Rheological Properties and Antioxidant Activity of Gelatin-Based Edible Coating Incorporating Tomato (Solanum lycopersicum L.) Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Rheology of Coating Solutions
2.2. Interaction of Added Coating Solution with Tomato Extract
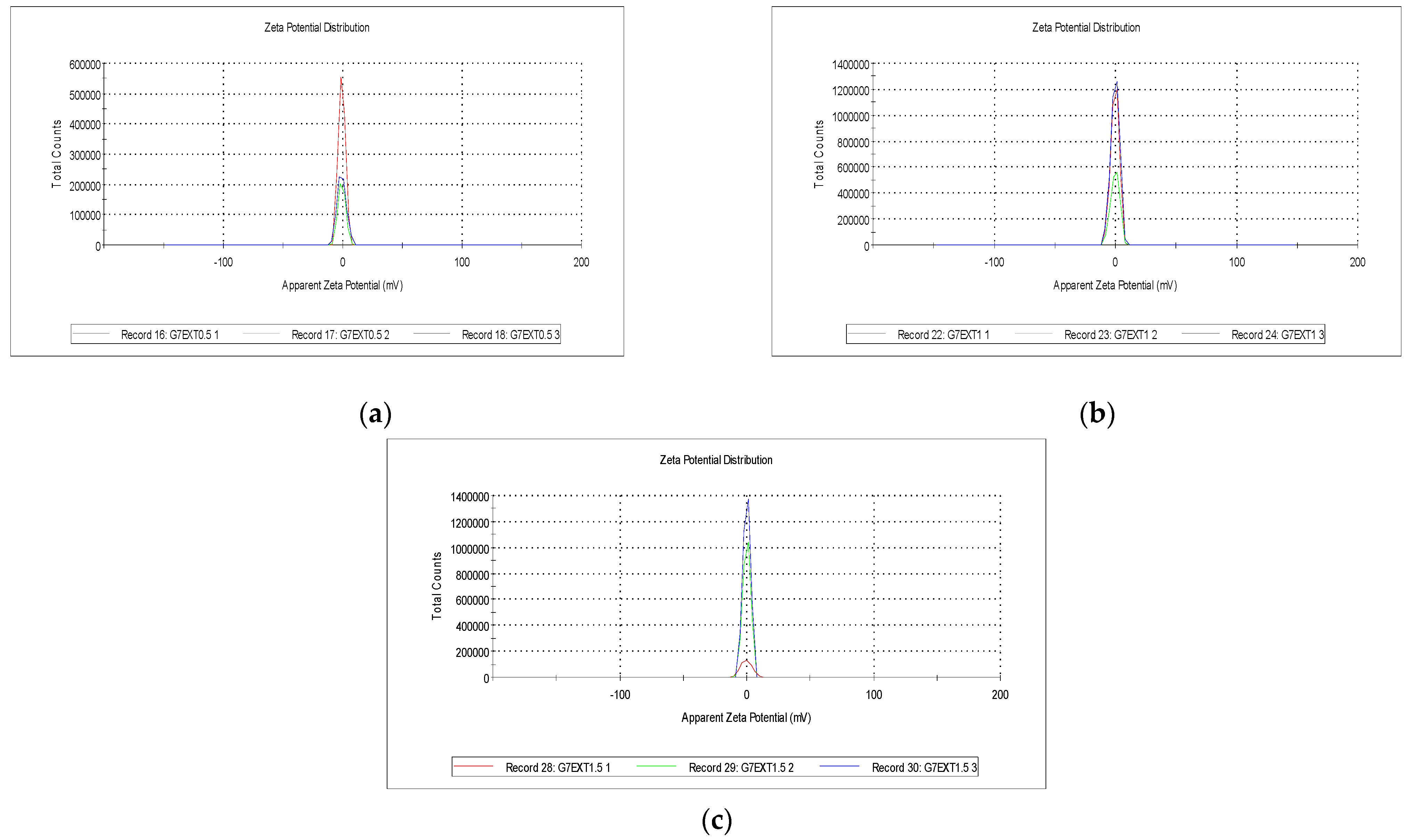
2.3. Zeta Potential
2.4. Antioxidant Capacity
2.5. Physicochemical Characterization
3. Conclusions
4. Materials and Methods
4.1. Obtaining Materials and Reagents
4.2. Ultrasound-Assisted Preparation of Tomato Extract
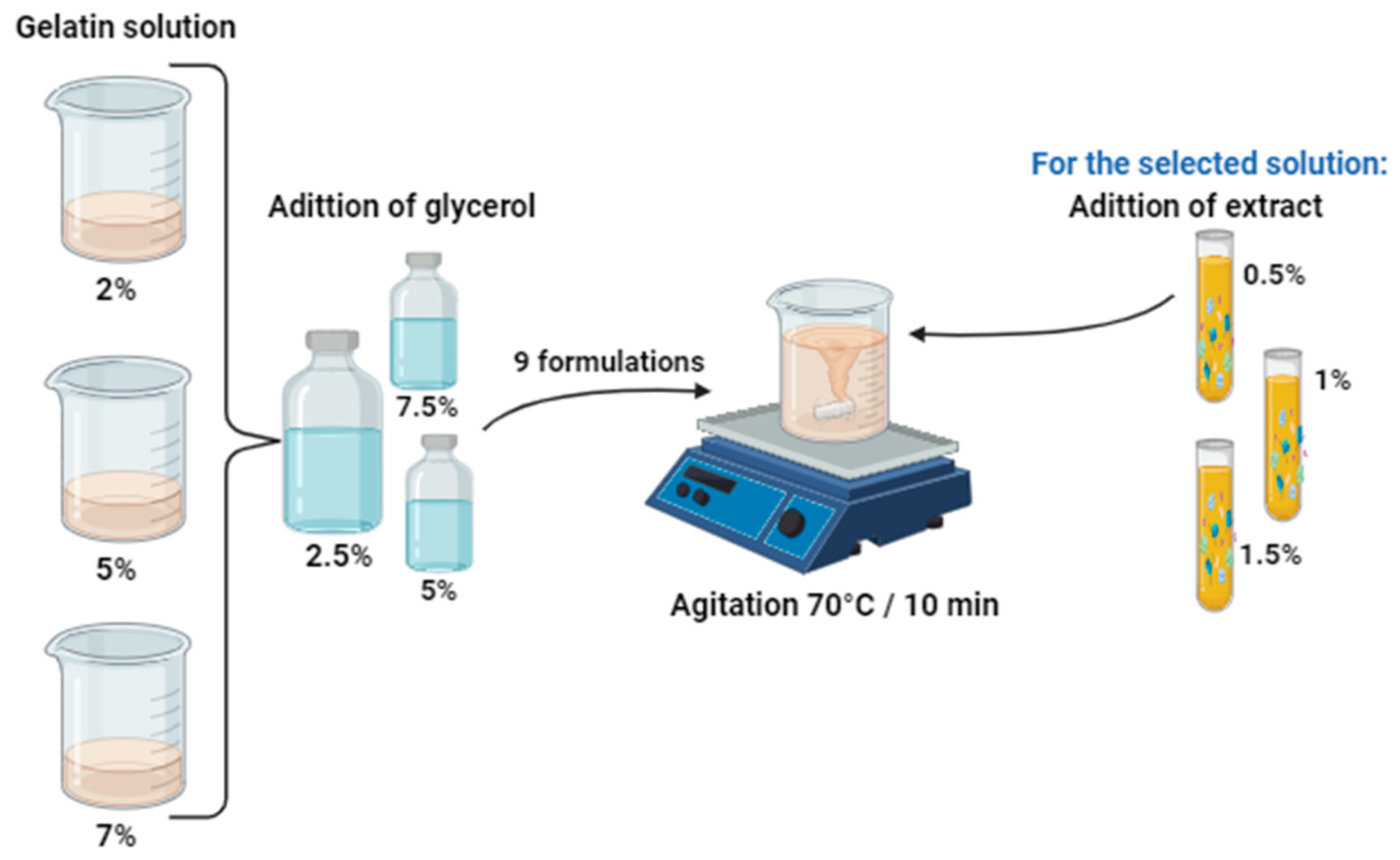
4.3. Preparation of the Edible Coating
4.4. Rheology of Coating Solutions
4.5. Fourier Transform Infrared Spectroscopy (FT-IR)
4.6. Zeta Potential
4.7. Antioxidant Activity
4.8. Physicochemical Characterization of Coating Solutions
4.8.1. Color
4.8.2. pH
4.9. Experimental Design and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Priya, K.; Thirunavookarasu, N.; Chidanand, D.V. Recent advances in edible coating of food products and its legislations: A review. J. Agric. Food Res. 2023, 12, 100623. [Google Scholar] [CrossRef]
- Hamann, D.; Puton, B.M.S.; Comin, T.; Colet, R.; Valduga, E.; Zeni, J.; Steffens, J.; Junges, A.; Backes, G.T.; Cansian, R.L. Active edible films based on green tea extract and gelatin for coating of fresh sausage. Meat Sci. 2020, 194, 108966. [Google Scholar] [CrossRef] [PubMed]
- Hamann, D.; Puton, B.M.S.; Colet, R.; Steffens, J.; Ceni, G.C.; Cansian, R.L.; Backes, G.T. Active edible films for application in meat products. Res. Soc. Dev. 2021, 10, e13610716379. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of gelatin in food packaging: A review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Gelatin-based composite films and their application in food packaging: A review. J. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Alcântara, L.O.; Martins, M.D.O.; Juliana Sousa, R.; Cerqueira, M.Â.; Silva Filho, A.L.C.; Souza, B.W.S. Wettability of edible coatings on Nile tilapia fillets (Oreochromis niloticus). J. Food Eng. 2019, 247, 152–159. [Google Scholar] [CrossRef]
- Salsabiela, S.; Sukma Sekarina, A.; Bagus, H.; Audiensi, A.; Azizah, F.; Heristika, W.; Manikharda; Susanto, E.; Munawaroh, H.S.H.; Show, P.L.; et al. Development of edible coating from gelatin composites with the addition of Black Tea extract (Camellia sinensis) on minimally processed watermelon (Citrullus lanatus). Polymers 2022, 14, 2628. [Google Scholar] [CrossRef]
- Katırcı, N.; Işık, N.; Güpür, Ç.; Guler, H.O.; Gursoy, O.; Yilmaz, Y. Differences in antioxidant activity, total phenolic and flavonoid contents of commercial and homemade tomato pastes. J. Saudi Soc. Agric. Sci. 2020, 19, 249–254. [Google Scholar] [CrossRef]
- Silva, L.P. Green synthesis of silver nanoparticles using tomato leaf extract and their entrapment in chitosan nanoparticles to control bacterial wilt. J. Sci. Food Agric. 2019, 99, 4248–4259. [Google Scholar]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramirez-Pimentel, J.G.; Vargas-Torres, A.; Hernández-Fuentes, A.D. Physicochemical and antioxidant properties of gelatin-based films containing oily tomato extract (Solanum lycopersicum L.). CyTA-J. Food 2019, 17, 142–150. [Google Scholar] [CrossRef]
- Gallego, M.; Arnal, M.; Talens, P.; Toldrá, F.; Mora, L. Efecto del recubrimiento de gelatina enriquecido con subproductos antioxidantes del tomate sobre la calidad de la carne de cerdo. Polímeros 2020, 12, 1032. [Google Scholar]
- Canché-López, K.C.; Toledo-López, V.M.; Vargas y Vargas, M.D.L.; Chan-Matú, D.I.; Madera-Santana, T.J. Caracterización de recubrimientos comestibles de quitosano elaborados con extractos naturales de Solanum lycopersicum y Moringa oleifera para la conservación de lomo de cerdo fresco. J. Food Meas. Charact. 2023, 17, 2233–2246. [Google Scholar] [CrossRef]
- Romanelli Vicente Bertolo, M.; da Conceição Amaro Martins, V.; de Guzzi Plepis, A.M.; Bogusz Junior, S. Rheological study of the incorporation of grape seed extract in chitosan and gelatin coatings. J. Appl. Polym. Sci. 2021, 138, 50052. [Google Scholar] [CrossRef]
- Dai, J.; Bai, M.; Li, C.; Cui, H.; Lin, L. The improvement of sodium dodecyl sulfate on the electrospinning of gelatin O/W emulsions for production of core-shell nanofibers. Food Hydrocoll. 2023, 145, 109092. [Google Scholar] [CrossRef]
- Szabo, K.; Teleky, B.E.; Mitrea, L.; Călinoiu, L.F.; Martău, G.A.; Simon, E.; Varvara, R.-A.; Vodnar, D.C. Active packaging—Poly (vinyl alcohol) films enriched with tomato by-products extract. Coatings 2020, 10, 141. [Google Scholar] [CrossRef]
- Andrade, R.D.; Skurtys, O.; Osorio, F.; Zuluaga, R.; Gañán, P.; Castro, C. Propiedades reológicas y físicas de suspensiones de gelatina que contienen nanofibras de celulosa para recubrimientos potenciales. Food Sci. Technol. Int. 2015, 21, 332–341. [Google Scholar] [CrossRef]
- Taktak, W.; Hamdi, M.; Chentir, I.; Boughriba, S.; Azaza, Y.B.; Li, S.; Nasri, M.; Karra-Chaâbouni, M.; Nasri, R. Development of emulsion gelatin gels for food application: Physicochemical, rheological, structural and thermal characterization. Int. J. Biol. Macromol. 2021, 182, 1–10. [Google Scholar] [CrossRef]
- Riva, S.C.; Opara, U.O.; Fawole, O.A. Recent developments on postharvest application of edible coatings on stone fruit: A review. Sci. Hortic. 2020, 262, 109074. [Google Scholar] [CrossRef]
- Schmitt, H.; Guidez, A.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Studies on the effect of storage time and plasticizers on the structural variations in thermoplastic starch. Carbohydr. Polym. 2015, 115, 364–372. [Google Scholar] [CrossRef]
- Nunes, J.C.; Melo, P.T.S.; Lorevice, M.V.; Aouada, F.A.; de Moura, M.R. Effect of green tea extract on gelatin-based films incorporated with lemon essential oil. J. Food Sci. Technol. 2021, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Hou, P.F.; Li, Y.X.; Yang, W.Y.; Shu, M.; Wu, G.P. Characterization, antioxidant and antibacterial activities of gelatin film incorporated with protocatechuic acid and its application on beef preservation. LWT 2021, 151, 112154. [Google Scholar] [CrossRef]
- Chou, M.Y.; Osako, K.; Lee, T.A.; Wang, M.F.; Lu, W.C.; Wu, W.J.; Ho, J.H. Characterization and antibacterial properties of fish skin gelatin/guava leaf extract bio-composited films incorporated with catechin. LWT 2023, 178, 114568. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2023, 17, 25–39. [Google Scholar] [CrossRef]
- Pérez-Marroquín, X.A.; Aguirre-Cruz, G.; Campos-Lozada, G.; Callejas-Quijada, G.; León-López, A.; Campos-Montiel, R.G.; Aguirre-Álvarez, G. Green synthesis of silver nanoparticles for preparation of gelatin films with antimicrobial activity. Polymers 2022, 14, 3453. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Rojas Benites, D.S.; Repo de Carrasco, R.; Encina Zelada, C.R. Determinación de la máxima retención de compuestos bioactivos y capacidad antioxidante en el néctar de tomate de árbol (Solanum betaceaum Cav.). Rev. Soc. Química Perú 2017, 83, 174–186. [Google Scholar] [CrossRef]
- Plaza, A.; Rodríguez, L.; Concha-Meyer, A.A.; Cabezas, R.; Zurob, E.; Merlet, G.; Palomo, I.; Fuentes, E. Effects of extraction methods on phenolic content, antioxidant and antiplatelet activities of tomato pomace extracts. Plants 2023, 12, 1188. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin-sodium alginate edible films with tea polyphenols. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef]
- Hanani, Z.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of pomegranate (Punica granatum L.) peel powder on the antioxidant and antimicrobial properties of fish gelatin films as active packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Kang, Y.R.; Lee, Y.K.; Kim, Y.J.; Chang, Y.H. Characterization and storage stability of chlorophylls microencapsulated in different combination of gum Arabic and maltodextrin. Food Chem. 2019, 272, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chiou, B.S.; Avena-Bustillos, R.J.; Zhang, Y.; Li, Y.; McHugh, T.H.; Zhong, F. Study of combined effects of glycerol and transglutaminase on properties of gelatin films. Food Hydrocoll. 2017, 65, 1–9. [Google Scholar] [CrossRef]
- Zaritzky, N. Edible coatings to improve food quality and safety. In Food Engineering Interfaces; Springer: New York, NY, USA, 2011; pp. 631–659. [Google Scholar]
- Canizales-Rodríguez, D.F.; Rodríguez-Félix, F.; Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Ruíz-Cruz, S.; Aubourg, S.P.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Burruel-Ibarra, S.E.; Pompa-Ramos, J.L.; et al. Food grade nanofiber of polylactic acid by electrospinning: Physicochemical characterization of solutions and parameters of the technique. J. Food Qual. 2024, 2024, 5579613. [Google Scholar] [CrossRef]
- Estrella-Osuna, D.E.; Tapia-Hernández, J.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A. Nanoencapsulation of eggplant (Solanum melongena L.) peel extract in electrospun gelatin nanofiber: Preparation, characterization, and in vitro release. Nanomaterials 2022, 12, 2303. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Anaya, D.G.; Madera-Santana, T.J.; Aguirre-Mancilla, C.L.; Grijalva-Verdugo, C.; Gonzales-Garcia, G.; Nuñez-Colín, C.A.; Rodriguez-Nuñez, J.R. Physicochemical characterization of residual potato (Solanum tuberosum) starch recovered from the potato chips industry in Mexico. Biotecnia 2023, 25, 60–72. [Google Scholar]
- Zhang, Y.L.; Cui, Q.L.; Wang, Y.; Shi, F.; Liu, Y.P.; Liu, J.L.; Nie, G.W. Effect of carboxymethyl chitosan-gelatin-based edible coatings on the quality and antioxidant properties of sweet cherry during postharvest storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflowers (Carthamus tinctorius L.) by-products: Antioxidant properties, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, e15765. [Google Scholar] [CrossRef]
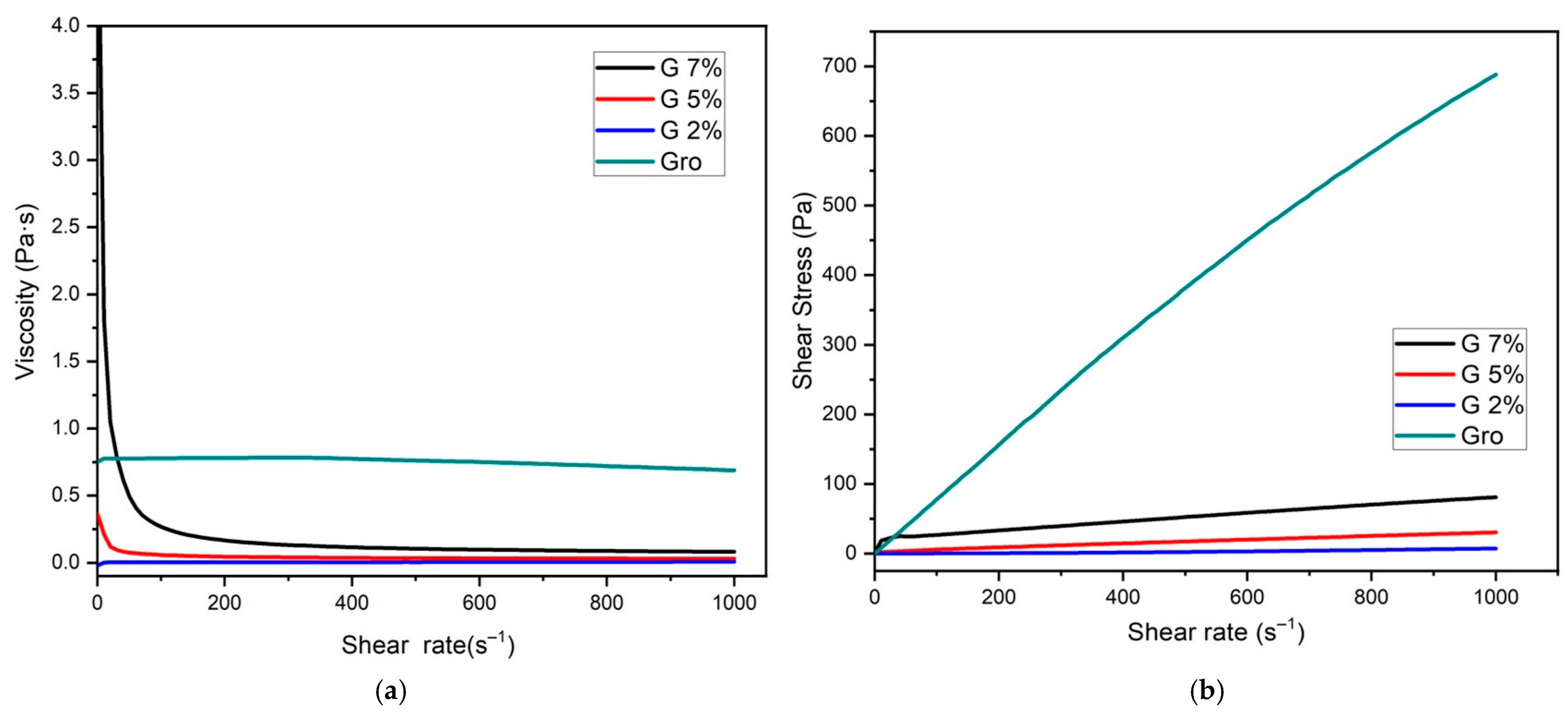




| Material Concentration (w/v) | K (passn) | N | R2 |
|---|---|---|---|
| Glycerol | 0.8315 ± 0.001 a | 0.9827 ± 0.123 a | 0.9992 ± 0.023 a |
| Gelatin 2% | 0.0129 ± 0.011 d | 0.8345 ± 0.002 b | 0.8923 ± 0.019 d |
| Gelatin 5% | 0.2382 ± 0.042 c | 0.6953 ± 0.031 d | 0.9918 ± 0.022 b |
| Gelatin 7% | 0.5621 ± 0.029 b | 0.7884 ± 0.008 c | 0.9408 ± 0.320 c |
| Formulation Gelatin/Glycerol (v/v) | K (passn) | N | R2 |
|---|---|---|---|
| 2%/2.5% | 0.2706 ± 0.0026 b | 0.6929 ± 0.0091 c | 0.9711 ± 0.0022 ab |
| 2%/5% | 0.1836 ± 0.0816 c | 0.6442 ± 0.0075 c | 0.9735 ± 0.0148 ab |
| 2%/7.5% | 0.1012 ± 0.0147 c | 0.6831 ± 0.0089 c | 0.9874 ± 0.0036 a |
| 5%/2.5% | 0.0885 ± 0.0024 c | 0.7865 ± 0.0035 b | 0.9751± 0.0037 ab |
| 5%/5% | 0.0643 ± 0.3212 cd | 0.7934 ±0.0052 b | 0.9259 ± 0.0336 b |
| 5%/7.5% | 0.5097 ± 0.0058 a | 0.7755 ± 0.0078 b | 0.9830 ± 0.0787 a |
| 7%/2.5% | 0.0297 ± 0.078 d | 1.0622 ± 0.9812 a | 0.9910 ± 0.7912 a |
| 7%/5% | 0.0620 ± 0.0091 cd | 0.9610 ± 0.0156 a | 0.9974 ± 0.0339 a |
| 7%/7.5% | 0.0586 ± 0.0872 cd | 1.0238 ± 0.0369 a | 0.9924 ± 0.0085 a |
| Formulation Gelatin/Glycerol/Extract (v/v) | K (passn) | N | R2 |
|---|---|---|---|
| 7%/7.5%/0.5% | 0.0113 ± 0.002 b | 1.0901 ± 0.0297 a | 0.9866 ± 0.0311 a |
| 7%/7.5%/1.0% | 0.0126 ± 0.001 b | 1.0675 ± 0.0336 a | 0.9828 ± 0.0862 a |
| 7%/7.5%/1.5% | 0.1432 ± 0.008 a | 1.0725 ± 0.0785 a | 0.9893 ± 0.0741 a |
| Material and Formulation (Gelatin/Glycerol/Extract) | Color | pH | ||
|---|---|---|---|---|
| L | a* | b* | ||
| Gelatin 7% | 37.58 ± 0.01 d | 0.61 ± 0.12 b | 2.43 ± 0.71 e | 5.46 ± 0.25 d |
| Glycerol | 20.56 ± 0.07 e | −4.58 ± 0.32 d | 7.91 ± 0.32 b | 5.23 ± 0.21 e |
| Tomato extract | 40.62 ± 0.91 c | 21.76 ± 1.48 a | 10.0 ± 0.59 a | 6.32 ± 0.55 a |
| Gelatin/Glycerol/Ext 0.5% | 42.27 ±0.02 a | 0.27 ± 0.02 c | 3.56 ± 0.23 d | 5.65 ± 0.12 b |
| Gelatin/Glycerol/Ext 1.0% | 41.82 ± 0.03 b | 0.32 ± 0.36 c | 3.61 ± 0.25 d | 5.52 ± 0.15 c |
| Gelatin/Glycerol/Ext 1.5% | 40.12 ± 0.03 c | 0.39 ± 0.14 c | 3.85 ± 0.14 c | 5.42 ± 0.13 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrella-Osuna, D.E.; Ruiz-Cruz, S.; Rodríguez-Félix, F.; Figueroa-Enríquez, C.E.; González-Ríos, H.; Fernández-Quiroz, D.; Márquez-Ríos, E.; Tapia-Hernández, J.A.; Pérez-Álvarez, J.Á.; Suárez-Jiménez, G.M. Rheological Properties and Antioxidant Activity of Gelatin-Based Edible Coating Incorporating Tomato (Solanum lycopersicum L.) Extract. Gels 2024, 10, 624. https://doi.org/10.3390/gels10100624
Estrella-Osuna DE, Ruiz-Cruz S, Rodríguez-Félix F, Figueroa-Enríquez CE, González-Ríos H, Fernández-Quiroz D, Márquez-Ríos E, Tapia-Hernández JA, Pérez-Álvarez JÁ, Suárez-Jiménez GM. Rheological Properties and Antioxidant Activity of Gelatin-Based Edible Coating Incorporating Tomato (Solanum lycopersicum L.) Extract. Gels. 2024; 10(10):624. https://doi.org/10.3390/gels10100624
Chicago/Turabian StyleEstrella-Osuna, Danya E., Saul Ruiz-Cruz, Francisco Rodríguez-Félix, Cielo E. Figueroa-Enríquez, Humberto González-Ríos, Daniel Fernández-Quiroz, Enrique Márquez-Ríos, José Agustín Tapia-Hernández, José Ángel Pérez-Álvarez, and Guadalupe Miroslava Suárez-Jiménez. 2024. "Rheological Properties and Antioxidant Activity of Gelatin-Based Edible Coating Incorporating Tomato (Solanum lycopersicum L.) Extract" Gels 10, no. 10: 624. https://doi.org/10.3390/gels10100624
APA StyleEstrella-Osuna, D. E., Ruiz-Cruz, S., Rodríguez-Félix, F., Figueroa-Enríquez, C. E., González-Ríos, H., Fernández-Quiroz, D., Márquez-Ríos, E., Tapia-Hernández, J. A., Pérez-Álvarez, J. Á., & Suárez-Jiménez, G. M. (2024). Rheological Properties and Antioxidant Activity of Gelatin-Based Edible Coating Incorporating Tomato (Solanum lycopersicum L.) Extract. Gels, 10(10), 624. https://doi.org/10.3390/gels10100624









