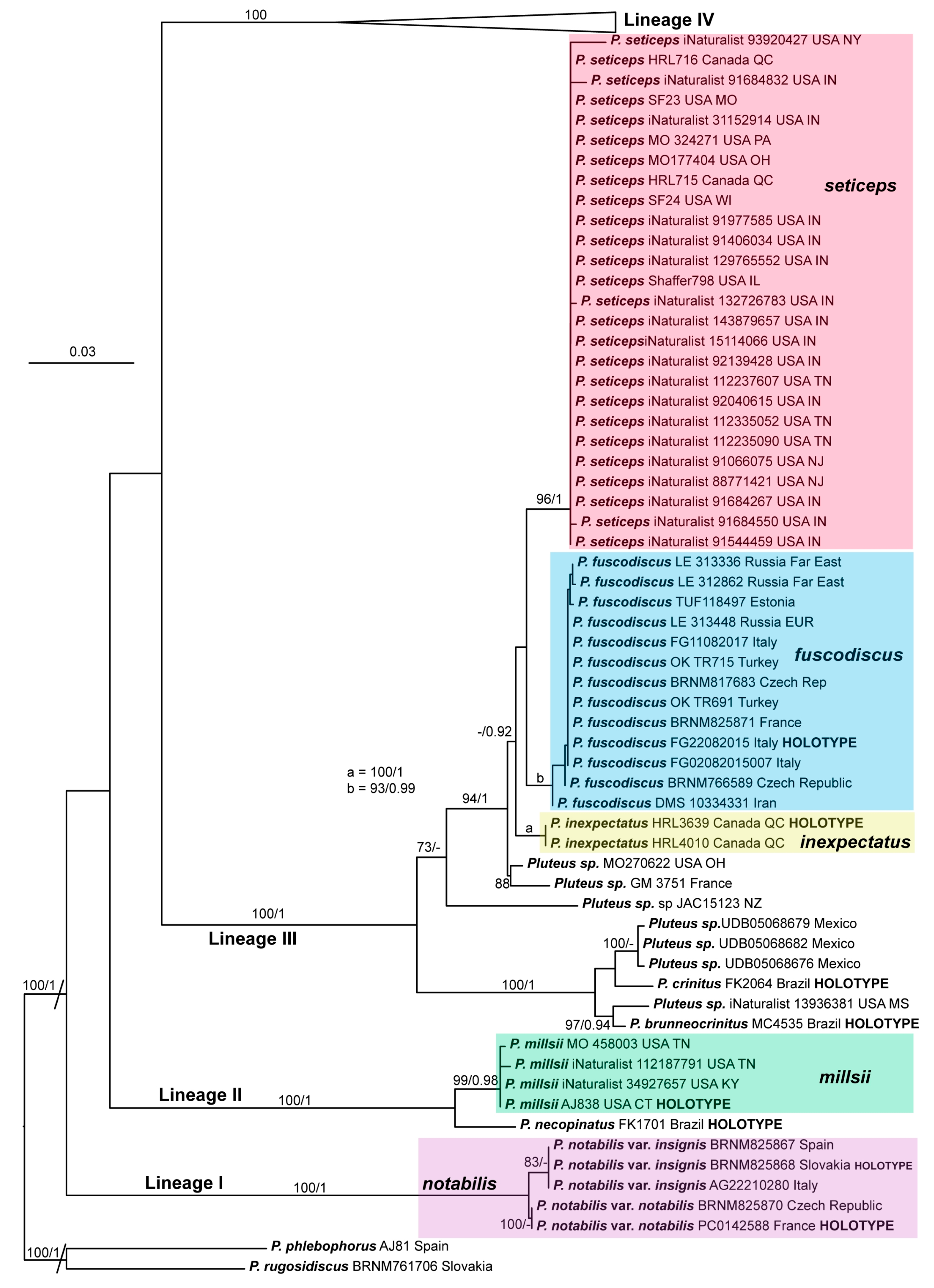
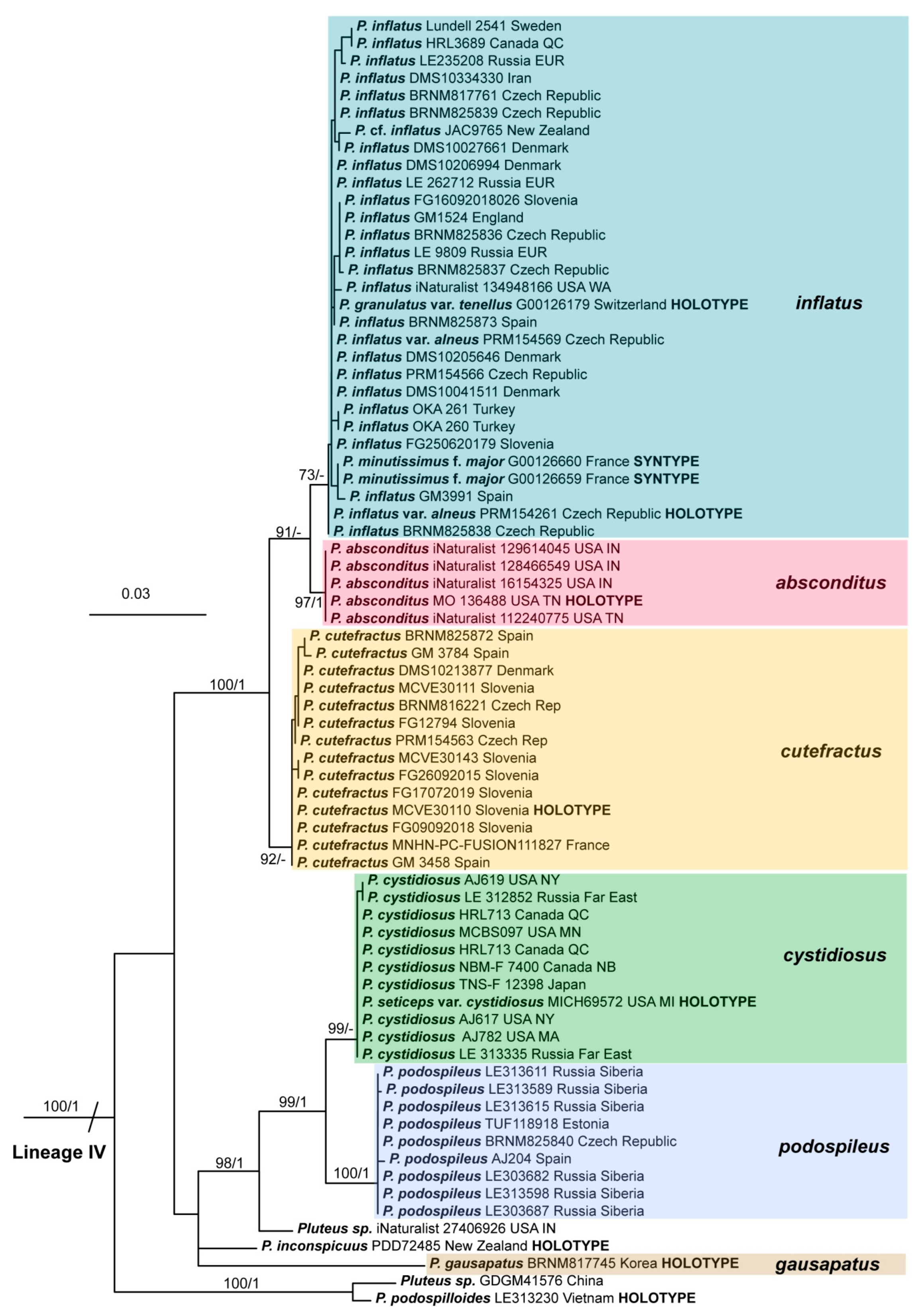
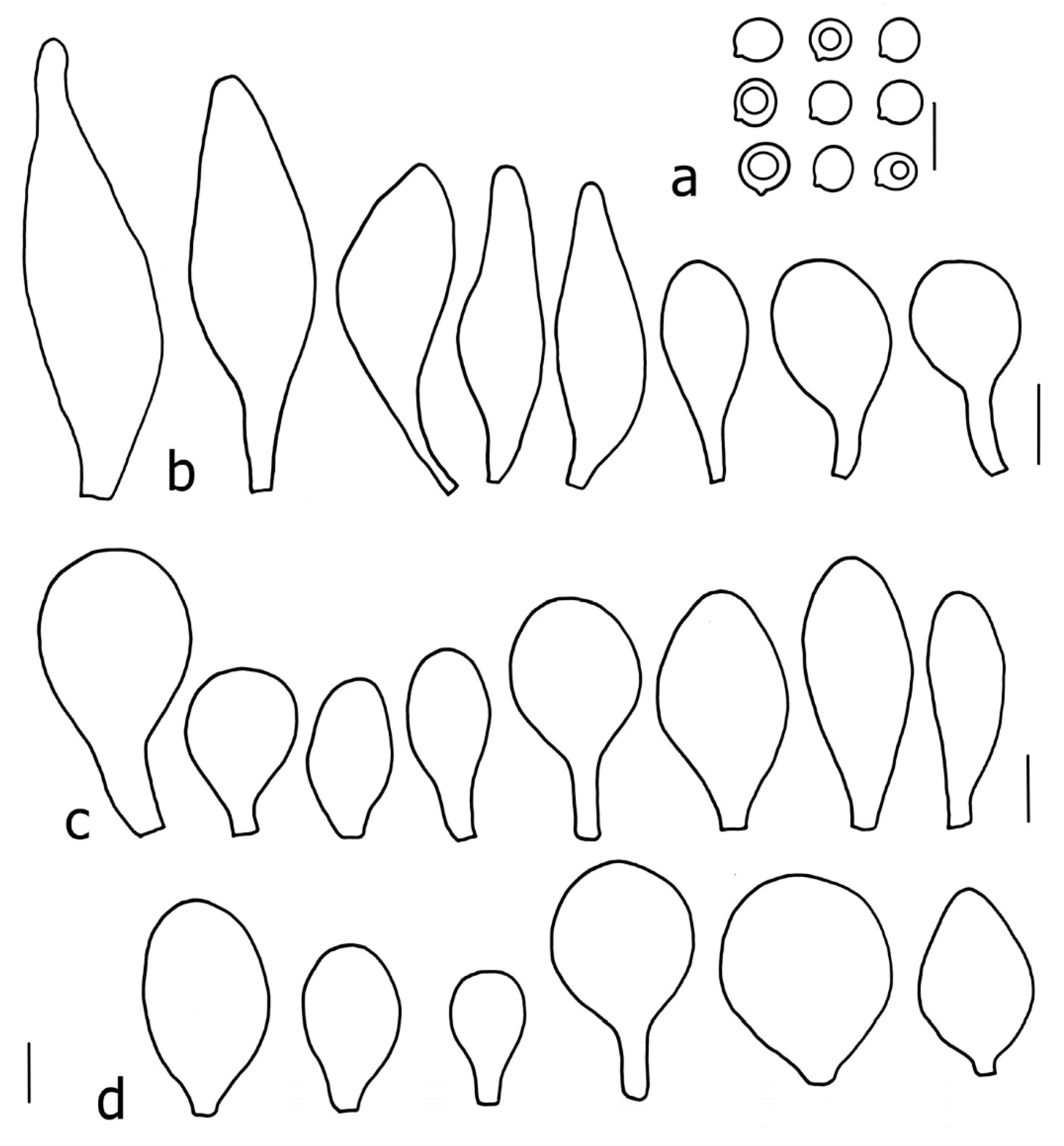
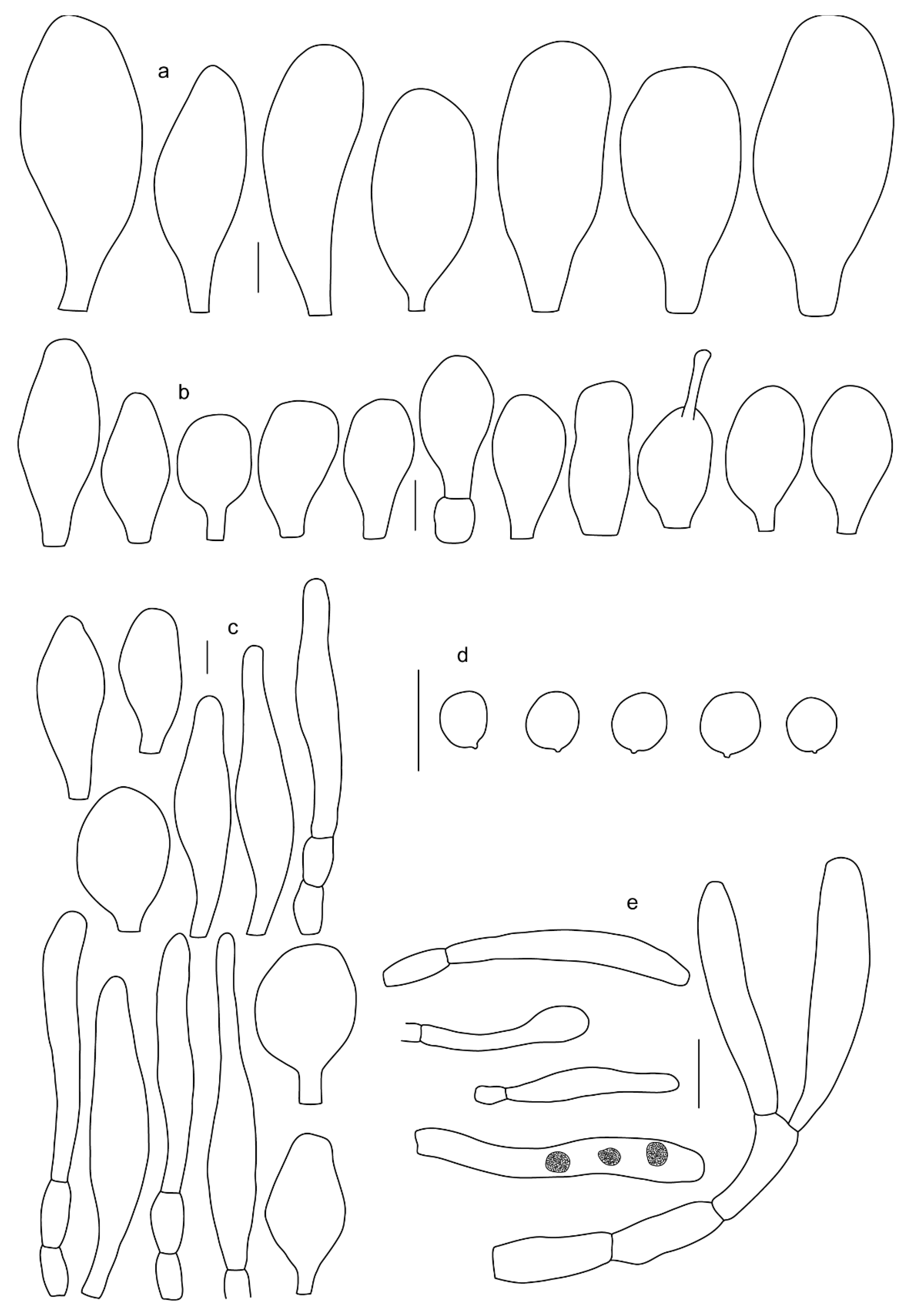
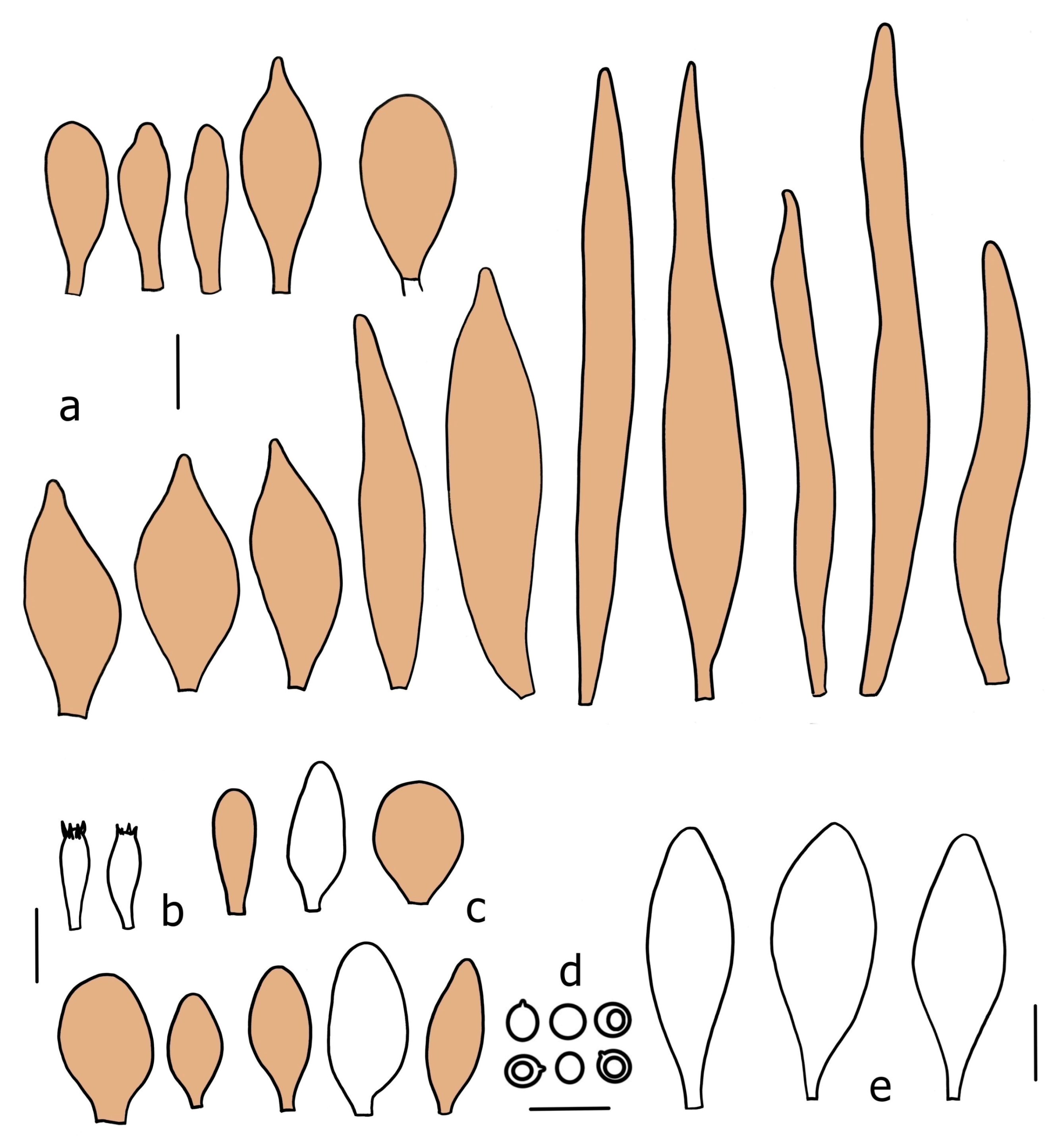
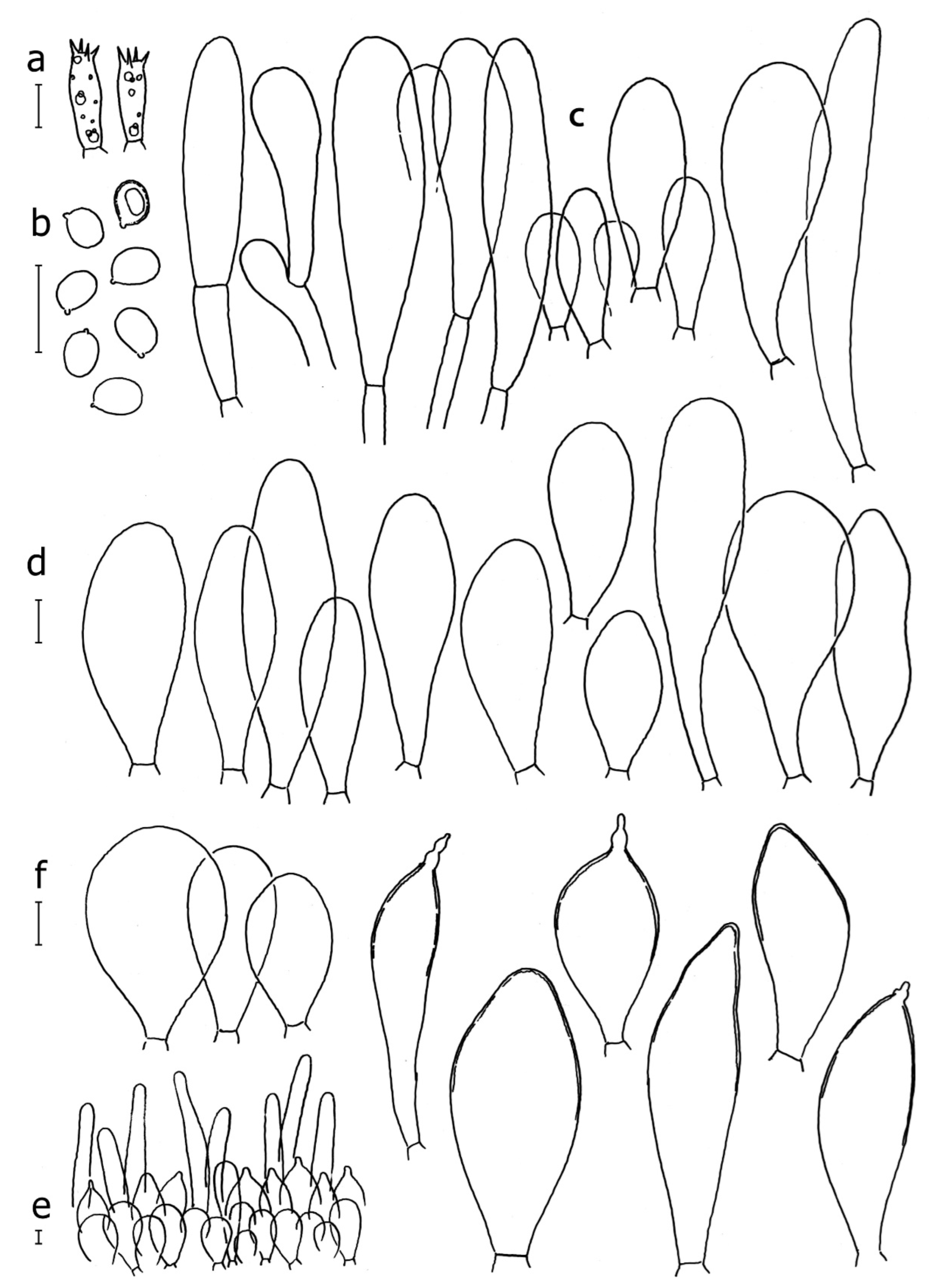
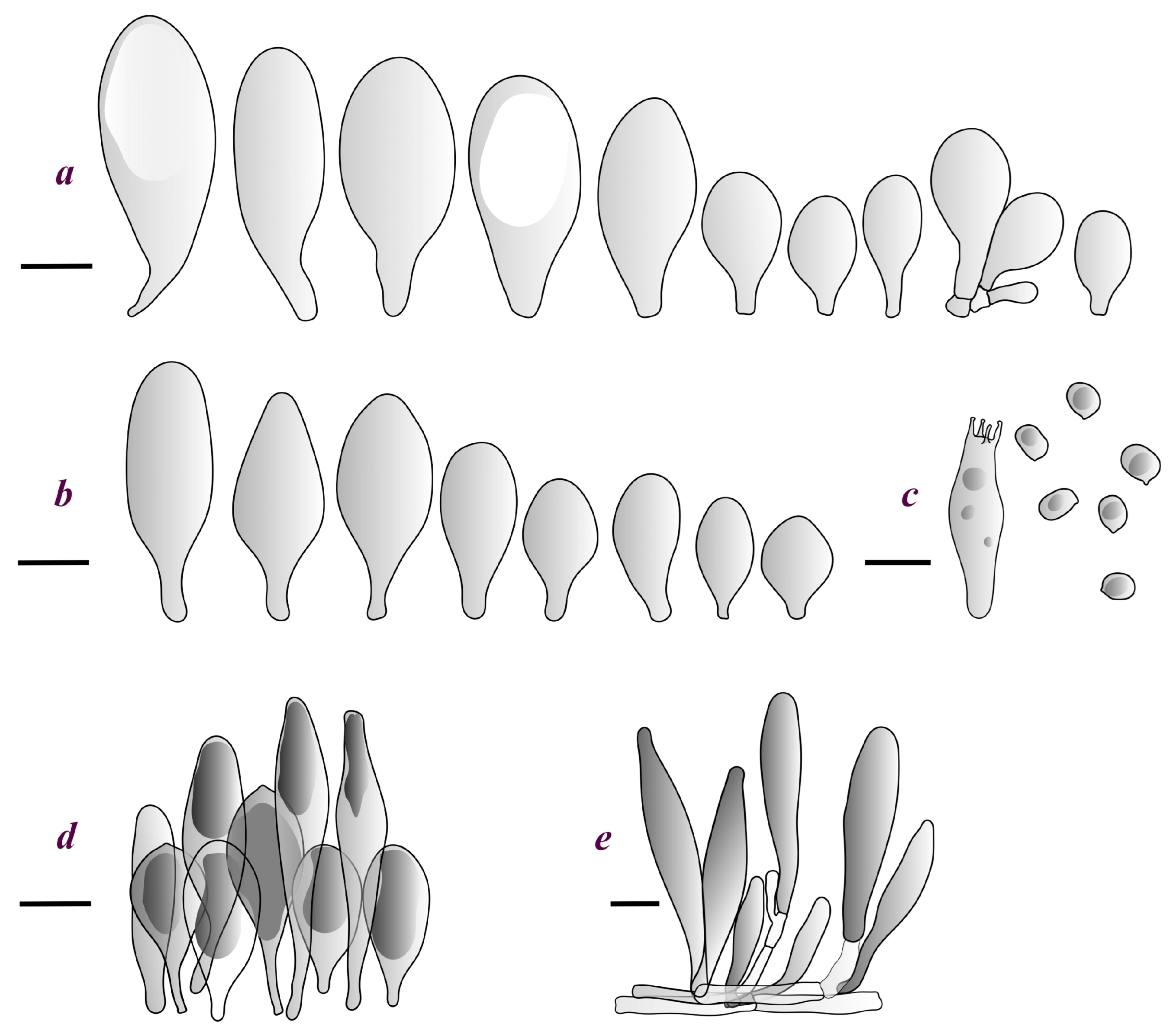
3.2. Taxonomy
Here we present the descriptions of the eleven species and one distinct variety currently known to occur in the Holarctic region. We consider that the P. podospileus group contains two well-known species, Pluteus podospileus and P. seticeps; the recently described P. cutefractus; P. seticeps var. cystidiosus elevated to species rank as P. cystidiosus; the forgotten P. inflatus, which is rediscovered and molecularly confirmed; and six new species (P. absconditus, P. fuscodiscus, P. gausapatus, P. inexpectatus, P. millsii; and P. notabilis with its new variety Pluteus notabilis var. insignis). Moreover, there are at least four other undescribed species that occur in the Holarctic region.
Sylloge Fungorum
5: 672 (1887) [
8].
Agaricus spilopus Berk. and Broome Ann. Mag. nat. Hist., Ser. 5 (7: 126, 1881) Nom. illeg., Art. 53.1; non Agaricus spilopus Berk. & Broome 1871; Pluteus spilopus sensu Berkeley and Broome (1881); non Pluteus spilopus (Berk. and Broome) Sacc., Syll. fung. (Abellini) 5: 669 (1887); Pluteus nanus var. podospileus (Sacc. and Cub.) Rick, Lilloa 3: 444 (1938); Hyporrhodius podospileus (Sacc. and Cub.) Henn., in Engler and Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1(1**): 258 (1898).
Excluded: Pluteus seticeps (G.F. Atk.) Singer, Lloydia 21(4): 272 (1959); Leptonia seticeps G.F. Atk., Journal of Mycology (8(3): 116, 1902); Pluteus psychiophorus var. seticeps (G.F. Atk.) Singer, Trans. Br. Mycol. Soc. 39(2): 214 (1956); Nom. inval., Art. 36.1 Ex. 6 (Shenzhen Code).
Pluteus seticeps var. cystidiosus Minnis and Sundb., N. Amer. Fung. 5(1): 13 (2010).
Pluteus minutissimus f. major Kühner, Bull. Soc. Mycol. France 72(3): 182 (1956); Pluteus minutissimus f. major Kühner, in Kühner and Romagnesi, Fl. Analyt. Champ. Supér. (Paris): 422 (1953). Nom. inval., Art. 39.1 (Shenzhen Code).
Uncertain: Pluteus minutissimus Maire, Publicacions del Instituto Botánico Barcelona 3 (4): 94 (1937). Pluteus podospileus f. minutissimus (Maire) Vellinga, in Vellinga and Schreurs, Persoonia 12(4): 362 (1985); Pluteus podospileus var. minutissimus Wasser, Flora Gribov Ukrainy, Bazidiomitsety, Amanital’nye Griby (Kiev): 83 (1992) Nom. inval., Art. 39.1, Art. 40.1 (Shenzhen Code). Pluteus psychiophorus var. minutissimus (Maire) Singer, Trans. Br. Mycol. Soc. (1956, 39(2): 214), Nom. inval., Art. 36.1 Ex. 6 (Shenzhen Code).
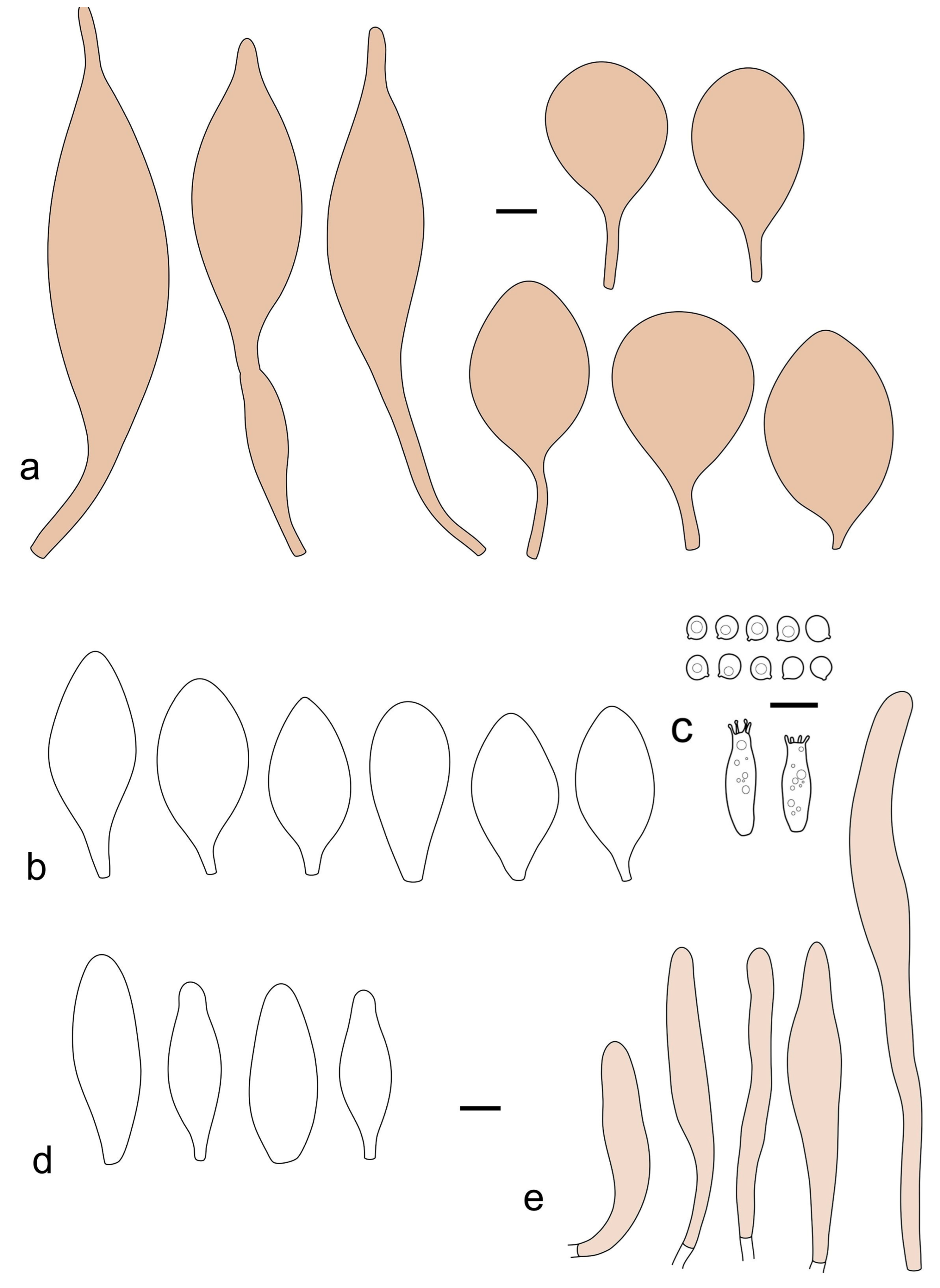
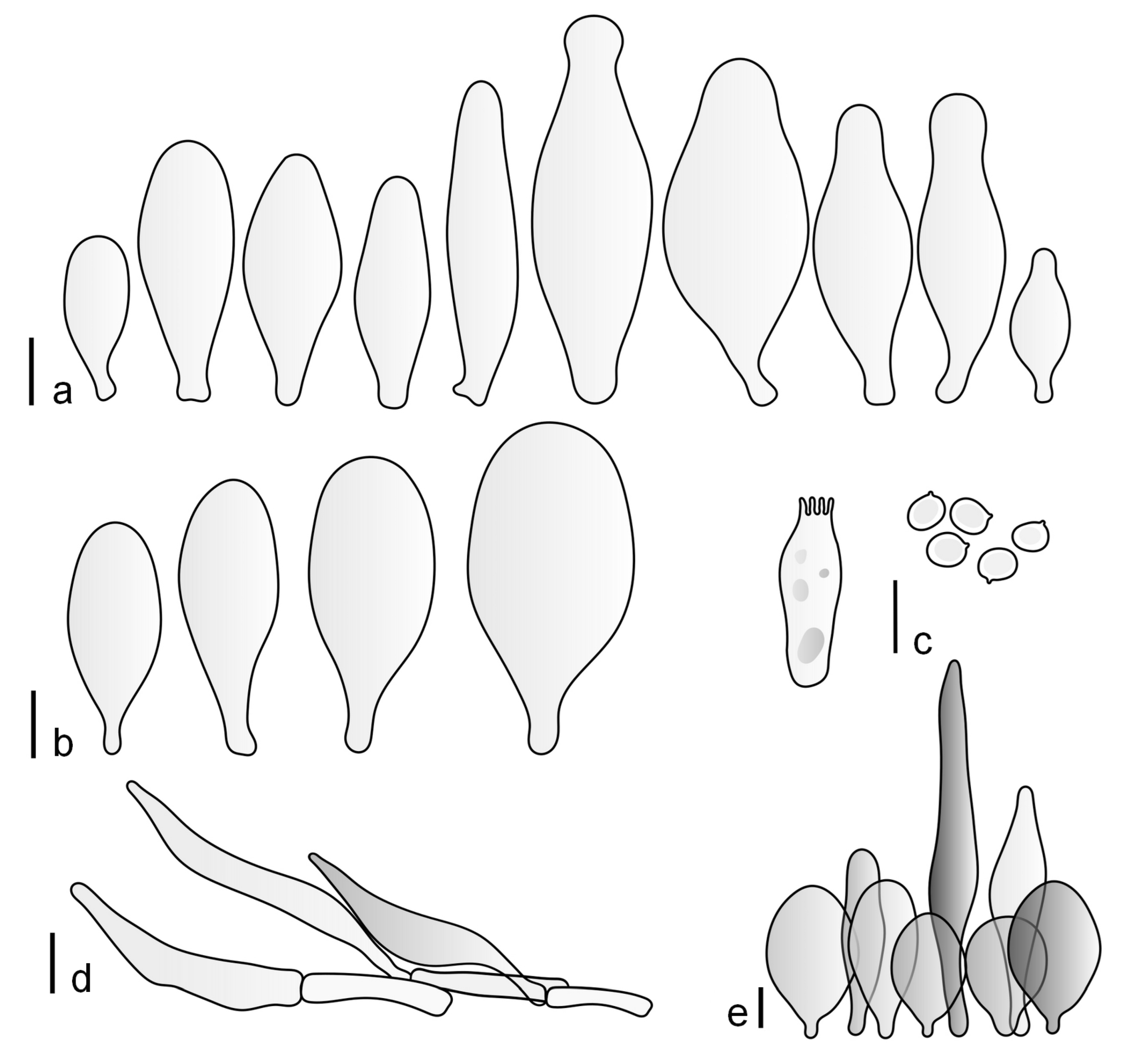
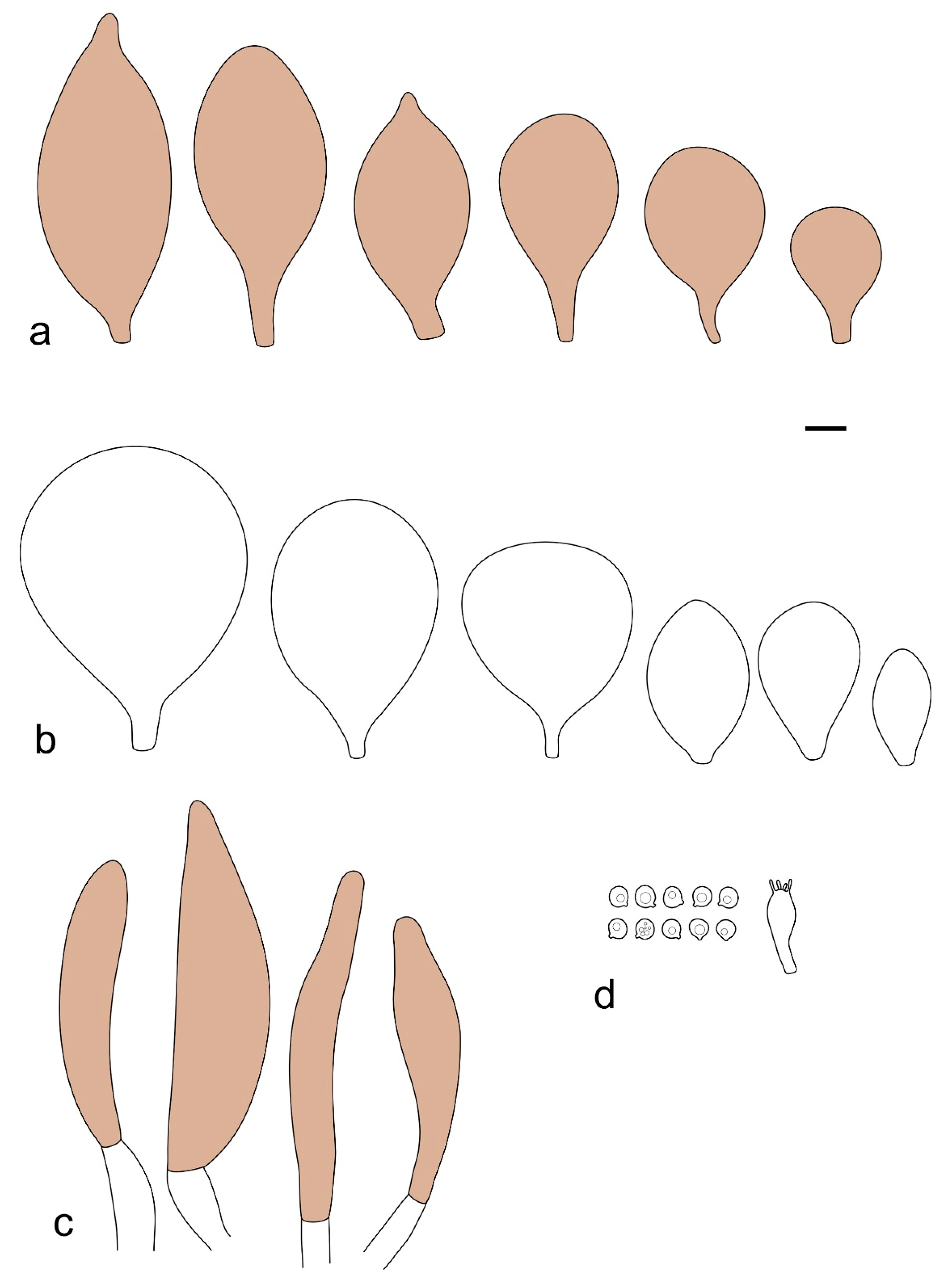
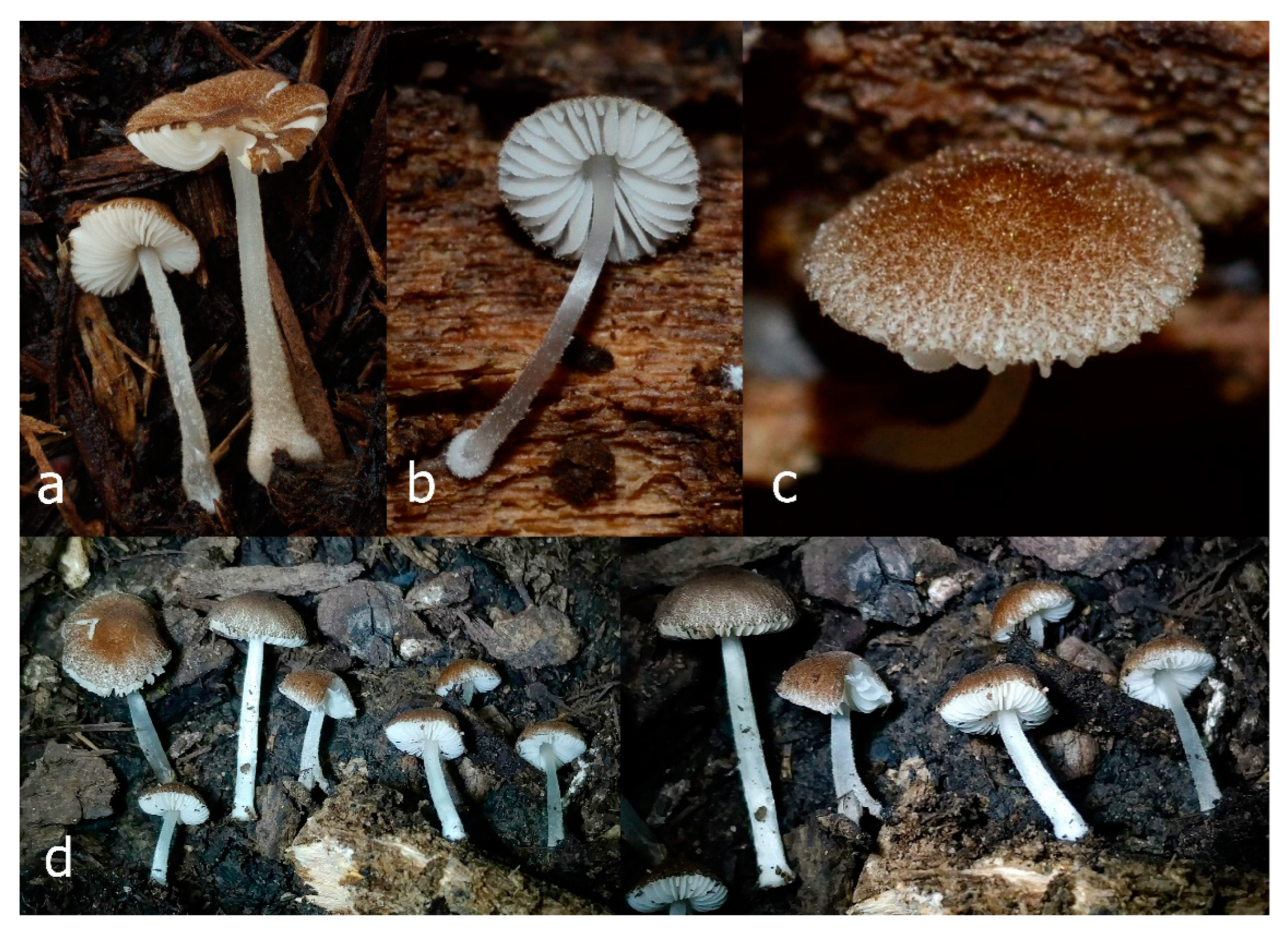
Pileus 15–36 mm in diam., hemispherical or campanulate when young, expanding to convex or plano-convex, then applanate, often with a low, broad umbo; not or slightly hygrophanous, not striate or slightly translucently striate at margin; surface dry, pruinose, slightly to strongly velvety, becoming granulose-squamulose especially around the centre, in some specimens strongly rugose at least in the centre, rarely almost smooth, for the most part not cracked, or with some cracks revealing the white context towards the margin, especially in older specimens; with predominant brown or red-brown colors (Munsell: 2.5YR 4/3–4/8, 3/6; 5YR 5/4–5/8, 4/4–4/6; RAL color chart: plane brown (RAL 060 40 40), wood brown (RAL 050 30 20), tobacco brown (RAL 050 30 30), copper brown (RAL 8004) or tropical wood brown (RAL 050 30 20), usually darker in the centre. Lamellae free, crowded, ventricose, up to 5 mm broad, white when young, later pink, with even, serrated, or flocculose edges, white or concolorous. Stipe 20–47 × 2–6 mm, cylindrical, slightly thickened downwards but without distinct basal bulb, white, whitish, very pale yellowish or beige, entirely minutely squamulose with dark or black-brown squamules, more densely in the lower half. Context in stipe and pileus white. Smell indistinct; taste not recorded.
Basidiospores [190/7/7] (4.4–)4.5–6.0(–6.4) × (2.7–)3.5–5.1(–5.2) μm, avl × avw = 5.2–5.7 × 4.1–4.7 μm, Q = (1.06–)1.10–1.30(–1.41), Q* = 1.20, subglobose to broadly ellipsoid, rarely ellipsoid or ovoid, very rarely globose, thick-walled. Basidia 15–33 × 6.0–9.0 μm, 4-spored, very rarely 2-spored, clavate, rarely almost cylindrical. Pleurocystidia scarce, (34–)36–52 × 13–26 μm, ovoid, broadly clavate to clavate or narrowly to broadly utriform; hyaline, thin-walled; scattered or rare over lamellar faces; rarely absent. Lamellar edge sterile. Cheilocystidia (20–)26–58(–63) × (9–)11–25 μm, narrowly to broadly clavate, narrowly utriform to utriform or ovoid; hyaline, thin-walled, crowded, usually forming a well-developed strip. Pileipellis a hymeniderm or epithelioid hymeniderm made up of two types of elements: (i) spheropedunculate, pyriform, or broadly clavate, (34–)43–65(–72) × 25–41 µm; (ii) narrowly fusiform to fusiform, inflated-fusiform, lanceolate, narrowly utriform, often mucronate, 80–160(–188) × 10–34(–40) µm; all elements with brown, red-brown, or yellow-brown, intracellular pigment, often aggregated in spots, slightly thick-walled. Stipitipellis a cutis of cylindrical, thin to slightly thick-walled, 6–9 µm wide hyphae, hyaline, or some with very pale yellow or brown pigment. Caulocystidia numerous, often in clusters, 36–146(–176) × 6–26 µm, cylindrical, narrowly clavate, narrowly fusiform, rarely spheropedunculate, often septate; most with brown or yellow-brown pigment, sometimes aggregated into spots. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: often gregarious, growing on well-decayed wood of angiosperms (e.g., Betula). In temperate or transitional hemiboreal forests. August–November.
Distribution: Eurasia: Europe (molecularly confirmed from Spain, the Czech Republic, and Estonia), and as far east as Siberia. It is likely distributed all over Europe e.g., [
4,
11], but is apparently less frequent than
Pluteus inflatus.
Pluteus podospileus has been cited from Japan [
15], but it is possible the Japanese record corresponds to
Pluteus cystidiosus. Confusion with similarly-looking taxa makes it hard to interpret older records in the absence of well-annotated voucher specimens.
Collections examined: CZECH REPUBLIC: Vysoké Chvojno, decaying deciduous trunk, 13 August 2015, leg. P. Včelička (BRNM825840). ESTONIA: Võru Co., Varstu Comm., Krabi, Paganamaa Landscape Reserve, 57.59608° N, 26.85878° E, on rotten wood, 5 September 2014, leg. V. Liiv (TUF118918, originally as Pluteus pallescens). RUSSIA: South Siberia, Krasnoyarsk Territory, Sayano–Shushensky Biosphere Reserve, vicinity of Kerema Reserve Field Station, at the mouth of the Bolshaya Kerema river, 52°07′07.6″ N, 92°13′35.8″ E, Betula pendula forest with Pinus sylvestris and Larix sibirica, on large fallen trunk of Betula, 23 August 2015, leg. E.F. Malysheva (LE F-303687); same area, 52°07′07.6″ N, 92°13′35.8″ E, Betula pendula forest with Pinus sylvestris and Larix sibirica, on decayed wood of Betula, 23 August 2015, leg. E.F. Malysheva (LE F-303682); same area, floodplain of the Malye Ury river, mixed forest (dominated by Larix sibirica, Picea abies and Betula pendula), on branches or on very decayed wood of Betula, 14 August 2020, leg. E.F. Malysheva (LE F-313589, LE F-313611); same area, floodplain of the Sarly river, mixed forest (with Larix sibirica, Picea abies, Pinus sylvestris and Betula pendula), on large fallen trunk of Betula, 16 August 2020, leg. E.F. Malysheva (LE F-313615). SPAIN: Seville, Constantina, on unidentified angiosperm wood, 5 November 2003, leg. J.M. Fernández-Rodríguez, COFC-F 2929 (duplicate AJ 204, NBM-F-009808).
Notes: In the concept accepted here,
Pluteus podospileus agrees well with the original description by Berkeley and Broome (1881, as
Agaricus spilopus): a relatively small species with a brown, rugose pileus and a stipe covered with brown squamules. Modern authors [
2,
4] have accepted
P. podospileus as a species with a hymenidermal pileipellis composed of both short and long elements. Collections of
P. podospileus studied here have brown-squamulose stipes and were collected directly on wood.
The exact relationship between
Pluteus podospileus and
P. minutissimus Maire is not currently clear, and different interpretations of these taxa exist in the literature.
Pluteus minutissimus was originally described as a very small species, growing solitary under
Quercus in northeastern Spain [
19]. The following characters were specifically mentioned in the original description: small basidioma (pileus 5 mm diam, stipe 10 × 1 mm), pileus without rugulosity and striation, basidiospores subglobose 5 × 4.0–4.5 μm, pleurocystidia rare but present near the lamellar edge, clavate cheilocystidia, and pileipellis composed of two types of elements (short and long). Traditionally, the main character separating
P. minutissimus from
P. podospileus (as accepted here) is the glabrous stipe surface without dark squamules. Kühner and Romagnesi [
10] attributed to
P. minutissimus (as forma “typicus”) a cap of 9–11 mm in diameter, a glabrous white stipe, and a preference for growing terrestrially, without direct connection to wood. At the same time, they described
P. minutissimus f.
major as differing from the typical morphology in its larger size (cap 12–37 mm), stipe dotted with brown squamules, and growth directly connected to decayed wood of angiosperms. Orton [
2] recognized
P. podospileus as a species with brown squamulose stipe growing on wood and
P. minutissimus as a species with glabrous stipe growing terrestrially, dismissing differences in size as useful characters to separate them. Vellinga and Schreurs [
1] reduced
P. minutissimus to a form of
P. podospileus, with the lack of brown squamules on the stipe as the only separating character. It should be noted that in the original description of
P. minutissimus, the only information on habitat is “under
Quercus”, but there are no specifics as to whether the species was growing terrestrially or connected to wood. The original collection of
P. minutissimus exists, but it is in poor condition and not available for study. However, curator Caroline Loup kindly revised the holotype. The conserved material consists of a single basidioma with a stipe 10 mm tall and a pileus 4 mm in diameter with only two visible pieces of protruding lamellae, less than 0.5 mm each. By her revision, basidiospores are globose, about 5–6 μm, a few clumps of cystidia are present, mostly clavate to (sub)fusiform; and basidia are collapsed. In the absence of modern collections from the type locality (Vallvidrera, Spain) or from similar habitats nearby, we are not in a position to accept or refute the synonymy of
P. podospileus and
P. minutissimus. It is possible that the taxon described by Kühner and Romagnesi as
P. minutissimus f.
typicus and by Orton as
P. minutissimus is indeed a separate species from
P. podospileus by virtue of the terrestrial habitat and the non-squamulose stipe. Whether
P. minutissimus would be the correct name for such a species would have to be confirmed. In the present study, we recognize six species in the
P. podospileus complex occurring in Europe, five of them molecularly confirmed from Spain or southern France:
Pluteus podospileus (NBM-F-009808),
P. cutefractus (BRNM825872, GM 3784, GM3458),
P. inflatus (BRNM 825873),
Pluteus sp. (GM3751), and
P. notabilis var.
notabilis (BRNM825867). Two of these species (
P. inflatus and
Pluteus sp. GM 3751) can have white, non-squamulose stipes and also grow terrestrially.
Pluteus sp. GM 3751 differs from the original description of
Pluteus minutissimus, at least in the strongly rugose-venose pileus.
Pluteus inflatus is a geographically widespread species also confirmed to occur in Spain, and some collections of
P. inflatus match the
P. minutissimus protologue. It should be noted that
P. minutissimus does not have priority over either
P. podospileus or
P. inflatus, the two species accepted here that more closely match its original description. However, we prefer to verify the taxonomic value of
P. minutissimus strictly by molecular analysis of the holotype collection in the future when less invasive methods become available.
České Houby
3: 609 (1921) [
20].
Syn.: Pluteus inflatus var. alneus Velen., Novitates Mycologicae: 145 (1939).
Pluteus granulatus var. tenellus J. Favre, Beiträge zur Kryptogamenflora der Schweiz 10 (3): 101 (Favre 1948).
Pluteus minutissimus f. major Kühner, Bull. Soc. Mycol. France 72 (3): 182 (1956); Pluteus minutissimus f. major Kühner, in Kühner and Romagnesi, Fl. Analyt. Champ. Supér. (Paris): 422 (1953). Nom. inval., Art. 39.1 (Shenzhen Code).
Holotype of Pluteus inflatus var. alneus: Czech Republic, Mnichovice, on trunk of Alnus, 7 August 1939, leg. J. Velenovský (PRM 154261).
Syntypes of Pluteus minutissimus f. major: France, Val de Marne, Bois de Vincennes, on a big stump near rivers, 21 September 1929, leg. and det. R. Kühner (G00126660; G00126659).
Holotype of Pluteus granulatus var. tenellus: Switzerland: Jura, haut-marais, tourbière du Bélieu pr. du Russey, Doubs, in association with Filipendula ulmaria, 15 August 1938 (G K9933, G00126179).
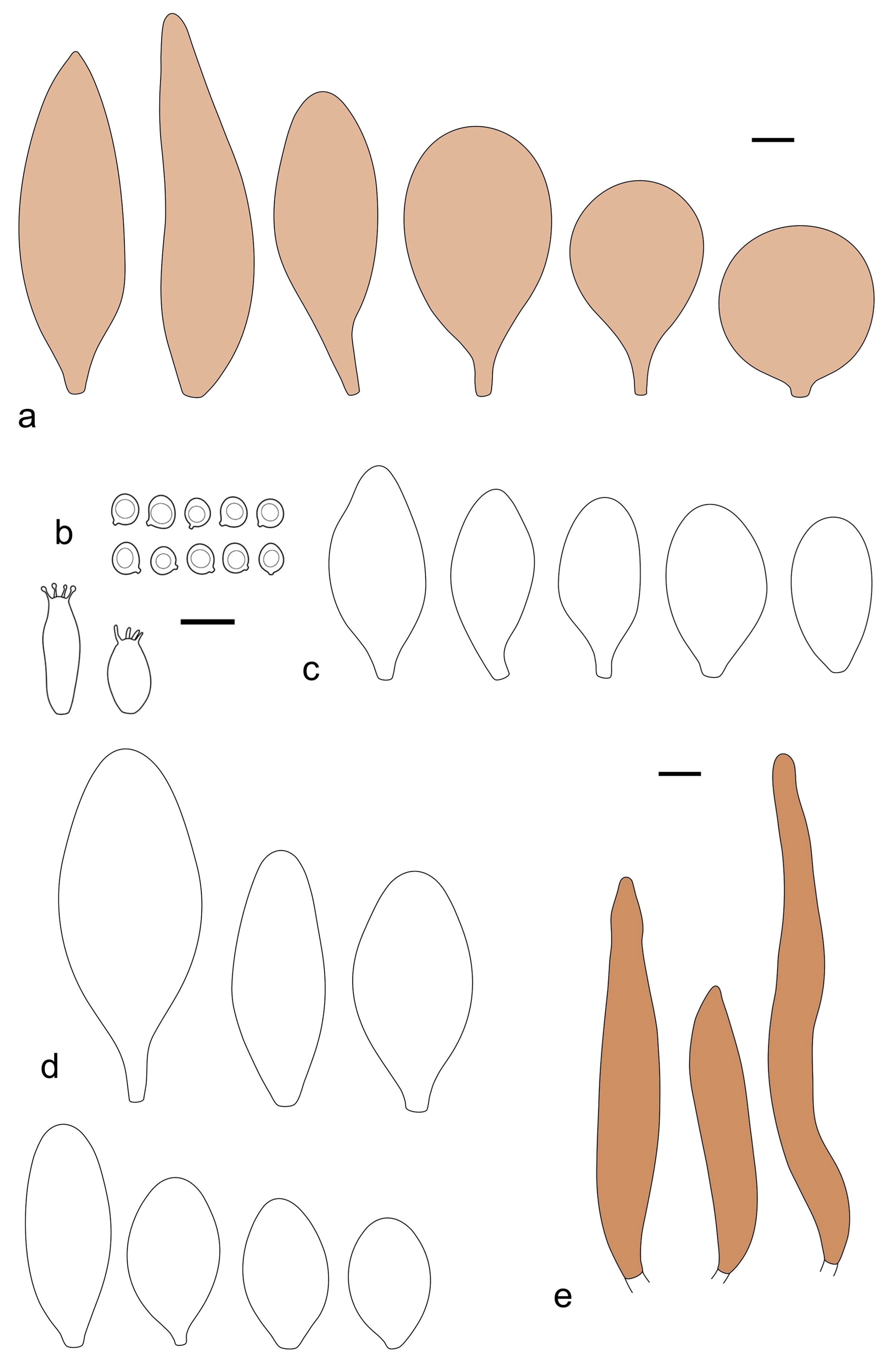
Pileus 10–30 mm in diameter, hemispherical or campanulate when young, expanding to convex to plano-convex, rarely plane, with or without indistinct, low and broad umbo, only rarely with distinct umbo, not or very rarely hygrophanous, smooth or slightly rugose at center, without striation or rarely translucently striate from margin up to half the radius of pileus, mostly not cracked, rarely with some cracks revealing the white context towards the margin, one collection cracked almost to the center. Surface dry, slightly to strongly velvety, becoming entirely minutely to distinctly granulose-squamulose (RAL 040 40 30), root brown (RAL 040 30 30), or wild brown (RAL 040 20 19), brown (S00 C00 Y60–S00 C00 Y70), red brown or chestnut brown, dark brown to almost back in the center (S00 C00 Y50). Lamellae free, crowded to moderately distant, mostly ventricose, up to 4 mm broad, white (Y10 M00 C00) or grey-white, later pinkish (Y10 M00 C00) to pink, with a concolorous, even, slightly pubescent to distinctly flocculose edge. Stipe 25–45(–60) × 1–2(–4) mm, cylindrical, sometimes slightly broadened at base, sometimes (finely) longitudinally fibrillose, whitish or greyish, entirely white pubescent or only in the lower half, sometimes with minute distinct brown scales in the lower part, tomentum at the base white. Context in stipe and pileus white or grey-white. Smell and taste indistinct.
Basidiospores [410/14/14] (4.5–)5.0–7(–7.7) × (4.0–)4.5–6(–6.5) µm, avl × avw = 5.3–6.2 × 4.8–5 µm, Q = (1.0–)1.1–1.3(–1.6); Q* = 1.13–1.30, subglobose to broadly ellipsoid, rarely globose or ovoid, thick-walled. Basidia 15–34 × 6–12 µm, 4-spored, rarely 2-spored, mostly clavate, less frequently almost cylindrical or subfusiform. Pleurocystidia absent or rare, rarely common all-over lamellar faces, 36–73(–80) × (10–)15–30 µm, narrowly clavate to broadly clavate or oviform, some ellipsoid, narrowly utriform or inflated-fusiform, hyaline, thin-walled. Lamellar edges heterogeneous and fertile, with cheilocystidia, basidia, and basidioles, with cheilocystidia crowded in places in clusters but not forming a continuous strip. Cheilocystidia 20–60(–65) × (10–)15–30 µm, numerous, narrowly to broadly clavate or cylindrical, ovoid or rarely narrowly utriform to utriform or subfusiform, hyaline, thin-walled. Pileipellis a hymeniderm, or rarely an epithelioid hymeniderm, made up of two types of elements: (i) spheropedunculate or broadly clavate to clavate, rarely subovoid 28–63(–66) × 16–42 µm; (ii) narrowly to broadly fusiform or cylindrical, rarely narrowly clavate to clavate or narrowly utriform, 72–130(–190) × (10–)16–30(–34) µm; all elements with pale brown to brown or yellow-brown intracellular pigment often aggregated into spots, thin to thick-walled. Stipitipellis a cutis of 5–12 µm wide cylindrical hyphae, slightly thick-walled, hyaline, rarely with pale brown pigment. Caulocystidia absent to numerous, often in clusters, narrowly to broadly fusiform, rarely clavate or flexuose, some with 2–3 basal septa, 40–145 × (7–)8–20 µm, colorless or with pale brown to brown, unevenly distributed intracellular pigment. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: mostly solitary, on very decayed wood of deciduous trees, on litter, or on soil. May–October.
Distribution: Eurasia and North America (Canada). Widespread in Europe (Czech Republic, Denmark, Russia, Slovenia, Sweden, Switzerland, UK—England) and Western Asia (Turkey, Iran).
Collections examined: EURASIA. CZECH REPUBLIC: Mirošovice, in the forest, August 1940, leg. J. Velenovský (PRM 154566); Mnichovice, Hrusice, Crataegus, 30 September 1940, leg. J. Velenovský (PRM 154565 without sequences, poor condition); Mnichovice, trunk of Alnus, August 1944, leg. and det. J. Velenovský (PRM 154569, originally as P. inflatus var. alneus); Ládví, between creek and pond, mixed floodplain forest (Alnus, Betula, Quercus, Fagus, Populus tremula, Picea), on soil between deciduous twigs, 25 August 2018, leg. J. Herčík (BRNM 817761); Olomouc, Černovírské slatiniště forest, about 220 m a.s.l., Alnetorum with Ulmus minor and Quercus robur, near wetland with Typha, wet deciduous wood, 26 August 2019, leg. V. Halasů (BRNM825839); Olomouc, ul. Václava III. Street 28, grass place in front of the house, in the grass, where the tree used to stand (probably Fraxinus), about 220 m a.s.l., 28 May 2019, leg. V. Halasů (BRNM825836); Štiřín, Alnetorum with Quercus and Tilia, on soil between deciduous twigs, 25 August 2018, leg. J. Herčík (BRNM825838); Valtice, Rendez-vous Nature Reserve, Quercetum with Juglans nigra on soil near footpath, 48°44,801′ 16°47,386′, 11 September 2016, leg. P. Včelička and V. Halasů (BRNM825837). DENMARK: Æbelø, on very rooty wood of Alnus glutinosa in deciduous forest, 1 September 2021, leg T. Læssøe and M. Schier Christiansen, DMS-10206994 (C); Brobæk Mose at Lyngby, N. of Copenhagen, on dead wood of Betula in bog woodland, 22 September 2016, leg T. Kehlet, DMS-10027661 (C); E Jutland, Havskov N. of Aarhus, on rotten wood of Fagus sylvatica in deciduous forest, 30 August 2021, leg. U. Nygaard and T. Kehlet, DMS-10205646 (C); North Zealand, Glostrup, 30 September 2019, leg. Thomas Kehlet, on soil on roadside in suburban area, DMS-10041511 (C). IRAN: Golestan, Gorgon, more or less virgin beech forest at 950 m elevation, on rotten log of Fagus orientalis, 3 October 2016, leg. J. Heilmann–Clausen and C. Bässler, DMS-10334330 (C). RUSSIA: Leningrad Region, Saint Petersburg, Botanical Garden, 22 September 1946, leg. B.P. Vassilkov (LE F-9809); Orenburg Region, Belyaevsky District, Orenburg Nature Reserve, site «Burtinskaya Steppe», Alnus glutinosa spinney near Kainar stream, on soil and woody debris among moss, 22 July 2004, leg. O.A. Desyatova (LE F-235208); Caucasus, Karachayevo-Circassian Republic, Teberda Biosphere Reserve, vicinity of Teberda, Dzhemagat Gorge, mixed forest (Pinus, Betula, Populus), on soil among moss or on very decayed deciduous wood, 15 August 2009, leg. E.F. Malysheva (LE F-262712). SLOVENIA: Nova Goricâ, Soča Park, 16 September 2018, leg. G. Ferisin (FG16092018026); ibid., 25 June 2017 (FG 250620179). SPAIN: Cataluña, Villalonga de Ter, Abella, in a humid valley, in a mixed forest, under Corylus avellana, Quercus pyrenaica, Fagus sylvatica, etc., on muddy ground, 1200 m, 30 August 2022, leg. S. Gibert, À. Torrent and G. Muñoz (GM3991); Espinavell, Molló (city), in riparian forest with Alnus glutinosa, Corylus avellana, Fagus sylvatica, and Betula pendula, 1400 m a.s.l., on acid soil, 15 July 2021, leg. S. Gibert (det. as P. minutissimus BRNM 825873). WESTERN ASIA. TURKEY. Artvin Province, Borçka Region, Camili Biosphere Reserve Area, on broken pieces of trunks and branches of Fagus orientalis, 1485 m a.s.l., 2 October 2011, leg. O. Kaygusuz (OKA-TR260); Rize Province, Çamlihemşin Region, near Ayder Upland, on decayed wood of F. orientalis, 1250 m a.s.l., 28 September 2012, leg. O. Kaygusuz (OKA-TR261). UNITED KINDGDOM. ENGLAND: London, Kew Botanical Gardens, terrestrial, under Celtis occidentalis, next to a cluster of Psathyrella lacrymabunda, 21 July 2009, leg. G. Muñoz (GM1524). NORTH AMERICA. CANADA: Québec, Saint-Marc-des-Carrières, 48 m a.s.l., at the edge of a parking lot surrounded by large Picea sp., in rocky-sandy soil, 28 June 2022, leg. R. Lebeuf (HRL3689).
Notes: Collection PRM 154569 was labeled as a holotype of
P. inflatus var.
alneus on a fungarium sheet but was collected after the publication of the protologue. The curator of PRM, J. Holec, confirmed (pers. comm.) that this collection was not labeled as a holotype by Velenovský but was labeled as such by a later worker. The original material of
Pluteus inflatus mentioned in the protologue was also collected by Velenovský from the village of Mnichovice on an
Alnus trunk, but earlier (7 August 1939, PRM 154261). This collection is the only material that perfectly matches the protologue. Thus, we recognize PRM 154261 to be the true holotype. The holotype of
P. inflatus does not exist. The holotype of
Pluteus inflatus var.
alneus (PRM 154261) as well as PRM154569 and
Pluteus inflatus PRM154566 fall into the same/
inflatus clade, while one collection (PRM154563 as
Pluteus inflatus) falls into the/
cutefractus clade. Velenovský [
20] described
P. inflatus var.
inflatus with a non-cracked pileus without striation, growing on deciduous (
Carpinus) stumps. This description confirms the/
inflatus clade, supported by three collections, as the best candidate for the original concept of
P. inflatus. Velenovský [
21] described a variety of
Pluteus inflatus var.
alneus only with the short note that it grows on the trunk of
Alnus and that it is easily recognized. Because species of the genus
Pluteus often grow on substrates of various deciduous trees (e.g., [
1,
2,
7,
53]), we do not believe that growth on
Alnus versus
Carpinus is sufficient to describe a separate variety. Thus, we synonymize
Pluteus inflatus var.
alneus under
Pluteus inflatus.
Both sequenced syntypes of
Pluteus minutissimus f.
major (G00126660 and G00126659) belong to
P. inflatus, which has priority. Thus,
Pluteus minutissimus f.
major is only a later synonym of
P. inflatus. Surprisingly, our molecular studies show that the holotype of
Pluteus granulatus var.
tenellus J. Favre (G K9933, G00126179) also represents
P. inflatus, even though Favre [
54] described this variety as having a pileipellis composed of three types of elements, while there are only two significant element types in a typical basidioma of
P. inflatus. Unfortunately, the size and condition of the
Pluteus granulatus var.
tenellus holotype do not allow a precise verification of the pileipellis structure in numerous parts of the pileipellis, but, based on our studies, there are various elements that can be interpreted as representing two or three types by their similarities.
Pluteus inflatus is a highly variable species that is difficult to distinguish from related species and has a broad ecology, being recorded from both soil, plant litter, and decaying wood.
Pluteus inflatus is widely distributed in Eurasia, and even two North American collections have been molecularly confirmed. Both collections were made in disturbed, man-made habitats (urban gardens, parking lots), which could indicate a recent, human-mediated introduction to North America, but more collections are needed to confirm this hypothesis.
Pluteus absconditus Justo, Kalichman and S.D. Russell
sp. nov. Figure 8 and
Figure 9.
MB 849337.
Etymology: absconditus, meaning hidden or concealed, because of the difficulty separating this species from P. inflatus without molecular data.
Diagnosis: Closely related to P. inflatus, differing in the more abundant pleurocystidia, shorter cheilocystidia, and different nrITS (5–8 evolutionary changes) and TEF1-α sequences (5 evolutionary changes).
Holotype: USA, Tennessee: Loudon Co., Greenback, East Lakeshore Trails, on soil in mixed forest with Juniperus virginiana, Pinus, and hardwoods (Acer, Quercus, Cornus florida, Juglans nigra, etc.), 15 June 2013, leg. C. Braaten, Mushroom Observer 136488 (NBM-F-009787).
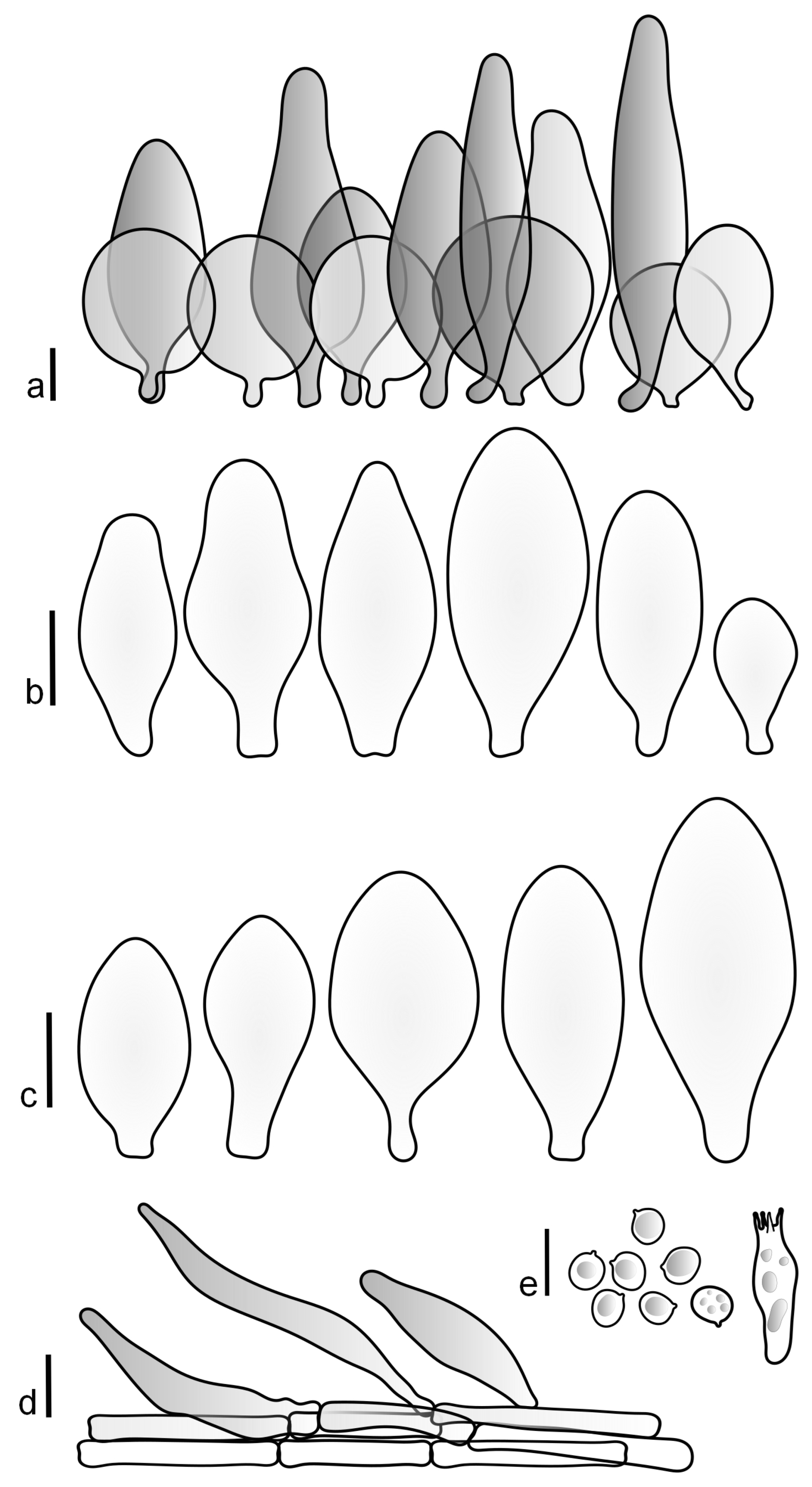
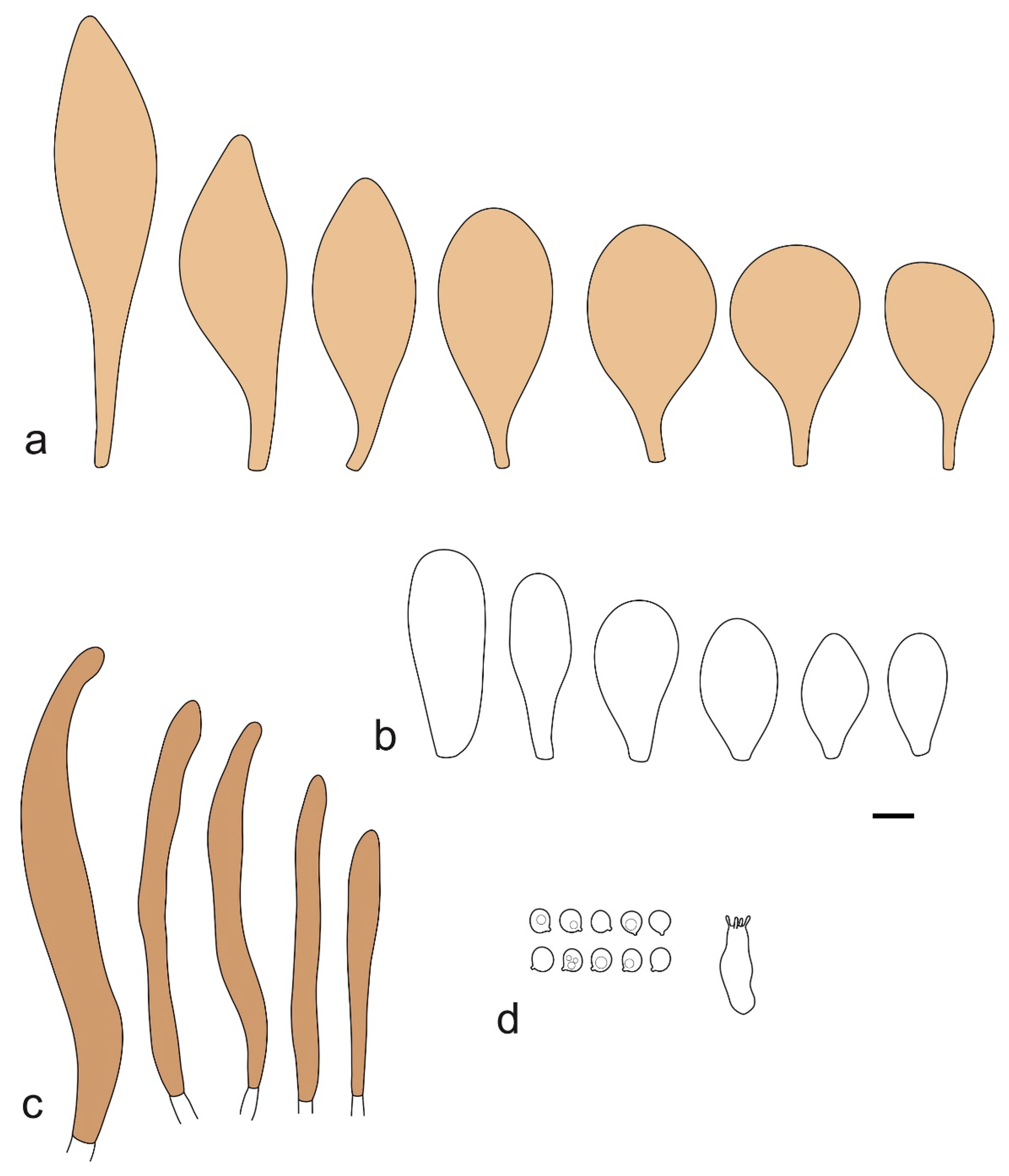
Pileus 15–40 mm in diameter, hemispherical when young, expanding to convex or plano-convex, with a low, broad umbo; surface strongly granulose-squamulose all over, with cracks that reveal the white context underneath, sometimes with a pulverulent-powdery aspect and/or with white-translucent pubescence; rugulose at center in young specimens; with predominant brown to reddish brown colors (Munsell: 5YR 4/6, 5/6–5/8, 6/6–6/8; 2.5YR 4/8, 5/8), distinctly darker at center; dry, not hygrophanous; margin rimose or (translucently) striate. Lamellae crowded, free, ventricose, up to 5 mm broad, white when young, later pink, with white or concolorous, flocculose, or smooth edges. Stipe 25–45 × 2–4 mm, cylindrical, with a slightly broadened base; surface white, silvery-white, shiny, or sometimes with a translucent aspect; pubescent-hairy all over, at least in young specimens; hairs sometimes grouped in mats with pale brown tips. Context in stipe and pileus white. Smell and taste none.
Basidiospores [60/2/2] 5.0–6.8 × 4.3–5.8 μm, avl × avw = 5.7–6.0 × 4.7–5.3 μm, Q = 1.00–1.26, Q* = 1.13–1.23, globose to broadly ellipsoid. Basidia 16–27 × 6.5–9 μm, predominantly 4-spored, but 2-spored and 1-spored basidia are also relatively common, clavate. Pleurocystidia 41–66 × 18–22(–26) μm, mostly narrowly clavate, ovoid, or ellipsoid; hyaline; very rarely with pale-brown intracellular pigment; thin-walled; common to scattered, all over lamellar faces. Lamellar edges heterogeneous, with cheilocystidia, basidia, and basidioles. Cheilocystidia 22–42(–49) × 11–22 μm, mostly (narrowly) clavate, subglobose, or spheropedunculate, rarely ovoid, hyaline, thin-walled, scattered, or in clusters, not forming a well-developed strip. Pileipellis a hymeniderm made up of two types of elements: (i) spheropedunculate or (narrowly) clavate, narrowly utriform or fusiform, 36–61 × 19–42 µm; (ii) fusiform, narrowly utriform, cylindrical, 75–127 × 19–28 µm; all elements with brown intracellular pigment, slightly thick-walled. Stipitipellis a cutis of cylindrical, slightly thick-walled, 6–10 µm wide hyphae, hyaline, or rarely with pale brown pigment. Caulocystidia common, often in loosely arranged clusters, 36–125 × 6–25 µm, cylindrical, narrowly clavate, narrowly fusiform, sometimes with a flexuous and/or rounded apex; often with 2–3 shortly-septate basal elements, 14–24 × 9–15 µm; hyaline or with pale brown intracellular pigment, sometimes with parietal pigment at the apex. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: solitary to subgregarious, growing on soil, often among leaves, twigs, and other plant matter, but without apparent connection to wood. June-August.
Distribution: Eastern North America: known from the USA (Indiana, Tennessee).
Additional collections examined: USA. Indiana: Monroe Co., Bloomington, Griffy Woods Nature Preserve, among woody debris, 28 August 2018, leg. Ron Kerner, iNaturalist 16154325; Brown Co., Nashville, 29 July 2022, leg. Brian Hunt, iNaturalist 128466549; ibid., 5 August 2022, iNaturalist 129614045. Tennessee: Anderson Co., Oak Ridge, Haw Ridge Park, 20 July 2019, leg. J. Kalichman, iNaturalist 112240775 (NBM-F-009786).
Notes: Pluteus absconditus is recovered in the phylogenetic analysis as a sister to Pluteus inflatus. Their nrITS sequences differ in 5–8 evolutionary changes, and their TEF1-α sequences in 5. Morphologically, both species are very similar, with P. inflatus differing in the rare or absent pleurocystidia and the more abundant and slightly larger cheilocystidia (20–60(–65) × (10–)15–30 µm). P. absconditus has 2-spored and 1-spored basidia, which is not a common character for Pluteus species, where most taxa have exclusively 4-spored basidia. The pileus of Pluteus absconditus seems to be paler than typical P. inflatus; however, the latter is an extremely variable species, and the variability of P. absconditus has not been well investigated yet.
Pluteus cutefractus Ferisin, Dovana, and Justo Sydowia 71: 285 (2020); Ferisin et al. [
27]
Figure 10 and
Figure 11.
Pluteus cutefractus Ferisin, Dovana, and Justo Sydowia 71: 182 (2019), in Song et al. [
55], Nom. inval.
Original diagnosis. Basidiomata with a markedly cracked pileipellis, globose to broadly ellipsoid basidiospores, predominantly ovoid to broadly clavate cheilocystidia and pleurocystidia, and trichohymeniderm pileipellis consisting of broadly utriform and fusiform elements.
General description: Pileus 10–29 mm broad, plano-convex when young, later plane, plano-concave to concave, not hygrophanous, venose or smooth in center, striate at margin, often cracked from margin up to 2/4–3/4 of pileus, more conspicuously when mature, showing whitish flesh underneath; fawn brown (RAL 8007), nut brown (RAL 8011), beige brown (RAL 8024), darker in the center. Lamellae free, moderately crowded, slightly ventricose, up to 4 mm broad, whitish when young, later light to dirty pink, sometimes with a yellowish tinge, with a whitish or concolorous flocculose edge. Stipe 29–51 × 2–5 mm, cylindrical, not bulbous, but sometimes 0.5–2 mm broadened, pubescent, sometimes finely longitudinally fibrillose, whitish or hyaline grayish, sometimes squamulose to densely covered with distinct rusty brown to brown floccules more conspicuous (more closely covered) in the lower part. Context whitish. Smell and taste indistinct.
Basidiopores [360/14/14] 4.9–7.1(–7.3) × (4.2–)4.5–5.8(–6.4) μm; avl × avw = 5.7–6.0 × 5–5.4 µm; Q = (1.00–)1.04–1.20(–1.33); Q* = 1.12–1.14, globose to broadly ellipsoid, thick-walled, inamyloid. Basidia 21–30 × 8–10 μm, clavate, 4-spored, very rarely 2-spored. Pleurocystidia (33–)40–55 × 20–25(–26) μm, scattered, ovoid, clavate to broadly clavate, rarely subfusiform with an obtuse apex, thin-walled, hyaline. Lamellar edges sterile, rarely heterogeneous. Cheilocystidia (18–)26–76 × 12–26 μm, ovoid, clavate to broadly clavate, rarely broadly subfusiform with obtuse apex, thin-walled, hyaline. Pileipellis a hymeniderm made up of broadly utriform (45–60 × 20–35 μm) and narrowly fusiform (100–150 × 11–35 μm) elements; pigment intracellular, vacuolar, light brown or brown. Stipitipellis a cutis of whitish hyphae 4–10(–12) μm wide. Caulocystidia present, descending to about 1/3 of the length of the stipe (36–)40–60 × 12–18 μm, variable in shape from clavate to fusiform, sometimes filled with evenly dissolved brownish intracellular pigment. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: solitary or in groups, on soil or on broadleaved trunks and stumps, from summer to autumn.
Distribution: known only from Europe (Czech Republic, Denmark, France, Slovenia, Spain).
Holotype: Slovenia: Nova Goricâ, Panoveč Park, with Abies alba and Fagus, on deciduous stump, 8 July 2017, leg. G. Ferisin (MCVE 30110).
Additional collections examined: CZECH REPUBLIC: Mnichovice, Jidášky, on a fallen trunk of Betula, September 1940, leg. J. Velenovský (PRM 154563 as Pluteus inflatus); Grygov, Království forest, under Alnus glutinosa, Fraxinus excelsior, Tilia, Quercus, Padus racemosa, on soil and deciduous stump, 23 June 2019, leg. V. Halasů and H. Ševčíková (BRNM 816221). DENMARK: Northen Zealand, Højbjerg Hegn, on soil in deciduous forest, leg. M. Sonniks, DMS-10213877 (C). FRANCE: Oise, in a muddy rut of a trail, deciduous forest, 3 August 1980, leg. and det. H. Romagnesi (MNHN-PC-FUSION111827, originally as Pluteus minutissimus). SLOVENIA: Harije, under Fagus, on deciduous stump, 17 July 2019, leg. G. Ferisin (FG 17072019); Nova Goricâ, Panoveč Park, with Abies alba and Fagus, on deciduous stump, 26 September 2015, leg. G. Ferisin (FG 26092015); ibid., 8 July 2017 (MCVE 30111); ibid., 8 July 2017 (FG12794); ibid., 2 June 2018, leg. G. Ferisin (MCVE 30143), 9 September 2018 (FG 09092018). SPAIN: Fitor (city), in riparian forest with Corylus avellana and Alnus glutinosa (also Quercus spp.?), on acid soil, 400 m a.s.l., 7 June 2008, leg. C. Roqué (det. as P. podospileus by C. Roqué, BRNM 825872); Navarra, Oieregi, Señorío de Bértiz, on the sand deposited at the root of a completely fallen broadleaved unidentified tree, among many Coprinellus disseminatus, in a riverside forest with abundant allochthonous vegetation (Platanus x hispanica, Quercus rubra, Trachycarpus fortune, Prunus laurocerasus, Diospyros virginiana, Phyllostachys viridiglaucescens, etc.), 25 September 2021, leg. G. Muñoz (GM3784); Cataluña, Sant Hilari Sacalm, under Alnus glutinosa, Corylus avellana and Fraxinus excelsior, apparently on soil, 14 September 2019, leg. S. Gibert (GM3458, duplo in SGS20190914.10).
Notes:
Pluteus cutefractus was described as having a markedly cracked pileus margin by Song et al. [
55]. This feature can be relatively indistinct in some young basidiomata. Moreover, many other species may have cracked pileus in extreme weather conditions. Thus, this feature must be considered carefully, and microscopic features and ecology must be taken into account.
MB 849803.
Etymology: fuscodiscus refers to the dark central disc on the pileus.
Diagnosis: Differs from other known species of the Pluteus podospileus complex by having a pileus with a distinctly darker disk in the center.
Holotype: Italy: Friuli Venezia Giulia, Farra d’Isonzo, and Bosco di Sotto, in small groups, on twigs of broadleaved trees (Salix, Quercus, and Populus), 22 August 2015, leg. G. Ferisin (FG22082015).
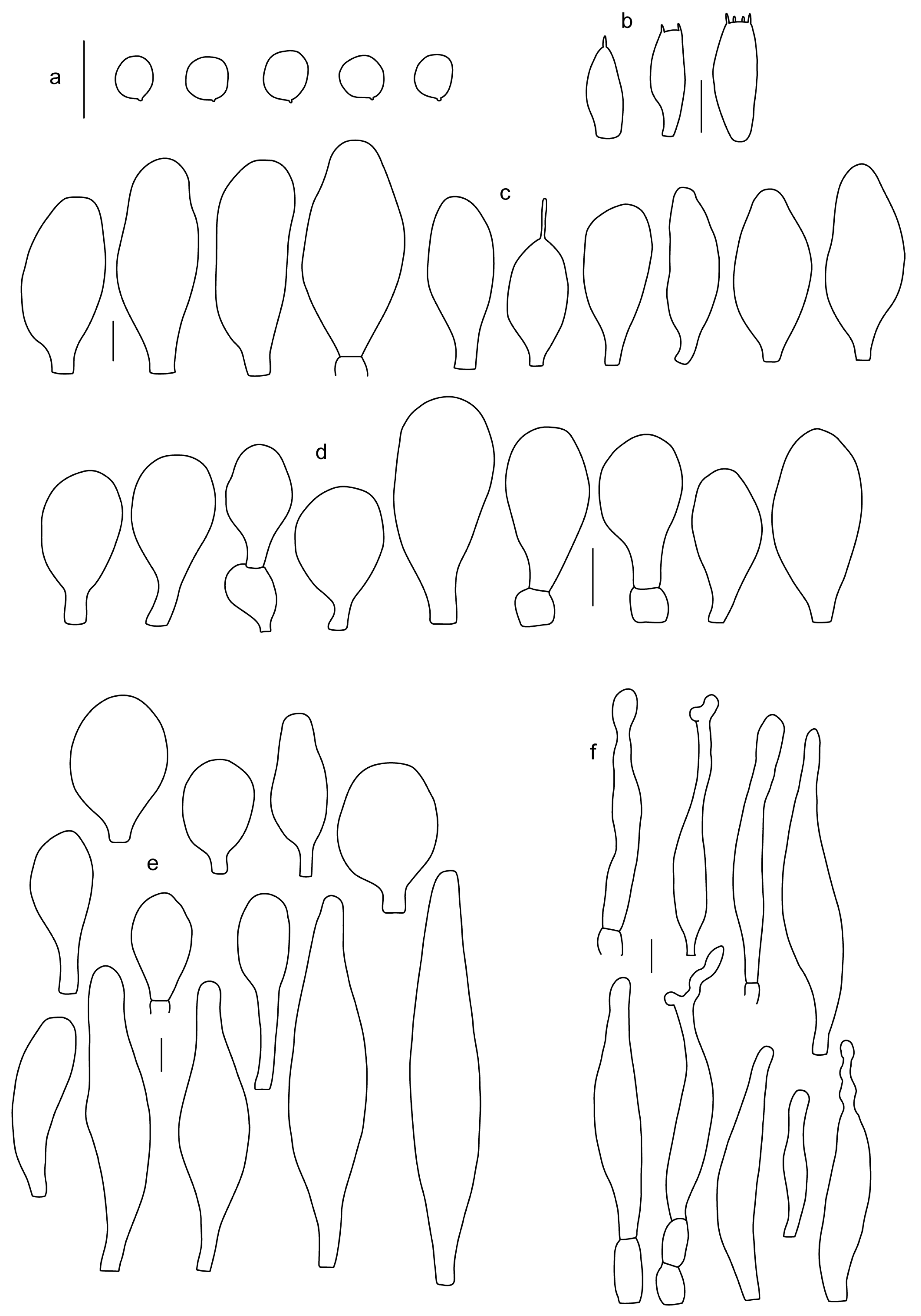
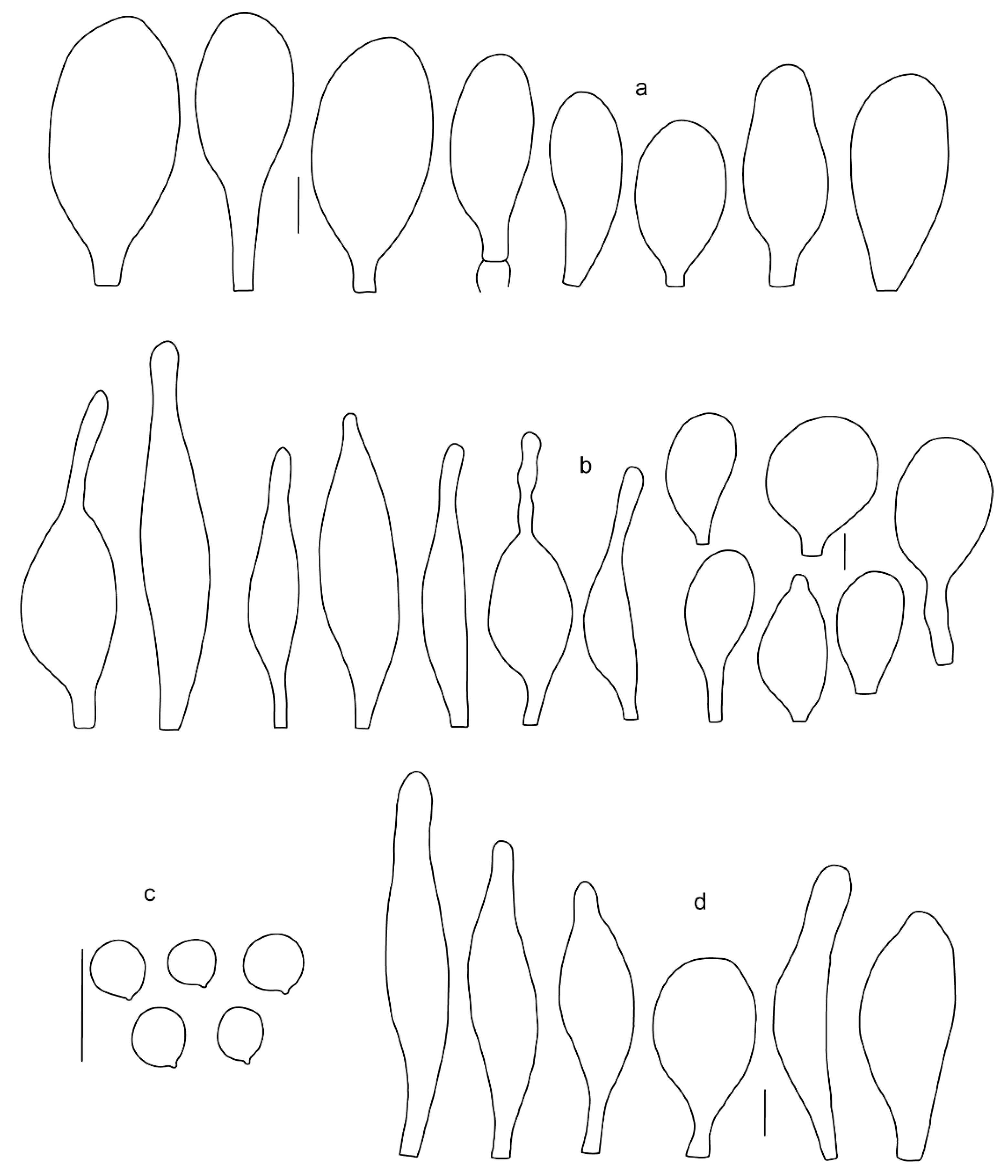
Pileus 5–30 mm in diameter, campanulate or convex to plano-convex, then applanate, with indistinct umbo, not or slightly hygrophanous, indistinctly striate near the pileus margin to striate up to half of radius, margin slightly eroded; surface granulose-pruinose to granulose-squamulose, velvety, cracking at the margin to show underlying whitish context or not, darkly colored night brown (RAL 050 20 16) or granite brown (RAL 050 20 10) when young, then golden brown (RAL 050 50 30), brazilian brown (RAL 060 40 309), coffee bean brown (RAL 060 40 20), madeira brown (RAL 050 40 40), chalk beige (RAL 075 80 10) to chestnut brown (RAL 040 40 30), with a distinctly darker disk in the centre: mahogany brown, chocolate brown, terra brown or darker, slightly paler towards the margin. Lamellae free, crowded to moderately crowded, ventricose, whitish, later pinkish, with concolorous flocculose eroded edges. Stipe 12–45 × 1–4 mm, cylindrical or faintly thickened downwards, often with a slightly broadened base; surface white, whitish pellucid, or greyish brown, at least on the lower half covered with brown to almost black floccules, often more densely at the base. Context whitish. Smell indistinct; taste not recorded.
Basidiospores [435/13/13] (4.5–)4.8–6.5(–6.8) × (3.46–)4.0–5.5(–5.9) µm, avl × avw = 5.3–5.7 × 4.2–4.8 μm, Q (1.0–)1.10–1.36(–1.47), Q* = 1.10–1.26, subglobose to ellipsoid, rarely globose, thick-walled. Basidia 16–32 × 6–12 µm, 4-spored. Pleurocystidia absent or very rare, 34–36 × 10–13 µm, utriform to spathulate, hyaline, thin-walled. Lamellar edge sterile. Cheilocystidia 25–76 × 10–30(–55) µm, clavate to almost cylindrical, broadly clavate, oviform, broadly utriform, in some collections frequently mucronate, hyaline, or with light brown intracellular pigment, rarely with oily contents, thin-walled. Pileipellis a hymeniderm made up of two types of elements: (i) spheropedunculate or broadly clavate, (30–)37–62 × 21–55 µm; (ii) (narrowly) fusiform, inflated-fusiform, sometimes mucronate, (57–)63–180 × (10–)12.5–33 µm; all elements with light to dark brown or yellow-brown intracellular pigment, rarely with oily contents, thick-walled. In some collections, there are not two types of elements but rather variable short-to-long elements. Stipitipellis a cutis of cylindrical, hyaline, thin-walled to slightly thick-walled hyphae, 6–10 µm wide. Caulocystidia numerous or rare, throughout full length, in tufts or solitary, 30–143 × 6–22(–24) µm, cylindrical, narrowly to narrowly fusiform, broadly clavate or broadly utriform, with yellow-brown to brown intracellular pigment, in some collections often aggregated into spots. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: solitary or in small groups, growing on decayed deciduous or coniferous wood, sawdust, or other plant litter. In July–October.
Distribution: Europe: Czech Republic, Estonia, France, Italy, European part of Russia; and Asia: Iran, Turkey, Far East Russia.
Additional collections examined: EUROPE. CZECH REPUBLIC: Lanžhot, Ranšpurk Nature Reserve, floodplain forest, fallen deciduous trunk, 3 October 2014, leg. H. Ševčíková (BRNM 766589); ibid., 15 October 2019, leg. H. Ševčíková (BRNM 817683). FRANCE: Saint-Gatien-des-Bois, Calvados, Fagus, 13 July 2017, leg. T. Duchemin (BRNM 825871). ESTONIA: Lääne Co., Taebla Comm., Palivere skiing track, 58.96537° N, 23.90702° E, on sawdust, 27 September 2012, leg. V. Liiv (TUF118497). ITALY: Friuli Venezia Giulia, Farra d’Isonzo, 2 August 2015, leg. G. Ferisin (DOV 653, FG 02082015007); ibid., 20 August 2015, leg G. Ferisin (FG 12152); ibid., 11 August 2017, leg. G. Ferisin (ALV 12977-FG 11082017). RUSSIA: European part, Samara Region, Zhiguli Biosphere Reserve, vicinity of Bakhilova Polyana, birch forest, on decayed wood of deciduous trees, 15 August 2004, leg. E.F. Malysheva (LE F-313448). ASIA. RUSSIA: Far East, Primorye Territory, Sikhote-Alin Nature Biosphere Reserve, vicinity of Ust’-Shandui Reserve Field Station, plateau near Zabolochennaya river, mixed forest with Tilia sp. and Pinus koraiensis, on wood of coniferous tree, 23 August 2012, leg. E.F. Malysheva (LE F-313336); Primorye Territory, Sikhote-Alin Nature Biosphere Reserve, vicinity of Yasny Reserve Field Station, floodplain of Yasnaya river, mixed forest with Pinus koraiensis, Tilia sp., Quercus mongolica, Populus maximowiczii, on litter in fern thickets, 26 August 2013, leg. O.V. Morozova (LE F-312862). WESTERN ASIA. IRAN: Golestan, Gorgon, +/−virgin beech forest, 950 m a.s.l., on rotten log of Fagus orientalis, 3 October 2016, leg. Jacob Heilmann-Clausen and Claus Bässler (DMS-10334331). TURKEY: Muğla Province, Köycegiz District, near Dögüşbelen town, on decayed wood of Liquidambar orientalis, 6 m a.s.l., 18 November 2010, leg. O. Kaygusuz (OKA-TR691); ibid., on decayed wood of L. orientalis, 10 m a.s.l., 16 December 2010, leg. O. Kaygusuz (OKA-TR695); ibid., on rotten wood of L. orientalis, 9 m a.s.l., 22 November 2011, leg. O. Kaygusuz (OKA-TR715); ibid., on fallen trunk or well-decayed wood of L. orientalis, 5 m a.s.l., 18 March 2012, leg. O. Kaygusuz (OKA-TR749); ibid., on well-decayed wood of L. orientalis, 8 m a.s.l., 25 March 2012, leg. O. Kaygusuz (OKA-TR750).
Notes: Typical basidiomata of Pluteus fuscodiscus have a granulose-pruinose, velvety pileus with a distinctly darker disk in the center, a white to greyish brown stipe with at least the lower half covered with brown to almost black floccules, a pileipellis made up of two types of elements, and an absence of utriform to spathulate pleurocystidia.
Lloydia 21(4): 272 (1959)1958.
Basionym: Leptonia seticeps G.F. Atk., J. Mycol. 8(3): 116 (1902).
Syn.: Leptoniella seticeps (G.F. Atk.) Murrill, North American Flora 10: 92. 1917.
Pluteus psychiophorus var. seticeps (G.F. Atk.) Singer, Trans. Br. Mycol. Soc. 39(2): 214 (1956) (nom. inval; published as “ad. int.” Art. 36.1).
Pluteus nanellus Murrill, N. Amer. Fl. (New York) 10(2): 130 (1917) (see Minnis and Sundberg 2010) [
13].
Missapl. Pluteus podospileus Sacc. and Cub., Syll. fung. (Abellini) 5: 672 (1887).
Excluded. Pluteus seticeps sensu Homola (1972) (probably Pluteus cystidiosus).
Pileus 8–24 mm in diameter, hemispherical or campanulate when young, expanding to convex or plano-convex, often with a low, broad umbo; surface soon becoming granulose-squamulose all over, often becoming minutely rimulose with age, showing the white context underneath, especially towards the margin, with the center remaining smooth or rugose; with predominant brown colors (Munsell: 5YR 4/6, 5/6–5/8, 6/6–6/8; 7.5YR 4/6, 5/6–5/8, 6/6–6/8), darker at center; dry, not hygrophanous; margin often translucently striate up to half the radius of pileus, often rimose. Lamellae crowded, free, ventricose, up to 4 mm broad, white when young, later pink, with even, serrated, or flocculose edges, white or concolorous. Stipe 15–28 × 1–2 mm, cylindrical, with slightly broadened base; surface white, shiny, sometimes with grey, grey-brown hues at base; surface smooth or with some appressed brown fibrils in the lower part of the stipe. Context in stipe and pileus white. Smell and taste none.
Basidiospores [200/11/9] 4.0–6.0(–6.5) × (3.5–)4.0–5.5 μm, avl × avw = 4.6–5.0 × 4.3–4.8 μm, Q = 1.00–1.26, Q* = 1.03–1.13, globose to broadly ellipsoid, some ovoid. Basidia 18–26 × 6.5–8 μm, 4-spored, clavate. Pleurocystidia absent from lamellar faces; very rarely, some cystidia present near the lamellar edge. Cheilocystidia 25–56(–91) × 10–30 μm, mostly clavate, narrowly clavate or ellipsoid, some narrowly utriform or ovoid; hyaline, thin-walled, crowded, forming a well-developed strip or in discrete clusters, making the lamellar edge heterogeneous. Pileipellis a hymeniderm or epithelioid hymeniderm, made up of two types of elements: (i) spheropedunculate, pyriform, or broadly clavate, 30–69 × 13–38 µm; (ii) fusiform, lageniform, narrowly utriform, often mucronate, (70–)80–111 × 11–27 µm; all elements with brown intracellular pigment, evenly dissolved or aggregated in spots, slightly thick-walled. Stipitipellis a cutis of cylindrical, slightly thick-walled, 6–10 µm wide hyphae; hyaline, or some with pale brown pigment; at the base of the stipe with groups of slightly irregular, adnate hyphae, heavily pigmented. Caulocystidia scattered, mostly not occurring in clusters, 44–89 × 13.5–27 µm, narrowly utriform, lageniform, fusiform, clavate, some mucronate; most with brown pigment, often densely aggregated. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: solitary or gregarious, growing on well-decayed wood of angiosperms (e.g.,
Acer). In temperate or transitional boreal/temperate forests. July–August. According to Minnis and Sundberg [
13], also June and September.
Distribution: North America: widely distributed in eastern North America. Molecularly confirmed from Canada (Québec) and the USA (Illinois, Indiana, Missouri, New Jersey, Ohio, Pennsylvania, Tennessee, and Wisconsin).
Collections examined: CANADA. Québec: Saint-Laurent, on decayed angiosperm wood, 5 July 2011, leg. Renée Lebeuf, HRL0715 (NBM-F-009796); ibid., 7 July 2011, HRL0716 (NBM-F-009797). USA. New Jersey: Gloucester County, Mantua, Chestnut Branch Park, on decayed hardwood, 8 August 2021, leg. M. Patiño, iNaturalist 91066075 (NBM-F-009802); Morris County, Mendham, Meadowood Park, on decayed hardwood, 24 July 2021, leg. M. Patiño, iNaturalist 88771421 (NBM-F-009801). Ohio: Franklin County, Gahanna, on a well-rotted, mossy log in floodplain near Platanus occidentalis, Populus deltoides, Asimina triloba, Acer negundo, and Aesculus flava, 17 July 2014, leg. D. Grootmyers, MO 177404 (NBM-F-009809). Pennsylvania: Luzerne County, Sweet Valley, on a pile of wood chips and other woody debris, 27 July 2018, leg. D. Wasilewski, MO 324271 (NBM-F-009810). Tennessee: Anderson Co., Oak Ridge, Haw Ridge Park, 14 June 2019, leg. J. Kalichman, iNaturalist 112235090 (NBM-F-009798); ibid., 23 June 2019, iNaturalist 112237607 (NBM-F-009799); ibid., 1 July 2021, iNaturalist 112335052 (NBM-F-009800).
Notes:
Pluteus seticeps has been considered by some authors to be a synonym of
P. podospileus [
2,
4], but detailed revision of the type collection, modern North American collections, and molecular data clearly indicate these are separate species [
7,
13]. Both species have non-overlapping geographical ranges, with
P. podospileus occurring only in Eurasia and
P. seticeps only present in eastern North America. Minnis and Sundberg [
13] confirmed the usage of the name
Pluteus seticeps for North American collections lacking pleurocystidia and established
Pluteus seticeps var.
cystidiosus Minnis and Sundb. for North American collections similar to
P. seticeps with pleurocystidia. Molecular data from the type collection of
P. seticeps var.
cystidiosus indicate that this taxon is not closely related to
P. seticeps but instead represents a sister taxon to the European
P. podospileus. The new combination
P. cystidiosus is adopted here for this species. Morphologically,
P. seticeps can be distinguished from
P. cystidiosus by the smaller basidiomata, pileus surface more markedly rimulose, stipe surface not covered in brown squamules, absence of pleurocystidia, and smaller caulocystidia that mostly do not occur in clusters.
Pluteus nanellus Murrill was described from New York as a species with small basidiomata, pale bay (pale reddish brown), minutely tomentose (visible only under the lens) pileus turning castaneous on drying, and a snow-white stipe [
25]. Singer [
22] and Homola [
24,
56] synonymized
Pluteus nanellus and
P. seticeps mostly based on the similarity of the pileipellis elements. Minnis and Sundberg [
13] revised the type collections of both taxa and considered them to be identical. The only significant difference in the original descriptions of these species is the presence of some brown fibrils at the base of the stipe of
P. seticeps, while this character is not mentioned in
P. nanellus. Unfortunately, stipes are absent in both types of collections. Many of the specimens examined here have some brown fibrils at the base, but completely glabrous stipes also occur. We agree with Minnis and Sundberg [
13] and consider
P. seticeps and
P. nanellus synonymous.
Pluteus cystidiosus (Minnis and Sundberg) Justo, Malysheva and Lebeuf
comb. & stat. nov. Figure 18 and
Figure 19.
MB 849340.
Basionym: Pluteus seticeps var. cystidiosus Minnis and Sundberg N. Amer. Fung. 5(1): 13 (2010).
Holotype: USA: Michigan, Chippewa Co., White House Landing, on deciduous wood, 5 July 1965, leg. R. Homola, coll. RL Homola 1340 (MICH 69572).
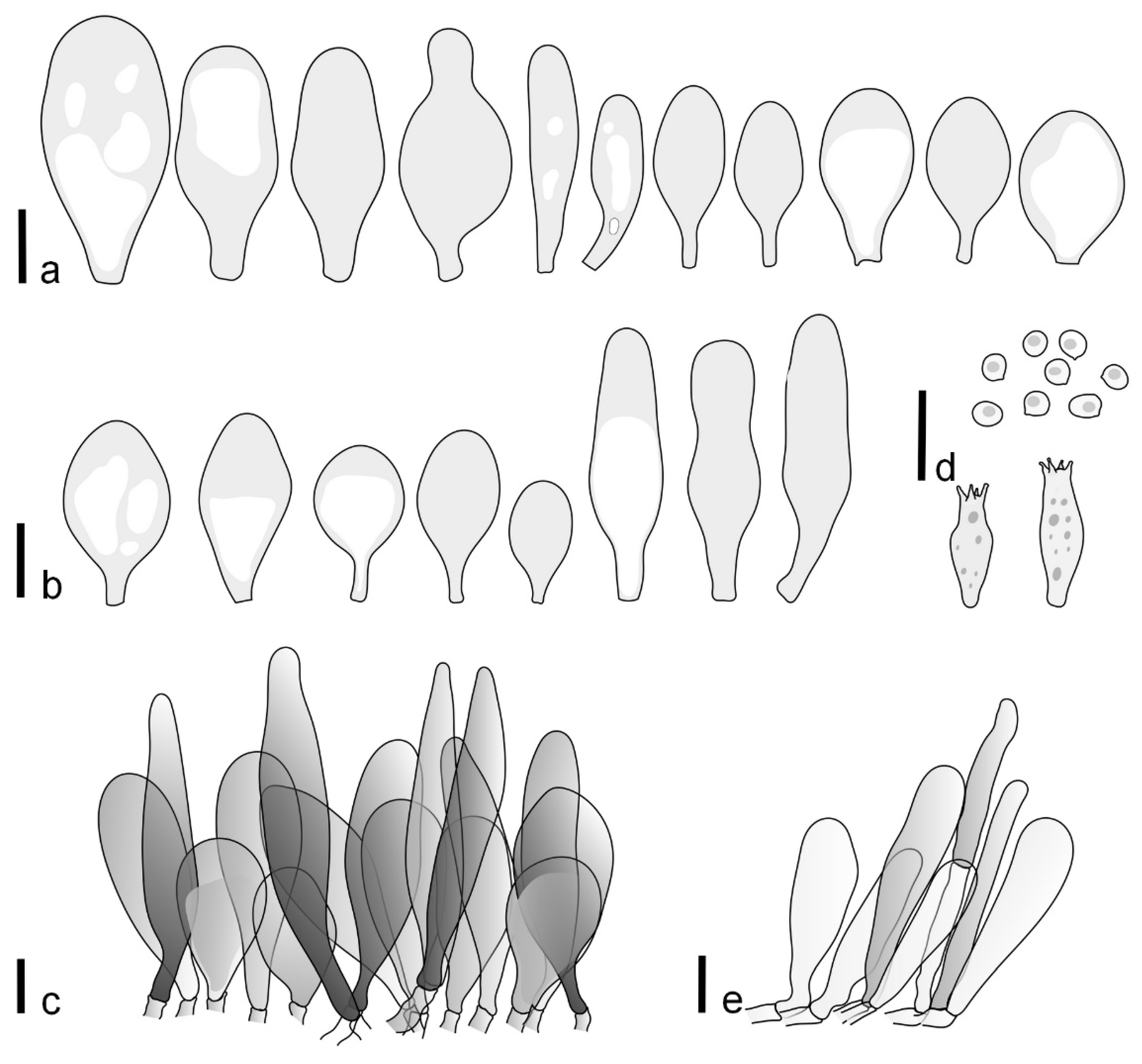
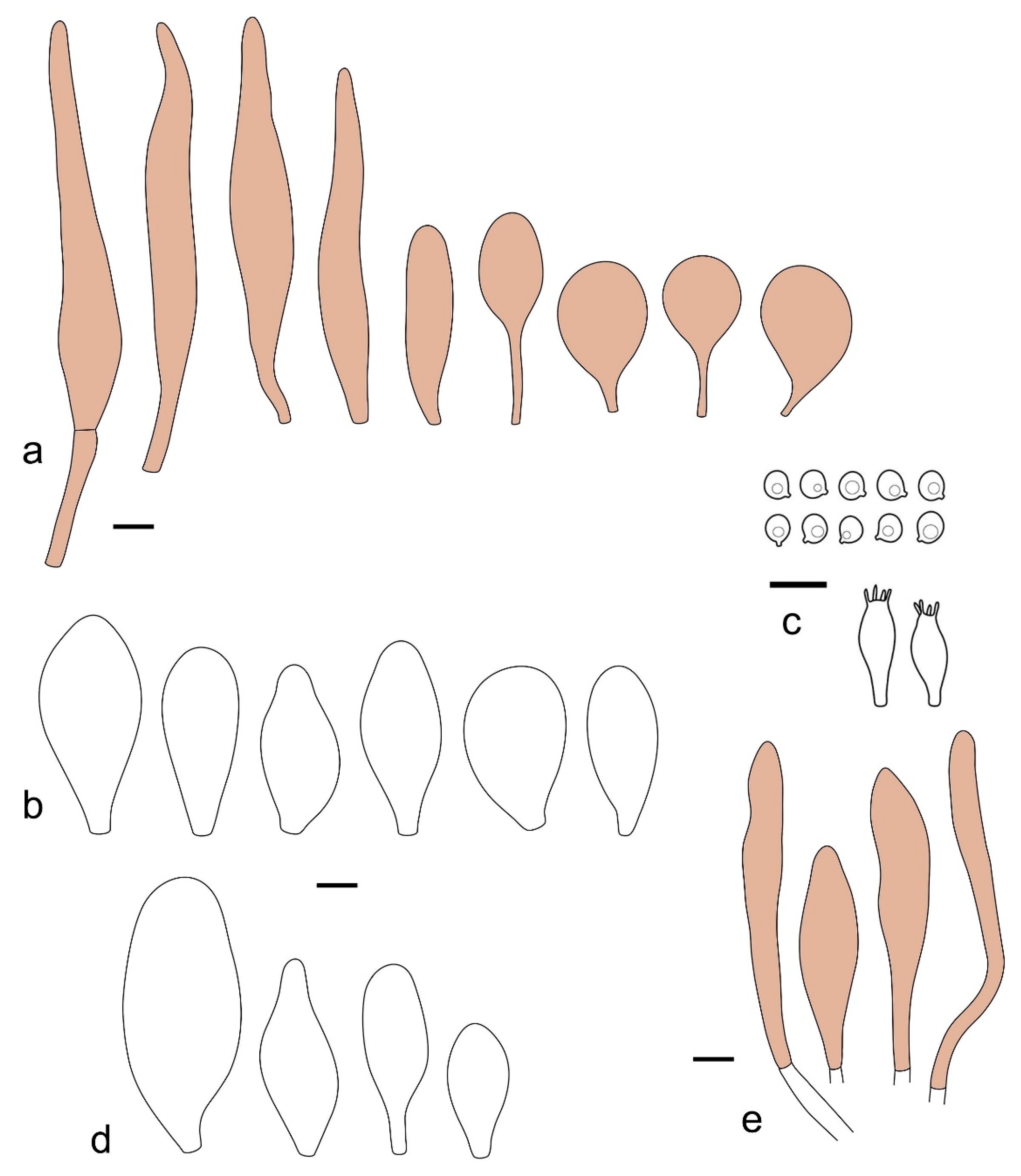
Pileus 14–35(–40) mm in diameter, hemispherical or campanulate when young, expanding to convex or plano-convex, often with a low, broad umbo; surface pruinose, slightly to strongly velvety, becoming granulose-squamulose especially around center, smooth or strongly rugose all over when young, in older specimens smooth or rugose at center, for the most part not cracked, or with some cracks revealing the white context towards the margin, especially in older specimens; with predominant brown or red-brown colors (Munsell: 2.5YR 4/6–4/8, 3/4–3/6; 5YR 5/4–5/8, 4/4–4/6; when young 5YR 3/3, 2.5/2; RAL color chart: chestnut brown (RAL 040 40 30), red brown (RAL 020 20 20) or wild brown (RAL 040 20 19), usually darker at center; dry, hygrophanous; margin entire, serrate, slightly rimose or translucently striate. Lamellae crowded, free, ventricose, up to 5 mm broad, white when young, later pink, with even, serrated, or flocculose edges, white or concolorous. Stipe 15–40 × 1–5 mm, cylindrical, with a slightly broadened base; surface white, entirely minutely squamulose with dark or black-brown squamules, more densely so in the lower half. Context in stipe and pileus white. Smell and taste none.
Basidiospores [210/7/7] 4.5–5.5(–6.2) × 3.5–5.0 μm, avl × avw = 5.1–5.3 × 4.2–4.6 μm, Q = (1.00–)1.07–1.30, Q* = 1.11–1.24, subglobose to broadly ellipsoid, rarely globose or ovoid. Basidia 18.5–26 × 5.5–9 μm, 4-spored, clavate. Pleurocystidia 35–73(–82) × 11–31 μm, mostly (narrowly) utriform, also ovoid, clavate, or (broadly) fusiform; hyaline, thin-walled; scattered or rare over lamellar faces; rarely absent. Lamellar edge sterile. Cheilocystidia 26–63(–78) × 10–22(–26) μm, narrowly to broadly clavate, (narrowly) utriform or ovoid; hyaline, thin-walled, crowded, forming a well-developed strip. Pileipellis a hymeniderm or epithelioid hymeniderm, made up of two types of elements: (i) spheropedunculate, pyriform, or broadly clavate (30–)36.5–70 × 20–32 µm; (ii) broadly fusiform, inflated-fusiform, lanceolate, narrowly utriform, often mucronate, 66–121.5 × 9–17(–32) µm; all elements with brown or yellow-brown intracellular pigment, often aggregated in spots, slightly thick-walled. Stipitipellis a cutis of cylindrical, slightly thick-walled, 7–10 µm wide hyphae, hyaline, or some with pale brown pigment. Caulocystidia common, often in clusters, 36–125 × 6–25 µm, cylindrical, narrowly clavate, narrowly fusiform, spheropedunculate, often septate; most with brown or yellow-brown pigment, sometimes aggregated. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: often gregarious, growing on well-decayed wood of angiosperms (e.g., Acer, Betula), also on still-standing trees. In temperate or transitional boreal/temperate forests. July–October.
Distribution: North America: molecularly confirmed from Canada (New Brunswick, Québec) and the USA (Massachusetts, Michigan, Minnesota, and New York). Eurasia: molecularly confirmed from Japan and the Russian Far East (Primorye Territory).
Additional collections examined: NORTH AMERICA. CANADA. New Brunswick: Albert County, Caledonia Gorge Protected Natural Area, 6 km NNW of Riverside Albert, where Crooked Creek meets Caledonia Brook, 45.79731, -64.77409, on decayed wood, 6 July 2011, leg. E. Duke, EKD2011-91 (NBM-F 07400). Québec: Saint-Laurent, on angiosperm wood, 5 July 2011, leg. R. Lebeuf, HRL0713 (NBM-F-009792); Saint-Narcisse, Parc de la rivière Batiscan, Secteur Barrage, trail La P’tite vite, mixed forest dominated by Tsuga canadensis, Fagus grandifolia, and Pinus strobus, on the lying trunk of Betula, 16 July 2019, leg. R. Lebeuf and André Paul, HRL2941 (NBM-F-009791). USA. Massachusetts: Berkshire County, Savoy Mountain State Forest, at the base of a standing maple tree, 4 October 2012, leg. A. Justo, AJ 782 (NBM-F-009790). New York; Essex Co., Adirondack Ecological Center, Huntington Wildlife Forest, mixed forest, on decayed angiosperm wood, 15 August 2012, leg. A. Justo, AJ 617 (NBM-F-009788); ibid., 14 August 2012, AJ 619 (NBM-F-009789). EURASIA. JAPAN. Hokkaido Pref., Shiribeshi Prov., Otaru-shi, Nagahashi, on decayed broad-leaved wood, 17 July 2005, leg. K. Yamamoto (TNS-F-12398). RUSSIA: Far East, Primorye Territory, Sikhote–Alin Biosphere Reserve, vicinity of Yasny Reserve Field Station, floodplain of Yasnaya river, Larix cajanderi forest with Betula platyphylla, on rotten trunk of Betula, 14 August 2012, E.F. Malysheva (LE F-312852); Primorye Territory, Land of the Leopard National Park, plateau near Ananjevka river, mixed forest (Quercus mongolica, Carpinus cordata, Ulmus japonica, Abies holophylla), on mossy trunk of deciduous tree, 1 September 2011, leg. A.E. Kovalenko (LE F-313335).
Notes: Pluteus cystidiosus is recovered in the phylogenies as the sister species of Pluteus podospileus, and both species show an almost total overlap in their morphological variation, with P. cystidiosus having slightly larger pleurocystidia (35–73(–82) × 11–31 μm vs. 36–51 × 13.4–24 μm in P. podospileus) and smaller caulocystidia (36–125 × 6–25 µm vs. 36–140(–176) × 6–25 µm). There are 10 evolutionary changes between the nrITS sequences of P. cystidiosus and P. podospileus and 18 evolutionary changes in their TEF1-α sequences.
Based on current data, P. podospileus is not expected to occur in North America, and P. cystidiosus is not expected to occur in Europe, but it is not known if their geographical ranges overlap in Asia. Pluteus cystidiosus occurs in Japan and the Russian far east, while P. podospileus has been confirmed to occur as far east as Siberia (Krasnoyarsk Territory).
Pluteus psychiophorus (Berk. and Broome) Sacc. and
Pluteus stigmatophorus (Berk. and Broome) Sacc. are two tropical species, originally described from Sri Lanka, for which molecular data are not currently available [
18].
Pluteus psychiophorus differs from
P. cystidiosus in the smaller basidiomata (pileus 7–20 mm), a smooth, non-squamulose stipe, larger basidiospores 6–8 × 5–6.5 μm, avl × avw = 6.5 × 5.7 μm, pleurocystidia predominantly clavate and smaller in size (35–48 × 18–23 μm), a heterogeneous lamellar edge not completely covered in cheilocystidia, and smaller elongated elements in the pileipellis, only up to 70 μm long [
18].
Pluteus stigmatophorus differs in the pigmented lamellar edges and larger basidiospores 5.2–7.5 × 4.5–6 μm, avl × avw = 6.0 × 5.7 μm that are predominantly subglobose (Q = 1.05), predominantly clavate, pigmented, and shorter (up to 48 μm long) pleurocystidia, and pigmented, predominantly clavate, and shorter (up to 47 μm long) cheilocystidia [
18].
Pluteus delicatulus C.K. Pradeep and K.B. Vrinda, originally described from India, differs in the smaller basidiomes (pileus 7–10 mm), smooth stipe without squamules, predominantly globose basidiospores, predominantly clavate and shorter (up to 40.5 μm) pleurocystidia, and the absence of caulocystidia [
57].
MB 849342.
Etymology: Named for the unexpected discovery of this unknown species in the Pluteus podospileus complex.
Diagnosis: Closely related to P. seticeps, differing in the presence of pleurocystidia, caulocystidia occurring in clusters, and different nrITS (14 evolutionary events) and TEF1-α (11 evolutionary events) sequences.
Holotype: Canada, Québec: Grondines, route Lefebvre, close to the railway track, on wood chips of angiosperm in a clear-cut area, 24 October 2021, leg. R. Lebeuf and A. Paul, HRL3639 (NBM-F-009793).
Pileus 10–21 mm in diameter, convex when young, expanding to applanate often with a low, broad umbo; surface uniformly granulose, sometimes rugose in the center, for the most part not cracked, rarely with a few cracks revealing the white context towards the margin or at mid-radius; with reddish brown to reddish blond colors (RAL color chart: beige (RAL 1211), mahogany brown (RAL 8016), darker at center; dry, not hygrophanous; margin entire, slightly sulcate; Lamellae close, free, convex, 2–3 mm broad, white when young, later pink, with even, serrate white edges. Stipe 12–21 × 1–2 mm, cylindrical, equal; surface white, almost smooth in the upper half and whitish-squamulose in the lower half, or minutely squamulose with dark or blackish brown squamules, more densely so in the lower half. Context in stipe and pileus greyish to concolorous with the outer surface, very thin in pileus. Smell and taste none.
Basidiospores [69/2/2] (4.2–)4.5–5.8(–6.0) × (4.0–)4.5–5.5(–5.8) μm, avl × avw = 5.0–5.1 × 4.7–5.0 μm, Q = 1.00–1.11, Q* = 1.03–1.07, globose to subglobose. Basidia 12–22 × 5–8 μm, 4-spored, clavate. Pleurocystidia mostly present close to the lamellar edge and then large, subglobose with or without pedicel, ovoid, ellipsoid; rare to absent elsewhere and then much smaller, clavate, ~23 × 12 µm; hyaline, thin-walled. Lamellar edge sterile. Cheilocystidia 18–45 × 8–24(–35) µm, mostly ellipsoid, (broadly) clavate or spheropedunculate, also ovoid or broadly fusiform; hyaline, thin-walled, crowded, forming a well-defined strip. Pileipellis a hymeniderm made up of two types of elements: (i) spheropedunculate, subglobose, ovoid-pedicellate, ellipsoid-pedicellate, 22–75 × 22–47 µm; (ii) (narrowly) fusiform, fusiform-pedicellate, lanceolate, broadly fusiform, narrowly utriform-pedicellate, conical, 67–165 × 13–39 µm; all elements with intracellular brown pigment aggregated in spots, thin-walled. Stipitipellis a cutis of cylindrical, thin-walled, 3–10 µm wide hyphae; hyaline. Caulocystidia of two types: (i) at stipe apex, scarce, isolated, or in small groups, 26–40(–80) × 11–16(–36) µm, ellipsoid, clavate, fusiform, sometimes catenulate, hyaline, also as brown-pigmented cylindrical terminal cells 2–3 µm wide with oblong or clavate extremity 62–190 × 11–19 µm; (ii) in lower stipe occasional or common, erect, often in clusters, more rarely isolated, 48–150 × 9–19 µm, (narrowly) fusiform, oblong, cylindrical, narrowly clavate, with rounded or attenuate apices, with intracellular brown pigment aggregated in spots. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: isolated or gregarious, growing on well-decayed wood or wood chips of angiosperms. Thus far, it has been collected in forests or open areas of the temperate zone in September and October.
Distribution: North America: molecularly confirmed from Canada (Québec).
Additional collections examined: CANADA. Québec: Saint-Narcisse, Parc de la rivière Batiscan, Secteur Barrage, along the road leading to trail Le Buis, predominantly deciduous forest of Quercus rubra, Pinus strobus, Acer sp., and Abies balsamea, on well-decayed wood of angiosperm, 8 September 2022, leg. R. Lebeuf and A. Paul, HRL4010 (NBM-F-009794).
Notes: Pluteus podospileus differs from P. inexpectatus in its subglobose to broadly ellipsoid basidiospores and distribution restricted to Eurasia; some collections of P. podospileus have larger basidiomata, but the variability in size of P. inexpectatus is not well known yet. Pluteus cystidiosus can have a more robust habit, a more or less velvety pileus, and subglobose to broadly ellipsoid basidiospores. In North America, confusion is most likely with Pluteus seticeps, which can be distinguished by the absence or very rare pleurocystidia even close to the lamellar edge and caulocystidia mostly not occurring in clusters. Another North American species, P. absconditus, differs by its pulverulent-powdery and/or white-translucent pubescent pileus, larger basidiospores, heterogeneous lamellar edge, and growth on soil.
Pluteus millsii Justo, Borovička, Grootmyers, Kalichman and S.D. Russell
sp. nov. Figure 22 and
Figure 23.
MB 849343.
Etymology: At the request of the original collector, Jan Borovička, this species is dedicated to Michael “Mike” Edward Mills, basist, pianist, and background vocalist of the band R.E.M. Mike is considered R.E.M.’s little genius, and we describe this little American Pluteus in his honor.
Diagnosis: Closely related to Pluteus necopinatus, differing in the presence of pleurocystidia, shorter cheilocystidia, occurrence in the Northern Hemisphere, and different nrITS sequences (17 evolutionary events).
Holotype: USA. Connecticut: New Haven, Yale University Campus, Morse College Garden, on very humid, decayed plant matter, 15 July 2012, leg. Jan Borovička, AJ 838 (NBM-F-009803). The holotype was found at the venue of the Annual Meeting of the Mycological Society of America.
Pileus 5–10 mm in diameter, hemispherical when young, expanding to convex or plano-convex, with a low, broad umbo; surface strongly granulose-squamulose all over, with cracks revealing the white context underneath, except at the center; with predominant brown colors (Munsell: 5YR 4/6, 3/4; 7.5YR 5/6–5/8, 4/6), sometimes gray-brown (7.5YR 6/3–6/6), darker at center; dry, not hygrophanous; margin entire, rimose, or very slightly translucently striate, Lamellae crowded, free, ventricose, up to 2 mm broad, white when young, later pink, with white flocculose edges. Stipe 10–20 × 1–2 mm, cylindrical, with a slightly broadened subbulbous base (up to 3 mm wide); surface white, silvery-white, shiny, and entirely hairy-woolly, covered with white hairs, sometimes grouped in definite mats with pale brown tips. Context in stipe and pileus white. Smell and taste none.
Basidiospores [90/4/3] (4.5–)4.9–5.8(–6.2) × (4–)4.4–5.8 μm, avl × avw = 5.2–5.6 × 4.4–5.1 μm, Q = 1.00–1.26, Q* = 1.10–1.18, globose to broadly ellipsoid. Basidia 16–24 × 6–9 μm, 4-spored, clavate. Pleurocystidia 36–47 × 17–22 μm, mostly clavate or narrowly clavate, some ovoid or ellipsoid; hyaline, thin-walled; scarce and scattered all over lamellar faces. Lamellar edge sterile. Cheilocystidia 22–39 × 10–21 μm, mostly (narrowly) clavate or (narrowly) utriform, more rarely ovoid, in some collections a few rostrate, with an apical projection up to 14 μm long; hyaline, thin-walled, crowded, forming a well-developed strip. Pileipellis a hymeniderm or trichohymeniderm, made up of two types of elements: (i) narrowly clavate, narrowly utriform, or fusiform, 30–85 × 17–25 µm; (ii) fusiform, narrowly utriform, cylindrical, 90–170 × 10–22 µm, often with 1–3 septa at the base; all elements with brown intracellular pigment, slightly thick-walled. Stipitipellis a cutis of cylindrical, slightly thick-walled, 7–10 µm wide hyphae, hyaline, or rarely some with pale brown pigment. Caulocystidia absent, but above the external layer of the stipitipellis often with loosely arranged tufts of hair-like, parallel to ascending, hyphal elements, with terminal elements 65–107 × 12–22 µm, cylindrical, narrowly clavate, narrowly utriform, mostly hyaline, a few with pale brown pigment, some with oily, slightly refractive content; thin-walled to slightly thick-walled. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: subgregarious, growing on well-decomposed wood and other plant matter. June–September.
Distribution: Eastern North America: so far, only known from the USA (Connecticut, Kentucky, and Tennessee).
Additional collections examined: USA. Kentucky: Bell Co., Clear Creek Springs, in a dry section of a creek bed, 20 September 2019, leg. Jack Steven Smith, iNaturalist 34927657. Tennessee: Knox Co., Knoxville, Knoxville Botanical Garden and Arboretum, 22 September 2018, leg. Jacob Kalichman, iNaturalist 112187791 (NBM-F-009804); ibid., Third Creek Greenway Park, on a well-rotted, decorticated hardwood log with Trechispora regularis, Thanatephorus sp., and Oliveonia pauxilla, within a stand of Phyllostachys aurea, 29 June 2021, leg. Django Grootmyers, Mushroom Observer 458003 (NBM-F-009805).
Notes: Pluteus millsii stands out macroscopically by the densely hairy-woolly surface of the stipe. Pluteus absconditus can also have a relatively hairy stipe, but this species differs in the presence of 2-spored and 1-spored basidia, slightly larger pleurocystidia (up to 66 µm long), a heterogeneous lamellar edge, and common caulocystidia.
Molecularly, P. millsii is quite distinct from all other taxa in the/podospileus clade: its nrITS sequences differ by 19 evolutionary changes from those of its closest relative, Pluteus necopinatus (96.9% sequence similarity). All other Pluteus nrITS sequences sampled in this study are less than 90% similar to P. millsii.
Pluteus necopinatus differs from
P. millsii by the non-hairy-woolly stipe surface, the absence of pleurocystidia, the larger cheilocystidia (37–74 × 12.5–30 μm) that are predominantly lageniform or utriform, and a pileipellis with shorter elongated elements (up to 125 μm long). Both taxa have similar tufts of hyphal elements above the stipitipellis, which we do not consider to be caulocystidia in the sense the term is applied in
Pluteus, as they are loosely arranged bundles of hyphae that lay on top of the tightly arranged hyphae of the stipitipellis. In other taxa of
Pluteus with true caulocystidia, these arise directly from the stipitipellis.
Pluteus necopinatus was originally described and is thus far only known from the Atlantic Forest of Brazil, growing directly out of a concrete wall [
46].
MB 849804.
Etymology: gausapatus refers to the plush pileus and stipe surface. “Gausapatus” also means “happy”, which well expresses the authors feelings about enjoying this beautiful species.
Diagnosis: Pluteus gausapatus notably differs from Pluteus podospilloides by the absence of the following features: khaki, dark brown, or olive-brown squamules and white short hairs on a pileus surface; dark brown squamules of the stipe; utriform or broadly lageniform colored pleurocystidia with a short neck; and by a distinct phylogenetic position (nrITS, TEF1-α) among the Pluteus species in the/podospileus clade.
Holotype: Republic of Korea: Taean Peninsula, Deoksung, Sudeoksa Temple, mixed forest, on decaying wood of broadleaved tree, 197 a.s.l., 8 July 2014, leg. V. Antonín (BRNM 817745).
Pileus 9–10 mm broad, convex with slightly depressed center and inflexed margin, not hygrophanous, smooth, entirely pruinose-tomentose to “plush”, hairy at the margin, indistinctly striate only up to 1 mm from the margin, nut brown (RAL 8011), slightly paler towards the margin. Lamellae free, L = 32, l = 1–3, distant, ventricose, up to 3 mm broad, whitish with a pale pinkish tinge, with an irregularly nut-brown flocculose edge. Stipe 10–12 × 1–1.5 mm, cylindrical, not bulbous, curved, entirely finely pubescent to “plush”, finely longitudinally fibrillose, white, in the lower part minutely squamulose with indistinct, pale brown floccules. Context whitish. Smell and taste indistinct.
Basidiopores [60/1/2] (4.8–)5.0–6.0(–6.5) × (4.0–)4.2–5.5 μm, avl × avw = 5.4–5.7 × 4.7–5.2 µm, Q = 1.00–1.25(–1.33), Q* = 1.13; globose to broadly ellipsoid, thick-walled, inamyloid. Basidia 19–26 × 7–10(–11) μm, clavate to subfusiform, 4-spored, very rarely 2-spored. Pleurocystidia (43–)51–62(–68) × (20–)23–27(–31) μm, scattered, mostly fusiform, thin-walled, hyaline. Lamellar edge sterile. Cheilocystidia 25–76 × 12–26 μm, variable in shape: vesiculose, ovoid, clavate to broadly clavate, subfusiform to broadly fusiform, mostly with a short neck, thin-walled, with brown intracellular pigment, rarely hyaline. Pileipellis composed of abundant elongate elements 100–170 × (10–)11–17(–20) μm, cylindrical with subacute apex or subfusiform, mixed with shorter broadly fusiform elements with obtuse or subacute apex 40–90 × 22–28 μm and short clavate to ovoid elements 20–45 × 9–26 μm; all elements thin-walled with brown intracellular pigment. Stipitipellis a cutis of whitish hyphae 4–9(–11) μm wide. Caulocystidia abundant, in tufts, 40–60(–90) × 12–18 μm, clavate to fusiform, hyaline, in the lower part also with pale brownish intracellular pigment. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: solitary, on a decayed thick branch of a broadleaved tree, July.
Distribution: Known only from Asia: Republic of Korea.
Notes: Pluteus gausapatus is characterized by its small basidiomata with a plush, brown pileus, brown lamellar edges, a whitish, finely pubescent to plush stipe, mostly fusiform pleurocystidia, and growth on decayed deciduous trees.
Pluteus podospilloides E.F. Malysheva and O.V. Morozova, originally described from Vietnam, differ by their pileus surface covered with minute khaki, dark brown, or olive-brown squamules and white short hairs; a stipe with a distinct dark brown squamule along its entire length; utriform or broadly lageniform colored pleurocystidia with a short neck; slightly thick-walled cheilocystidia; and pileipellis elements [
28].
Pluteus delicatulus C.K. Pradeep and K.B. Vrinda is somewhat similar to
P. gausapatus by its small basidiomata and pileipellis, but it differs by its lamellae without brown edges, glabrous stipe without caulocystidia, slightly broader basidiospores, and vesiculose to clavate pleurocystidia [
57].
Pluteus stigmatophorus (Berk. and Broome) Sacc. has similar pileipellis elements to
P. gausapatus,
but its pileus is larger and depressed at the center, its stipe is brownish yellow and black-dotted, its basidiospores are slightly larger, and its pleurocystidia are clavate-pedicellate to submucronate [
8,
16,
18].
Pluteus stigmatophorus is known from Sri Lanka [
17].
Pluteus extremiorientalis E.F. Malysheva and Malysheva also have a brownish-colored and tomentose-squamulose pileus, brownish to fuscous lamellar edges, and a stipe (lower part) covered by dark brown squamules (or fibrils). However, this Russian Far East species differs by having a fibrillose-squamulose pileus margin, larger basidiospores, predominantly lageniform or utriform pleurocystidia, and a pileipellis consisting of only two types of shorter elements [
58].
MB 849805.
Etymology: from the Latin adjective notabilis, “noteworthy, remarkable”.
Diagnosis: Pluteus notabilis differs from Pluteus inflatus by its pileipellis with fusoid to utriform elements with a long mucronate apex, some brownish flocculose lamellar edges, smaller basidiospores on average, and a sterile lamellar edge.
Holotype: France. Doubs. Les Fourgs, on soil with woody debris and sawdust of co-nifers (Abies and/or Picea), a heap formed to store snow piled up during the winter, according to the “snowfarming” technique, with Psathyrella scanica, Psathyrella suavissima, and Cystoderma superbum among other species, 25 September 2018, leg. G. Eyssartier, holotype PC0142588, isotype BRNM 825869.
Pileus 18–45 mm broad, convex or hemispherical when young, later plano-convex; surface completely and remarkably felted, often fairly coarsely radially wrinkled, not striate or only shortly translucently striate on overmature specimens, with fimbriate or denticulate margin, not or very weakly hygrophanous, warm date brown to almost blackish brown when young (RAL 8022), paler and with chestnut brown to reddish brown tinges on adult specimens, dark brown with distinctly paler margin on very old specimens. Lamellae moderately distant, free, ventricose, white when young, then pinkish, with an eroded-denticulate edge, with a whitish, less frequently brownish flocculose edge, especially near the pileus margin. Stipe 17–48 × 2–5 mm, cylindrical, with a slightly enlarged base, whitish to cream, with a very pale, indistinct ochre tinge on very old specimens, densely covered with distinct brown and very fine floccules, often aligned in longitudinal stripes. Context white to whitish; smell and taste indistinct; basal mycelium white. Spore print pink.
Basidiospores (4.5–)4.7–5.4(–5.8) × (3.4–)3.6–4.5(–5.0) μm, avl × avw = 5 × 4 μm, Q = 1.1–1.5, Q* = 1.26, broadly ellipsoid to ellipsoid or subglobose, rarely globose. Basidia (18–)20–28(–32) × 6–9(–11) μm, 4-spored, narrowly clavate or narrowly fusiform, hyaline. Pleurocystidia (29–)43–80(–90) × (17–)17–25(–35) μm, scattered, moderately abundant, broadly fusiform to utriform or clavate, rarely almost spheropedunculate or subovoid. Lamellar edge sterile. Cheilocystidia (18–)25–90(–105) × (10–)11–25(–30) μm, subcylindrical to narrowly clavate or clavate, less frequently subutriform, hyaline, or with brown intracellular pigment. Pileipellis a hymeniderm composed of three types of elements, all colored brown by intracellular pigment: (i) clavate to spheropedunculate elements measuring (28–)30–50 × 20–40 µm, composing a fairly regular sublayer; (ii) fusiform to (sub-)utriform elements, 50–82 × 15–27 µm, with a more or less conical or distinctly mucronate apex, mucron up to 8 μm long, some mucrons constricted; frequently with thickened wall on upper part; (iii) long subcylindrical emerging elements up to 180(–250) × 15 µm, thin-walled on exsiccata, giving a fluffy appearance to areas of the pileus where they are abundant. Stipitipellis composed of cylindrical and thin-walled hyaline hyphae (3–)5–10(–15) μm wide. Caulocystidia numerous, in tufts, (40–)45–145 × 15–30(–35) μm, thin-walled, predominantly long, subcylindrical, and reminding the long elements of the pileipellis, but also clavate or (sub-)utriform. Clamp connections absent in all studied tissues.
Habit, habitat, and phenology: on soil with woody debris and sawdust of conifers and on strongly decomposed wood near fallen trunks of Abies alba. August–September.
Distribution: Europe: France and the Czech Republic.
Additional collections examined: CZECH REPUBLIC: Beskydy Mountains, Razula Nature Reserve, Abieto-Fagetum with Picea abies, on strongly decomposed wood near a fallen trunk of Abies alba, near also P. granulatus, 15 August 2019, leg. H. Ševčíková, BRNM 825870.
Pluteus notabilis var. insignis Ševčíková, Cáfal, Ferisin and Galbusera
var. nov. Figure 28 and
Figure 29.
MB 849806.
Etymology: from the Latin adjective insignis, “remarkable, noteworthy”.
Diagnosis: Differs from Pluteus notabilis var. notabilis by having lamellae without brown edges, larger basidiospores on average, shorter pleurocystidia, and pileipellis elements without a distinct mucronate apex.
Holotype: SLOVAKIA: Chvojnická pahorkatina, Vieska-Horáreň, on decaying deciduous wood, 28 August 2016, leg. R. Cáfal (BRNM 825868).
Pileus 20–35 mm broad, convex to campanulate when young, later plano-convex to almost plane, with obtuse center without or with low umbo, dull; surface finely granulose to granulose-velvety, some finely tomentose in the center, not or indistinctly wrinkled or sparsely veined radially up to the edge, without striation, with slightly inflexed margin, not hygrophanous, warm date brown, reddish brown, dark brown to almost warm blackish brown when young (RAL 8017 or darker, RAL 8022), then slightly paler. Lamellae L = 36–48, l = 1–5, free, crowded to moderately distant to almost distant, ventricose, white when young, then pinkish to flesh pink, with an eroded-denticulate, concolorous, finely flocculose edge. Stipe 38 × 1.5–2.5 mm, cylindrical, slightly broadened toward the lower part, with a slightly enlarged base, solid, innately fibrillose, twisted or not, hyaline, whitish, or cream, graying with age, entirely minutely to densely covered with distinct brown fine floccules, sometimes aligned in short longitudinal stripes, at least on the lower part. Context whitish in pileus, whitish or hyaline in stipe. Smell and taste indistinct. Spore print pinkish.
Basidiospores [93/3/3] (4.0–)4.5–6.6(–7.0) × 3.5–5.6(–6.5) μm, avl × avw = 5.65 × 4.4 μm, Q = 1.0–1.5, Q* = 1.21–1.32, subglobose to broadly ellipsoid, rarely globose or ellipsoid, thick-walled. Basidia (17–)18–29 × 6–10 μm, 4-spored, narrowly clavate to narrowly fusiform, hyaline, thin-walled. Pleurocystidia 26–60(–62) × 14–30(–32) μm, scattered, rare, broadly clavate to clavate, subovoid to ovale-oblong or narrowly to broadly fusiform to utriform or subutriform, often pedunculate, hyaline, thin-walled. Lamellar edge sterile. Cheilocystidia (17–)20–82(–93) × (10–)11–30(–34) μm, numerous, subcylindrical to narrowly clavate to broadly clavate or vesiculose, subfusiform or fusiform with short neck and obtuse apex, hyaline, thin-walled. Pileipellis a hymeniderm composed of short and elongate elements: (i) (28–)30–55(–82) × 20–40(–45) µm elements broadly clavate to clavate or spheropedunculate, rarely subfusiform to fusiform, some submucronate; (ii) narrowly (sub)fusiform or narrowly cylindrical elements 90–175 × (16–)18–23(–26) µm, some with long neck up to 35 µm; all elements with brown intracellular pigment, with thin or slightly thickened wall. Stipitipellis a cutis composed of cylindrical hyphae (2.5–)5–10(–14) μm wide, hyaline, thin-walled, or with slightly thickened hyphae. Caulocystidia (54–)75–170 × 6.5–15(–28) µm, numerous, in tufts, mostly narrowly to broadly fusiform, more rarely clavate, cylindrical or subcylindrical, with brown intracellular pigment, thin-walled. Clamp connections absent in all examined tissues.
Habit, habitat, and phenology: on soil in riparian forests and on decaying deciduous wood, including Populus tremula. May–August.
Distribution: Europe: Italy, Slovakia, and Spain.
Additional collections examined: ITALY: Matelica, Marche, on stump of Populus tremula, 28 October 2022, leg. Sofie and A. Galbusera (AG 22210280). SPAIN: Gavarres mountains, Fitor (city), in riparian forest with Corylus avellana, Alnus glutinosa, and Quercus ilex, on siliceous soil, about 400 m a.s.l., 18 May 2006, leg. C. Roqué (originally det. as P. podospileus, BRNM825867).
Notes: fusiform to utriform pileipellis elements with a mucronate apex up to 8 μm are confirmed only for Pluteus notabilis var. notabilis and for Pluteus seticeps. The later pleurocystidia are absent or extremely rare, and this species occurs in North America. Pluteus notabilis var. insignis differs from Pluteus notabilis var. notabilis by its concolorous lamellar edges, somewhat larger basidiospores on average, pleurocystidia only up to 58 µm long, and pileipellis without elements displaying a distinct, long mucronate apex. Moreover, Pluteus notabilis var. notabilis has a distinctly radially wrinkled pileus, while some basidiomata lack this feature and some are less distinctly wrinkled. However, the variability of this feature needs to be confirmed. Phylogenetically, based on nrITS and TEF1-α, both taxa are similar.
Macroscopically,
Pluteus notabilis somewhat resembled the original description of
Pluteus granulatus Bres. by its granulose brownish pileus and its growth on
Abies alba [
59]. However, our holotype study confirms that this species belongs to section
Hispidoderma. The similar
Pluteus stigmatophorus (Berk. and Broome) Sacc. also has fusoid to utriform pileipellis elements, sometimes resembling the pileocystidia of
Pluteus notabilis var.
notabilis, but they are never mucronate [
18]. Moreover,
Pluteus stigmatophorus has broader, subglobose to globose basidiospores; cheilo- and pleurocystidia with a dark pigment; a pileus depressed at the center; a black-dotted stipe on a brownish yellow ground [
8,
17,
18], and it is known from tropical Sri Lanka [
17].
Pluteus anomocystidiatus Menolli and de Meijer [
60], known from Brazil, has short (29–33 × 16.2–20 µm) clavate or spheropedunculate pleurocystidia, long (50–116 × 12.5–25 µm) narrowly fusiform or filiform cheilocystidia with distinct brownish pigment macroscopically visible as a distinct dark brown lamellar edge, and a different pileipellis.
Excluded, insufficiently known and doubtful taxa
Pluteus praestabilis (Britzelm.) Sacc.
Sylloge Fungorum 5: 672, 1883.
Basionym: Agaricus praestabilis Britzelm., Berichte des Naturhistorischen Vereins Augsburg 27: 193, 1883.
This species was originally described as having a blackish brown velvety pruinose pileus 45 mm in diameter, a white stipe measuring 45 × 5 mm with brown floccules, basidiospores measuring 6 × 4–5 µm and as growing on soil near
Fagus sylvatica [
8,
61]. Stangl and Bresinsky [
62] considered
Pluteus praestabilis a synomym of
P. plautus. Although this taxon may belong to the
Pluteus plautus complex, it may also represent a species related to the
P. podospileus complex [
53]. All the characteristics mentioned resemble several species belonging to the
Pluteus podospileus group. Unfortunately, the protologue is vague, the holotype does not exist, and, therefore, its identity cannot be satisfactorily clarified.