1. Introduction
Candida spp. infections have progressively increased over the past decades and become a major cause of morbidity and mortality in critical care patients. This phenomenon is mainly associated with the increasing rate of invasive procedures, the extensive use of broad-spectrum antimicrobials, the higher rate of immunocompromised patients admitted to Intensive Care Units (ICUs), and the length of ICU stay [
1]. Although
C. albicans accounts for the majority of
Candida infections, several non-
albicans Candida species have been associated with an increasing incidence of invasive infection with high rates of therapeutic failure [
2].
In 2022, the WHO launched the first-ever list of priority pathogens (WHO FPPL) as the first global effort to systematically prioritize fungal pathogens, considering their unmet research and development (R&D) needs and perceived public health importance. Of the 19 fungi that represent the greatest threat to public health,
Aspergillus fumigatus,
Candida albicans,
Cryptococcus neoformans, and
Candida auris were considered as of critical priority [
3].
C. auris was first reported in Japan in 2009, and since then, it has emerged worldwide, being associated with nosocomial outbreaks in intensive care settings. In Europe, according to the European Centre for Disease Prevention and Control (ECDC),
C. auris has been identified in more than a dozen countries. The last outbreak was reported in 2019–2021 in northern Italy with at least 277 cases, some of them in patients with severe COVID-19 infections [
1,
4]. Between 2013 and 2021, 1812 cases of
C. auris infections or carriage were reported by 15 EU/EEA countries, and the number of reported cases almost doubled from 2020 (335 cases) to 2021 (655 cases) [
5]. In the USA, the number of
C. auris cases increased by 44% in 2019 and by 95% in 2021 and the number of cases resistant to echinocandins in 2021 was about three times higher than in each of the two previous years [
6].
Public health concerns regarding this infection occur because it combines critical characteristics, such as the potential to spread through horizontal transmission, the ability to cause life-threatening infections, the multi-resistance profile, and the limitations for optimal treatment [
7,
8]. The crude in-hospital mortality rate for
C. auris candidemia is estimated to range from 30 to 72%, even in patients receiving antifungal treatment [
1]. Higher rates are associated with patients admitted to ICU and with candidemia, although these may be mainly attributable to the severity of the underlying diseases [
4,
7].
Given the clinical importance and global spread of this species, we report and characterize the first case of C. auris identified in Portugal, including a brief phylogenetic study of this isolate.
2. Case Presentation
A 59-year-old male from Angola was admitted to a hospital in Luanda, and 10 days prior to admission, he had a positive SARS-CoV-2 antigen test with mild symptoms. He presented rapid progressive cough, shortness of breath, and fatigue, and was then transferred to the hospital Intensive Care Unit (ICU) with respiratory failure and cardiogenic shock. After a short period of invasive ventilatory and vasopressor support, he developed multiorgan failure with acute kidney injury and presumed acute liver failure (ALF), as he had hyperbilirubinemia, cytolysis, and coagulopathy.
Considering the possible eligibility for liver transplantation, the patient was transferred to Lisbon, Portugal, and admitted directly to the ICU in a hospital, which is a reference center in transplantation. At admission, he was under sedation, with invasive mechanical ventilation and vasopressor support with noradrenaline. Continuous renal replacement therapy (CRRT) was prescribed, coagulopathy was corrected with blood products transfusions, and he initiated Ceftriaxone as empirical treatment for presumed infection with an elevation of inflammatory markers. He was immediately submitted for trans-jugular liver biopsy in order to investigate the etiology of ALF. Hemodynamic evaluation with echocardiogram showed reduced ejection fraction (20%), with an estimated cardiac index of 2.8 L/m2/min. The clinical presentation did not fulfill the criteria for liver transplantation. In fact, the investigation on infectious and autoimmune causes for acute hepatitis was negative, and the biopsy revealed necrotic liver tissue, leading the physicians to assume the diagnosis of ischemic hepatitis due to cardiogenic shock. The investigation went on, and etiologies for myocardiopathy as a cause of cardiogenic shock were searched. The coronary angiogram was normal, and all potentially infectious agents were excluded (the diagnostic tests included various real-time PCR for SARS-CoV-2). There was a consensus on the diagnosis of viral myocardiopathy despite the fact the proper virus could not be identified.
During his stay in the ICU, the patient evolved in sudden primary severe acute respiratory distress syndrome (ARDS) due to ventilator-associated pneumonia with a need for extracorporeal membrane oxygenation (ECMO) support. Multidrug-resistant (MDR) Acinetobacter baumanii was isolated in bronchoalveolar lavage (BAL) and blood cultures, and he was treated with colistin, according to the antimicrobial susceptibility test (AST). The patient had support with veno-venous ECMO for four days. After decannulation, there was a clinical reaggravation with septic shock. Before these clinical findings, after 4 days of treatment with colistin for pneumoniae and bacteriemia with A. baumannii, another BAL was performed, in which, in addition to A. baumannii, C. auris was also isolated.
Despite the continued treatment with colistin for 16 days and also with caspofungin for 10 days, there was progressive clinical deterioration with ARDS, refractory shock, and multiorgan failure. Even with all organ support and treatments, the patient died one month after initial admission in the ICU in Luanda with no other plausible reason to justify the multiorgan failure except the lack of response of both etiological agents to the treatment.
3. Materials and Methods
The isolate CaurisPT_1_2022 was identified as C. auris by MALDI-TOF_MS at the hospital laboratory and was then sent to the Mycology Reference Laboratory at the Portuguese National Institute of Health (INSA) for further tests, including molecular identification for species confirmation, determination of the antifungal susceptibility profile, and genomic analysis.
Colonies were isolated in Sabouraud dextrose agar supplemented with cloranphenicol (0.05%). Total genomic DNA was extracted from the purified colonies. For species confirmation, the D1–D2 region of 28S ribosomal DNA (rDNA) was amplified using the primer set NL1 and NL4. Sanger sequencing of both strands was performed and nucleotide sequences were edited using the program Chromas Lite v 2.01 and aligned with the program CLUSTALX v 2.1 [
9]. A BLASTn was performed in GenBank (Bethesda, MD, USA) and CBS-KNAW Fungal Biodiversity Centre databases (Utrecht, the Netherlands) for highly similar sequences (megablast) and to all CBS databases, respectively.
Antifungal susceptibility was performed by microdilution using the MICRONAUT-AM AFST system (MERLIN Diagnostika GmbH, Bornheim, Germany), according to manufacturer’s instructions, and minimal inhibitory concentrations (MICs) were determined according to EUCAST procedures. The antifungals included in this test were anidulafungin (0.002 mg/L, 0.015–8 mg/L), micafungin (0.002 mg/L, 0.015–8 mg/L), caspofungin (0.002 mg/L, 0.015–8 mg/L), fluconazole (0.002 mg/L, 0.25–128 mg/L), posaconazole (0.0078–8 mg/L), voriconazole (0.0078–8 mg/L), itraconazole (0.031–4 mg/L), amphotericin B (0.031–16 mg/L), and 5-flucytosine (0.0625–32 mg/L).
For whole-genome analysis, total DNA was subjected to Nextera XT library preparation and paired-end sequenced on an Illumina NextSeq 2000 (2× 150 bp), according to manufacturer’s instructions. Sequencing reads were inspected with FastQC v0.11.9 [
10] and filtered with Trimmomatic v0.39 [
11]. A K-mer Analysis Toolkit (KAT) was used to count the
k-mer frequency and GC content and to estimate the expected genome size [
12], using default parameters. SPAdes v3.13.0 was used to perform the genome assembly [
13]. The quality of the assembly was inspected with Quast v5.2.0 [
14]. As contamination with
Cellulosimicrobium cellulans was detected, KAT was used to obtain the
k-mer hashes that corresponded to the possible contaminant and to remove their contigs in the assembly. A final step of removal of redundant contigs and scaffolding was performed with Redundans [
15].
In order to place the
C. auris isolate among the previously defined genetic clades of this species, a comparative genomics analysis including other 24
C. auris samples was performed. These samples were carefully selected from a recent population genomics study of this pathogen to ensure the representativeness of clades I–IV of multiple continents (including all available European isolates) and of different antifungal-resistance profiles (
Table S1) [
16]. Whole-genome sequencing (WGS) data were retrieved from the Sequence Read Archive (SRA). Read mapping and variant calling were performed with the HaploTypo pipeline v1.0.1, using default parameters [
17]. Briefly, BWA-MEM 0.7.17-r1188 was used to align the sequencing reads in the reference genome of
C. auris (BP8441; accession: GCA_002759435.2) [
18]. GATK 4.2.6.1 was used to perform variant calling considering a minimum depth of coverage of 20 reads and a haploid genome [
19]. The genomic sequence of each sample was then obtained with GATK FastaAlternateReferenceMaker tool. Positions with INDELs or covered by less than 20 reads in at least one sample were excluded. SNP-sites was used to obtain the variant sites [
20]. Maximum-likelihood tree reconstruction of the alignment comprising 193,870 variant sites was performed with RAxML v8 [
21]. Following the same procedure, after the association of the Portuguese
C. auris isolate to clade III, we retrieved all isolates of this clade used in the above-mentioned population genomics study from SRA [
16] and reconstructed an additional maximum-likelihood tree considering only these isolates (and the Portuguese sample) and their 3278 variant sites.
4. Results
4.1. Molecular Identification and Antifungal Susceptibility
The obtained sequences from the D1–D2 region were compared with sequences deposited in the GenBank and CBS-KNAW Fungal Biodiversity Centre databases. In GenBank, the similarity and coverage were 99.62% and 100%, respectively, with the sequence accession number CP043535; in the CBS-KNAW Fungal Biodiversity Centre database, the similarity and coverage were 99.80% and 97.70%, respectively, with the sequence accession number CBS 15615_ex94611_LSU.
The susceptibility profile of the isolate, CaurisPT_1_2022, is reported in
Table 1.
4.2. Whole-Genome Analysis
The genomic characterization of CaurisPT_1_2022 was performed with a comparative genomics approach including other publicly available samples (
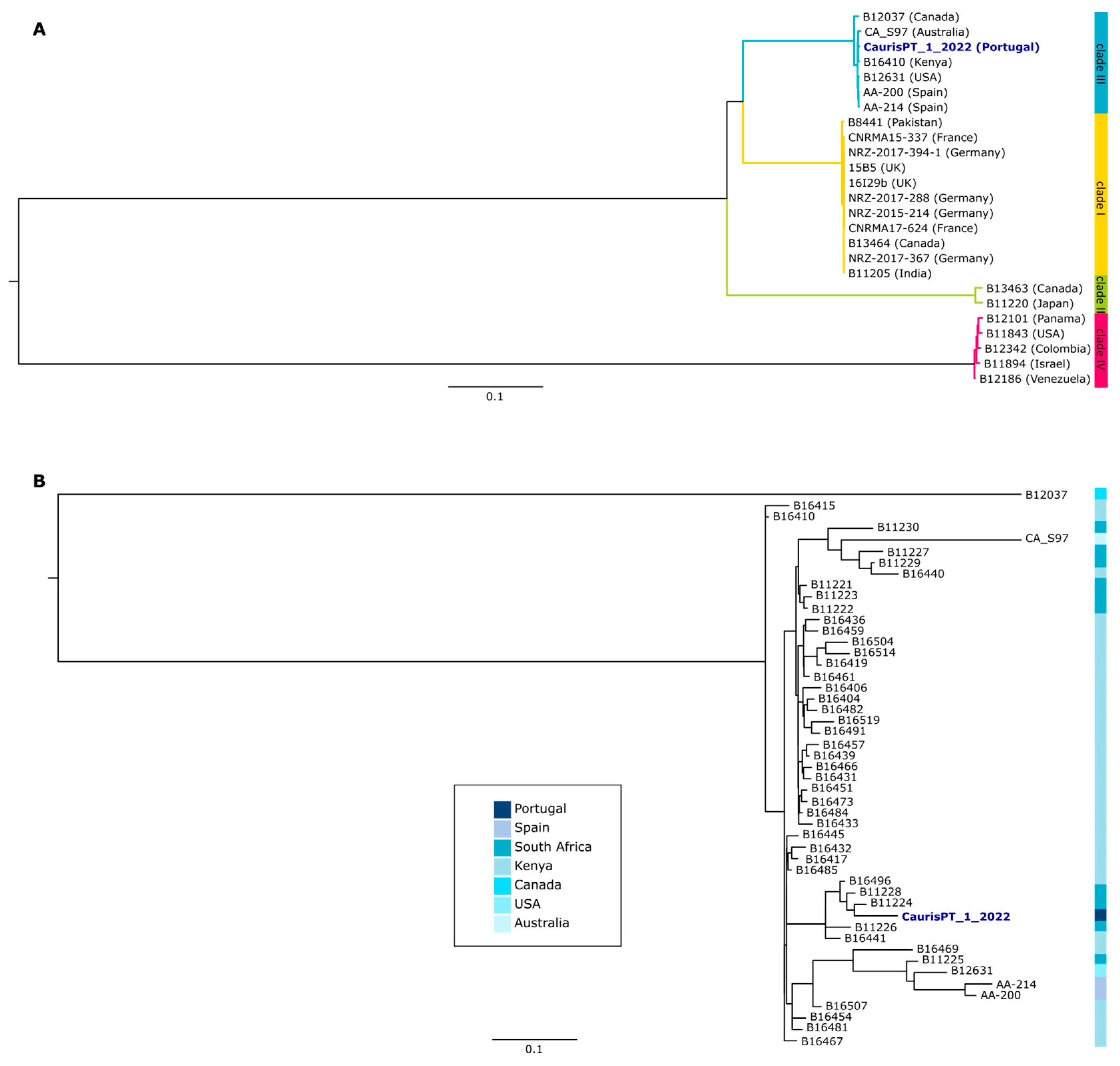
Table S1 and Methods for more details). Our results showed that this isolate belongs to clade III, which has previously been associated with Africa, but also enrolls strains from different continents, including Europe (
Figure 1A) [
16,
23]. Indeed, the three strains sequenced so far in Spain also belong to the same clade. Nevertheless, the Portuguese isolate seems to be more closely related to isolates from Africa than to the European ones (
Figure 1B). Furthermore, we assessed the presence of non-synonymous mutations of the potential association with antifungal resistance and detected the presence of C125A and F126L mutations in
ERG11, both of them potentially associated with azole resistance (
Table S2) [
24]. These results are in agreement with the phenotypic susceptibility tests.
5. Discussion
Due to climatic changes and temperature increases, new pathogenic fungi that cause invasive fungal diseases are emerging, and the yeast
C. auris, although only described in 2009, is now boasting a near-global distribution [
25].
Candida auris is an emerging fungus that presents a serious global health threat; it is often multidrug-resistant and frequently causes impacting outbreaks in healthcare settings. For this reason, it is important to quickly identify
C. auris in a hospitalized patient so that healthcare facilities can take special precautions to stop its spread [
22].
The identification of the
C. auris reported in the present study is consonant with other reported cases in the literature [
26]. In fact, it happened in a critically ill patient, who presented multiple organ failure and had a prolonged length of stay in ICU. This patient underwent a large range of invasive techniques and multiorgan support, such as invasive mechanical ventilation, CRRT, ECMO, and transfusions, among others. Each of these procedures was likely to have contributed to a worse impact on the susceptibility to infection and outcome in terms of mortality. In the present case, the detection of
C. auris in BAL happened on day 4 of treatment with colistin for
A. baumannii according to AST, and it coincided with a clinical reaggravation after ECMO decannulation, with septic shock. Despite bacteriemia and persistent pneumoniae due to
A. baumanni, the
C. auris isolate could justify the clinical worsening. Although the plausible hypothesis of colonization with
C. auris in a critically ill patient transferred from Angola, with risk factors for fungal infection, this isolate was considered to be an etiological agent of infection by the clinicians. The therapeutical decision of giving caspofungin, though, did not change the final outcome. The literature supports the high mortality rate associated with
C. auris infection [
1], but in this case, it remains unclear the role of
C. auris in clinical aggravation with multiorgan failure and death. In fact, there was a co-infection with
C. auris and
A. baumannii, with both microorganisms associated with high mortality [
1,
8,
27], which cannot be neglected, but what is unclear is its influence on the patient’s outcome, as there were 16 days of proper treatment with colistin according to AST and 10 days with caspofungin. Despite the reported association between
C. auris and other bloodstream infections and the use of broad-spectrum antibiotics [
1], there is a lack of literature with no other cases of co-infection with
A. baumannii reported. On the other hand, and despite the negative real-time PCR, the previously reported COVID-19 infection is noteworthy. Indeed, although there was no identification of the proper virus, there was a consensus on the diagnosis of a viral myocardiopathy likely caused by SARS-CoV-2. In addition, there are other cases of
C. auris described in COVID-19 patients [
28], and there is no information regarding their susceptibility to
C. auris infections.
Since there was previous isolation of an MDR A. baumannii, measures to stop the spreading of this agent were already taken into account: the patient stayed in an isolation room, whose staff were using personal protective equipment; the patient was already under surveillance by the local Infection Prevention and Control and Antimicrobial Stewardship when C. auris was isolated. Then, the safety measures were reinforced, with regular sanitizing of the whole ICU, which successfully avoided its transmission, with no other isolates of C. auris reported.
No antifungal susceptibility breakpoints for
C. auris are currently standardized for the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or the Clinical and Laboratory Standards Institute (CLSI). However, the Centers for Disease Control and Prevention (CDC) defined tentative resistance breakpoints, as indicated in
Table 1. According to these values, our isolate is resistant to only one antifungal class (azoles), mirroring other reports [
29]. Genomic analysis revealed the presence of genetic markers known to be associated with this phenotype. It also placed this isolate among
C. auris clade III, which has already been reported in Europe [
30]. Curiously, the closest sequences to this isolate are not the available Spanish sequences, but rather sequences from Africa. Considering that this patient was initially admitted to an ICU in Angola before being transferred to the ICU in Portugal, one cannot discard the hypothesis that the infection/colonization took place in the Angola hospital, which is likely characterized by the presence of populations of microbial pathogens considerably different from the ones we are used to dealing with in our ICU. Nevertheless, population genomic studies corroborate the global spread of
C. auris, considering the phylogenetic clustering of strains with a diverse geographical origin [
16]. The development of rapid genomic analysis has been key to understanding the international and local-scale transmission of
C. auris, including the emergence of multidrug-resistant variants [
31].
6. Conclusions
To date, this is the first and single case of C. auris identified in Portugal. However, due to the multiple comorbidities and the identification of other infecting agents, the role of C. auris in the patient’s disease progression and outcome cannot be disclosed. Considering the genomic data and the hospital admission history, the African origin for this C. auris isolate is plausible. This case reinforces the need for continuous surveillance in the hospital setting and for a better understanding of the risk factors, the mechanisms of virulence, and preventive and treatment measures.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/jof9080837/s1, Table S1: List of samples used in the genomic analysis study, with information about their respective metadata; Table S2: List of all non-synonymous mutations identified in CaurisPT_1_2022 when compared to the genome of
C. auris B8441.
Author Contributions
Conceptualization, J.H., T.I.D., T.S. and N.G.; methodology, L.D., S.D., R.S., C.V. and V.M.; software, V.M.; validation, S.C., N.G. and L.B.; data curation, R.S. and V.M.; writing—original draft preparation, J.H., T.I.D., J.C., R.S., C.V., V.M. and J.P.G.; writing—review and editing, J.H., T.S., N.G., L.D., R.S., C.V., V.M. and J.P.G.; supervision, T.S., N.G., L.B., R.S. and J.P.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the European Union EU4Health Programme under grant agreement no. 101113460 (GENEO) and by national funds through the Foundation for Science and Technology (FCT), I.P., under Individual CEEC 2022.00851.CEECIND/CP1748/CT0001.
Institutional Review Board Statement
Ethical review and approval were waived for this study because it was a clinical case and informed consent was available.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
The obtained sequence of the isolate, CaurisPT_1_2022, was deposited in Genebank with the accession number OQ349558. Whole-genome sequencing data are available at the European Read Archive (ENA) under BioProject PRJEB64175.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cortegiani, A.; Misseri, G.; Fasciana, T.; Giammanco, A.; Giarratano, A.; Chowdhary, A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J. Intensive Care 2018, 6, 69. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022.
- European Centre for Disease Prevention and Control. Candida auris Outbreak in Healthcare in Northern Italy, 2019–2021; ECDC: Stockholm, Sweden, 2022.
- Kohlenberg, A.; Monnet, D.L.; Plachouras, D. Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Eurosurveillance 2022, 27, 2200846. [Google Scholar] [CrossRef] [PubMed]
- Lyman, M.; Forsberg, K.; Sexton, D.J.; Chow, N.A.; Lockhart, S.R.; Jackson, B.R.; Chiller, T. Worsening Spread of Candida auris in the United States, 2019 to 2021. Ann. Intern. Med. 2023, 176, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Corcione, S.; Montrucchio, G.; Shbaklo, N.; De Benedetto, I.; Sales, G.; Cedrone, M.; Vita, D.; Costa, C.; Zozzoli, S.; Zaccaria, T.; et al. First Cases of Candida auris in a Referral Intensive Care Unit in Piedmont Region, Italy. Microorganisms 2022, 10, 1521. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Taori, S.K.; Schelenz, S.; Jeffery, K.; Johnson, E.M.; Borman, A.; Manuel, R.; Brown, C.S. Candida auris: A Review of the Literature. Clin. Microbiol. Rev. 2018, 31, e00029-17. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Babraham Bioinformatics—FastQC. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 August 2022).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Mapleson, D.; Accinelli, G.G.; Kettleborough, G.; Wright, J.; Clavijo, B.J. KAT: A K-mer analysis toolkit to quality control NGS datasets and genome assemblies. Bioinformatics 2017, 33, 574–576. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Pryszcz, L.P.; Gabaldón, T. Redundans: An assembly pipeline for highly heterozygous genomes. Nucleic Acids Res. 2016, 44, e113. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020, 11, e03364-19. [Google Scholar] [CrossRef] [PubMed]
- Pegueroles, C.; Mixão, V.; Carreté, L.; Molina, M.; Gabaldón, T. HaploTypo: A variant-calling pipeline for phased genomes. Bioinformatics 2020, 36, 2569–2571. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Page, A.J.; Taylor, B.; Delaney, A.J.; Soares, J.; Seemann, T.; Keane, J.A.; Harris, S.R. SNP-sites: Rapid efficient extraction of SNPs from multi-FASTA alignments. Microb. Genom. 2016, 2, e000056. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Candida auris: Antifungal Susceptibility Testing and Interpretation. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 3 May 2023).
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Williamson, B.; Wilk, A.; Guerrero, K.D.; Mikulski, T.D.; Elias, T.N.; Sawh, I.; Cancino-Prado, G.; Gardam, D.; Heath, C.H.; Govender, N.P.; et al. Impact of Erg11 Amino Acid Substitutions Identified in Candida auris Clade III Isolates on Triazole Drug Susceptibility. Antimicrob. Agents Chemother. 2022, 66, e0162421. [Google Scholar] [CrossRef]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdi, A.; Al-Maani, A.; Al-Wahaibi, A.; Alqayoudhi, A.; Al-Jardani, A.; Al-Abri, S. Characteristics, Risk Factors, and Survival Analysis of Candida auris Cases: Results of One-Year National Surveillance Data from Oman. J. Fungi 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef] [PubMed]
- Goravey, W.; Ali, G.A.; Ali, M.; Ibrahim, E.B.; Al Maslamani, M.; Hadi, H.A. Ominous combination: COVID-19 disease and Candida auris fungemia—Case report and review of the literature. Clin. Case Rep. 2021, 9, e04827. [Google Scholar] [CrossRef]
- Sekyere, J.O. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef]
- Chybowska, A.D.; Childers, D.S.; Farrer, R.A. Nine Things Genomics Can Tell Us about Candida auris. Front. Genet. 2020, 11, 351. [Google Scholar] [CrossRef]
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).