Ecological Barriers for an Amphibian Pathogen: A Narrow Ecological Niche for Batrachochytrium salamandrivorans in an Asian Chytrid Hotspot
Abstract
1. Introduction
2. Materials and Methods
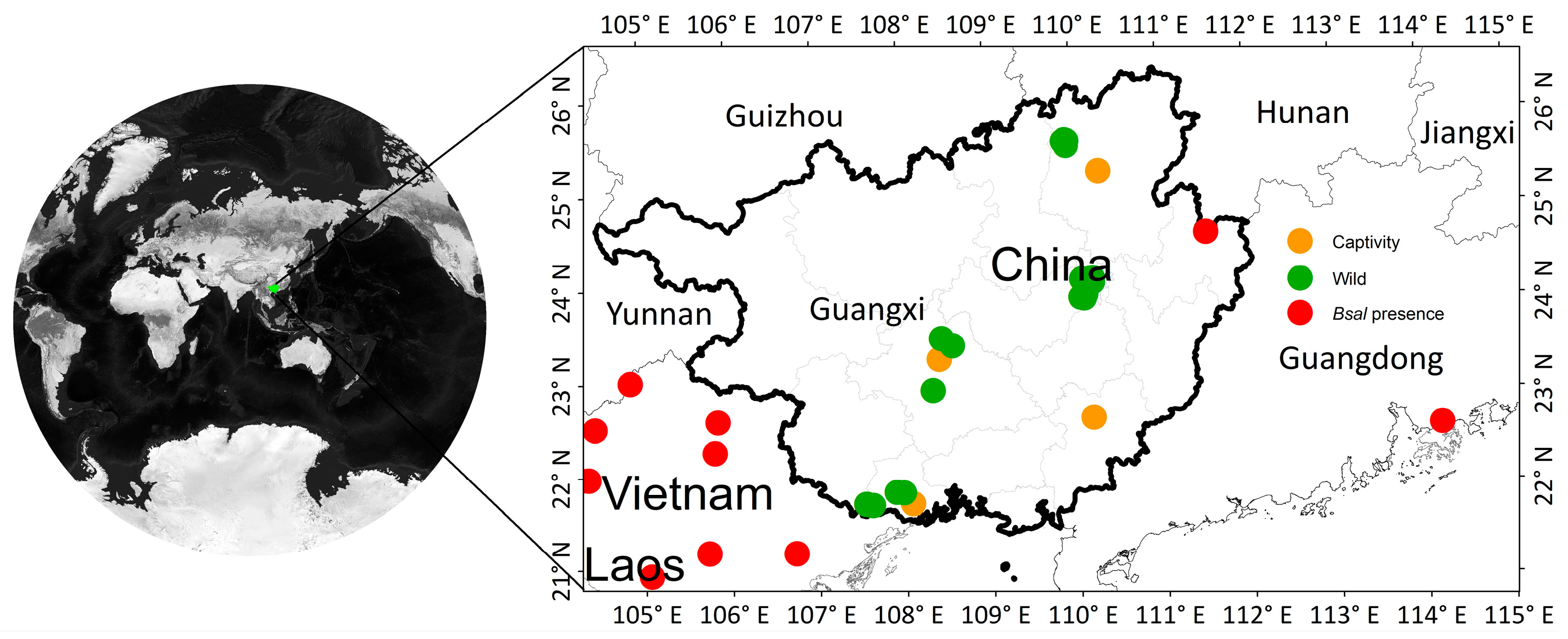
2.1. Survey Region
2.2. Sample Collection
2.3. Detection of Bsal
2.4. Ecological Niche Modelling
| Variable | Hypotheses | Source | |
|---|---|---|---|
| Climate factor | Annual mean temperature (bio_1) | Temperature and moisture affect Bsal life history and pathogenicity [3,22,67]. | WorldClim v.2.1 [68] |
| Mean diurnal temperature range (bio_2) | |||
| Isothermality (bio_3) | |||
| Temperature seasonality (bio_4) | |||
| Max temperature of warmest month (bio_5) | |||
| Min temperature of coldest month (bio_6) | |||
| Annual temperature range (bio_7) | |||
| Mean temperature of wettest quarter (bio_8) | |||
| Mean temperature of driest quarter (bio_9) | |||
| Mean temperature of warmest quarter (bio_10) | |||
| Mean temperature of coldest quarter (bio_11) | |||
| Annual precipitation (bio_12) | |||
| Precipitation of wettest month (bio_13) | |||
| Precipitation of driest month (bio_14) | |||
| Precipitation seasonality (bio_15) | |||
| Precipitation of wettest quarter (bio_16) | |||
| Precipitation of driest quarter (bio_17) | |||
| Precipitation of warmest quarter (bio_18) | |||
| Precipitation of coldest quarter (bio_19) | |||
| Landscape factor | Altitude | It is expected that these factors have an important influence on Bsal distribution, with human-related factors having positive relationships with probabilities of Bsal occurrence. | http://srtm.csi.cgiar.org/srtmdata/ (accessed on 22 June 2023) |
| Soil water stress | [69] | ||
| Human footprint | [56] | ||
| Human population density | [70] | ||
| Normalized difference vegetation index | https://www.earthdata.nasa.gov/ (accessed on 26 June 2023) | ||
| Enhanced vegetation index | https://www.earthdata.nasa.gov/ (accessed on 26 June 2023) | ||
| Net primary productivity | https://www.earthdata.nasa.gov/ (accessed on 26 June 2023) | ||
| Biotic factor | Amphibian species’ richness | Dilution or amplification effects of biodiversity have profound influence on pathogen transmission [38,71]. Higher amphibian species richness might pose dilution effect since it possibly includes resistant species, whereas higher caudate species richness may pose an amplification effect since it may include more susceptible species. | [72] |
| Caudate species’ richness | [72] |
3. Results
3.1. Bsal Absence in Wild and Captive Amphibians
3.2. Bsal Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AmphibiaWeb. University of California, Berkeley, CA, USA. Available online: https://amphibiaweb.org (accessed on 4 August 2023).
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mol. Ecol. Resour. 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M.C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; et al. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc. Natl. Acad. Sci. USA 2013, 110, 15325–15329. [Google Scholar] [CrossRef] [PubMed]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Martel, A.; Blooi, M.; Adriaensen, C.; Van Rooij, P.; Beukema, W.; Fisher, M.C.; Farrer, R.A.; Schmidt, B.R.; Tobler, U.; Goka, K.; et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 2014, 346, 630–631. [Google Scholar] [CrossRef]
- O’Hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M.; et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, G.; Herath, J.; Dan, S.; Pie, M.R.; Murray, K.A.; Pethiyagoda, R.; Hanken, J.; Meegaskumbura, M. Climatic niche evolution of infectious diseases driving amphibian declines. Glob. Chang. Biol. 2023; submitted. [Google Scholar]
- Ghose, S.L.; Yap, T.A.; Byrne, A.Q.; Sulaeman, H.; Rosenblum, E.B.; Chan-Alvarado, A.; Chaukulkar, S.; Greenbaum, E.; Koo, M.S.; Kouete, M.T.; et al. Continent-wide recent emergence of a global pathogen in African amphibians. Front. Conserv. Sci. 2023, 4, 1069490. [Google Scholar] [CrossRef]
- Towe, A.E.; Gray, M.J.; Carter, E.D.; Wilber, M.Q.; Ossiboff, R.J.; Ash, K.; Bohanon, M.; Bajo, B.A.; Miller, D.L. Batrachochytrium salamandrivorans can devour more than salamanders. J. Wildl. Dis. 2021, 57, 942–948. [Google Scholar] [CrossRef]
- Castro Monzon, F.; Rödel, M.-O.; Ruland, F.; Parra-Olea, G.; Jeschke, J.M. Batrachochytrium salamandrivorans’ amphibian host species and invasion range. EcoHealth 2023, 19, 475–486. [Google Scholar] [CrossRef]
- Fisher, M.C.; Garner, T.W.J. Chytrid fungi and global amphibian declines. Nat. Rev. Microbiol. 2020, 18, 332–343. [Google Scholar] [CrossRef]
- Carter, E.D.; Miller, D.L.; Peterson, A.C.; Sutton, W.B.; Cusaac, J.P.W.; Spatz, J.A.; Rollins-Smith, L.; Reinert, L.; Bohanon, M.; Williams, L.A.; et al. Conservation risk of Batrachochytrium salamandrivorans to endemic lungless salamanders. Conserv. Lett. 2020, 13, e12675. [Google Scholar] [CrossRef]
- Crawshaw, L.; Buchanan, T.; Shirose, L.; Palahnuk, A.; Cai, H.Y.; Bennett, A.M.; Jardine, C.M.; Davy, C.M. Widespread occurrence of Batrachochytrium dendrobatidis in Ontario, Canada, and predicted habitat suitability for the emerging Batrachochytrium salamandrivorans. Ecol. Evol. 2022, 12, e8798. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, A.; Basanta, M.D.; García-Castillo, M.G.; Zumbado-Ulate, H.; Neam, K.; Rovito, S.; Searle, C.L.; Parra-Olea, G. Anticipating the potential impacts of Batrachochytrium salamandrivorans on Neotropical salamander diversity. Biotropica 2022, 54, 157–169. [Google Scholar] [CrossRef]
- Moubarak, M.; Fischhoff, I.R.; Han, B.A.; Castellanos, A.A. A spatially explicit risk assessment of salamander populations to Batrachochytrium salamandrivorans in the United States. Divers. Distrib. 2022, 28, 2316–2329. [Google Scholar] [CrossRef]
- North American Bsal Task Force, A North American Strategic Plan to Prevent and Control Invasions of the Lethal Salamander Pathogen Batrachochytrium salamandrivorans. 2022. Available online: https://salamanderfungus.org (accessed on 4 June 2023).
- Yap, T.A.; Koo, M.S.; Ambrose, R.F.; Wake, D.B.; Vredenburg, V.T. Averting a North American biodiversity crisis. Science 2015, 349, 481–482. [Google Scholar] [CrossRef]
- Gray, M.J.; Carter, E.D.; Piovia-Scott, J.; Cusaac, J.P.W.; Peterson, A.C.; Whetstone, R.D.; Hertz, A.; Muniz-Torres, A.Y.; Bletz, M.C.; Woodhams, D.C.; et al. Broad host susceptibility of North American amphibian species to Batrachochytrium salamandrivorans suggests high invasion potential and biodiversity risk. Nat. Commun. 2023, 14, 3270. [Google Scholar] [CrossRef]
- Lötters, S.; Wagner, N.; Albaladejo, G.; Boning, P.; Dalbeck, L.; Dussel, H.; Feldmeier, S.; Guschal, M.; Kirst, K.; Ohlhoff, D.; et al. The amphibian pathogen Batrachochytrium salamandrivorans in the hotspot of its European invasive range: Past-present-future. Salamandra 2020, 56, 173–188. [Google Scholar]
- Nguyen, T.T.; Nguyen, T.V.; Ziegler, T.; Pasmans, F.; Martel, A. Trade in wild anurans vectors the urodelan pathogen Batrachochytrium salamandrivorans into Europe. Amphib. Reptil. 2017, 38, 554–556. [Google Scholar] [CrossRef]
- Schulz, V.; Schulz, A.; Klamke, M.; Preissler, K.; Sabino-Pinto, J.; Musken, M.; Schlupmann, M.; Heldt, L.; Kamprad, F.; Enss, J.; et al. Batrachochytrium salamandrivorans in the Ruhr District, Germany: History, distribution, decline dynamics and disease symptoms of the salamander plague. Salamandra 2020, 56, 189–214. [Google Scholar]
- Stegen, G.; Pasmans, F.; Schmidt, B.R.; Rouffaer, L.O.; Van Praet, S.; Schaub, M.; Canessa, S.; Laudelout, A.; Kinet, T.; Adriaensen, C.; et al. Drivers of salamander extirpation mediated by Batrachochytrium salamandrivorans. Nature 2017, 544, 353–356. [Google Scholar] [CrossRef]
- Martel, A.; Vila-Escale, M.; Fernández-Giberteau, D.; Martinez-Silvestre, A.; Canessa, S.; Van Praet, S.; Pannon, P.; Chiers, K.; Ferran, A.; Kelly, M.; et al. Integral chain management of wildlife diseases. Conserv. Lett. 2020, 13, e12707. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Martel, A.; Asselberghs, J.; Bales, E.K.; Beukema, W.; Bletz, M.C.; Dalbeck, L.; Goverse, E.; Kerres, A.; Kinet, T.; et al. Expanding distribution of lethal amphibian fungus Batrachochytrium salamandrivorans in Europe. Emerg. Infect. Dis. 2016, 22, 1286–1288. [Google Scholar] [CrossRef] [PubMed]
- González, D.L.; Baláž, V.; Solský, M.; Thumsová, B.; Kolenda, K.; Najbar, A.; Najbar, B.; Kautman, M.; Chajma, P.; Balogová, M.; et al. Recent findings of potentially lethal salamander fungus Batrachochytrium salamandrivorans. Emerg. Infect. Dis. 2019, 25, 1416–1418. [Google Scholar] [CrossRef] [PubMed]
- Laking, A.E.; Ngo, H.N.; Pasmans, F.; Martel, A.; Nguyen, T.T. Batrachochytrium salamandrivorans is the predominant chytrid fungus in Vietnamese salamanders. Sci. Rep. 2017, 7, 44443. [Google Scholar] [CrossRef] [PubMed]
- Farrer, R.A.; Weinert, L.A.; Bielby, J.; Garner, T.W.J.; Balloux, F.; Clare, F.; Bosch, J.; Cunningham, A.A.; Weldon, C.; du Preez, L.H.; et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc. Natl. Acad. Sci. USA 2011, 108, 18732–18736. [Google Scholar] [CrossRef]
- Wacker, T.; Helmstetter, N.; Wilson, D.; Fisher, M.C.; Studholme, D.J.; Farrer, R.A. Two-speed genome evolution drives pathogenicity in fungal pathogens of animals. Proc. Natl. Acad. Sci. USA 2023, 120, e2212633120. [Google Scholar] [CrossRef]
- Lötters, S.; Wagner, N.; Kerres, A.; Vences, M.; Steinfartz, S.; Sabino-Pinto, J.; Seufer, L.; Preissler, K.; Schulz, V.; Veith, M. First report of host co-infection of parasitic amphibian chytrid fungi. Salamandra 2018, 54, 287–290. [Google Scholar]
- Longo, A.V.; Fleischer, R.C.; Lips, K.R. Double trouble: Co-infections of chytrid fungi will severely impact widely distributed newts. Biol. Invasions 2019, 21, 2233–2245. [Google Scholar] [CrossRef]
- Ribas, M.P.; Cabezón, O.; Velarde, R.; Estruch, J.; Serrano, E.; Bosch, J.; Thumsová, B.; Martínez-Silvestre, A. Coinfection of chytrid fungi in urodeles during an outbreak of chytridiomycosis in Spain. J. Wildl. Dis. 2022, 58, 658–663. [Google Scholar] [CrossRef]
- Farrer, R.A.; Martel, A.; Verbrugghe, E.; Abouelleil, A.; Ducatelle, R.; Longcore, J.E.; James, T.Y.; Pasmans, F.; Fisher, M.C.; Cuomo, C.A. Genomic innovations linked to infection strategies across emerging pathogenic chytrid fungi. Nat. Commun. 2017, 8, 14742. [Google Scholar] [CrossRef]
- Byrne, A.Q.; Vredenburg, V.T.; Martel, A.; Pasmans, F.; Bell, R.C.; Blackburn, D.C.; Bletz, M.C.; Bosch, J.; Briggs, C.J.; Brown, R.M.; et al. Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation. Proc. Natl. Acad. Sci. USA 2019, 116, 20382–20387. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, X.; Fisher, M.C.; Garner, T.W.J.; Li, Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers. Distrib. 2012, 18, 307–318. [Google Scholar] [CrossRef]
- Sun, D.; Ellepola, G.; Herath, J.; Liu, H.; Liu, Y.; Murray, K.; Meegaskumbura, M. Climatically specialized lineages of Batrachochytrium dendrobatidis and Asian origins. Ecohealth, 2023; submitted. [Google Scholar]
- Peterson, A.T. Ecologic niche modeling and spatial patterns of disease transmission. Emerg. Infect. Dis. 2006, 12, 1822–1826. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ellepola, G.; Herath, J.; Meegaskumbura, M. The two chytrid pathogens of amphibians in Eurasia—Climatic niches and future expansion. BMC Ecol. Evol. 2023, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Searle, C.L.; Biga, L.M.; Spatafora, J.W.; Blaustein, A.R. A dilution effect in the emerging amphibian pathogen Batrachochytrium dendrobatidis. Proc. Natl. Acad. Sci. USA 2011, 108, 16322–16326. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.D.; Kuenzi, A.J.; Mills, J.N. Species diversity concurrently dilutes and amplifies transmission in a zoonotic host–pathogen system through competing mechanisms. Proc. Natl. Acad. Sci. USA 2018, 115, 7979–7984. [Google Scholar] [CrossRef]
- Beukema, W.; Erens, J.; Schulz, V.; Stegen, G.; Spitzen-van der Sluijs, A.; Stark, T.; Laudelout, A.; Kinet, T.; Kirschey, T.; Poulain, M.; et al. Landscape epidemiology of Batrachochytrium salamandrivorans: Reconciling data limitations and conservation urgency. Ecol. Appl. 2021, 31, e02342. [Google Scholar] [CrossRef]
- Murray, K.A.; Retallick, R.W.R.; Puschendorf, R.; Skerratt, L.F.; Rosauer, D.; McCallum, H.I.; Berger, L.; Speare, R.; VanDerWal, J. Assessing spatial patterns of disease risk to biodiversity: Implications for the management of the amphibian pathogen, Batrachochytrium dendrobatidis. J. Appl. Ecol. 2011, 48, 163–173. [Google Scholar] [CrossRef]
- Rohr, J.R.; Halstead, N.T.; Raffel, T.R. Modelling the future distribution of the amphibian chytrid fungus: The influence of climate and human-associated factors. J. Appl. Ecol. 2011, 48, 174–176. [Google Scholar] [CrossRef]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.P.; Sousa, R.; Gonçalves, D.V.; Miranda, R.; Reis, J.; Teixeira, A.; Varandas, S.; Lopes-Lima, M.; Filipe, A.F. Streams in the Mediterranean Region are not for mussels: Predicting extinctions and range contractions under future climate change. Sci. Total Environ. 2023, 883, 163689. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Martel, A.; Wu, J.; Van Praet, S.; Canessa, S.; Pasmans, F. Widespread occurrence of an emerging fungal pathogen in heavily traded Chinese urodelan species. Conserv. Lett. 2018, 11, e12436. [Google Scholar] [CrossRef]
- Cunningham, A.A.; Beckmann, K.; Perkins, M.; Fitzpatrick, L.; Cromie, R.; Redbond, J.; O’Brien, M.F.; Ghosh, P.; Shelton, J.; Fisher, M.C. Emerging disease in UK amphibians. Vet. Rec. 2015, 176, 468. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, L.D.; Pasmans, F.; Martel, A.; Cunningham, A.A. Epidemiological tracing of Batrachochytrium salamandrivorans identifies widespread infection and associated mortalities in private amphibian collections. Sci. Rep. 2018, 8, 13845. [Google Scholar] [CrossRef]
- Sabino-Pinto, J.; Bletz, M.; Hendrix, R.; Perl, R.G.B.; Martel, A.; Pasmans, F.; Lötters, S.; Mutschmann, F.; Schmeller, D.S.; Schmidt, B.R.; et al. First detection of the emerging fungal pathogen Batrachochytrium salamandrivorans in Germany. Amphib. Reptil. 2015, 36, 411–416. [Google Scholar] [CrossRef]
- Boyle, D.G.; Boyle, D.B.; Olsen, V.; Morgan, J.A.T.; Hyatt, A.D. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Org. 2004, 60, 141–148. [Google Scholar] [CrossRef]
- Gaertner, J.P.; Forstner, M.R.J.; O’Donnell, L.; Hahn, D. Detection of Batrachochytrium dendrobatidis in endemic salamander species from Central Texas. EcoHealth 2009, 6, 20–26. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, F.; Bai, C.; Liu, X.; Wang, S.; Gao, X.; Yan, S.; Li, X.; Liu, Z.; Li, Y. A survey for Batrachochytrium salamandrivorans in Chinese amphibians. Curr. Zool. 2014, 60, 729–735. [Google Scholar] [CrossRef][Green Version]
- Blooi, M.; Pasmans, F.; Longcore, J.E.; Spitzen-van der Sluijs, A.; Vercammen, F.; Martel, A. Duplex real-time PCR for rapid simultaneous detection of Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans in amphibian samples. J. Clin. Microbiol. 2013, 51, 4173–4177, Correction to J. Clin. Microbiol. 2016, 54, 246–246. [Google Scholar] [CrossRef]
- Basanta, M.D.; Rebollar, E.A.; Parra-Olea, G. Potential risk of Batrachochytrium salamandrivorans in Mexico. PLoS ONE 2019, 14, e0211960. [Google Scholar] [CrossRef] [PubMed]
- Beukema, W.; Martel, A.; Nguyen, T.T.; Goka, K.; Schmeller, D.S.; Yuan, Z.; Laking, A.E.; Nguyen, T.Q.; Lin, C.F.; Shelton, J.; et al. Environmental context and differences between native and invasive observed niches of Batrachochytrium salamandrivorans affect invasion risk assessments in the Western Palaearctic. Divers. Distrib. 2018, 24, 1788–1801. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 2017, 67, 534–545. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P.; Anderson, B. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Ringwaldt, E.M.; Brook, B.W.; Buettel, J.C.; Cunningham, C.X.; Fuller, C.; Gardiner, R.; Hamer, R.; Jones, M.; Martin, A.M.; Carver, S. Host, environment, and anthropogenic factors drive landscape dynamics of an environmentally transmitted pathogen: Sarcoptic mange in the bare-nosed wombat. J. Anim. Ecol. 2023, 92, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Sun, P.; Wang, T.; Wang, G.; Zhang, X.; Wang, L. Consensus forecasting of species distributions: The effects of niche model performance and niche properties. PLoS ONE 2015, 10, e0120056. [Google Scholar] [CrossRef] [PubMed]
- Naimi, B.; Araújo, M.B. sdm: A reproducible and extensible R platform for species distribution modelling. Ecography 2016, 39, 368–375. [Google Scholar] [CrossRef]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Carter, E.D.; Bletz, M.C.; Le Sage, M.; LaBumbard, B.; Rollins-Smith, L.A.; Woodhams, D.C.; Miller, D.L.; Gray, M.J. Winter is coming–Temperature affects immune defenses and susceptibility to Batrachochytrium salamandrivorans. PLoS Pathog. 2021, 17, e1009234. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J. Global High-Resolution Soil-Water Balance. Figshare. 2019. Available online: https://doi.org/10.6084/m9.figshare.7707605.v3 (accessed on 12 February 2019).
- Center for International Earth Science Information Network-CIESIN-Columbia University. Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 11. Palisades, New York: NASA Socioeconomic Data and Applications Center (SEDAC). 2018. Available online: https://doi.org/10.7927/H49C6VHW (accessed on 22 June 2023).
- Halliday, F.W.; Heckman, R.W.; Wilfahrt, P.A.; Mitchell, C.E. A multivariate test of disease risk reveals conditions leading to disease amplification. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171340. [Google Scholar] [CrossRef]
- International Union for Conservation of Nature-IUCN, and Center for International Earth Science Information Network-CIESIN-Columbia University. Gridded Species Distribution: Global Amphibian Richness Grids, 2015 Release. Palisades, New York: NASA Socioeconomic Data and Applications Center (SEDAC). 2015. Available online: https://doi.org/10.7927/H4RR1W66 (accessed on 26 April 2023).
- Zhu, W.; Bai, C.; Wang, S.; Soto-Azat, C.; Li, X.; Liu, X.; Li, Y. Retrospective survey of museum specimens reveals historically widespread presence of Batrachochytrium dendrobatidis in China. EcoHealth 2014, 11, 241–250. [Google Scholar] [CrossRef]
- Blooi, M.; Martel, A.; Haesebrouck, F.; Vercammen, F.; Bonte, D.; Pasmans, F. Treatment of urodelans based on temperature dependent infection dynamics of Batrachochytrium salamandrivorans. Sci. Rep. 2015, 5, 8037. [Google Scholar] [CrossRef]
- Wang, S.P.; Zhu, W.; Fan, L.Q.; Li, J.Q.; Li, Y.M. Amphibians testing negative for Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans on the Qinghai-Tibetan Plateau, China. Asian Herpetol. Res. 2017, 8, 190–198. [Google Scholar] [CrossRef]
- Wang, Y.; Verbrugghe, E.; Meuris, L.; Chiers, K.; Kelly, M.; Strubbe, D.; Callewaert, N.; Pasmans, F.; Martel, A. Epidermal galactose spurs chytrid virulence and predicts amphibian colonization. Nat. Commun. 2021, 12, 5788. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.; Pasmans, F.; Muñoz, J.F.; Shea, T.P.; Carranza, S.; Cuomo, C.A.; Martel, A. Diversity, multifaceted evolution, and facultative saprotrophism in the European Batrachochytrium salamandrivorans epidemic. Nat. Commun. 2021, 12, 6688. [Google Scholar] [CrossRef] [PubMed]
- Spitzen-van der Sluijs, A.; Stark, T.; DeJean, T.; Verbrugghe, E.; Herder, J.; Gilbert, M.; Janse, J.; Martel, A.; Pasmans, F.; Valentini, A. Using environmental DNA for detection of Batrachochytrium salamandrivorans in natural water. Environ. DNA 2020, 2, 565–571. [Google Scholar] [CrossRef]
- Olson, D.H.; Ronnenberg, K.L.; Glidden, C.K.; Christiansen, K.R.; Blaustein, A.R. Global patterns of the fungal pathogen Batrachochytrium dendrobatidis support conservation Urgency. Front. Vet. Sci. 2021, 8, 685877. [Google Scholar] [CrossRef] [PubMed]
- DiRenzo, G.V.; Longo, A.V.; Muletz-Wolz, C.R.; Pessier, A.P.; Goodheart, J.A.; Lips, K.R. Plethodontid salamanders show variable disease dynamics in response to Batrachochytrium salamandrivorans chytridiomycosis. Biol. Invasions 2021, 23, 2797–2815. [Google Scholar] [CrossRef]
- Spitzen-van der Sluijs, A.; Stegen, G.; Bogaerts, S.; Canessa, S.; Steinfartz, S.; Janssen, N.; Bosman, W.; Pasmans, F.; Martel, A. Post-epizootic salamander persistence in a disease-free refugium suggests poor dispersal ability of Batrachochytrium salamandrivorans. Sci. Rep. 2018, 8, 3800. [Google Scholar] [CrossRef]
- Bolte, L.; Goudarzi, F.; Klenke, R.; Steinfartz, S.; Grimm-Seyfarth, A.; Henle, K. Habitat connectivity supports the local abundance of fire salamanders (Salamandra salamandra) but also the spread of Batrachochytrium salamandrivorans. Landsc. Ecol. 2023, 38, 1537–1554. [Google Scholar] [CrossRef]
- Bozzuto, C.; Canessa, S. Impact of seasonal cycles on host-pathogen dynamics and disease mitigation for Batrachochytrium salamandrivorans. Glob. Ecol. Conserv. 2019, 17, e00551. [Google Scholar] [CrossRef]
- Grisnik, M.; Gray, M.J.; Piovia-Scott, J.; Carter, E.D.; Sutton, W.B. Incorporating caudate species susceptibilities and climate change into models of Batrachochytrium salamandrivorans risk in the United States of America. Biol. Conserv. 2023, 284, 110181. [Google Scholar] [CrossRef]
- Thomas, V.; Wang, Y.; Van Rooij, P.; Verbrugghe, E.; Baláž, V.; Bosch, J.; Cunningham, A.A.; Fisher, M.C.; Garner, T.W.J.; Gilbert, M.J.; et al. Mitigating Batrachochytrium salamandrivorans in Europe. Amphib. Reptil. 2019, 40, 265–290. [Google Scholar] [CrossRef]


| Family | Species | Individual | Bd | Bsal |
|---|---|---|---|---|
| Dicroglossidae | Fejervarya multistriata | 14 | 0 | 0 |
| Dicroglossidae | Hoplobatrachus chinensis | 16 | 0 | 0 |
| Dicroglossidae | Limnonextes bannaensis | 9 | 0 | 0 |
| Dicroglossidae | Quasipaa boulengeri | 34 | 1 | 0 |
| Dicroglossidae | Quasipaa spinosa | 66 | 0 | 0 |
| Dicroglossidae | Hoplobatrachus chinensis * | 15 | 0 | 0 |
| Megophryidae | Brachytarsophrys carinense | 2 | 0 | 0 |
| Megophryidae | Leptobrachella liui | 19 | 3 | 0 |
| Megophryidae | Leptobrachella shiwadashanensis | 7 | 0 | 0 |
| Megophryidae | Leptobrachium guangxiense | 2 | 0 | 0 |
| Megophryidae | Ophryophryne microstoma | 11 | 0 | 0 |
| Megophrorida | Xenophrys major | 3 | 0 | 0 |
| Microhylidae | Kaloula pulchra | 1 | 1 | 0 |
| Microhylidae | Microhyla heymonsi | 13 | 0 | 0 |
| Microhylidae | Microhyla pulchra | 11 | 0 | 0 |
| Ranidae | Amolops chunganensis | 22 | 13 | 0 |
| Ranidae | Amolops ricketti | 271 | 1 | 0 |
| Ranidae | Hylarana latouchii | 2 | 0 | 0 |
| Ranidae | Hylarana maosonensis | 17 | 0 | 0 |
| Ranidae | Hylarana guentheri | 40 | 0 | 0 |
| Ranidae | Odorrana exiliversabilis | 12 | 0 | 0 |
| Ranidae | Odorrana graminea | 53 | 2 | 0 |
| Ranidae | Odorrana lungshengensis | 22 | 6 | 0 |
| Ranidae | Odorrana nasuta | 26 | 0 | 0 |
| Ranidae | Odorrana versabilis | 20 | 3 | 0 |
| Ranidae | Rana hanluica | 4 | 1 | 0 |
| Ranidae | Lithobates catesbeiana * | 75 | 0 | 0 |
| Rhacophorida | Kurixalus odontotarsus | 42 | 2 | 0 |
| Rhacophorida | Liuixalus shiwandashan | 3 | 0 | 0 |
| Rhacophorida | Polypedates megacephalus | 131 | 1 | 0 |
| Rhacophorida | Rhacophorus minimus | 37 | 3 | 0 |
| Rhacophorida | Theloderma rhododiscus | 38 | 8 | 0 |
| Rhacophorida | Zhangixalus dennysi | 32 | 1 | 0 |
| Rhacophorida | Zhangixalus pinglongensis | 1 | 0 | 0 |
| Salamandrida | Pachytriton inexpectatus | 20 | 2 | 0 |
| Salamandrida | Pachytriton moi | 5 | 0 | 0 |
| Salamandridae | Andrias davidianus (Larva) * | 5 | 3 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, D.; Ellepola, G.; Herath, J.; Meegaskumbura, M. Ecological Barriers for an Amphibian Pathogen: A Narrow Ecological Niche for Batrachochytrium salamandrivorans in an Asian Chytrid Hotspot. J. Fungi 2023, 9, 911. https://doi.org/10.3390/jof9090911
Sun D, Ellepola G, Herath J, Meegaskumbura M. Ecological Barriers for an Amphibian Pathogen: A Narrow Ecological Niche for Batrachochytrium salamandrivorans in an Asian Chytrid Hotspot. Journal of Fungi. 2023; 9(9):911. https://doi.org/10.3390/jof9090911
Chicago/Turabian StyleSun, Dan, Gajaba Ellepola, Jayampathi Herath, and Madhava Meegaskumbura. 2023. "Ecological Barriers for an Amphibian Pathogen: A Narrow Ecological Niche for Batrachochytrium salamandrivorans in an Asian Chytrid Hotspot" Journal of Fungi 9, no. 9: 911. https://doi.org/10.3390/jof9090911
APA StyleSun, D., Ellepola, G., Herath, J., & Meegaskumbura, M. (2023). Ecological Barriers for an Amphibian Pathogen: A Narrow Ecological Niche for Batrachochytrium salamandrivorans in an Asian Chytrid Hotspot. Journal of Fungi, 9(9), 911. https://doi.org/10.3390/jof9090911







