Effect of Experiment Warming on Soil Fungi Community of Medicago sativa, Elymus nutans and Hordeum vulgare in Tibet
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experiment Design
2.2. Soil Sampling and Analysis
2.3. Statistical Analyses
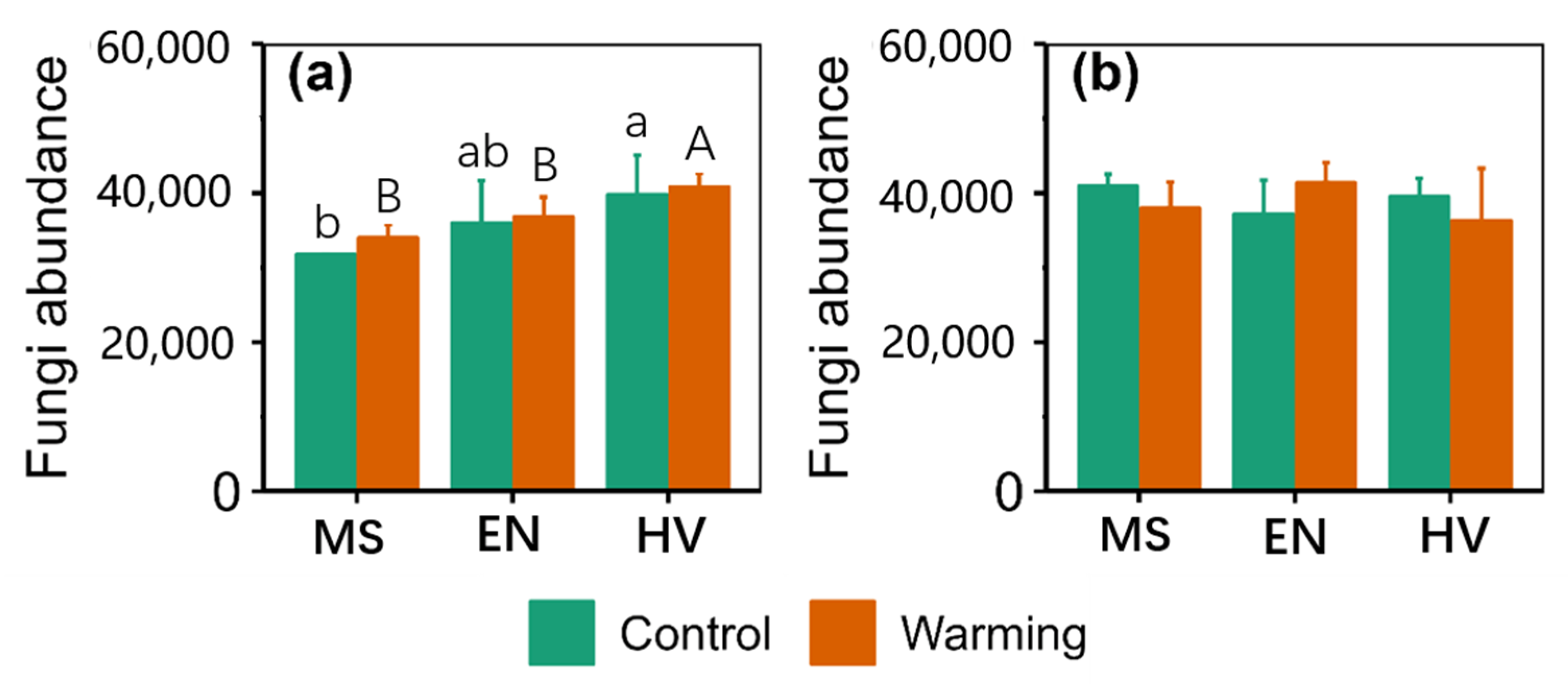
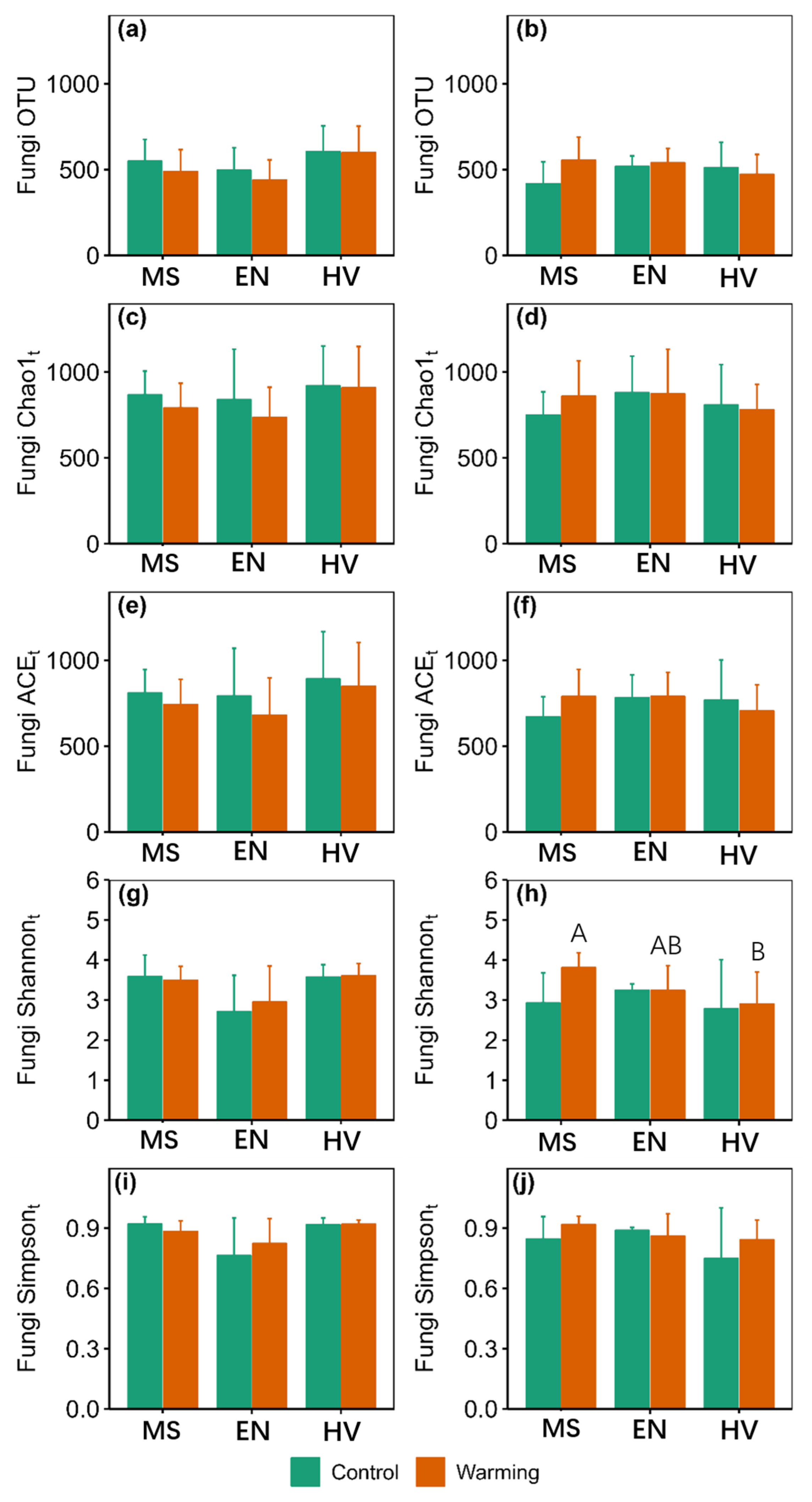
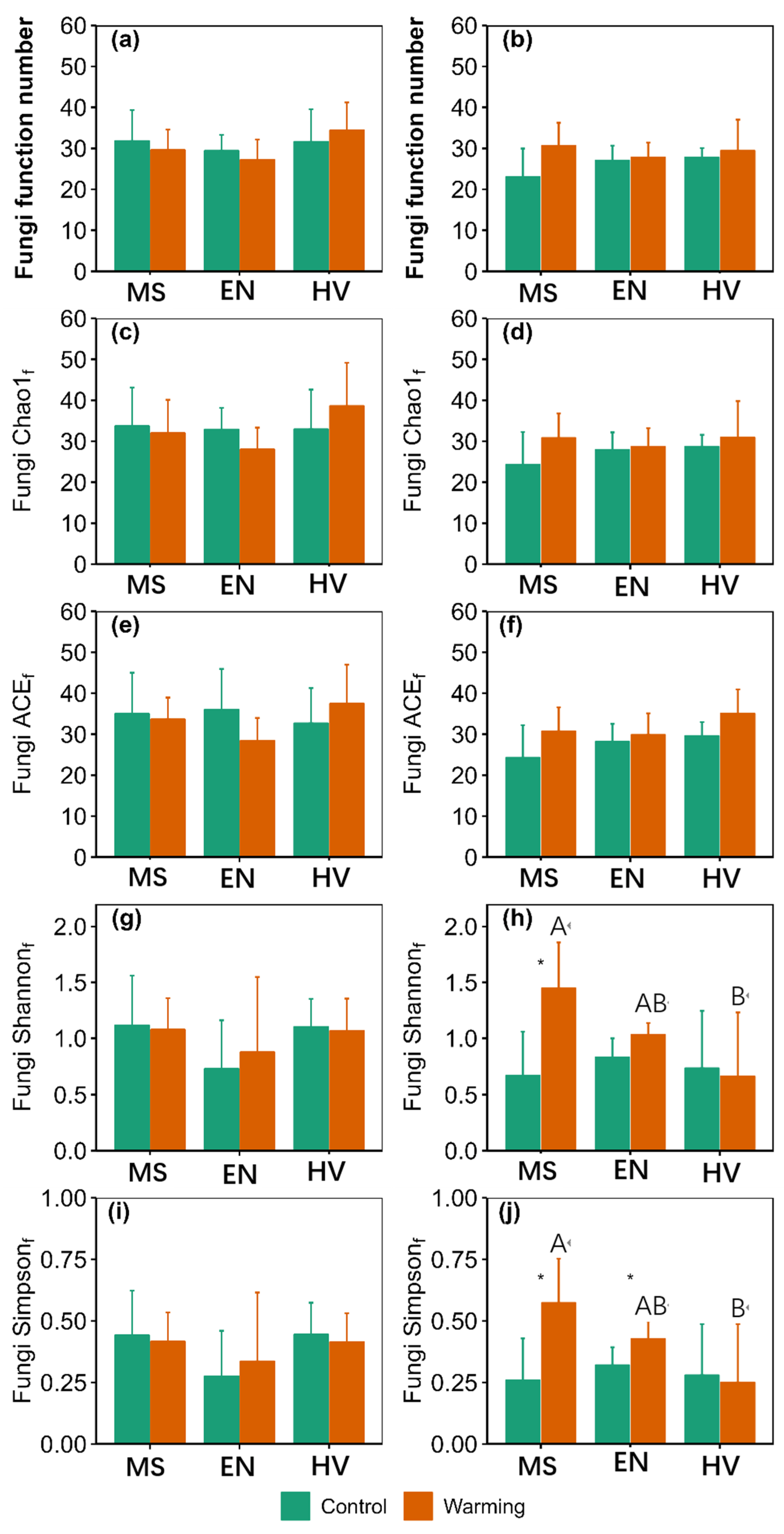
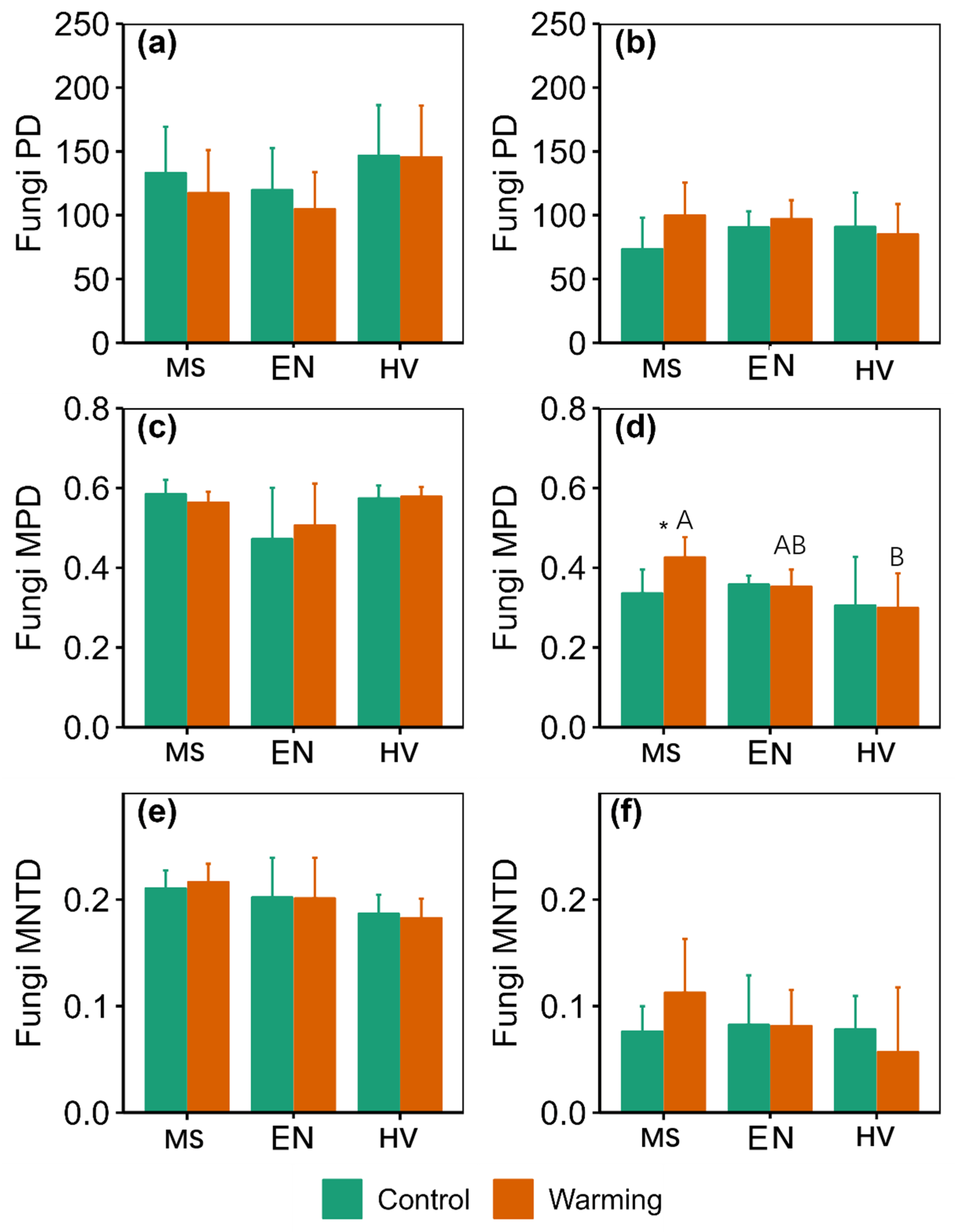
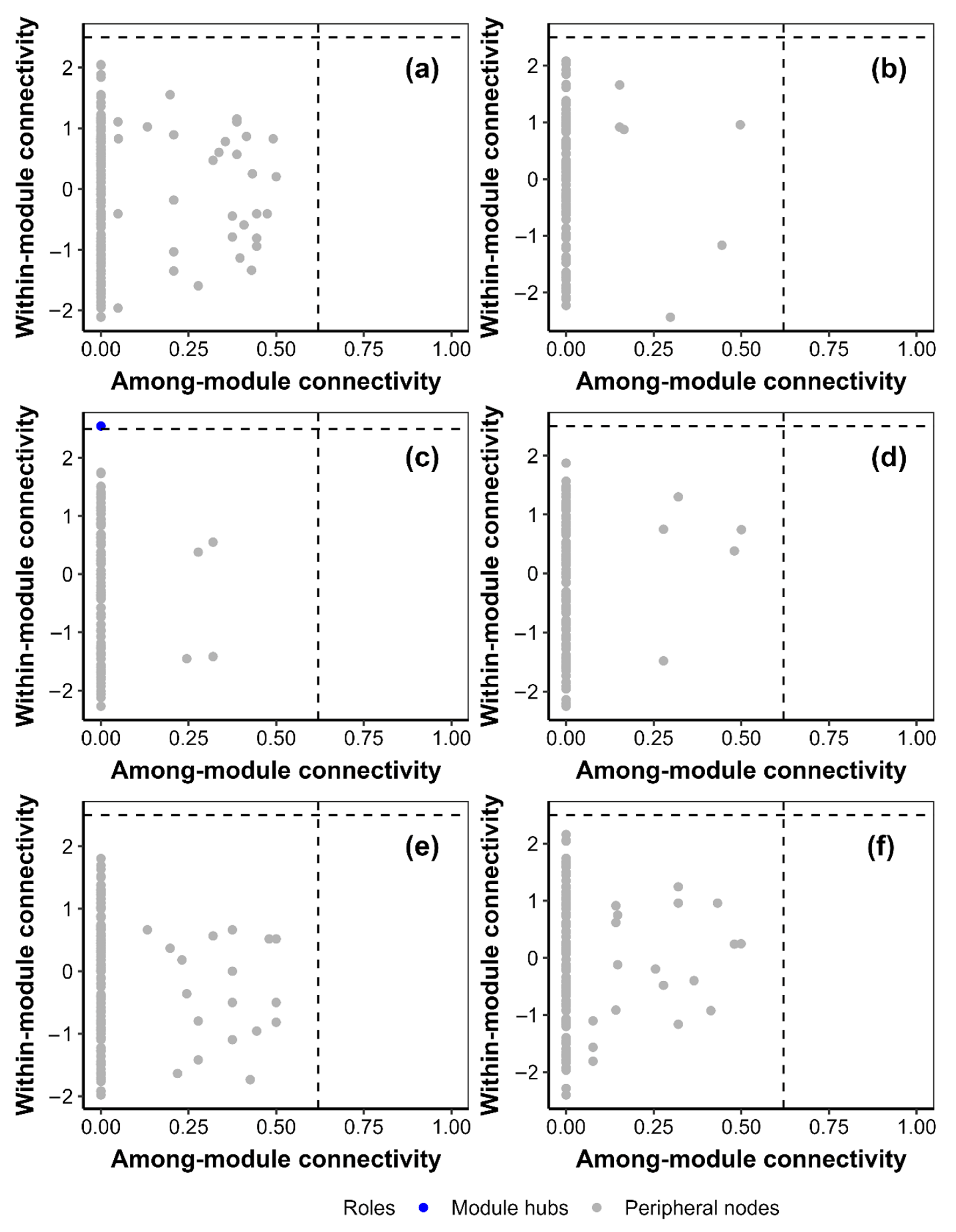
3. Results
3.1. Total Abundance
3.2. α-Diversity
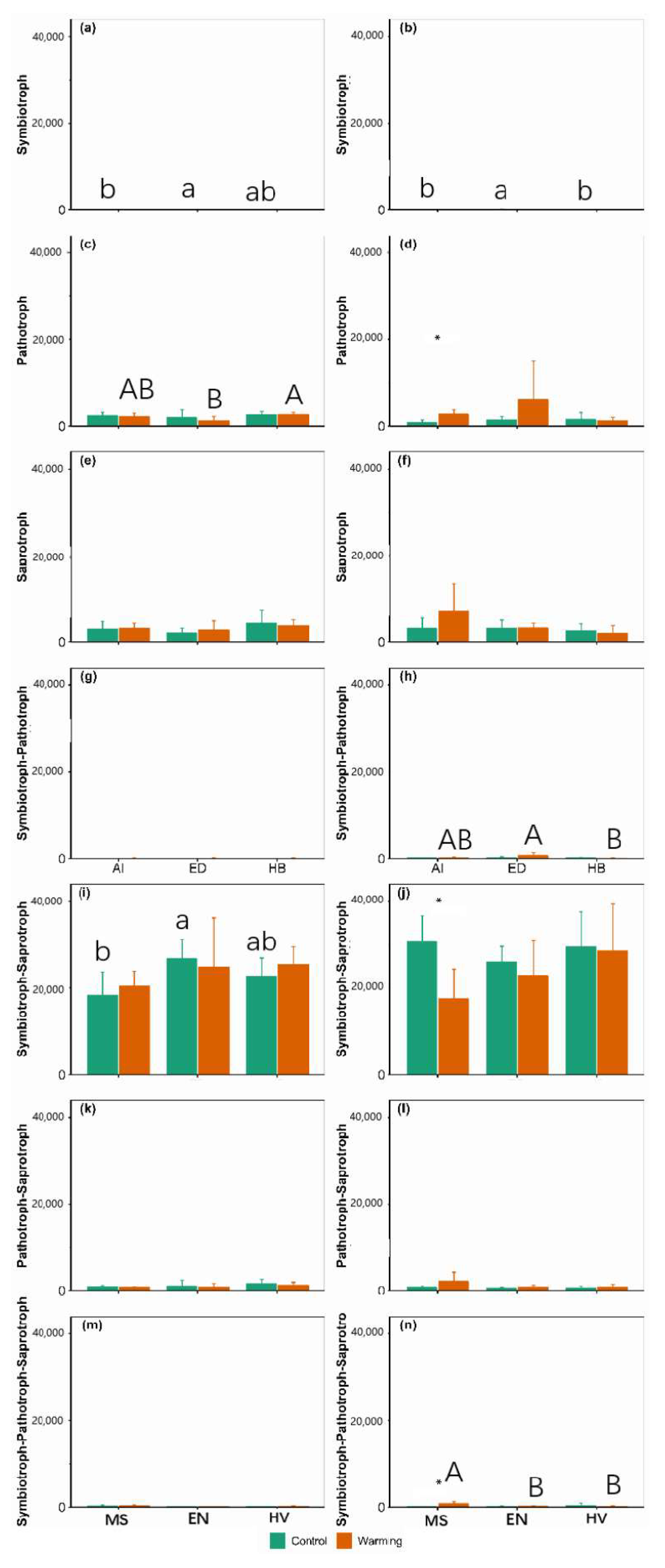
3.3. Community Composition
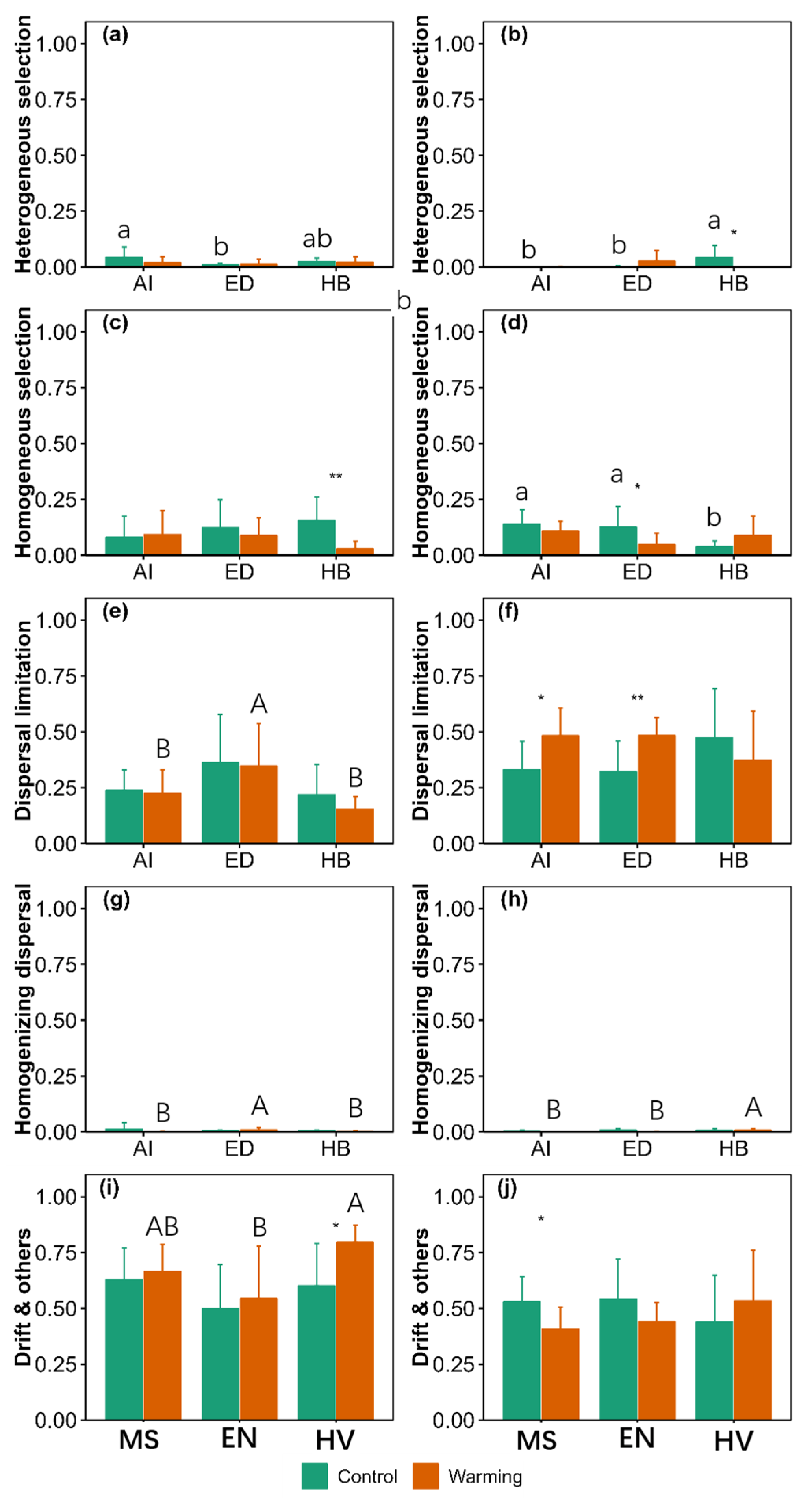
3.4. Community Assembly
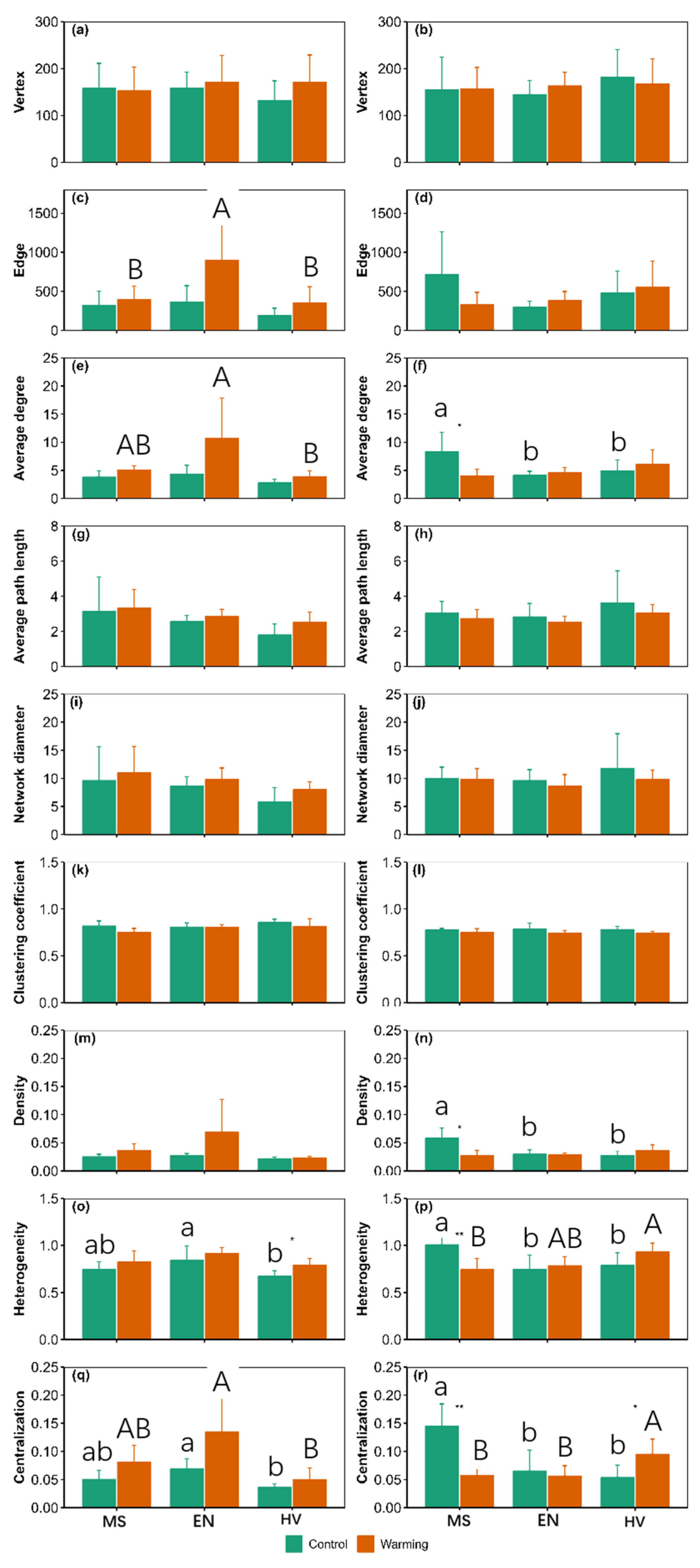
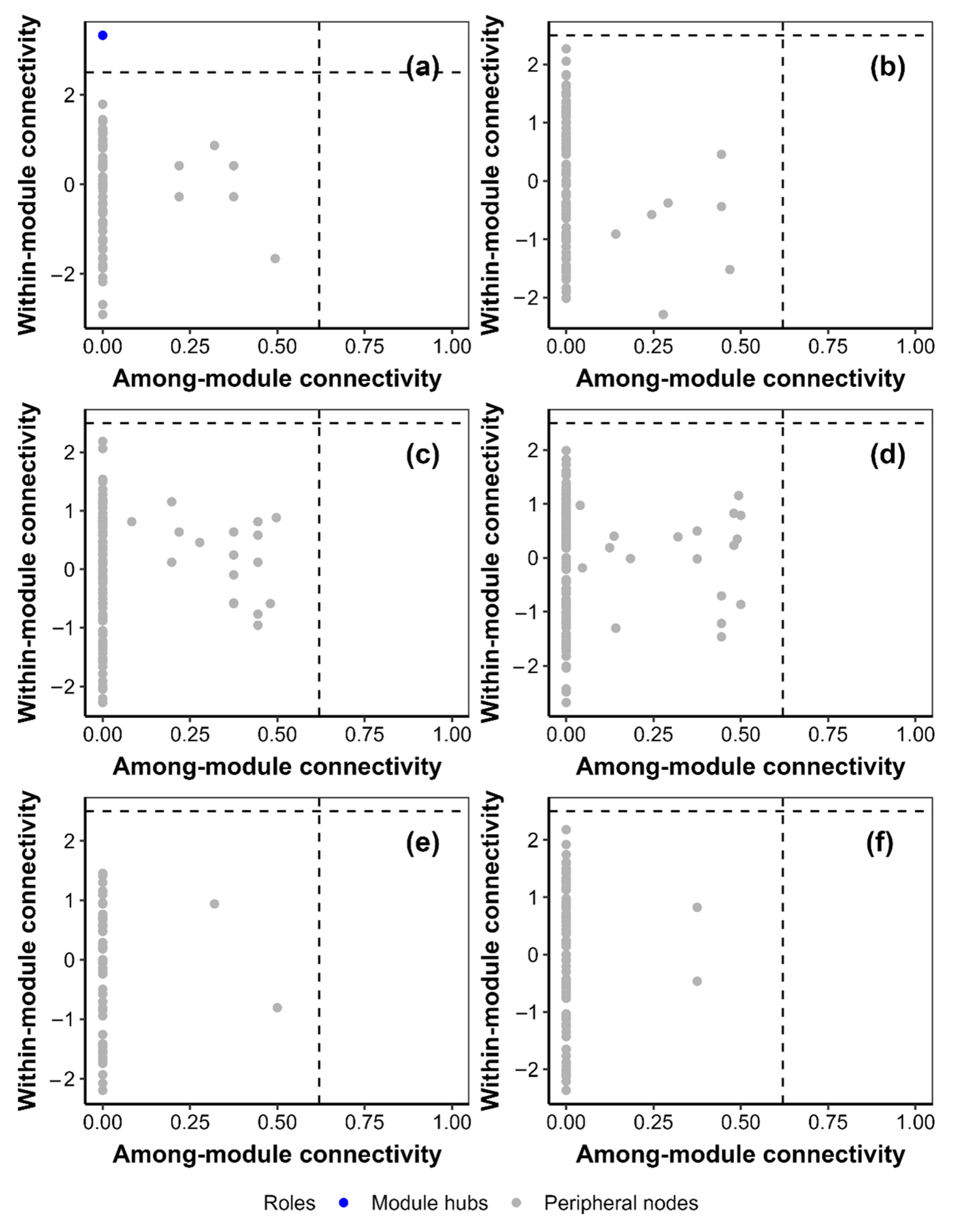
3.5. Cooccurrence Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Wang, M.Y.; Khan, N.; Tan, L.L.; Yang, S. Potentials, Utilization, and Bioengineering of Plant Growth-Promoting Methylobacterium for Sustainable Agriculture. Sustainability 2021, 13, 3941. [Google Scholar] [CrossRef]
- Roell, M.S.; Zurbriggen, M.D. The impact of synthetic biology for future agriculture and nutrition. Curr. Opin. Biotechnol. 2020, 61, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Fu, G. Impacts of anthropogenic activities and climate change on forage nutrition storage in Tibetan grasslands. Plants 2023, 12, 2735. [Google Scholar] [CrossRef]
- Zhong, Z.; Fu, G. Response of soil fungal species, phylogenetic and functional diversity to diurnal asymmetric warming in an alpine agricultural ecosystem. Agric. Ecosyst. Environ. 2022, 335, 107993. [Google Scholar] [CrossRef]
- Zhu, Y.; Peng, J.; Wei, Z.; Shen, Q.; Zhang, F. Linking the soil microbiome to soil health. Sci. Sin. Vitae 2021, 51, 1–11. [Google Scholar]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Zhong, Z.M.; Shen, Z.X.; Fu, G. Response of soil respiration to experimental warming in a highland barley of the Tibet. SpringerPlus 2016, 5, 137. [Google Scholar] [CrossRef][Green Version]
- Zheng, C.; Chen, C.; Zhang, X.; Song, Z.; Deng, A.; Zhang, B.; Wang, L.; Mao, N.; Zhang, W. Actual impacts of global warming on winter wheat yield in Eastern Himalayas. Int. J. Plant Prod. 2016, 10, 159–174. [Google Scholar]
- Wang, F.; Tang, J.; Li, Z.; Xiang, J.; Wang, L.; Tian, L.; Jiang, L.; Luo, Y.; Hou, E.; Shao, X. Warming reduces the production of a major annual forage crop on the Tibetan Plateau. Sci. Total Environ. 2021, 798, 149211. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Ning, D.L. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, 10–1128. [Google Scholar] [CrossRef]
- Hu, J.J. Effects of Warming, Altered Precipitation and Fertilization on Phyllosphere Bacterial Community Structure in an Alpine Meadow; Lanzhou University: Lanzhou, China, 2022. [Google Scholar]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V. Assessing the role of interseeding alfalfa into grass on improving pasture soil health in semi-arid Texas High Plains. Appl. Soil Ecol. 2020, 147, 103399. [Google Scholar] [CrossRef]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant-plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gomez-Cadenas, A.; Perez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Guimera, R.; Amaral, L.A.N. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling Interactions in the Microbiome: A Network Perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Z.; Guo, J.; Tong, L.; Ren, L.; Han, B.; Wu, Q. Principle and application of co-occurrence networks for freshwater ecosystem assessment. Hupo Kexue 2022, 34, 1765–1787. [Google Scholar]
- Bascompte, J. Networks in ecology. Basic Appl. Ecol. 2007, 8, 485–490. [Google Scholar] [CrossRef]
- Barberan, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Gao, K.; Mao, Z.B.; Meng, E.X.; Li, J.; Liu, X.Y.; Zhang, Y.Y.; Zhang, L.; Wang, G.L.; Liu, Y. Effects of elevated CO2 and warming on the root-associated microbiota in an agricultural ecosystem. Environ. Microbiol. 2022, 24, 6252–6266. [Google Scholar] [CrossRef]
- Corneo, P.E.; Pellegrini, A.; Cappellin, L.; Gessler, C.; Pertot, I. Moderate warming in microcosm experiment does not affect microbial communities in temperate vineyard soils. Microb. Ecol. 2014, 67, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Wang, Y.Q.; Cheng, H.; Chang, S.X.; Liang, C.; An, S.S. Negative effects of multiple global change factors on soil microbial diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Jing, Y.M.; Xiang, Y.Z.; Zhang, R.D.; Lu, H.B. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Lienhard, P.; Terrat, S.; Mathieu, O.; Leveque, J.; Prevost-Boure, N.C.; Nowak, V.; Regnier, T.; Faivre, C.; Sayphoummie, S.; Panyasiri, K.; et al. Soil microbial diversity and C turnover modified by tillage and cropping in Laos tropical grassland. Environ. Chem. Lett. 2013, 11, 391–398. [Google Scholar] [CrossRef]
- Cai, L.J.; Guo, Z.H.; Zhang, J.T.; Gai, Z.J.; Liu, J.Q.; Meng, Q.Y.; Liu, X.H. No tillage and residue mulching method on bacterial community diversity regulation in a black soil region of Northeastern China. PLoS ONE 2021, 16, e0256970. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Sanchez-Ramirez, S.; Koljalg, U.; Bahram, M.; Doring, M.; Schigel, D.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Stegen, J.C.; Kim, M.; Dong, K.; Adams, J.M.; Lee, Y.K. Soil pH mediates the balance between stochastic and deterministic assembly of bacteria. ISME J. 2018, 12, 1072–1083. [Google Scholar] [CrossRef]
- Maguire, V.G.; Bordenave, C.D.; Nieva, A.S.; Llames, M.E.; Colavolpe, M.B.; Garriz, A.; Ruiz, O.A. Soil bacterial and fungal community structure of a rice monoculture and rice-pasture rotation systems. Appl. Soil Ecol. 2020, 151, 103535. [Google Scholar] [CrossRef]
- Tovar, C.; Melcher, I.; Kusumoto, B.; Cuesta, F.; Cleef, A.M.; Meneses, R.I.; Halloy, S.; Llambi, L.D.; Beck, S.; Muriel, P.; et al. Plant dispersal strategies of high tropical alpine communities across the Andes. J. Ecol. 2020, 108, 1910–1922. [Google Scholar] [CrossRef]
- Yin, H.J.; Li, Y.F.; Xiao, J.; Xu, Z.F.; Cheng, X.Y.; Liu, Q. Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Glob. Chang. Biol. 2013, 19, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Zhang, G.; Fu, G. Effect of Experiment Warming on Soil Fungi Community of Medicago sativa, Elymus nutans and Hordeum vulgare in Tibet. J. Fungi 2023, 9, 885. https://doi.org/10.3390/jof9090885
Zhong Z, Zhang G, Fu G. Effect of Experiment Warming on Soil Fungi Community of Medicago sativa, Elymus nutans and Hordeum vulgare in Tibet. Journal of Fungi. 2023; 9(9):885. https://doi.org/10.3390/jof9090885
Chicago/Turabian StyleZhong, Zhiming, Guangyu Zhang, and Gang Fu. 2023. "Effect of Experiment Warming on Soil Fungi Community of Medicago sativa, Elymus nutans and Hordeum vulgare in Tibet" Journal of Fungi 9, no. 9: 885. https://doi.org/10.3390/jof9090885
APA StyleZhong, Z., Zhang, G., & Fu, G. (2023). Effect of Experiment Warming on Soil Fungi Community of Medicago sativa, Elymus nutans and Hordeum vulgare in Tibet. Journal of Fungi, 9(9), 885. https://doi.org/10.3390/jof9090885





