Rheology Analysis
4.5.4. Texture Analysis
4.6. UV-Protective Activity of the Semi-Solid Samples
4.6.1. Calculation of SPF of Semi-Sold Samples Using Mansur Equation
4.6.2. Calculation of SPF and UVA-PF of Semi-Sold Samples Using PMMA Plates
4.6.3. In Silico SPF Prediction
4.7. Antioxidant Activity of Semi-Solid Samples
4.7.1. DPPH Radical Scavenging Activity
4.7.2. Ferric Ion Reducing Antioxidant Power (FRAP Assay)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based Active Photoprotectants for Sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural Products and Extracts from Plants as Natural UV Filters for Sunscreens: A Review. Anim. Model. Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Xu, C.-C.; Wang, B.; Pu, Y.-Q.; Tao, J.-S.; Zhang, T. Advances in Extraction and Analysis of Phenolic Compounds from Plant Materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A Novel Approach in Skin Care: By-Product Extracts as Natural UV Filters and an Alternative to Synthetic Ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of Phenols Recovered from Olive Mill Wastewater as UV Booster in Cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Lorquin, F.; Lorquin, J.; Claeys-Bruno, M.; Rollet, M.; Robin, M.; Di Giorgio, C.; Piccerelle, P. Lignosulfonate Is an Efficient SPF Booster: Application to Eco-Friendly Sunscreen Formulations. Sustain. Chem. Pharm. 2021, 24, 100539. [Google Scholar] [CrossRef]
- Aguilera, J.; Vicente-Manzanares, M.; de Gálvez, M.V.; Herrera-Ceballos, E.; Rodríguez-Luna, A.; González, S. Booster Effect of a Natural Extract of Polypodium Leucotomos (Fernblock®) That Improves the UV Barrier Function and Immune Protection Capability of Sunscreen Formulations. Front. Med. 2021, 8, 684665. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.P.; Kinghorn, A.D. Extraction of Plant Secondary Metabolites. In Natural Products Isolation; Methods in Molecular Biology; Sarker, S.D., Nahar, L., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 864, pp. 341–366. ISBN 978-1-61779-623-4. [Google Scholar]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J. Multi-Functioning Deep Eutectic Solvents as Extraction and Storage Media for Bioactive Natural Products That Are Readily Applicable to Cosmetic Products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Martinović, M.; Krgović, N.; Nešić, I.; Žugić, A.; Tadić, V.M. Conventional vs. Green Extraction Using Natural Deep Eutectic Solvents—Differences in the Composition of Soluble Unbound Phenolic Compounds and Antioxidant Activity. Antioxidants 2022, 11, 2295. [Google Scholar] [CrossRef] [PubMed]
- Milutinov, J.; Krstonošić, V.; Ćirin, D.; Pavlović, N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers 2023, 15, 2302. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Tadić, V.M.; Žugić, A.; Martinović, M.; Stanković, M.; Maksimović, S.; Frank, A.; Nešić, I. Enhanced Skin Performance of Emulgel vs. Cream as Systems for Topical Delivery of Herbal Actives (Immortelle Extract and Hemp Oil). Pharmaceutics 2021, 13, 1919. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Moturi, V.; Lee, Y. Thixotropic Property in Pharmaceutical Formulations. J. Control. Release 2009, 136, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Calixto, L.S.; Maia Campos, P.M.B.G.; Savary, G.; Picard, C. Interactions Between UV Filters and Active Substances in Emulsion: Effect on Microstructure, Physicochemical and In-Vivo Properties. Int. J. Pharm. 2018, 553, 220–228. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Law, J.C.F.; Lam, T.K.; Leung, K.S.Y. Risks of Organic UV Filters: A Review of Environmental and Human Health Concern Studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef] [PubMed]
- Stojiljković, D.; Arsić, I.; Tadic, V. Oil Extracts of Wild Apple Fruit as Active Substances in Uv Protection Preparations. RAD Assoc. J. 2016, 1, 187–192. [Google Scholar] [CrossRef][Green Version]
- Zou, W.; Ramanathan, R.; Urban, S.; Sinclair, C.; King, K.; Tinker, R.; Bansal, V. Sunscreen Testing: A Critical Perspective and Future Roadmap. Trends Anal. Chem. 2022, 157, 116724. [Google Scholar] [CrossRef]
- Dimitrovska Cvetkovska, A.; Manfredini, S.; Ziosi, P.; Molesini, S.; Dissette, V.; Magri, I.; Scapoli, C.; Carrieri, A.; Durini, E.; Vertuani, S. Factors affecting SPF in vitro measurement and correlation with in vivo results. Int. J. Cosmet. Sci. 2017, 39, 310–319. [Google Scholar] [CrossRef]
- Dutra, E.A.; Gonçalves da Costa e Oliveira, D.A.; Kedor-Hackmann, E.R.M.; Santoro, M.I.R.M. Determination of Sun Protection Factor (SPF) of Sunscreens by Ultraviolet Spectrophotometry. Rev. Bras. Cienc. Farm. 2004, 40, 381–385. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Janotková, L.; Bendová, H.; Jedličková, L.; Hamranová, V.; Martiniaková, S. The Real UVB Photoprotective Efficacy of Vegetable Oils: In Vitro and In Vivo Studies. Photochem. Photobiol. Sci. 2021, 20, 139–151. [Google Scholar] [CrossRef] [PubMed]
- ISO 24443:2021; Cosmetics. Determination of Sunscreen UVA Photoprotection In Vitro. BSI: Geneva, Switzerland, 2001.
- Rizikiyan, Y.; Suharyani, I.; Falya, Y.; Amelia, R.; Nuh, M.; Sulastri, L.; Indawati, I. Nades Extract of Gedong Mango Leaves and Mulberry Leaves in Spray Gel as a Sunscreen. Int. J. Appl. Pharm. 2022, 14, 121–125. [Google Scholar] [CrossRef]
- Mattioli, R.; Di Risola, D.; Federico, R.; Ciogli, A.; Gasparrini, F.; Villani, C.; Fontana, M.; Maggiore, A.; d’Erme, M.; Mosca, L.; et al. Effect of Natural Deep Eutectic Solvents on Trans-Resveratrol Photo-Chemical Induced Isomerization and 2,4,6-Trihydroxyphenanthrene Electro-Cyclic Formation. Molecules 2022, 27, 2348. [Google Scholar] [CrossRef] [PubMed]
- Milošević, S.; Bebek Markovinović, A.; Teslić, N.; Mišan, A.; Pojić, M.; Brčić Karačonji, I.; Jurica, K.; Lasić, D.; Putnik, P.; Bursać Kovačević, D.; et al. Use of Natural Deep Eutectic Solvent (NADES) as a Green Extraction of Antioxidant Polyphenols from Strawberry Tree Fruit (Arbutus unedo L.): An Optimization Study. Microchem. J. 2024, 200, 110284. [Google Scholar] [CrossRef]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Kljakić, A.C.; Pojić, M.; Mandić, A.; Stupar, A.; Santos, F.; Duarte, A.R.C.; Mišan, A. Natural Deep Eutectic Solvent (NADES) Extraction Improves Polyphenol Yield and Antioxidant Activity of Wild Thyme (Thymus serpyllum L.) Extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D. Understanding Bland Altman Analysis. Biochem. Med. 2015, 25, 141. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Off. J. Eur. Union L 2009, 342, 59. [Google Scholar]
- Wakabayashi, M.; Okano, K.; Mukawa, T.; Maezawa, D.; Masaki, H.; Kuroda, A.; Asakura, K. Problems on the Evaluation of the Critical Wavelength of Sunscreens for “Broad Spectrum” Approval Brought on by Viscous Fingering During Sunscreen Application. Photochem. Photobiol. 2016, 92, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal Extracts, Lichens and Biomolecules as Natural Photo-Protection Alternatives to Synthetic UV Filters. A Systematic Review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef] [PubMed]
- Ayad, R.; Lefahal, M.; Makhloufi, E.H.; Akkal, S. Photoprotection Strategies with Antioxidant Extracts: A New Vision. Phys. Sci. Rev. 2024, 9, 2273–2286. [Google Scholar] [CrossRef]
- Gilbert, L.; Picard, C.; Savary, G.; Grisel, M. Rheological and Textural Characterization of Cosmetic Emulsions Containing Natural and Synthetic Polymers: Relationships between Both Data. Colloids Surf. A Physicochem. Eng. 2013, 421, 150–163. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture Profile Analysis of Bioadhesive Polymeric Semisolids: Mechanical Characterization and Investigation of Interactions between Formulation Components. J. Appl. Polym. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Szczesniak, A.S. Classification of Textural Characteristics. J. Food Sci. 1963, 28, 385–389. [Google Scholar] [CrossRef]
- Savary, G.; Gilbert, L.; Grisel, M.; Picard, C. Instrumental and Sensory Methodologies to Characterize the Residual Film of Topical Products Applied to Skin. Ski. Res. Technol. 2019, 25, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Trịnh, K.T.; Glasgow, S. On the Texture Profile Analysis Test. In Proceedings of the Chemeca 2012, Wellington, New Zealand, 23–26 September 2012. [Google Scholar]
- Gore, E.; Picard, C.; Savary, G. Spreading Behavior of Cosmetic Emulsions: Impact of the Oil Phase. Biotribology 2018, 16, 17–24. [Google Scholar] [CrossRef]
- Mochizuki, Y. Texture Profile Analysis. Curr. Protoc. Food Anal. Chem. 2001, H2.3.1–H2.3.7. [Google Scholar] [CrossRef]
- de Souza Mansur, J.; Breder, M.N.R.; Mansur, M.C. d’Ascençäo; Azulay, R.D. Correlaçäo entre a determinaçäo do fator de proteçäo solar em seres humanos e por espectrofotometria. An. Bras. Dermatol. 1986, 61, 167–172. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A Comparison of in Vivo and in Vitro Testing of Sunscreening Formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Stolić-Jovanović, A.; Martinović, M.; Nešić, I. The Influence of Selected Thickeners on the Textural Properties of Oil-in-Water Emulsions. Acta Fac. Med. Naissensis 2022, 39, 57–65. [Google Scholar] [CrossRef]
- Evageliou, V. Shear and Extensional Rheology of Selected Polysaccharides. Int. J. Food Sci. Technol. 2020, 55, 1853–1861. [Google Scholar] [CrossRef]
- Olivem® 1000. Available online: https://www.ulprospector.com/en/eu/PersonalCare/Detail/1652/73671/Olivem-1000 (accessed on 25 October 2024).
- CosIng-Cosmetics-GROWTH-European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/reference/annexes/list/VI (accessed on 25 October 2024).
- Ad Standards Council. List of UV Filters Which Cosmetic Products May Contain. Available online: https://asc.com.ph/our-standards/code-annexes/category-of-cosmetic-products/asean-handbook-of-cosmetic-ingredient/list-of-uv-filters-which-cosmetic-products-may-contain/ (accessed on 25 October 2024).
- Bradic, J.; Petrovic, A.; Nikolic, M.; Nedeljkovic, N.; Andjic, M.; Kladar, N.; Bolevich, S.; Jakovljevic, V.; Kocovic, A. Newly Developed Semi-Solid Formulations Containing Mellilotus Officinalis Extract: Characterization, Assessment of Stability, Safety, and Anti-Inflammatory Activity. Pharmaceutics 2024, 16, 1003. [Google Scholar] [CrossRef] [PubMed]
- Diffey, B.; Robson, J. A New Substrate to Measure Sunscreen Protection Factors throughout the Ultraviolet Spectrum. J. Soc. Cosmet. Chem. 1989, 40, 127–133. [Google Scholar]
- Villalobos-Hernández, J.R.; Müller-Goymann, C.C. In Vitro Erythemal UV-A Protection Factors of Inorganic Sunscreens Distributed in Aqueous Media Using Carnauba Wax-Decyl Oleate Nanoparticles. Eur. J. Pharm. Biopharm. 2007, 65, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
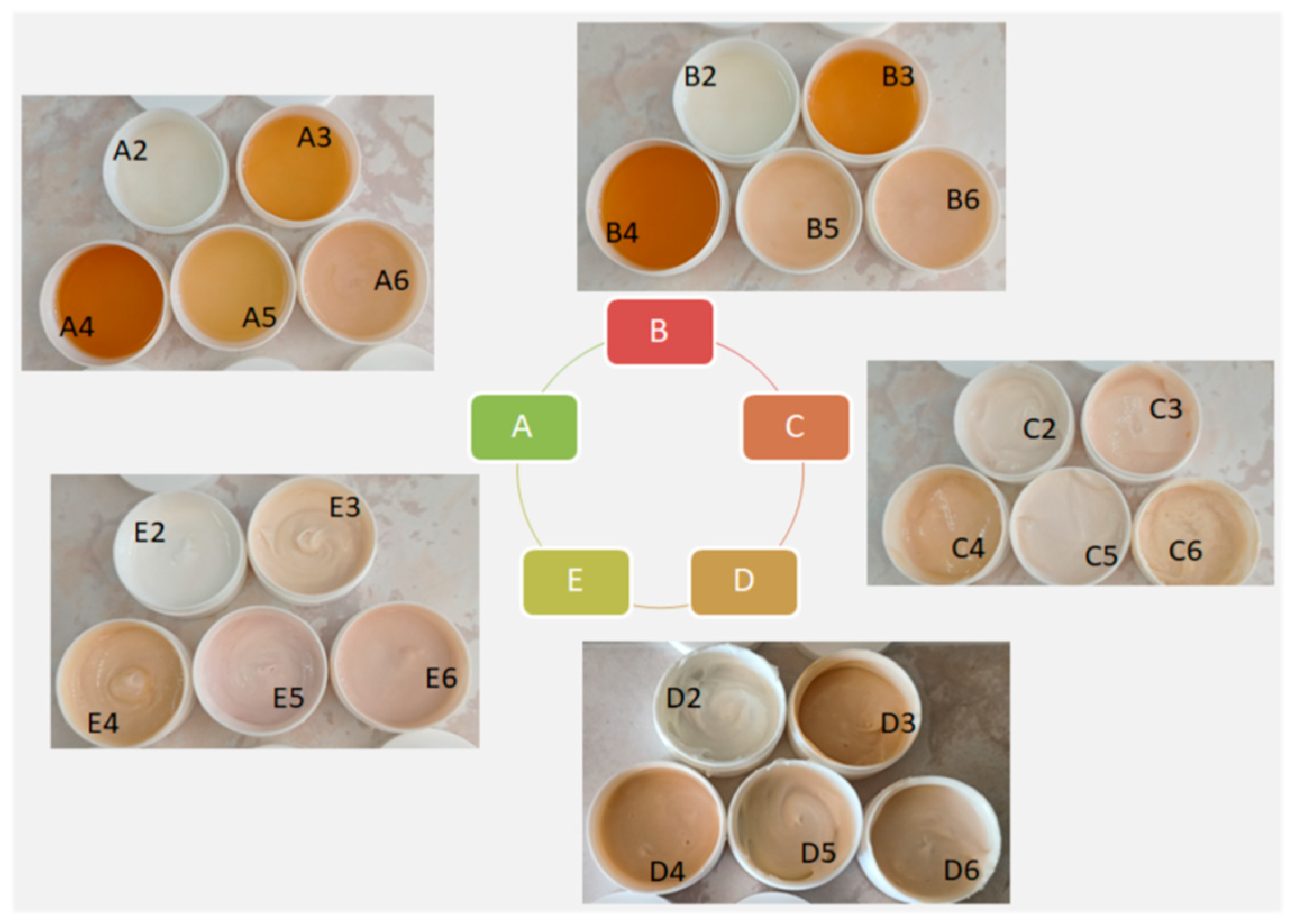

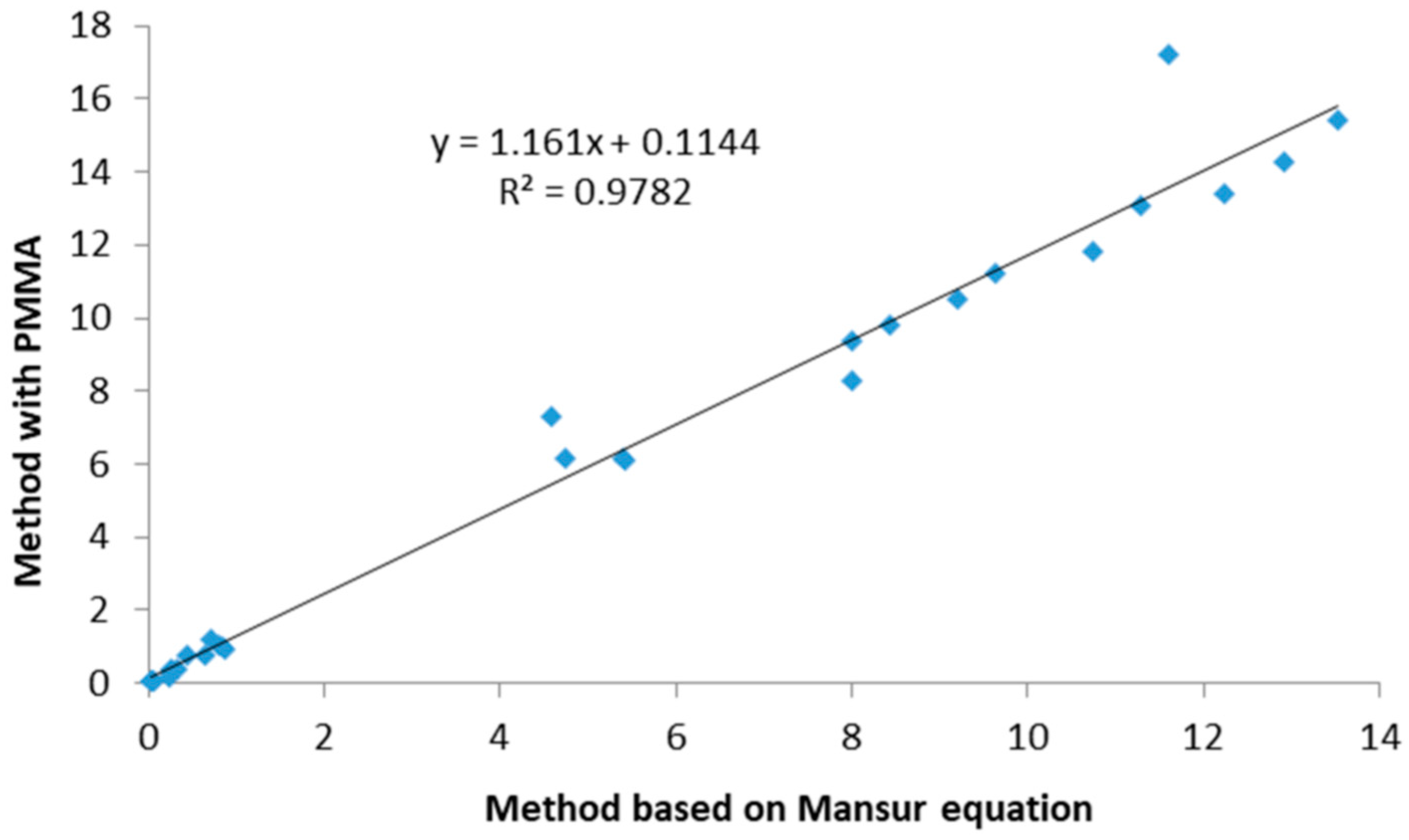
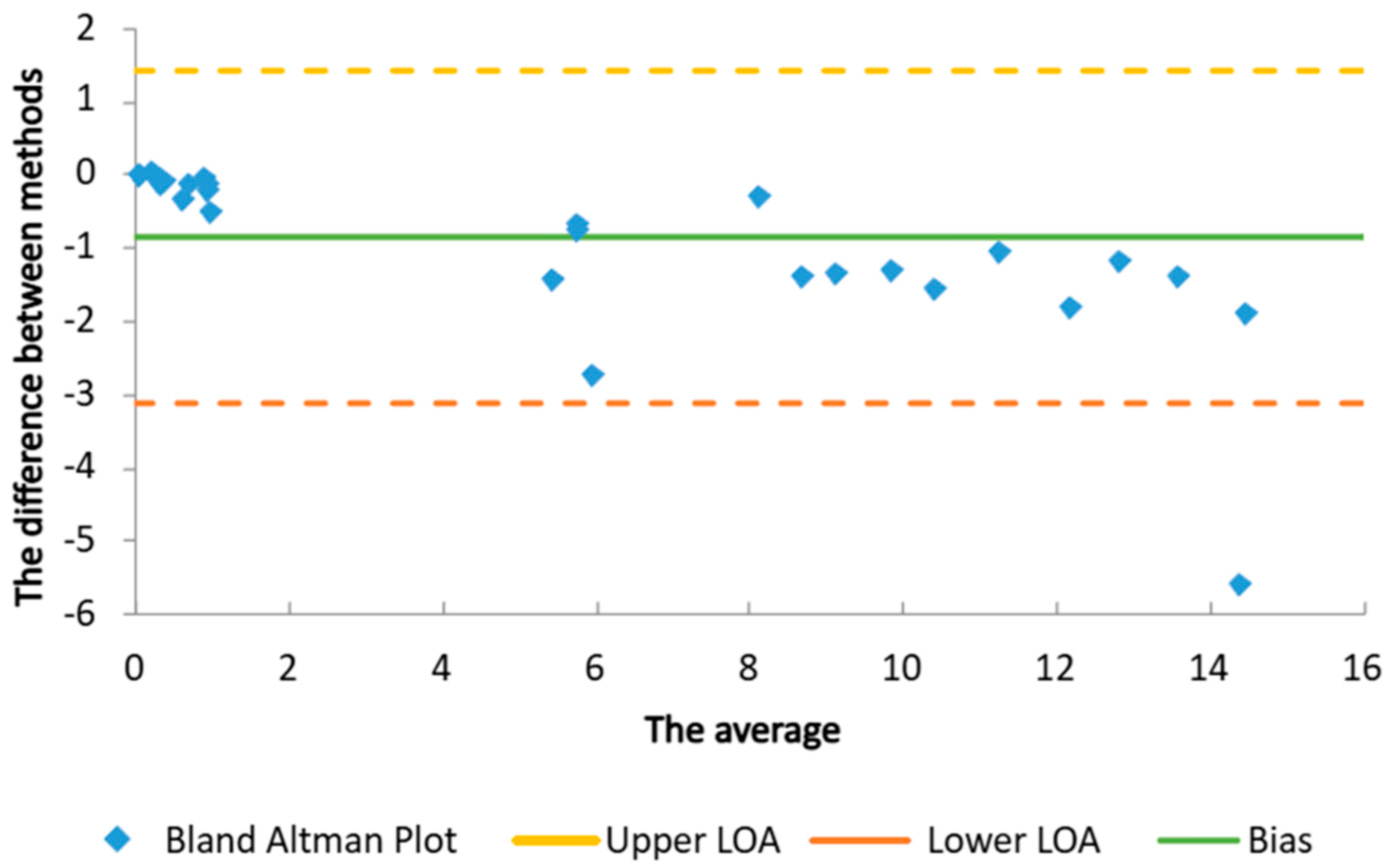

| Sample | SPF Mansur Equation | SPF PMMA Plates | UVA-PF (ISO 24443:2021 [24]) | UVA/SPF | UVA/UVB | Critical λ (nm) |
|---|---|---|---|---|---|---|
| Reference product | - | 20.3 ± 0.5 | ≈1/3 SPF | max 1 | ≈0.5 | min 370 nm |
| Samples A—Hydrogels with XG | ||||||
| A1 | 0.03 ± 0.01 | 0.05 ± 0.01 | <0.02 | - | - | 381 |
| A2 | 9.64 ± 0.2 | 11.2 ± 0.1 | 3.8 ± 0.1 | 0.339 | 0.51 | 380 |
| A3 | 0.25 ± 0.01 | 0.40 ± 0.01 | <0.10 | - | - | 379 |
| A4 | 0.70 ± 0.02 | 1.22 ± 0.01 | 0.45 ± 0.01 | 0.369 | 0.58 | 382 |
| A5 | 4.73 ± 0.1 | 6.15 ± 0.01 | 2.1 ± 0.1 | 0.341 | 0.52 | 379 |
| A6 | 11.60 ± 0.2 | 17.2 ± 0.1 | 5.7 ± 0.1 | 0.331 | 0.50 | 377 |
| Samples B—Hydrogels with HEC | ||||||
| B1 | 0.04 ± 0.01 | 0.06 ± 0.01 | <0.02 | - | - | 382 |
| B2 | 10.73 ± 0.3 | 11.8 ± 0.1 | 4.0 ± 0.1 | 0.339 | 0.51 | 385 |
| B3 | 0.33 ± 0.01 | 0.41 ± 0.01 | <0.10 | - | - | 380 |
| B4 | 0.81 ± 0.1 | 1.03 ± 0.01 | 0.34 ± 0.01 | 0.330 | 0.49 | 379 |
| B5 | 5.41 ± 0.01 | 6.1 ± 0.1 | 2.0 ± 0.1 | 0.328 | 0.49 | 379 |
| B6 | 11.28 ± 0.2 | 13.1 ± 0.1 | 4.4 ± 0.1 | 0.336 | 0.51 | 375 |
| Samples C—Emulsions | ||||||
| C1 | 0.05 ± 0.01 | 0.07 ± 0.01 | <0.02 | - | - | 380 |
| C2 | 9.20 ± 0.2 | 10.5 ± 0.1 | 3.3 ± 0.1 | 0.314 | 0.46 | 381 |
| C3 | 0.23 ± 0.01 | 0.18 ± 0.01 | <0.10 | - | - | 380 |
| C4 | 0.87 ± 0.1 | 0.92 ± 0.01 | 0.35 ± 0.02 | 0.380 | 0.61 | 377 |
| C5 | 5.39 ± 0.3 | 6.14 ± 0.01 | 2.3 ± 0.2 | 0.375 | 0.60 | 375 |
| C6 | 12.23 ± 0.2 | 13.4 ± 0.1 | 4.4 ± 0.1 | 0.328 | 0.49 | 377 |
| Samples D—Emulgels with XG | ||||||
| D1 | 0.06 ± 0.01 | 0.05 ± 0.01 | <0.02 | - | - | 377 |
| D2 | 7.99 ± 0.2 | 8.3 ± 0.1 | 2.7 ± 0.1 | 0.325 | 0.48 | 378 |
| D3 | 0.25 ± 0.01 | 0.35 ± 0.01 | <0.10 | - | - | 377 |
| D4 | 0.64 ± 0.01 | 0.76 ± 0.01 | 0.25 ± 0.1 | 0.329 | 0.49 | 378 |
| D5 | 4.58 ± 0.11 | 7.3 ± 0.1 | 2.5 ± 0.1 | 0.342 | 0.52 | 379 |
| D6 | 8.44 ± 0.31 | 9.8 ± 0.1 | 3.1 ± 0.1 | 0.316 | 0.46 | 379 |
| Samples E—Emulgels with HEC | ||||||
| E1 | 0.03 ± 0.01 | 0.04 ± 0.01 | <0.02 | - | - | 378 |
| E2 | 12.91 ± 0.2 | 14.3 ± 0.1 | 4.7 ± 0.1 | 0.329 | 0.49 | 377 |
| E3 | 0.44 ± 0.01 | 0.78 ± 0.01 | 0.23 ± 0.1 | 0.295 | 0.42 | 379 |
| E4 | 0.85 ± 0.01 | 0.99 ± 0.01 | 0.34 ± 0.1 | 0.343 | 0.53 | 375 |
| E5 | 8.00 ± 0.1 | 9.4 ± 0.1 | 3.2 ± 0.1 | 0.340 | 0.52 | 377 |
| E6 | 13.52 ± 0.4 | 15.4 ± 0.1 | 5.5 ± 0.1 | 0.357 | 0.56 | 378 |
| INCI Name | Function | A1 | A2 | A3 | A4 | A5 | A/B6 |
|---|---|---|---|---|---|---|---|
| Xanthan gum | Gel-forming agent | 1 | 1 | 1 | 1 | 1 | 1 |
| Glycerin | Humectant | 10 | 10 | 10 | 10 | 10 | 10 |
| Potassium sorbate | Preservative | 1 | 1 | 1 | 1 | 1 | 1 |
| Ethanol | Solvent | - | 8 | - | - | 4 | 8 |
| Homosalate | UV filter | - | 4 | - | - | 2 | 4 |
| Ethylhexyl methoxycinnamate | UV filter | - | 4 | - | - | 2 | 4 |
| Benzophenone-4 | UV filter | - | 4 | - | - | 2 | 4 |
| NaDES extracts | Cosmetic active ingredient | - | - | 12 | 24 | 6 | 12 |
| Sodium hydroxide, 10% | pH regulator | q.s. to adjust pH to 4.5. | |||||
| Aqua | Solvent | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * |
| INCI Name | Function | B1 | B2 | B3 | B4 | B5 | B6 |
|---|---|---|---|---|---|---|---|
| Hydroxyethyl cellulose | Gel-forming agent | 2 | 2 | 2 | 2 | 2 | 2 |
| Glycerin | Humectant | 10 | 10 | 10 | 10 | 10 | 10 |
| Potassium sorbate | Preservative | 1 | 1 | 1 | 1 | 1 | 1 |
| Ethanol | Solvent | - | 8 | - | - | 4 | 8 |
| Homosalate | UV filter | - | 4 | - | - | 2 | 4 |
| Ethylhexyl methoxycinnamate | UV filter | - | 4 | - | - | 2 | 4 |
| Benzophenone-4 | UV filter | - | 4 | - | - | 2 | 4 |
| NaDES extracts | Cosmetic active ingredient | - | - | 12 | 24 | 6 | 12 |
| Sodium hydroxide, 10% | pH regulator | q.s. to adjust pH to 4.5. | |||||
| Aqua | Solvent | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * |
| INCI Name | Function | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|---|
| Olivem 1000® (sorbitan olivate (and) cetearyl olivate) | Emulsifier | 5 | 5 | 5 | 5 | 5 | 5 |
| Olivem Feel® (cetearyl alcohol (and) cetyl palmitate (and) sorbitan palmitate (and) sorbitan oleate) | Co-emulsifier | 1 | 1 | 1 | 1 | 1 | 1 |
| Caprylic/capric triglyceride | Emollient | 20 | 20 | 20 | 20 | 20 | 20 |
| Glycerin | Humectant | 8 | 8 | 8 | 8 | 8 | 8 |
| Potassium sorbate | Preservative | 1 | 1 | 1 | 1 | 1 | 1 |
| Homosalate | UV filter | - | 4 | - | - | 2 | 4 |
| Ethylhexyl methoxycinnamate | UV filter | - | 4 | - | - | 2 | 4 |
| Benzophenone-4 | UV filter | - | 4 | - | - | 2 | 4 |
| NaDES extracts | Cosmetic active ingredient | - | - | 12 | 24 | 6 | 12 |
| Sodium hydroxide, 10% | pH regulator | q.s. to adjust pH to 4.5. | |||||
| Aqua | Solvent | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * |
| INCI Name | Function | D1 | D2 | D3 | D4 | D5 | D6 |
|---|---|---|---|---|---|---|---|
| Olivem 1000® (sorbitan olivate (and) cetearyl olivate) | Emulsifier | 5 | 5 | 5 | 5 | 5 | 5 |
| Olivem Feel® (cetearyl alcohol (and) cetyl palmitate (and) sorbitan palmitate (and) sorbitan oleate) | Co-emulsifier | 1 | 1 | 1 | 1 | 1 | 1 |
| Caprylic/capric triglyceride | Emollient | 20 | 20 | 20 | 20 | 20 | 20 |
| Glycerin | Humectant | 8 | 8 | 8 | 8 | 8 | 8 |
| Potassium sorbate | Preservative | 1 | 1 | 1 | 1 | 1 | 1 |
| Homosalate | UV filter | - | 4 | - | - | 2 | 4 |
| Ethylhexyl methoxycinnamate | UV filter | - | 4 | - | - | 2 | 4 |
| Benzophenone-4 | UV filter | - | 4 | - | - | 2 | 4 |
| NaDES extracts | Cosmetic active ingredient | - | - | 12 | 24 | 6 | 12 |
| Xanthan gum | Gel-forming agent | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Sodium hydroxide, 10% | pH regulator | q.s. to adjust pH to 4.5. | |||||
| Aqua | Solvent | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * |
| INCI Name | Function | E1 | E2 | E3 | E4 | E5 | E6 |
|---|---|---|---|---|---|---|---|
| Olivem 1000® (sorbitan olivate (and) cetearyl olivate) | Emulsifier | 5 | 5 | 5 | 5 | 5 | 5 |
| Olivem Feel® (cetearyl alcohol (and) cetyl palmitate (and) sorbitan palmitate (and) sorbitan oleate) | Co-emulsifier | 1 | 1 | 1 | 1 | 1 | 1 |
| Caprylic/capric triglyceride | Emollient | 20 | 20 | 20 | 20 | 20 | 20 |
| Glycerin | Humectant | 8 | 8 | 8 | 8 | 8 | 8 |
| Potassium sorbate | Preservative | 1 | 1 | 1 | 1 | 1 | 1 |
| Homosalate | UV filter | - | 4 | - | - | 2 | 4 |
| Ethylhexyl methoxycinnamate | UV filter | - | 4 | - | - | 2 | 4 |
| Benzophenone-4 | UV filter | - | 4 | - | - | 2 | 4 |
| NaDES extracts | Cosmetic active ingredient | - | - | 12 | 24 | 6 | 12 |
| Hydroxyethyl cellulose | Gel-forming agent | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Sodium hydroxide, 10% | pH regulator | q.s. to adjust pH to 4.5. | |||||
| Aqua | Solvent | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * | q.s. ad 100 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinović, M.; Nešić, I.; Bojović, D.; Žugić, A.; Blagojević, S.; Blagojević, S.; Tadić, V.M. Plant-Based Sunscreen Emulgel: UV Boosting Effect of Bilberry and Green Tea NaDES Extracts. Gels 2024, 10, 825. https://doi.org/10.3390/gels10120825
Martinović M, Nešić I, Bojović D, Žugić A, Blagojević S, Blagojević S, Tadić VM. Plant-Based Sunscreen Emulgel: UV Boosting Effect of Bilberry and Green Tea NaDES Extracts. Gels. 2024; 10(12):825. https://doi.org/10.3390/gels10120825
Chicago/Turabian StyleMartinović, Milica, Ivana Nešić, Dragica Bojović, Ana Žugić, Slavica Blagojević, Stevan Blagojević, and Vanja M. Tadić. 2024. "Plant-Based Sunscreen Emulgel: UV Boosting Effect of Bilberry and Green Tea NaDES Extracts" Gels 10, no. 12: 825. https://doi.org/10.3390/gels10120825
APA StyleMartinović, M., Nešić, I., Bojović, D., Žugić, A., Blagojević, S., Blagojević, S., & Tadić, V. M. (2024). Plant-Based Sunscreen Emulgel: UV Boosting Effect of Bilberry and Green Tea NaDES Extracts. Gels, 10(12), 825. https://doi.org/10.3390/gels10120825







