Abstract
Hydrogels have a wide range of medical applications, including use within implantable systems. However, when used in implants, their visibility under conventional medical imaging techniques is limited, creating safety risks for patients. In the current work, we assessed the possibility of enhancing hydrogels using Ln-based contrasting agents to facilitate their visualization in photon-counting computed tomography (PCCT). The contrast enhancement of gelatin, polyacrylamide (PAM), and silicone shells of implants was assessed. A novel synthetic route for producing cross-linked nanosized Ln2O3 with polyacrylamide was proposed and discussed in detail. Several prototypes of silicone implants, including silicone shell and gelatin or PAM filling with different combinations of contrasting agents, were produced and assessed in phantom PCCT studies.
1. Introduction
Hydrogels have a wide range of medical applications [1]. Their tunable physico-chemical properties, relative biocompatibility [2], stability, and biodegradability make them suitable for use as pharmaceutical substances, drug delivery systems [3], inner fillings of implantable systems, or even as shell-less implantable sensors [4].
Despite this, hydrogels have a significant disadvantage that introduces risk when used for medical purposes, especially as implant materials. They are only partially visible through conventional medical imaging methods such as ultrasound, X-ray, or magnetic resonance imaging (MRI) [5,6]. This complicates the regular assessment of a gel-filled implant and the timely diagnosis of its malfunctions, such as ruptures with extracapsular leakage of the inner material [7].
One potential solution for this can be the addition of compounds with high X-ray attenuation coefficients to hydrogels, enhancing their visibility with roentgenological and some other imaging methods [8,9]. The compound most commonly used as a contrasting agent for X-ray imaging is iodine [10]. Hydrogels or similar biomaterials can be contrasted by iodinated fragments of tyrosine residues [11], wholly aliphatic iodinated polyesters [12], iodinated polyurethane [13], or covalently iodinated polymethacrylate [14], facilitating their visualization in biomedical studies by computed tomography (CT). Hydrogels can also be contrasted by barium, bismuth sulfate [15], or even radioactive isotopes such as 111In3+, making them suitable for complex diagnostic studies using single-photon emission computed tomography (SPECT) and positron emission tomography (PET) [16].
The modification of hydrogels with elements possessing high X-ray attenuation coefficients [17] proves particularly effective when coupled with advanced diagnostic methods such as photon-counting computed tomography (PCCT) combining CT with elements of X-ray spectroscopy [18]. It provides a visualization of the inner structures of study objects, accounting not only for their average X-ray attenuation but also their unique X-ray attenuation spectra, giving insights into their chemical composition and making it possible to distinguish between areas with comparable roentgenological density but different elemental contents [19,20]. PCCT has been extensively used in biomedical research and is now being introduced into clinical practice. Over 30 clinical trials assessing PCCT capabilities in comparison with conventional (energy-integrative) CT scanners in different indications have been published [21,22]. PCCT allows the simultaneous use of multiple contrast agents (CAs), including elements not present in the human body (i.e., lanthanides La, Nd, Eu, Gd, Dy, Ho, Er, Yb, Lu [23,24,25,26,27,28], Hf [29], Ta [24], Bi [30], W [30], and Au [31]) for separate visualizations of different contrasted structures within a single assessment. This means that the implementation of such contrasting agents into the filling of the implants can not only enhance their visualization but also enable differentiation from components of the same or different implants, which can be critical for assessing their condition.
The CAs of special interest for PCCT studies are lanthanides (Ln). This group has similar chemical properties but different K-edge energies, leading to different X-ray attenuation spectra. So, the same synthetic processes can be used to produce different CAs and contrast-enhanced materials for subsequent simultaneous visualization by PCCT [23].
The most common CA belonging to lanthanides is Gd, which is actively used as a contrast enhancement in MRI studies [32] and is probed as a reserve CA for CT studies in patients with severe allergic reactions to standard iodinated contrasts [33]. It is also considered promising for dual-mode CT/MRI studies [34]. Gd contrasts used in clinical practice typically have the structure of chelate complexes of Gd3+ with polycarbonate acids [35]. More recently, considerable advances have also been made in developing Gd-based CAs in the form of nanoparticles, coated or stabilized by proteins, saccharides, lipids, or inorganic matrices consisting of carbon or SiO2 [36]. CA nanoparticles demonstrate comparable or higher contrasting properties in MRI or CT studies compared to molecular contrasts [37,38]. Such CAs are also considered to have low cytotoxicity [39,40]. This is attributed to their high chemical stability and low release of free Gd3+ ions which are considered the primary mechanism of Gd-induced toxicity [41]. However, it should be noted that most of the studies evaluated Gd3+ concentrations below 100 mg·L−1, while commercially available Gd contrasts (i.e., gadavist [42]) are administered in doses leading to blood Gd3+ concentrations exceeding 200 mg·L−1. Gd-based nanoparticle CAs may have different mechanisms of toxicity independent of the release of Gd3+ [43], indicating the need for further research on their possible toxic effects in different modes of administration. Other lanthanides have been probed and studied as potential CAs to a much lesser extent.
The goal of the current work was to assess the possibility and effectiveness of the contrast enhancement of prototype implants based on gelatin or polyacrylamide (PAM) fillings and silicone shells by Ln2O3 (Ln = La, Gd, and Yb). We assessed the possibility of introducing Ln2O3 into the PAM matrix during the co-polymerization of monomers or into the commercially available bi-component silicone during molding. We developed novel synthetic routes, making it possible to (1) decrease the size of Ln2O3 nanoparticles and (2) establish the covalent bonding of these particles to PAM to ensure stability and uniform distribution of the contrasts. The resulting compounds were assessed by physico-chemical methods and tested in PCCT studies of the prototype implants.
2. Results and Discussion
2.1. Calibration of Material Determination by PCCT and Material Choice for Contrasted Implants
Hybrid pixel detectors used in PCCT have a limited number of energy thresholds and relatively low energy resolution. Consequently, the resulting X-ray attenuation spectra significantly differ from those produced using theoretical data or more precise experimental methods [44]. This requires calibration of the PCCT device and its analysis software for the determination of specific materials or chemical elements anticipated in the study objects. During calibration, a unique attenuation profile for selected energy thresholds and their dependency on concentration is created for each material. These profiles are then compared with the actual profile of each voxel of the 3D reconstruction, and the material with the most fitting profile is defined and quantified [45].
Our pilot studies revealed that simple calibration using only water solutions of the contrasting elements was insufficient for the accurate determination of Ln2O3-containing silicones due to the impact of Si on the X-ray attenuation spectra of the Si-containing materials [46]. Therefore, for implant prototypes incorporating both contrasted gelatin fillings and silicone shells, separate calibration profiles of Ln-containing gelatins and silicones were produced using a phantom, including two samples of each material in two concentrations (Figure 1a). The concentrations were chosen based on our previous studies confirming that the lowest range for Ln detection and quantification by PCCT using reference samples is 2.5–5 mg·mL−1 [23,25]. Thirty reconstructed transversal slides were used. The analysis of the calibration applied reversely to the reconstruction phantom (Figure 1b–i) confirmed that all Ln2O3-containing gelatins could be determined accurately, as well as Gd2O3- and Yb2O3-containing silicones. La2O3-containing silicone demonstrated determination artifacts, likely due to the low K-edge energy of La diminishing the La component in the X-ray attenuation spectrum of the contrasted silicone (Figure 1d). This material was excluded from further studies.
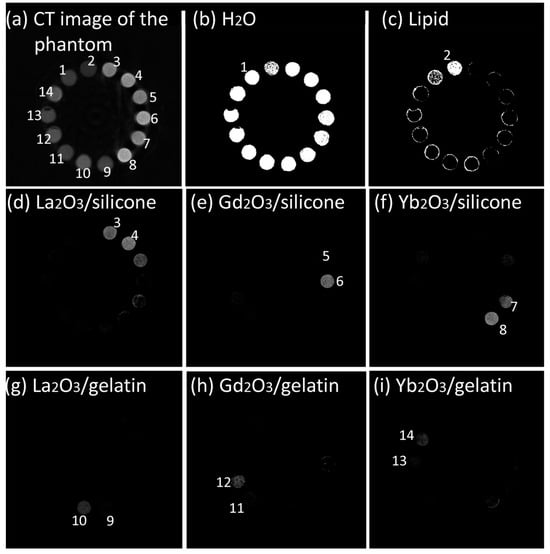
Figure 1.
(a) Reconstructed transversal slice of calibration phantom where 1 is H2O, 2 is lipid, 3 and 4 are La2O3/silicone with 10 and 40 mg·mL−1, 5 and 6 are Gd2O3/silicone with 10 and 40 mg·mL−1, 7 and 8 are Yb2O3/silicone with 10 and 40 mg·mL−1, 9 and 10 are La2O3/gelatin with 10 and 40 mg·mL−1, 11 and 12 are Gd2O3/gelatin with 10 and 40 mg·mL−1, and 13 and 14 are Yb2O3/gelatin with 10 and 40 mg·mL−1. (b–i) Results of application of material determination criteria for determination of H2O (b), lipid (c), La2O3/silicone (d), Gd2O3/silicone (e), Yb2O3/silicone (f), La2O3/gelatin (g), Gd2O3/gelatin (h), and Yb2O3/gelatin (i).
Studies using PAM-based implant prototypes not requiring the determination of contrasted silicones have been conducted with simper calibration using La(NO3)3 and Gd(NO3)3 solutions. The calibration process also included Ca(C2H3O2)2 solutions to support the determination of Ca structures (i.e., bones) in future studies, as well as H2O and lipid samples mandated by the calibration algorithm.
2.2. Preparation and Phantom PCCT Studies of Gelatin-Filled Implant Models
Silicone shells were prepared from two-component molding silicone, Best Mold PL 15, with a Pt catalyst (SP Polymer, Russia) cured at room temperature. The components were mixed in a 1:1 volume ratio and poured into polytetrafluoroethylene cylindrical custom molds with a diameter of 12 and a length of 25 mm. Ln2O3 (Ln = La, Gd, and Yb) was added to the liquid silicone with a Ln content of 20 mg·mL−1 in the silicone where required. In some cases, hard gelatin capsules of ~1 mL were used to fix the inner space. The shells were solidified for at least 24 h. Gelatin fillers were prepared by mixing 20 mg·mL−1 of La2O3 with 5 wt.% gelatin. Gelatin solutions were poured into the prepared silicone shells, refrigerated to ensure gelling, and then sealed with silicone or Ln2O3-doped silicone sheets.
The phantom PCCT studies were performed in two steps. First, the survey scans including the phantom with all implants were performed to confirm the possibility of simultaneous determination of the composition and visualization of the components of the multiple implants (Table 1 and Figure 2). Second, the chosen implants were scanned separately and reconstructed with a higher resolution. The resulting images were assessed in a 37–48 keV energy window (used as an illustration of an energy-integrative scan because this energy range prevails in the emission spectra of the X-ray source) and with qualitative material determination.

Table 1.
CT numbers of materials used in gelatin-filled implants (mean ± SD).
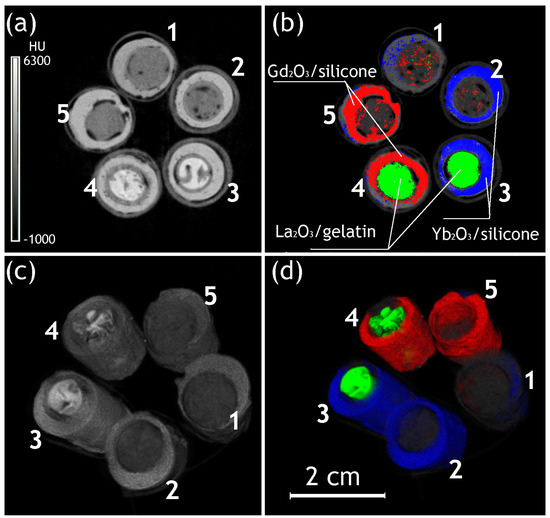
Figure 2.
Survey scans, including transversal slices (a,b) and 3D reconstructions (c,d) of the gelatin-filled implants in energy-integrative mode (energy window 37–48 keV, (a,c)) and material determination mode (b,d). Gd—red; La—Green; Yb—yellow; 1—gelatin@silicone; 2—gelatin@(Yb2O3/silicon); 3—(La2O3/gelatin)@(Yb2O3/silicon); 4—(La2O3/gelatin)@(Gd2O3/silicon); 5—gelatin@(Gd2O3/silicon).
The images of the 3D reconstruction of gelatin–silicone models are presented in Figure 2, and the CT numbers of the materials are summarized in Table 1. A review of the energy-integrative images produced without material determination confirmed the visualization and, to a certain extent, separation of the implant components, including the silicone shells, inner material (including heterogeneities potentially caused by gelatin shrinkage during gelling), and partially gelatin capsules, where they remained stable (Figure 2a,c). La-contrasted gelatin had a higher radiodensity than raw gelatin, but the different types of silicone shells could not be distinguished from each other.
The same images produced with the determination of materials provided higher visibility of all components, with a clear delineation of the shells from the inner materials (Figure 2b,d).
Despite the relatively positive results of the survey scans, the subsequent scans of individual gelatin–silicone implants revealed substantial abnormalities in contrast distribution in their components. The material determination scan of the implant with an Yb-contrasted silicone shell (Figure 3) revealed the presence of small particles of Yb2O3 not bound with the silicone at both the inner and outer sides of the shell (Figure 3b,c,e,f), potentially appearing as a result of the precipitation of Yb2O3 particles at the wall of the mold during the solidification of the silicone and indicating a risk to material stability and toxicity in the case of implementation. This approach to contrast enhancement was considered sub-optimal.
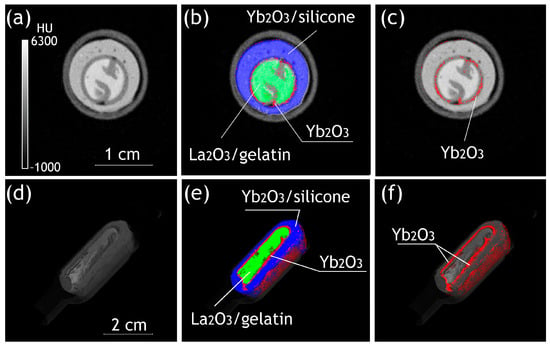
Figure 3.
The energy-integrative (a,d) and material determination (b,c,e,f) reconstructions ((a–c)—transversal slices; (d–f)—3D visualizations) of the La2O3 + gelatin/Yb2O3 + silicone implant. Yb2O3 absorbed at the interface is shown in red.
2.3. Synthesis and Characterization of Cross-Linked Ln2O3/PAM Samples
Following the study of gelatin-filled implants, the possibility of contrasting PAM was assessed. The introduction of Ln2O3 particles into the PAM matrix without chemical modification was not successful due to heavy oxides flaking from the reaction mixture (Figure S1, Supplementary Materials). It was concluded that the size of Ln2O3 particles should be reduced and that these particles should be subsequently coated by carbon shells forming a core–shell structure with functional surface reactive groups to ensure the covalent bonding and uniform distribution of Ln2O3 in the PAM matrix.
The concept of using carbon shells for a better distribution of Ni and Fe particles in PAM was previously presented in [47]. Hydrogels based on PAM with Ag@carbon nanotubes were described in [48]. Composites of fullerene [49], carbon dots [50], multi-walled carbon nanotubes [51,52], graphene oxide [53,54], and graphite [55] with PAM, including cross-linked carbon/PAM samples [56], have also been described. However, considering the physico-chemical properties of Ln2O3, we proposed functionalizing the carbon shell in Ln2O3@C core–shell nanoparticles with allylic groups. These groups containing the C=C bond react with acrylic acid and acrylamide via the radical polymerization process with the formation of covalent bonds.
The general scheme, including the seven steps of the synthetic route, is presented in Figure 4. Nanosized Ln2O3 particles could be produced by stabilization using graphene nanoflakes (GNFs). GNFs are graphitic-like flat particles with a size of ~15 × 15 nm (Figure 5a) consisting of 7–10 stacked graphene layers (Figure 5b).
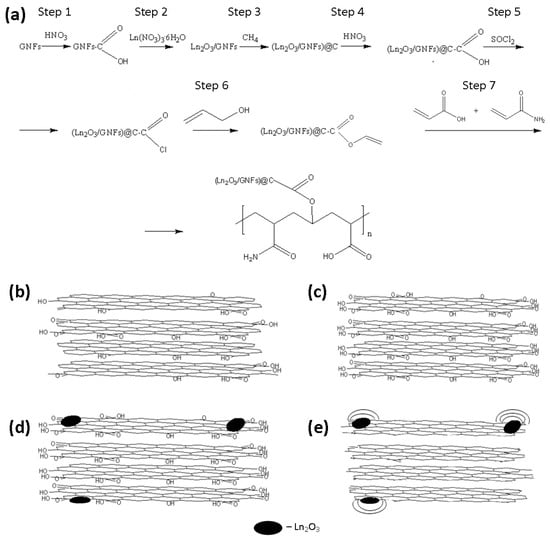
Figure 4.
The general scheme of the synthetic route (a). The models of GNFs (b), GNFs_ox (c), Ln2O3/GNFs (d), and (Ln2O3/GNFs)@C (e).
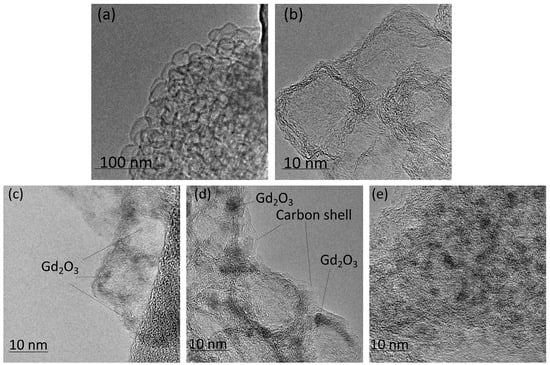
Figure 5.
TEM images of GNFs (a,b), Gd2O3/GNFs (c), (Gd2O3/GNFs)@C (d), and (Gd2O3/GNFs)@C_ox (e).
Composite Ln2O3/GNFs could be obtained through the impregnation of GNFs with a Ln(NO3)3·6H2O solution and the further decomposition of nitrate under an inert atmosphere, as described in our previous works [23,25]. The sizes of Ln2O3 particles synthesized through the above-mentioned route were 1–2 nm according to transmission electron microscopy (TEM) (Figure 5c). Subsequent graphitization by the pyrolytic decomposition of CH4 produced (Ln2O3/GNFs)@C core–shell particles, which was also confirmed by TEM (Figure 5d). The surface-oxidized (Ln2O3/GNFs)@C_ox samples were produced by (Ln2O3/GNFs)@C oxidation with HNO3 vapors. The core–shell structure and the size of the Ln2O3 core in the (Ln2O3/GNFs)@C_ox particles were preserved (Figure 5e). It should be noted that the GNF structure was saved after all the processes and surface reactions. The structure of GNF particles is clearly demonstrated in Figure S2 in the Supplementary Materials. The composition and physico-chemical properties of Ln2O3/GNFs, (Ln2O3/GNFs)@C, and (Ln2O3/GNFs)@C_ox (Ln = La and Gd), based on X-ray photoelectron spectroscopy (XPS), electron paramagnetic resonance (EPR), Raman spectra, TEM, and thermogravimetry/differential scanning calorimetry (TG/DSC) data, are partially reported in [23,57,58,59].
The surface of (Gd2O3/GNFs)@C_ox was further modified by reacting first with SOCl2 to produce an acyl chloride, and then with sodium alloxide to produce an ester. The synthesis of a nanodiamond and CNTs with surface acyl chloride groups was previously described in [60,61]. The product of the reaction of (Gd2O3/GNFs)@C-COCl with sodium alloxide was investigated by IR spectra (Figure 6). The spectra of (Gd2O3/GNFs)@C-COOCH2C2H3 were comparable with those of (Gd2O3/GNFs)@C_ox (Figure 6). The observed spectra were in good agreement with the spectral data for different oxidized carbon nanomaterials, such as graphene oxide [62,63], oxidized carbon nanotubes [64], and oxidized GNFs specifically (Figure 6). A wide line at 1710 cm−1 corresponds to the valent vibrations of the C=O fragment in the carboxyl groups. The lines at 1080 and 3130 cm−1 correspond to C-OH bonds in both carboxylic and hydroxyl groups. The lines at ~500–550 cm−1 should be associated with the Gd-O bond [65,66].
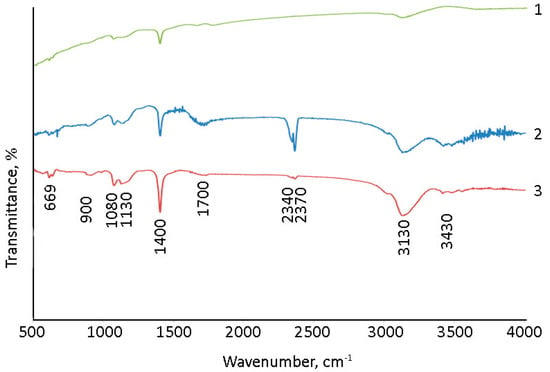
Figure 6.
IR spectra of 1—GNFs_ox; 2—(Gd2O3/GNFs)@C_ox; and 3—(Gd2O3/GNFs)@C-COOCH2C2H3.
The chemical composition and the nature of atoms in the (Gd2O3/GNFs)@C-COOCH2C2H3 composition were confirmed by XPS (Figure 7). The sample consisted of 57.4 at.% C, 16.1 at.% O, 3.5 at.% Gd, 7.4 at.% Na, 9.8 at.% Cl, 3.9 at.% N, and 1.8 at.% S (Figure 7a). The sample contained NaCl (Figure 7b,c) and Na2SO4 (Figure 7c) as by-products of a metathesis reaction between (Gd2O3/GNFs)@C-COCl and NaOCH2C2H3 according to step 6 and the formation of acyl chloride via step 5 (Figure 4). Nitrogen atoms were predominantly found in the form of oxides (Figure S3, Supplementary Materials) and remained in the product after the surface oxidation phase [57,59]. High-resolution deconvoluted C1s XPS spectra of (Gd2O3/GNFs)@C-COOCH2C2H3 (Figure 7d) contained different carbon species with binding energies of 284.4, 285.2, 286.4, 287.6, 288.8, and 290.3 eV corresponding to sp2- and sp3-hybridized C, C–O, ketone C=O, and hydroxyl C(O)O groups and CO32−. This confirmed the presence of all possible carbon groups expected in (Gd2O3/GNFs)@C-COOCH2C2H3. The CO32− group in the spectra was presumably related to Gd carbonate (Figure 7e) [67] which was formed as the primary Gd phase [23]. This assumption is supported by O1s XPS spectra showing binding energies of 531.2, 532.9, and 534.0 eV corresponding to CO32−, C=O/C-OH, and COO- groups (Figure 7b).
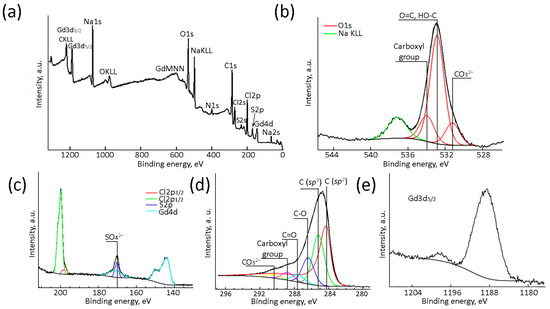
Figure 7.
Survey (a), O1s (b), S2p (c), C1s (d), and Gd3p (c,e) high-resolution XPS spectra of (Gd2O3/GNFs)@C-COOCH2C2H3.
TG/DSC data of (Gd2O3/GNFs)@C-COOCH2C2H3 with mass-spectra of evolved gasses for the measurements in the air and Ar atmosphere are depicted in Figure 8a,c and Figure 8b,d, respectively. During heating in an oxidizing atmosphere, adsorption water is released with heat absorption (Figure 8a,c). Then, the carbon shell burns with an intense release of heat and CO2 peaking at approximately 500 °C. This is followed by an endothermic peak caused by the melting of NaCl, a by-product of the synthesis process. At temperatures above 800 °C, the salt decomposes, releasing chlorine. The residual mass of 50% corresponds to Gd2O3. In an inert atmosphere, the composition of gaseous products is more complex (Figure 8b,d). Water (m/z = 18, 17, and 16) and carbon dioxide (m/z = 44, 12, and 45) are released in a wide temperature range. The peak of water release is observed at a temperature slightly above 110 °C. There are also peaks for m/z = 29 (C2H5+), 27 (C2H3+), and 26 (C2H2+). Amidst background noise, peaks are also observed for m/z = 57(C3H5O+) and 56 (C3H4O+). Above 130 °C, the m/z = 30 peak begins to appear, reaching its maximum at around 230 °C. At approximately 590 °C, the maximum ion current for m/z = 15 (CH3+) is observed. Between 600 and 700 °C, SO2 is released (m/z = 64, 48) due to the by-product of synthesis in accordance with XPS data. The maximum CO2 emission is observed near 770 °C, but it is released over a much broader temperature range compared to an oxidizing atmosphere (Figure 8d).
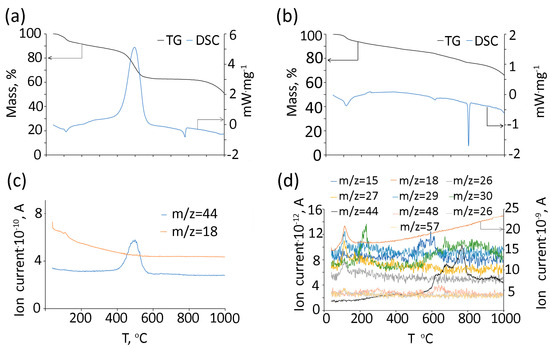
Figure 8.
TG and DSC curves (a,b) and gas products (c,d) of (Gd2O3/GNFs)@C-COOCH2C2H3 under air (a,c) and Ar (b,d) atmospheres.
The surface modification of (Gd2O3/GNFs)@C particles by allylic groups improves their degree of dispersion in solvents [60], and they serve as functional groups reacting with the monomers acrylic acid and acrylamide via radical mechanism polymerization [60]. The IR spectra of PAM and (Gd2O3/GNFs)@C-COOCH2C2H3/PAM are presented in Figure 9. The spectra of PAM and PAM with cross-linked Gd2O3, i.e., (Gd2O3/GNFs)@C-COOCH2C2H3 particles, are similar. The interpretation of the IR spectra was based on [68,69]. The wide lines at 3300 cm−1 correspond to H2O and amide NH2 asymmetric stretching groups. The lines at 2953 and 1406 cm−1 are associated with C-H asymmetric and symmetric stretching, respectively. The lines at 1660 and 1616 cm−1 correspond to the stretching of primary and secondary amide C=O groups. C–O–C groups could contribute to the lines at 1178 and 1178 cm−1. The lines at ~1350 cm−1 could be interpreted as unreacted C=C groups and C–O–H in plane bending because of their comparable wavenumbers [68].
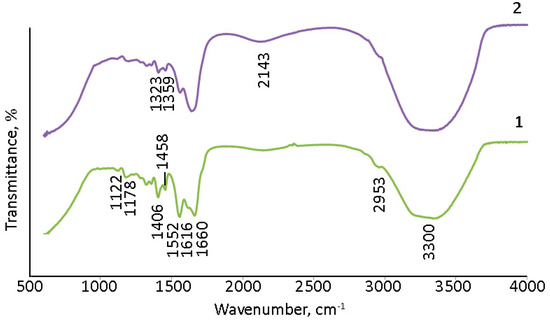
Figure 9.
IR spectra: 1—PAM; 2—(Gd2O3/GNFs)@C-COOCH2C2H3/PAM.
PAM has a unique property, namely the ability to adsorb water with swelling [70,71]. The swelling of PAM and the cross-linked Ln2O3/PAM composite was evaluated according to the increase in the weight of samples after water sorption. Two hundred milliliters of distilled water was added to a solid weighed portion of 0.50 g of the polymer sample and kept for 24 h, after which it was filtered through a sieve with a mesh size of 0.5 mm, and the weight of the swollen residue was determined. The swelling values of PAM and Gd2O3/PAM were 420 and 320 g·g−1. The densities of PAM and the (Ln2O3/GNFs)@C-COOCH2C2H3/PAM (Ln = La, Gd) composites determined as the ratio between their mass and volume were 1.27, 1.04, and 1.20 g·mL−1, accordingly. The qualitative determination of Gd in the water solution demonstrated the absence of Gd, which indirectly indicates the binding of Gd2O3 to the polymer matrix.
The uniformity of Ln2O3 distribution in the PAM matrix was confirmed by scanning electron microscopy (SEM) images and surface element mapping (Figure 10). The contents of La and Gd were 0.61 and 0.30 wt.% according to EDX data, which are in good agreement with the synthesis process.
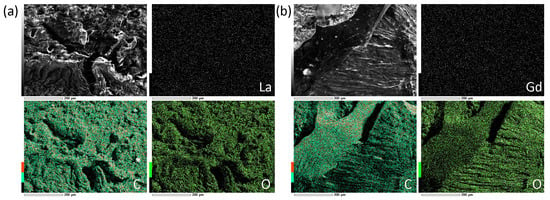
Figure 10.
SEM images and surface Ln, C, and O mapping of (La2O3/GNFs)@C-COOCH2C2H3/PAM (a) and (Gd2O3/GNFs)@C-COOCH2C2H3/PAM (b).
2.4. Preparation and Phantom PCCT Studies of PAM-Filled Implant Models
PAM implant models were produced from standard silicone tubing filled by (Ln2O3/GNFs)@C-COOCH2C2H3/PAM (Ln = La or Gd) or PAM and sealed using silicone. The implants were solidified for 24 h before studying them. The PCCT studies were performed in one step (without survey scans) due to a smaller number of implants requiring assessment. The energy-integrative reconstructions were produced from a separate scan with a single energy threshold of 30 keV.
The transversal slices of the reconstructed implant models with a silicone shell and (Ln2O3/GNFs)@C-COOCH2C2H3/PAM Ln = La (a) and Gd (b) filler are presented in Figure 11. In both cases, the fillers and shells could be visualized. No visible abnormalities were detected in the distribution of the CA. The average X-ray attenuation coefficient (Table 2) of both (Ln2O3/GNFs)@C-COOCH2C2H3/PAM fillers was lower compared to raw PAM, which could be explained by the presence of low-density GNFs compensating for the higher attenuation of the Ln component of the material. However, the contrasted fillers had characteristic attenuation spectra, making it possible to separate them from the silicone shells and from each other.
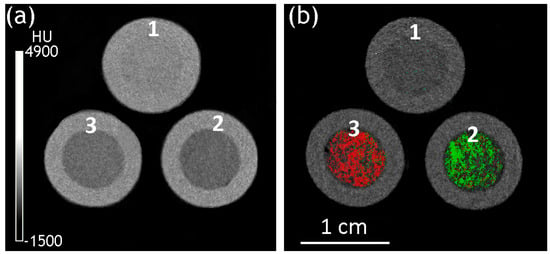
Figure 11.
Transversal slices of the reconstructed PAM-filled implants in energy-integrative (a) and material determination (b) modes: 1—PAM/silicone; 2—(La2O3/GNFs)@C-COOCH2C2H3/PAM/silicone; and 3—(Gd2O3/GNFs)@C-COOCH2C2H3/PAM/silicone. Green—La; red—Gd.

Table 2.
The CT numbers of the materials used in PAM-filled implants (mean ± SD).
The visualization and successful Ln determination in PAM fillings were performed with a Ln concentration in (Ln2O3/GNFs)@C-COOCH2C2H3/PAM of 4.5 mg·mL−1. In our past works, we demonstrated that the lower concentration threshold for Ln determination by PCCT was ~2.5 mg·mL−1 [23], while further optimization of material determination algorithms allows for it to be reduced to under ~1 mg·mL−1 [44]. This confirms the higher potential of Ln contrast enhancement compared to other approaches, such as iodine-based contrasts [72], requiring higher CA concentrations (>4 mg·mL−1 iodine in clinical applications) and not allowing the simplified production of multiple CAs for simultaneous visualization. This also confirms the high potential of the proposed materials from a biocompatibility perspective. Their core–shell structure provides additional shielding for Ln nanoparticles, which is expected to decrease their cytotoxicity compared to simpler nanoparticles with direct contact between Ln and biological media [73]. However, the biocompatibility of the contrasted materials requires further comprehensive studies due to the different nature of possible exposure compared to the commonly studied contrasts for short-term IV administration.
3. Conclusions
The results of this study demonstrate that Ln2O3 contrasting can be an effective method for the visualization of hydrogel-based implants for diagnostic studies involving CT or PCCT. Various combinations of Ln can be used, although the suitability and effectiveness of each individual combination should be confirmed through calibration experiments. The inclusion of CA particles into the components of the implant at the stage of polymerization without covalent bonding may give sub-optimal results due to the redistribution of the contrast, including the dissemination of the contrast at the surface of the implant. Covalent bonding of the CA into the hydrogel structure with the formation of the new cross-linked Ln2O3/PAM composite increases the uniformity of its distribution in the material and increases its stability. Ln2O3/PAM composites can be visualized with specific Ln determination in implantable systems with Ln concentrations below 5 mg·mL−1, demonstrating their high potential compared to other contrast enhancement approaches. The structure of the proposed materials allows for low cytotoxicity to be anticipated, although this requires further comprehensive studies due to their different uses and possible mechanisms of exposure compared to the Ln-based nanoparticle contrasts described in the literature.
4. Materials and Methods
4.1. Synthesis of Cross-Linked Ln2O3/PAM Samples
The general scheme of the synthetic route, including seven steps, and models of component structures are shown in Figure 3. The synthesis and product characterization of steps 1–4 are described in detail in our previous works [57,58,59]. Steps 5–7 were originally described for modifying the nanodiamond surface in [60] and customized for the composites produced in step 4.
4.1.1. Synthesis of Contrast Agents
In brief, GNFs were synthesized by the pyrolysis of hexane (99.8%, Reachim) at 900 °C in the presence of a MgO template according to [74]. MgO was removed by boiling in HCl (99.9%, Reachim). The obtained GNFs were washed with distilled water and dried at 110 °C.
Step 1. GNFs were oxidized by HNO3 (Component-Reaktiv) refluxing for 1 h to produce a GNFs_ox sample. The content of oxygen on the GNFs_ox surface was 11.1 at.% [59].
Step 2. Then, the GNFs_ox samples were impregnated with Ln(NO3)3·6H2O (Ln = La and Gd) (99%, China Northern Rare Earth Group High-Tech Co., Ltd., Baotou, China) ethanolic solution. The solvent was evaporated slowly using an ultrasound bath. After that, the solid sediment was heated at 400 °C with nitrogen (99.999%, NII KM, Moscow, Russia) flow for 45 min to decompose nitrate and obtain a Ln2O3/GNF composite [23].
Step 3. The core–shell structure (Ln2O3/GNFs)@C was achieved by methane decomposition over Ln2O3/GNFs particles [58]. The Ln2O3/GNFs sample was placed into the fixed-bed reactor, purged with nitrogen, and heated to 450 °C. After that, methane (99.99% Moscow Gas Processing Plant, Moscow, Russia) was passed through the reaction mixture for 15–20 min. The product (Ln2O3/GNFs)@C was used without purification.
Step 4. The surface oxidation of (Ln2O3/GNFs)@C particles was performed via HNO3 gas phase treatment according to recommendations [57,59]. The (Ln2O3/GNFs)@C sample was put into the open glass vessel and treated with HNO3 vapors for 1 h. Adsorbed HNO3 was washed by dt. H2O, and the resulting product (Ln2O3/GNFs)@C_ox was dried at 110 °C.
Step 5. The next surface modification process for (Ln2O3/GNFs)@C_ox involved the substitution of the hydroxyl group in a carboxylic group with a chlorine atom to produce acyl chloride. For this purpose, 1.0 g of (Ln2O3/GNFs)@C_ox was placed in a round-bottomed double-crested bowl equipped with a thermometer and a reflux condenser. Fifty milliliters of SOCl2 was added. The reaction mixture was refluxed at 80 °C for 24 h. The unreacted SOCl2 was evaporated to produce acyl chloride (Ln2O3/GNFs)@C-COCl.
Step 6. The synthesis of the ester was carried out through the reaction of acyl chloride (Ln2O3/GNFs)@C-COCl with sodium alloxide. Metallic Na in the amount of 0.46 g was added to 50 mL of allylic alcohol (>99%, Himkraft Ltd., Kaliningrad, Russia). After the reaction, the NaOCH2C2H3 solution was dropped to (Ln2O3/GNFs)@C-COCl under an Ar (99.993%, Logica Ltd., Moscow, Russia) atmosphere. The reaction mixture was refluxed for 8 h; the solid product (Ln2O3/GNFs)@C-COOCH2C2H3 was centrifugated at 6000 rpm and washed with ethylacetate two times. The product was kept at 0 °C in the dark until the next stage.
4.1.2. Synthesis of PAM
PAM was synthesized according to the standard procedure of the copolymerization of potassium acrylate, acrylamide, and the crosslinking agent N,N′-methylenebisacrylamide with (NH4)2S2O8 as a catalyst of the radical polymerization process [75,76,77]. Acrylic acid (99.98%, Vekton, St. Petersburg, Russia) in the amount of 7.1 g was dissolved in 20 mL of water and neutralized to pH 7 with 50 mL of a KOH solution. Acrylamide (99.9%, Vekton, St. Petersburg, Russia) in the amount of 9.12 g was dissolved in 40 mL of H2O. Both solutions were combined, and then 0.0270 g of N,N′-methylenebisacrylamide (99.99%, Amresco LLC, Boise, ID, USA) was added. The mixture was blown by Ar. Solid (NH4)2S2O8 (99%, Ruschem, Moscow, Russia) was added. The reaction mixture was heated to 50 °C to trigger the gelling reaction and then self-heated to 90 °C. The product was washed with water and dried at 130 °C for 10 h.
4.1.3. Synthesis of Ln2O3/PAM Composites
The composites of PAM with Ln2O3 (Ln = La, Gd, and Yb) (99.99%, China Northern Rare Earth Group High-Tech Co., Ltd., Baotou, China) were synthesized by sewing the suspended composites in the PAM matrix via the copolymerization of monomers according to [78]. Solid Ln2O3 was added to the water solution of potassium acrylate and acrylamide (the concentration of monomers was in accordance with the previous synthesis description) with vigorous stirring. The mass content of Ln2O3 varied from 5 to 25 wt.%. N2 was blown out, and a water solution of N,N′-methylenebisacrylamide was titrated to the reaction mixture. The polymerization reaction started after Na2S2O8 addition. However, in all cases, Ln2O3 flaked out.
Step 7. A cross-linked sample, (Gd2O3/GNFs)@C-COOCH2C2H3/PAM, was obtained by adding solid (Gd2O3/GNFs)@C-COOCH2C2H3 powder into the reaction mixture of potassium acrylate, acrylamide, and N,N′-methylenebisacrylamide before the copolymerization process. The mixture was intensively stirred and purged with Ar for 20 min. (NH4)2S2O8 was added as an initial agent. The reaction was generally similar to the synthesis of polyacrylamide. The contents of Ln in the composition of (Ln2O3/GNFs)@C-COOCH2C2H3/PAM were 0.39 and 0.30 wt.% for the La and Gd composites, respectively.
4.2. Methods
The thermal stability of (Gd2O3/GNFs)@C-COOCH2C2H3 was studied using a simultaneous thermal analyzer, STA 449 C Jupiter, combined with a quadrupole mass-spectrometer, QMS 403 C Aeolos (both NETZSCH, Selb, Germany). The material in the Al2O3 crucible was heated at a rate of 10 °C·min−1 from 40 to 1000 °C, both in oxidizing (airflow of 80 mL·min−1) and inert (Ar flow of 80 mL·min−1) atmospheres. The weighing block was purged with a flow of argon protective gas of 40 mL·min−1. The signals of thermogravimetry (TG), differential thermogravimetry (DTG), differential scanning calorimetry (DSC), and ion currents corresponding to certain m/z ratios of evolved gasses were recorded during the heating process. A baseline correction was applied for the TG/DSC curves using the measurement with an empty crucible.
The morphology and particle size were studied by SEM using JEOL JSM-6390LA and JEOL JCM-6000 instruments (JEOL Ltd., Tokyo, Japan). TEM images were obtained using JEOL 2100 F/Cs (Jeol, Tokyo, Japan) operating at 200 kV and equipped with a UHR pole tip as well as a spherical aberration corrector (CEOS, Heidelberg, Germany) and EEL spectrometer (Gatan, Munich, Germany).
The materials were studied using an FTIR spectrometer, FSM 1202 (Infraspek, Moscow, Russia). Transmission spectra were obtained by accumulating 30 scans with a resolution of 4 cm−1. Powdered samples were pressed into tablets with KBr (98.5%, BLD Pharmatech Ltd. China, Shanghai, China) using a hydraulic press (OMEC, Brescia, Italy). A reference spectrum was registered from a pure KBr pellet. Polyacrylamide samples were pressed to a ZnSe crystal of the multi-reflection ATR accessory.
The XPS spectra were recorded on an Axis Ultra DLD spectrometer (Kratos Analytical, Manchester, UK) using monochromatic AlKα radiation (1486.7 eV). The pass energies of the analyzer were 160 eV for survey spectra and 40 eV for high-resolution scans. The relative error for the element concentrations was about 5%.
4.3. PCCT Studies
PCCT studies were conducted using a MARS Bioimaging microtomograph (MARS Bioimaging Ltd., Christchurch, New Zealand) equipped with 2 Medipix 3RX CZT pixel detectors [79] with a sensor size of 14 mm × 14 mm and a thickness of 1 mm. The material determination scans of the gelatin-filled implant prototypes were performed with energy thresholds of 7, 25, 37, 48, 58, 72, 80, and 90 keV, which were chosen based on the K-edge energies of the studied Ln (38.9, 50.2, and 61.3 keV for La, Gd, and Yb, respectively). The scans of the PAM-filled implant prototypes were performed with energy thresholds of 7, 25, 36, 48, and 60 keV since only La and Gd were used. The images illustrating the energy-integrative reconstructions were produced using a 37–48 keV energy window (for gelatin-filled implant prototypes) or by separate scans within a single energy threshold at 30 keV (for PAM-filled implant prototypes). The scans were performed in continuous circular mode with automated flat field correction, a geometric magnification of 1.7, and rotation steps of 1° in survey and calibration scans and 0.5° in the scans of individual implants. The reconstruction was performed with a voxel size of 0.12 mm for survey scans and 0.06 mm for calibration scans and scans of individual implants. Because of the detector size, the total scanning time in all studies was approximately 70 min, including 55 min for acquiring the actual projections and 15 min for acquiring the flat field data for all camera positions (up to 4 for each position of the sample). Scanning was performed with temperature conditioning, keeping the detector temperature under 16 °C. Visualization was performed using the Mars Vision 2.0 software (MARS Bioimaging Ltd., Christchurch, New Zealand). The energy-integrative attenuation data were additionally translated into the Hounsfield scale using additional water samples scanned with the same parameters. The CT numbers (HU values) were measured from transversal slices for the area of at least 2 mm2 and presented as mean ± SD.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/gels10120807/s1, Figure S1: SEM images of PAM (a) and mixture of La2O3 (b) and Gd2O3 (c) with PAM synthesized during polymerization of acrylic acid and acrylamide in presence of Ln2O3. Figure S2: HRTEM (a,b) and HAADF-STEM (c) images with EELS spectra of (Gd2O3/GNFs)@C-COOCH2C2H3 (d). Figure S3: N1s XPS spectra of (Gd2O3/GNFs)@C-COOCH2C2H3.
Author Contributions
Conceptualization, E.V.S. and D.A.S.; methodology, K.I.M., S.Y.K. and T.O.L.; validation, D.A.S. and G.A.C.; formal analysis, E.V.S., K.I.M., S.Y.K. and T.O.L.; investigation, E.V.S., D.A.S. and T.O.L.; resources, G.A.C.; writing—original draft preparation, E.V.S., D.A.S. and K.I.M.; writing—review and editing, E.V.S., D.A.S., K.I.M., S.Y.K. and G.A.C.; visualization, E.V.S. and D.A.S.; supervision, G.A.C. and O.S.M.; project administration, O.S.M. and G.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 22-15-00072.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank Sergey Maximov and Dmitry Stolbov for the TEM and SEM images and acknowledge the support from the “Nanochemistry and Nanomaterials” Equipment Center under the Lomonosov Moscow State University Program of Development.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chamkouri, H.; Chamkouri, M. A Review of Hydrogels, Their Properties and Applications in Medicine. Am. J. Biomed. Sci. Res. 2021, 11, 485–493. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mat. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Hameed, H.; Faheem, S.; Paiva-Santos, A.C.; Sarwar, H.S.; Jamshaid, M. A Comprehensive Review of Hydrogel-Based Drug Delivery Systems: Classification, Properties, Recent Trends, and Applications. AAPS PharmSciTech 2024, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Martinea, G.; Begines, B.; Pajuelo, E.; Vazquez, J.; Rodriguez-Alberlo, L.M.; Cofini, D.; Torres, Y.; Alcudia, A. Versatile Biodegradable Poly(acrylic acid)-Based Hydrogels Infiltrated in Porous Titanium Implants to Improve the Biofunctional Performance. Biomacromolecules 2023, 24, 4743–4758. [Google Scholar] [CrossRef]
- Wong, T.; Lo, L.W.; Fung, P.Y.E.; Lai, H.Y.M.; She, H.L.H.; Ng, W.K.C.; Kwok, K.M.K.; Lee, C.M. Magnetic resonance imaging of breast augmentation: A pictorial review. Insights Imaging 2016, 7, 399–410. [Google Scholar] [CrossRef]
- Dong, Y.C.; Bouché, M.; Uman, S.; Burdick, J.A.; Cormode, D.P. Cormode Detecting and Monitoring Hydrogels with Medical Imaging. ACS Biomater. Sci. Eng. 2021, 7, 4027. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-H.; Weng, Y.-T.; Chou, Y.-Y.; Wang, C.-H. Rupture of 40-year-old silicone gel breast implants: A case report. BMC Geriatr. 2023, 23, 589. [Google Scholar] [CrossRef]
- Mottu, F.; Rüfenacht, D.A.; Doelker, E. Radiopaque polymeric materials for medical applications. Current aspects of biomaterial research. Investig. Radiol. 1999, 34, 323. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, J.T.; Wang, Z. Implementation of functional integration of hydrogel matrices with rare earth elements and related applications. J. Rare Earths2024, in press. [CrossRef]
- Koc, M.M.; Aslan, N.; Kao, A.P.; Barber, A.H. Evaluation of X-Ray tomography contrast agents: A review of production, protocols, and biological applications. Microsc. Res. Technol. 2019, 82, 812. [Google Scholar] [CrossRef]
- Becerra, C.F.; Silva, V.B.; Ahmed, E.; Bear, J.C.; Feng, Z.; Chau, D.Y.S.; Parker, S.G.; Halligan, S.; Lythgoe, M.F.; Stuckey, D.J.; et al. X-Ray Visible Protein Scaffolds by Bulk Iodination. Adv. Sci. 2024, 11, 2306246. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.R.; Brosnan, S.M.; Burk, L.M.; Lee, Y.Z.; Luft, J.C.; Ashby, V.S. Iodinated polyesters as a versatile platform for radiopaque biomaterials. J. Polym. Sci. A Polym. Chem. 2017, 55, 2171. [Google Scholar] [CrossRef]
- Kiran, S.; James, N.R.; Joseph, R.; Jayakrishnan, A. Synthesis and characterization of iodinated polyurethane with inherent radiopacity. Biomaterials 2009, 30, 5552. [Google Scholar] [CrossRef]
- Davy, K.W.M.; Anseau, M.R. Novel iodinated methacrylates as X-Ray opaque denture base polymers. J. Mater. Sci. Lett. 1996, 15, 686. [Google Scholar] [CrossRef]
- Barnett, B.P.; Arepally, A.; Stuber, M.; Arifin, D.R.; Kraitchman, D.L.; Bulte, J.W. Synthesis of magnetic resonance-, X-Ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nat. Protoc. 2011, 6, 1142–1151. [Google Scholar] [CrossRef]
- Patrick, P.S.; Bear, J.C.; Fitzke, H.E.; Zaw-Thin, M.; Parkin, I.P.; Lythgoe, M.F.; Kalber, T.L.; Stuckey, D.J. Radio-metal cross-linking of alginate hydrogels for non-invasive in vivo imaging. Biomaterials 2020, 243, 119930. [Google Scholar] [CrossRef]
- Dong, Y.C.; Kumar, A.; Rosario-Berrios, D.N.; Si-Mohamed, S.; Hsu, J.C.; Nieves, L.M.; Douek, P.; Noёl, P.B.; Cormode, D.P. Ytterbium Nanoparticle Contrast Agents for Conventional and Spectral Photon-Counting CT and Their Applications for Hydrogel Imaging. ACS Appl. Mater. Interfaces 2022, 14, 39274. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ye, Z.; Chen, J.; Deng, L.; Song, B. Photon Counting CT: Technical Principles, Clinical Applications, and Future Prospects. Acad. Radiol. 2023, 30, 2362–2382. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Douek, P.C.; Boccalini, S.; Oei, E.; Cormode, D.; Pourmorteza, A.; Boussel, L.; Si-Mohamed, S.; Budde, R. Clinical Applications of Photon-counting CT: A Review of Pioneer Studies and a Glimpse into the Future. Radiology 2023, 309. [Google Scholar] [CrossRef]
- Bie, J.; Straten, M.; Booij, R.; Bos, D.; Dijkshoorn, M.L.; Hirsch, A.; Sharma, S.P.; Oei, E.H.G.; Budde, R.P.J. Photon-counting CT: Review of initial clinical results. Eur. J. Radiol. 2023, 163, 110829. [Google Scholar] [CrossRef] [PubMed]
- Lachance, C.; Horton, J. Photon-Counting CT: High Resolution, Less Radiation: Emerging Health Technologies; Report No.: EH0124; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, USA, 2024. [Google Scholar] [PubMed]
- Suslova, E.V.; Kozlov, A.P.; Shashurin, D.A.; Rozhkov, V.A.; Sotenskii, R.V.; Maximov, S.V.; Savilov, S.V.; Medvedev, O.S.; Chelkov, G.A. New Composite Contrast Agents Based on Ln and Graphene Matrix for Multi-Energy Computed Tomography. Nanomaterials 2022, 12, 4110. [Google Scholar] [CrossRef]
- Kim, J.; Bar-Ness, D.; Si-Mohamed, S.; Coulon, P.; Blevis, I.; Douek, P.; Cormode, D.P. Assessment of candidate elements for development of spectral photon-counting CT specific contrast agents. Sci. Rep. 2018, 8, 12119. [Google Scholar] [CrossRef]
- Suslova, E.; Shashurin, D.; Kozlov, A.; Maximov, S.V.; Rozhkov, V.A.; Sotenskii, R.V.; Savilov, S.V.; Medvedev, O.S.; Chelkov, G.A. Development of La-graphene composite contrasting agents for photon-counting computed tomography. Funct. Mater. Lett. 2022, 15, 2250029. [Google Scholar] [CrossRef]
- Dunning, C.A.S.; O’connell, J.; Robinson, S.M.; Murphy, K.J.; Frencken, A.L.; van Veggel, F.C.J.M.; Iniewski, K.; Bazalova-Carter, M. Multi energy Computed Tomography of Lanthanide Contrast Agents with a High-Flux 330-Mm-Pitch Cadmium Zinc Telluride Detector in a Table-Top System. J. Med. Imaging 2022, 7, 033502. [Google Scholar] [CrossRef]
- Smith, K.; Getzin, M.; Garfield, J.J.; Suvarnapathaki, S.; Camci-Unal, G.; Wang, G.; Gkikas, M. Nanophosphor-Based Contrast Agents for Spectral X-Ray Imaging. Nanomaterials 2019, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Richtsmeier, D.; Dunning, C.A.S.; Iniewski, K.; Bazalova-Carter, M. Multi-Contrast K-Edge Imaging on a Bench-Top Multi energy CT System: Acquisition Parameter Study. J. Instrum. 2020, 15, 10029. [Google Scholar] [CrossRef]
- Ostadhossein, F.; Tripathi, I.; Benig, L.; LoBato, D.; Moghiseh, M.; Lowe, C.; Raja, A.; Butler, A.; Panta, R.; Anjomrouz, M.; et al. Multi-“Color” Delineation of Bone Microdamages Using Ligand-Directed Sub-5 Nm Hafnia Nanodots and Photon Counting CT Imaging. Adv. Funct. Mater. 2020, 30, 1904936. [Google Scholar] [CrossRef]
- Amato, C.; Klein, L.; Wehrse, E.; Rotkopf, L.T.; Sawall, S.; Maier, J.; Ziener, C.H.; Schlemmer, H.; Kachelrieß, M. Potential of Contrast Agents Based on High-Z Elements for Contrast-enhanced Photon-counting Computed Tomography. Med. Phys. 2020, 47, 6179. [Google Scholar] [CrossRef]
- Cormode, D.P.; Roessl, E.; Thran, A.; Skajaa, T.; Gordon, R.E.; Schlomka, J.-P.; Fuster, V.; Fisher, E.A.; Mulder, W.J.M.; Proksa, R.; et al. Atherosclerotic Plaque Composition: Analysis with Multicolor CT and Targeted Gold Nanoparticles. Radiology 2020, 256, 774. [Google Scholar] [CrossRef]
- Do, C.; DeAguero, J.; Brearley, A.; Trejo, X.; Howard, T.; Escobar, G.P.; Wagner, B. Gadolinium-Based Contrast Agent Use, Their Safety, and Practice Evolution. Kidney360 2020, 1, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Holmes, B.; Sanampudi, S.; Ananthakrishnan, L. Diagnostic CT cystography with diluted gadolinium-based contrast: A viable alternative to an iodinated contrast-based cystogram. Urol. Case Rep. 2024, 26, 102856. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Q.; Zang, Z.; Zhang, S.; Wang, Z.; Li, L.; Yu, X.; Yang, X.; Lu, Z.; Zhang, X. A dual-mode T1 MRI/CT contrast agent of Gd2O3/Au@MSNs for tumor imaging with high performance. Materialia 2022, 24, 101494. [Google Scholar] [CrossRef]
- Levine, D.; McDonald, R.J.; Kressel, H.Y. Gadolinium Retention After Contrast-Enhanced MRI. JAMA 2018, 320, 1853–1854. [Google Scholar] [CrossRef]
- Fatima, A.; Ahmad, M.W.; Al Saidi, A.K.A.; Choudhury, A.; Chang, Y.; Lee, G.H. Recent Advances in Gadolinium Based Contrast Agents for Bioimaging Applications. Nanomaterials 2021, 11, 2449. [Google Scholar] [CrossRef]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Pellico, J.; Ellis, C.M.; Davis, J.J. Nanoparticle-Based Paramagnetic Contrast Agents for Magnetic Resonance Imaging. Contrast Media Mol. Imaging 2019, 2019, 1845637. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Zhang, M.; Li, G.; Hao, L. Gadolinium-Coated Mesoporous Silica Nanoparticle for Magnetic Resonance Imaging. Front. Chem. 2022, 10, 837032. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Ho, S.L.; Tegafaw, T.; Cha, H.; Chang, Y.; Oh, I.T.; Yaseen, A.M.; Marasini, S.; Ghazanfari, A.; Yue, H.; et al. Stable and non-toxic ultrasmall gadolinium oxide nanoparticle colloids (coating material = polyacrylic acid) as high-performance T1 magnetic resonance imaging contrast agents. RSC Adv. 2018, 8, 3189–3197. [Google Scholar] [CrossRef]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evans, P. Gadolinium: Pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef]
- Gadavist (Gadobutrol) Injection, for Intravenous Use. Labelling Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201277s000lbl.pdf (accessed on 28 November 2024).
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.; Alrokayan, S. Toxicity Mechanism of Gadolinium Oxide Nanoparticles and Gadolinium Ions in Human Breast Cancer Cells. Curr. Drug Metab. 2019, 20, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Sotenskii, R.V.; Rozhkov, V.A.; Shashurin, D.A.; Suslova, E.V.; Chelkov, G.A. Novel algorithm for qualitative and quantitative material analysis by the K-edges for photon-counting computed tomography. JINST 2024, 19, P04009. [Google Scholar] [CrossRef]
- Bateman, C.J.; Knight, D.; Brandwacht, B.; Mc Mahon, J.; Healy, J.; Panta, R.; Aamir, R.; Rajendran, K.; Moghiseh, M.; Ramyar, M.; et al. MARS-MD: Rejection based image domain material decomposition. JINST 2018, 13, P05020. [Google Scholar] [CrossRef]
- Suslova, E.; Shashurin, D.; Zoirova, Z.; Shumyantsev, A.; Medvedev, O.; Chelkov, G. Gd2O3-based contrasting agents for photon-counting computed tomography: Effect of structure, composition, and particle size. Mater. Chem. Phys. 2024, 313, 128733. [Google Scholar] [CrossRef]
- Mikhnevich, E.A.; Safronov, A.P.; Beketov, I.V.; Medvedev, A.I. Carbon coated Nickel Nanoparticles in Polyacrylamide Ferrogels: Interaction with Polymeric Network and Impact on Swelling. Chim. Techno Acta 2020, 7, 116. [Google Scholar] [CrossRef]
- Olăreț, E.; Voicu, Ș.I.; Oprea, R.; Miculescu, F.; Butac, L.; Stancu, I.C.; Serafim, A. Nanostructured Polyacrylamide Hydrogels with Improved Mechanical Properties and Antimicrobial Behavior. Polymers 2022, 14, 2320. [Google Scholar] [CrossRef] [PubMed]
- Porwal, S.; Diwedi, A.; Kamal, M. 13C NMR and Raman Studies of Fullerene-Based Poly (Acrylamides). Int. J. Org. Chem. 2012, 2, 377–386. [Google Scholar] [CrossRef]
- Wu, W.; Wu, X.; He, M.; Yuan, X.; Lai, J.; Sun, H. A novel carbon dot/polyacrylamide composite hydrogel film for reversible detection of the antibacterial drug ornidazole. RSC Adv. 2021, 11, 22993. [Google Scholar] [CrossRef]
- Abo-Zahra, S.F.; Abdelmonem, I.M.; Siyam, T.E.; El-Masry, A.M.; Abdel-Aziz, H.M. Radiation synthesis of polyacrylamide/functionalized multiwalled carbon nanotubes composites for the adsorption of Cu(II) metal ions from aqueous solution. Polym. Bull. 2022, 79, 4395. [Google Scholar] [CrossRef]
- El-Sweify, F.H.; Abdelmonem, I.M.; El-Masry, A.M.; Siyam, T.E.; Abo-Zahra, S.F. Adsorption Behavior of Co(II) and Eu(III) on Polyacrylamide/Multiwalled Carbon Nanotube Composites. Radiochemistry 2019, 61, 323. [Google Scholar] [CrossRef]
- Cheng, M.-M.; Huang, L.-J.; Wang, Y.-X.; Zhao, Y.-C.; Tang, J.-G.; Wang, Y.; Zhang, Y.; Hedayati, M.; Kipper, M.J.; Wickramasinghe, S.R. Synthesis of graphene oxide/polyacrylamide composite membranes for organic dyes/water separation in water purification. J. Mater. Sci. 2019, 54, 252. [Google Scholar] [CrossRef]
- Yu, S.; Li, N.; Higgins, D.; Li, D.; Li, Q.; Xu, H.; Spendelow, J.S.; Wu, G. Self-Assembled Reduced Graphene Oxide/Polyacrylamide Conductive Composite Films. ACS Appl. Mater. Interfaces 2014, 6, 19783. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, K.; Palanisamy, N. Methylene blue adsorption onto an eco-friendly modified polyacrylamide/graphite composites: Investigation of kinetics, equilibrium, and thermodynamic studies. Sep. Sci. Technol. 2020, 55, 266. [Google Scholar] [CrossRef]
- Xie, S.; Chen, Y.; Guo, Z.; Luo, Y.; Tan, H.; Xu, L.; Xu, J.; Zheng, J. Agar/carbon dot crosslinked polyacrylamide double-network hydrogels with robustness, self-healing, and stimulus-response fluorescence for smart anti-counterfeiting. Mater. Chem. Front. 2021, 5, 5418–5428. [Google Scholar] [CrossRef]
- Suslova, E.V.; Ulyanov, A.N.; Kozlov, A.P.; Shashurin, D.A.; Savilov, S.V.; Chelkov, G.A. Composition and Electronic Structure of La2O3/CNFs@C Core-Shell Nanoparticles with Variable Oxygen Content. Nanomaterials 2023, 13, 2945. [Google Scholar] [CrossRef]
- Kozlov, A.; Suslova, E.; Maksimov, S.; Isaikina, O.; Maslakov, K.; Shashurin, D.; Savilov, S.; Shelkov, G. The Preparation of Nanocomposite with a Core–Shell Structure Made of Carbon Matrices and Lanthanum Nanoparticles. Phys. Part. Nucl. Lett. 2023, 20, 1254. [Google Scholar] [CrossRef]
- Suslova, E.V.; Kozlov, A.P.; Shashurin, D.A.; Maximov, S.V.; Maslakov, K.I.; Savilov, S.V. La2O3-carbon composite with core–shell structure and features of its gas-phase oxidation. Mendeleev Commun. 2024, 34, 90. [Google Scholar] [CrossRef]
- Sivtsov, E.V.; Kalinin, A.V.; Gostev, A.I.; Smirnov, A.V.; Agibalova, L.V.; Shumilov, F.A. In Situ Preparation of Polymer Nanocomposites Based on Sols of Surface-Modified Detonation Nanodiamonds by Classical and Controlled Radical Polymerization. Polym. Sci. Ser. B 2020, 62, 734. [Google Scholar] [CrossRef]
- Hamon, M.A.; Chen, J.; Hu, H.; Chen, Y.; Itkis, M.E.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Dissolution of Single-Walled Carbon Nanotubes. Adv. Mater. 1999, 11, 834. [Google Scholar] [CrossRef]
- Sudesh; Kumar, N.; Das, S.; Bernhard, C.; Varma, G.D. Effect of graphene oxide doping on superconducting properties of bulk MgB2. Supercond. Sci. Technol. 2013, 26, 095008. [Google Scholar] [CrossRef]
- Brusko, V.; Khannanov, A.; Rakhmatullin, A.; Dimiev, A.M. Unraveling the infrared spectrum of graphene oxide. Carbon 2024, 229, 119507. [Google Scholar] [CrossRef]
- Savilov, S.V.; Ivanov, A.S.; Chernyak, S.A.; Kirikova, M.N.; Ni, J.; Lunin, V.V. Features of the oxidation of multiwalled carbon nanotubes. Russ. J. Phys. Chem. 2015, 89, 1989. [Google Scholar] [CrossRef]
- Il’ves, V.G.; Sokovnin, S.Y.; Uporov, S.A.; Zuev, M.G. Properties of the amorphous-nanocrystalline Gd2O3 powder prepared by pulsed electron beam evaporation. Phys. Solid State 2013, 55, 1262. [Google Scholar] [CrossRef]
- Gayathri, T.; Kumar, R.A.; Dhilipkumaran, S.; Jayasankar, C.K.; Saravanan, P.; Devanand, B. Microwave-assisted combustion synthesis of silica-coated Eu:Gd2O3 nanoparticles for MRI and optical imaging of cancer cells. J. Mater. Sci. Mater. Electron. 2019, 30, 6860. [Google Scholar] [CrossRef]
- Li, J.P.H.; Zhou, X.; Pang, Y.; Zhu, L.; Vovk, E.I.; Cong, L.; van Bavel, A.P.; Li, S.; Yang, Y. Understanding of Binding Energy Calibration in XPS of Lanthanum Oxide by In Situ Treatment. Phys. Chem. Chem. Phys. 2019, 21, 22351–22358. [Google Scholar] [CrossRef]
- Uranta, K.G.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the Effectiveness of Polyacrylamide (PAM) Application in Hydrocarbon Reservoirs at Different Operational Conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef]
- Gaabour, L.H. Spectroscopic and thermal analysis of polyacrylamide/chitosan (PAM/CS) blend loaded by gold nanoparticles. Res. Phys. 2017, 7, 2153. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Hu, J.; Liu, Z. Atomic Understanding of the Swelling and Phase Transition of Polyacrylamide Hydrogel. Int. J. Appl. Mech. 2016, 8, 1640002. [Google Scholar] [CrossRef]
- Dehkordi, N.K.; Shojaei, S.; Asefnejad, A.; Hassani, K.; Benisi, S.Z. The effect of three types of cross-linked hydrogels and volume fraction of polyacrylamide on the swelling and thermal behavior using molecular dynamics simulation. J. Mater. Res. Technol. 2023, 24, 4627–4638. [Google Scholar] [CrossRef]
- Sawall, S.; Amato, C.; Klein, L.; Wehrse, E.; Maier, J.; Kachelrieß, M. Toward molecular imaging using spectral photon-counting computed tomography? Curr. Opin. Chem. Biol. 2021, 63, 163–170. [Google Scholar] [CrossRef]
- Hemmer, E.; Yamano, T.; Kishimoto, H.; Venkatachalam, N.; Hyodo, H.; Soga, K. Cytotoxic aspects of gadolinium oxide nanostructures for up-conversion and NIR bioimaging. Acta Biomater. 2013, 9, 4734–4743. [Google Scholar] [CrossRef] [PubMed]
- Savilov, S.V.; Strokova, N.E.; Ivanov, A.S.; Arkhipova, E.A.; Desyatov, A.V.; Hui, X.; Aldoshin, S.M.; Lunin, V.V. Nanoscale Carbon Materials from Hydrocarbons Pyrolysis: Structure, Chemical Behavior, Utilisation for Non-Aqueous Supercapacitors. Mater. Res. Bull. 2015, 69, 13. [Google Scholar] [CrossRef]
- Shabadrov, P.A.; Safronov, A.P. Superswelling of Hydrogels Based on the Copolymer of Acrylamide and Methacrylic Acid. Polym. Sci. Ser. A 2018, 60, 628. [Google Scholar] [CrossRef]
- Neamtu, I.; Chiriac, A.P.; Nita, L.E. Characterization of poly(acrylamide) as temperature-sensitive hydrogel. J. Optoelectron. Adv. Mater. 2006, 8, 1939. [Google Scholar]
- Shen, J.; Yan, B.; Li, T.; Long, Y.; Li, N.; Ye, M. Study on graphene-oxide-based polyacrylamide composite hydrogels. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1476. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O. Synthesis, Characterization and Adsorption Studies of an Acrylic Acid-Grafted Sodium Alginate-Based TiO2 Hydrogel Nanocomposite. Adsorpt. Sci. Technol. 2018, 36, 458. [Google Scholar] [CrossRef]
- Marsh, J.F.; Jorgensen, S.M.; Rundle, D.S.; Vercnocke, A.J.; Leng, S.; Butler, P.H.; McCollough, C.H.; Ritman, E.L. Evaluation of a photon counting Medipix3RX cadmium zinc telluride spectral X-Ray detector. J. Med. Imaging 2018, 5, 043503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).