Influence of the Oil Structuring System on Lipid Hydrolysis and Bioaccessibility of Healthy Fatty Acids and Curcumin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Undigested Systems
2.1.1. General Appearance, Color, and Texture
2.1.2. Undigested Systems as Carriers of Fatty Acids with Implications on Human Health
2.2. Extent of Lipolysis during Static In Vitro Digestion
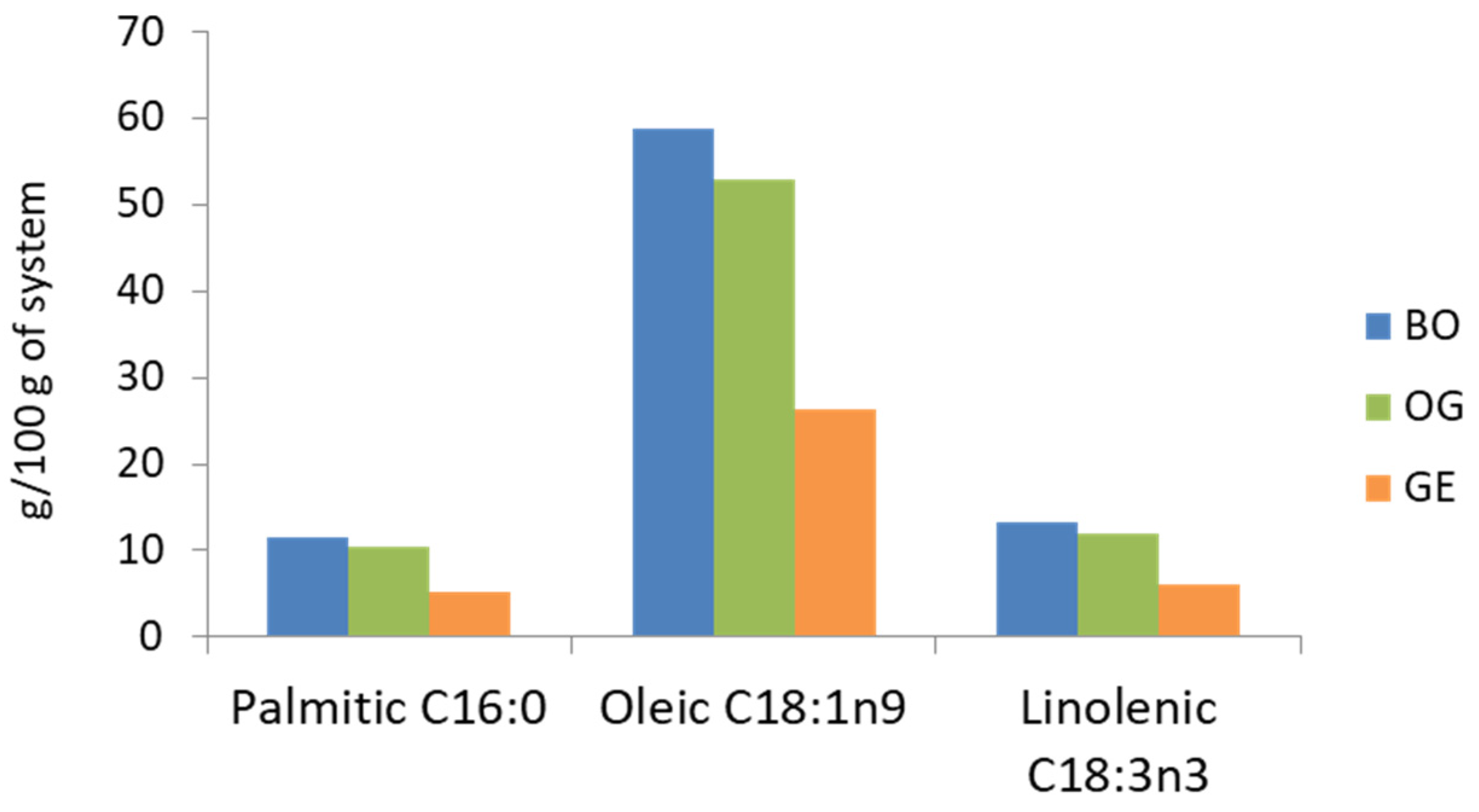
2.3. Fatty Acids Profile and Bioaccessibility of Evaluated Fatty Acids
2.4. Bioaccessibility of Curcumin
2.5. Correlations
3. Conclusions
4. Materials and Methods
4.1. System Design and Preparation
4.2. Characterization of the Undigested Systems
4.2.1. Color Parameters
4.2.2. Texture Analysis
4.3. Simulated Static In Vitro Gastrointestinal Digestion (GID) of the Systems
4.3.1. Extent of Lipolysis during In Vitro GID
4.3.2. Fatty Acids Profile and Bioaccessibility of the Evaluated Fatty Acids
4.3.3. Bioaccessibility of Curcumin
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrero, A.M.; Ruiz-Capillas, C. Novel lipid materials based on gelling procedures as fat analogues in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 39, 1–6. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Emulsion gels as potential fat replacers delivering beta-glucan and healthy lipid content for food applications. J. Food Sci. Technol. Mysore 2016, 53, 4336–4347. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Cofrades, S. Quality characteristics of healthy dry fermented sausages formulated with a mixture of olive and chia oil structured in oleogel or emulsion gel as animal fat replacer. Foods 2020, 9, 830. [Google Scholar] [CrossRef]
- FAO. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Alongi, M.; Lucci, P.; Clodoveo, M.L.; Schena, F.P.; Calligaris, S. Oleogelation of extra virgin olive oil by different oleogelators affects the physical properties and the stability of bioactive compounds. Food Chem. 2022, 368, 130779. [Google Scholar] [CrossRef]
- O’Sullivan, C.M.; Davidovich-Pinhas, M.; Wright, A.J.; Barbut, S.; Marangoni, A.G. Ethylcellulose oleogels for lipophilic bioactive delivery—Effect of oleogelation on in vitro bioaccessibility and stability of beta-carotene. Food Funct. 2017, 8, 1438–1451. [Google Scholar] [CrossRef]
- Robert, P.; Zamorano, M.; Gonzalez, E.; Silva-Weiss, A.; Cofrades, S.; Giménez, B. Double emulsions with olive leaves extract as fat replacers in meat systems with high oxidative stability. Food Res. Int. 2019, 120, 904–912. [Google Scholar] [CrossRef]
- Zhu, Q.M.; Gao, J.B.; Han, L.J.; Han, K.X.; Wei, W.; Wu, T.; Li, J.L.; Zhang, M. Development and characterization of novel bigels based on monoglyceride-beeswax oleogel and high acyl gellan gum hydrogel for lycopene delivery. Food Chem. 2021, 365, 130419. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef]
- Araiza-Calahorra, A.; Akhtar, M.; Sarkar, A. Recent advances in emulsion-based delivery approaches for curcumin: From encapsulation to bioaccessibility. Trends Food Sci. Technol. 2018, 71, 155–169. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced drug delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef]
- Tsuda, T. Curcumin as a functional food-derived factor: Degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Sun, H.; Lv, J.; Wang, Y.W.; Zhang, Y.; Wang, F.J. Effects of polysaccharide thickening agent on the preparation of walnut oil oleogels based on methylcellulose: Characterization and delivery of curcumin. Int. J. Biol. Macromol. 2023, 232, 123291. [Google Scholar] [CrossRef]
- Calligaris, S.; Alongi, M.; Lucci, P.; Anese, M. Effect of different oleogelators on lipolysis and curcuminoid bioaccessibility upon in vitro digestion of sunflower oil oleogels. Food Chem. 2020, 314, 126146. [Google Scholar] [CrossRef]
- Guo, Q.; Ye, A.; Bellissimo, N.; Singh, H.; Rousseau, D. Modulating fat digestion through food structure design. Prog. Lipid Res. 2017, 68, 109–118. [Google Scholar] [CrossRef]
- Wilde, P.J.; Chu, B.S. Interfacial & colloidal aspects of lipid digestion. Adv. Colloid Interface Sci. 2011, 165, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. The effect of household storage and cooking practices on quality attributes of pork burgers formulated with PUFA- and curcumin-loaded oleogels as healthy fat substitutes. LWT Food Sci. Technol. 2020, 119, 108909. [Google Scholar] [CrossRef]
- Li, X.L.; Meng, R.; Xu, B.C.; Zhang, B.; Cui, B.; Wu, Z.Z. Function emulsion gels prepared with carrageenan and zein/carboxymethyl dextrin stabilized emulsion as a new fat replacer in sausages. Food Chem. 2022, 389, 133005. [Google Scholar] [CrossRef]
- Borrin, T.R.; Georges, E.L.; Brito-Oliveira, T.C.; Moraes, I.C.F.; Pinho, S.C. Technological and sensory evaluation of pineapple icecreams incorporating curcumin-loaded nanoemulsions obtained by the emulsion inversion point method. Int. J. Dairy Technol. 2018, 71, 491–500. [Google Scholar] [CrossRef]
- Ciuffarin, F.; Alongi, M.; Plazzotta, S.; Lucci, P.; Schena, F.P.; Manzocco, L.; Calligaris, S. Oleogelation of extra virgin olive oil by different gelators affects lipid digestion and polyphenol bioaccesibility. Food Res. Int. 2023, 173, 113239. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.I.; Rigby, N.M.; Brodkorb, A.; Mackie, A.R. Dairy food structures influence the rates of nutrient digestion through different in vitro gastric behavior. Food Hydrocoll. 2017, 67, 63–73. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M.R. Lipolysis of soy protein and HPMC mixed emulsion as modulated by interfacial competence of emulsifiers. Food Hydrocoll. 2020, 99, 105328. [Google Scholar] [CrossRef]
- Cofrades, S.; Garcimartin, A.; Perez-Mateos, M.; Saiz, A.; Redondo-Castillejo, R.; Bocanegra, A.; Benedi, J.; Alvarez, M.D. Stabilized soy protein emulsion enriched with silicon and containing or not methylcellulose as novel technological alternatives to reduce animal fat digestion. Food Res. Int. 2023, 170, 112833. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fasolin, L.H.; Picone, C.S.F.; Pastrana, L.M.; Cunha, R.L.; Vicente, A.A. Structural and mechanical properties of organogels: Role of oil and gelator molecular structure. Food Res. Int. 2017, 96, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Toro-Vazquez, J.F.; Morales-Rueda, J.A.; Dibildox-Alvarado, E.; Charó-Alonso, M.A.; Alonzo-Macías, M.; González-Chávez, M.M. Development of organogels with candelilla wax and safflower oil with high triolein content. J. Am. Oil Chem. Soc. 2007, 84, 989–1000. [Google Scholar] [CrossRef]
- Bellesi, F.A.; Martinez, M.J.; Ruiz-Henestrosa, V.M.P.; Pilosof, A.M.R. Comparative behavior of protein or polysaccharide stabilized emulsion under in vitro gastrointestinal conditions. Food Hydrocoll. 2016, 52, 47–56. [Google Scholar] [CrossRef]
- Mcclements, D.J. Enhanced delivery of lipophilic bioactives using emulsions: A review of major factors affecting vitamin, nutraceutical, and lipid bioaccesibility. Food Funct. 2018, 9, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; McClements, D.J. New mathematical model for interpreting pH-Stat digestion profiles: Impact of lipid droplet characteristics on in vitro digestibility. J. Agric. Food Chem. 2010, 58, 8085–8092. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem 2012, 131, 48–54. [Google Scholar] [CrossRef]
- Zhang, S.W.; Xu, X.F.; Yang, J.G.; Ren, J. Impact of emulsifier structure and concentration on lipolysis dynamics and curcumin bioaccessibility in the nanoemulsions stabilized by polyglycerol fatty acid esters. Food Biophys. 2022, 17, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Wan, W.B.; Cheng, W.W.; Liu, G.Q.; Han, L.P. Oxidatively stable curcumin-loaded oleogels structured by beta-sitosterol and lecithin: Physical characteristics and release behaviour in vitro. Int. J. Food Sci. Technol. 2019, 54, 2502–2510. [Google Scholar] [CrossRef]
- Teixe-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martin-Belloso, O. Emulsion-based delivery systems to enhance the functionality of bioactive compounds: Towards the use of ingredients from natural, sustainable sources. Foods 2023, 12, 1502. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Zhang, G.; Xiao, H.; McClements, J. Influence of lipid phase composition of excipient emulsions on curcumin solubility, stability, and bioaccessibility. Food Biophys. 2016, 11, 213–225. [Google Scholar] [CrossRef]
- Jiang, T.; Liao, W.; Charcosset, C. Recent advances in encapsulation of curcumin in nanoemulsions: A review of encapsulation technologies, bioaccessibility and applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Laparra, J.M.; Velez, D.; Montoro, R.; Barbera, R.; Farre, R. Estimation of arsenic bioaccessibility in edible seaweed by an in vitro digestion method. J. Agric. Food Chem. 2003, 51, 6080–6085. [Google Scholar] [CrossRef]
- Dobarganes, M.C.; Velasco, J.; Dieffenbacher, A. Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats: Results of collaborative studies and the standardized method (Technical report). Pure Appl. Chem. 2000, 72, 1563–1575. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gavara, R.; Hernández-Munoz, P. Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications. Innov. Food Sci. Emerg. Technol. 2015, 29, 302–307. [Google Scholar] [CrossRef]
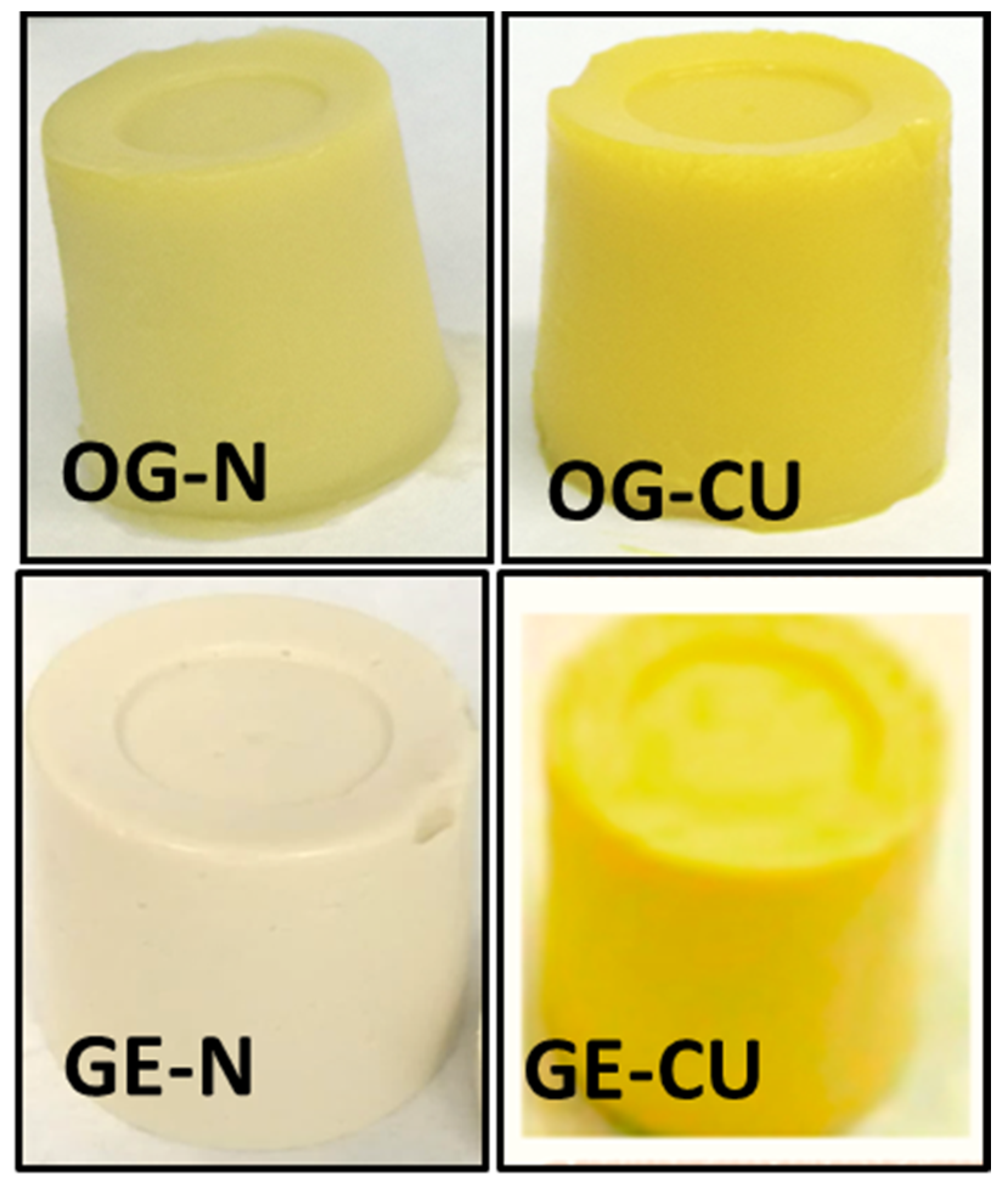

| Systems | Without CU | With CU | |
|---|---|---|---|
| L* | OG | 57.08 ± 0.68 a1 | 55.23 ± 2.54 a1 |
| GE | 80.02 ± 2.16 b2 | 72.14 ± 2.54 b1 | |
| a* | OG | −3.19 ± 0.07 a1 | −2.56 ± 0.27 a2 |
| GE | −0.20 ± 0.14 b1 | 1.45 ± 0.76 b2 | |
| b* | OG | 17.19 ± 0.51 a1 | 30.45 ± 2.88 a2 |
| GE | 16.06 ± 1.18 a1 | 53.36 ± 1.20 b2 | |
| Penetration Force (N) | OG | 0.96 ± 0.01b1 | 1.10 ± 0.02 b2 |
| GE | 0.31 ± 0.05 a1 | 0.27 ± 0.02 a1 |
| Systems | Without CU | With CU | |
|---|---|---|---|
|
TAG (g/100 g) | BO | 51.05 ± 0.52 b1 | 53.24 ± 3.68 b1 |
| OG | 48.79 ± 5.63 b1 | 72.56 ± 4.23 c2 | |
| GE | 26.42 ± 0.32 a2 | 13.71 ± 1.63 a1 | |
|
DAG (g/100 g) | BO | 22.32 ± 0.10 a1 | 21.47 ± 0.88 b1 |
| OG | 21.69 ± 2.96 a2 | 12.09 ± 0.56 b1 | |
| GE | 30.02 ± 1.38 b2 | 23.98 ± 0.49 b1 | |
|
MAG (g/100 g) | BO | 4.32 ± 0.26 a1 | 4.31 ± 0.51 a1 |
| OG | 5.01 ± 0.01 a2 | 3.23 ± 0.69 a1 | |
| GE | 18.15 ± 0.40 b2 | 12.44 ± 0.67 b1 | |
|
FFA (g/100 g) | BO | 22.33 ± 0.35 a1 | 20.98 ± 2.31 b1 |
| OG | 24.52 ± 2.66 a2 | 13.13 ± 2.98 a1 | |
| GE | 25.42 ± 1.46 a1 | 49.88 ± 0.48 c2 | |
|
FFA + MAG (g/100 g) | BO | 26.65 ± 0.62 a1 | 25.29 ± 2.81 b1 |
| OG | 29.53 ± 2.67 a2 | 15.36 ± 3.67 a1 | |
| GE | 43.57 ± 1.06 b1 | 62.32 ± 1.15 c2 | |
|
Lipid Digestibility (%) | BO | 48.90 ± 0.52 a1 | 46.70 ± 3.68 b1 |
| OG | 51.16 ± 5.63 a2 | 32.37 ± 4.23 a1 | |
| GE | 73.55 ± 0.32 b1 | 86.27 ± 1.63 c2 |
| Systems | Without CU | With CU | |
|---|---|---|---|
|
SFA (g/100 g) | BO | 6.58 ± 1.54 a1 | 6.24 ± 0.32 a1 |
| OG | 6.55 ± 0.58 a1 | 4.10 ± 1.35 a1 | |
| GE | 11.05 ± 0.42 b1 | 16.59 ± 0.66 b1 | |
|
MUFA (g/100 g) | BO | 17.36 ± 0.54 a1 | 11.54 ± 1.32 a1 |
| OG | 18.67 ± 2.36 a2 | 7.83 ± 2.70 a1 | |
| GE | 29.49 ± 2.64 b1 | 33.94 ± 0.44 b1 | |
|
PUFA (g/100 g) | BO | 6.01 ± 1.11 ab1 | 4.57 ± 0.48 a1 |
| OG | 5.37 ± 0.62 a1 | 3.16 ± 1.09 a1 | |
| GE | 8.33 ± 0.86 b1 | 12.84 ± 0.19 b2 |
| Systems | Without CU | With CU | |
|---|---|---|---|
| PA (C16:0) | BO | 32.94 ± 4.22 a1 | 35.19 ± 4.40 a1 |
| OG | 29.03 ± 6.48 a2 | 19.93 ± 6.86 a1 | |
| GE | 49.11 ± 1.03 b1 | 77.18 ± 2.51 b2 | |
| OA (C18:1n9c) | BO | 23.51 ± 2.98 a1 | 16.49 ± 2.34 a1 |
| OG | 21.91 ± 5.18 a2 | 9.60 ± 3.31 a1 | |
| GE | 34.24 ± 1.47 b1 | 38.96 ± 2.10 b1 | |
| ALA (C18:3n3) | BO | 20.99 ± 4.44 a1 | 18.23 ± 2.45 a1 |
| OG | 19.74 ± 4.61 a2 | 10.46 ± 3.76 a1 | |
| GE | 30.61 ± 1.77 b1 | 41.00 ± 2.39 b2 |
| TAG | MAG + FFA | Palmitic Bio | Oleic Bio | α-Linolenic Bio | System | |
|---|---|---|---|---|---|---|
| TAG | 1 | −0.993 ** | −0.837 * | −0.740 | −0.692 | BO-N/BO-CU |
| −0.997 ** | −0.946 ** | −0.941 ** | −0.942 ** | OG-N/OG-CU | ||
| −0.996 ** | −0.996 ** | −0.956 ** | −0.970 ** | GE-N/GE-CU | ||
| MAG + FFA | 1 | 0.829 * | 0.657 | 0.602 | BO-N/BO-CU | |
| 0.960 ** | 0.953 ** | 0.953 ** | OG-N/OG-CU | |||
| 0.999 ** | 0.975 ** | 0.986 ** | GE-N/GE-CU | |||
| Palmitic Bio | 1 | 0.727 | 0.539 | BO-N/BO-CU | ||
| 0.998 ** | 0.997 ** | OG-N/OG-CU | ||||
| 0.966 ** | 0.979 ** | GE-N/GE-CU | ||||
| Oleic Bio | 1 | 0.959 ** | BO-N/BO-CU | |||
| 1.000 ** | OG-N/OG-CU | |||||
| 0.998 ** | GE-N/GE-CU | |||||
| α-linolenic Bio | 1 | BO-N/BO-CU | ||||
| OG-N/OG-CU | ||||||
| GE-N/GE-CU | ||||||
| Curcumin Bio | BO-N/BO-CU | |||||
| OG-N/OG-CU | ||||||
| GE-N/GE-CU |
| Systems | Samples | Oil Mixture | CU | Beeswax | SPI | Gelatin | Water |
|---|---|---|---|---|---|---|---|
| BO | Without CU (BO–N) | 100.0 | - | - | - | - | - |
| With CU (BO–CU) | 99.8 | 0.2 | - | - | - | - | |
| OG | Without CU (OG–N) | 89.0 | - | 11.0 | - | - | - |
| With CU (OG–CU) | 88.82 | 0.178 | 11.0 | - | - | - | |
| GE | Without CU (GE–N) | 45.0 | - | - | 10.0 | 3.0 | 42.0 |
| With CU (GE–CU) | 44.91 | 0.09 | - | 10.0 | 3.0 | 42.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cofrades, S.; Gómez-Estaca, J.; Álvarez, M.D.; Garcimartín, A.; Macho-González, A.; Benedí, J.; Pintado, T. Influence of the Oil Structuring System on Lipid Hydrolysis and Bioaccessibility of Healthy Fatty Acids and Curcumin. Gels 2024, 10, 33. https://doi.org/10.3390/gels10010033
Cofrades S, Gómez-Estaca J, Álvarez MD, Garcimartín A, Macho-González A, Benedí J, Pintado T. Influence of the Oil Structuring System on Lipid Hydrolysis and Bioaccessibility of Healthy Fatty Acids and Curcumin. Gels. 2024; 10(1):33. https://doi.org/10.3390/gels10010033
Chicago/Turabian StyleCofrades, Susana, Joaquín Gómez-Estaca, María Dolores Álvarez, Alba Garcimartín, Adrián Macho-González, Juana Benedí, and Tatiana Pintado. 2024. "Influence of the Oil Structuring System on Lipid Hydrolysis and Bioaccessibility of Healthy Fatty Acids and Curcumin" Gels 10, no. 1: 33. https://doi.org/10.3390/gels10010033
APA StyleCofrades, S., Gómez-Estaca, J., Álvarez, M. D., Garcimartín, A., Macho-González, A., Benedí, J., & Pintado, T. (2024). Influence of the Oil Structuring System on Lipid Hydrolysis and Bioaccessibility of Healthy Fatty Acids and Curcumin. Gels, 10(1), 33. https://doi.org/10.3390/gels10010033







