Disseminated Cryptococcosis Is a Common Finding among Human Immunodeficiency Virus-Infected Patients with Suspected Sepsis and Is Associated with Higher Mortality Rates
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Culture
2.2. Minimum Inhibitory Concentration (MIC) Test
2.3. Cryptococcal Antigen (CrAg) Test
2.4. Ethical Considerations
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic of Participants
3.2. Cryptococcal Antigenemia
3.3. Blood Culture
3.4. Minimum Inhibitory of Concentration (MIC)
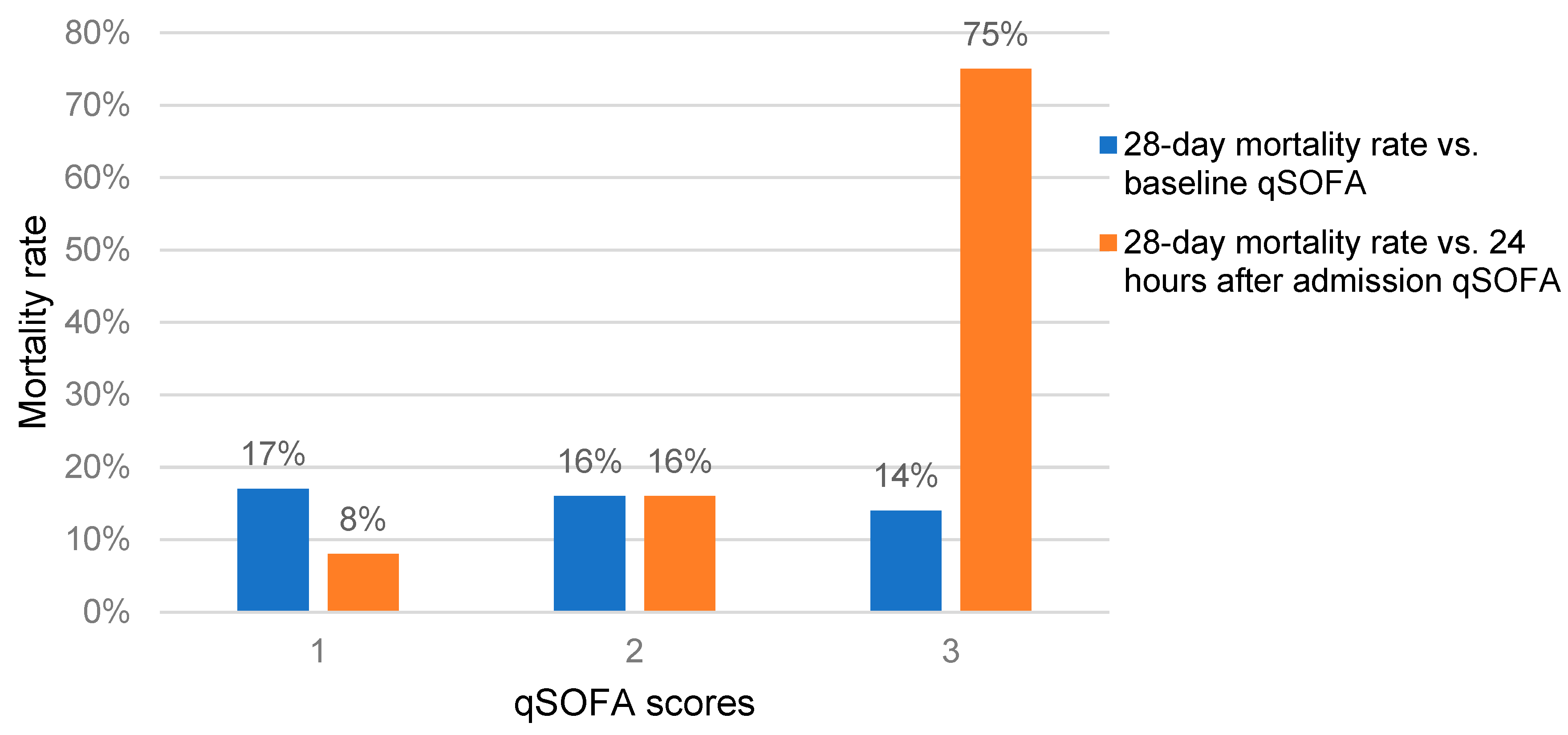
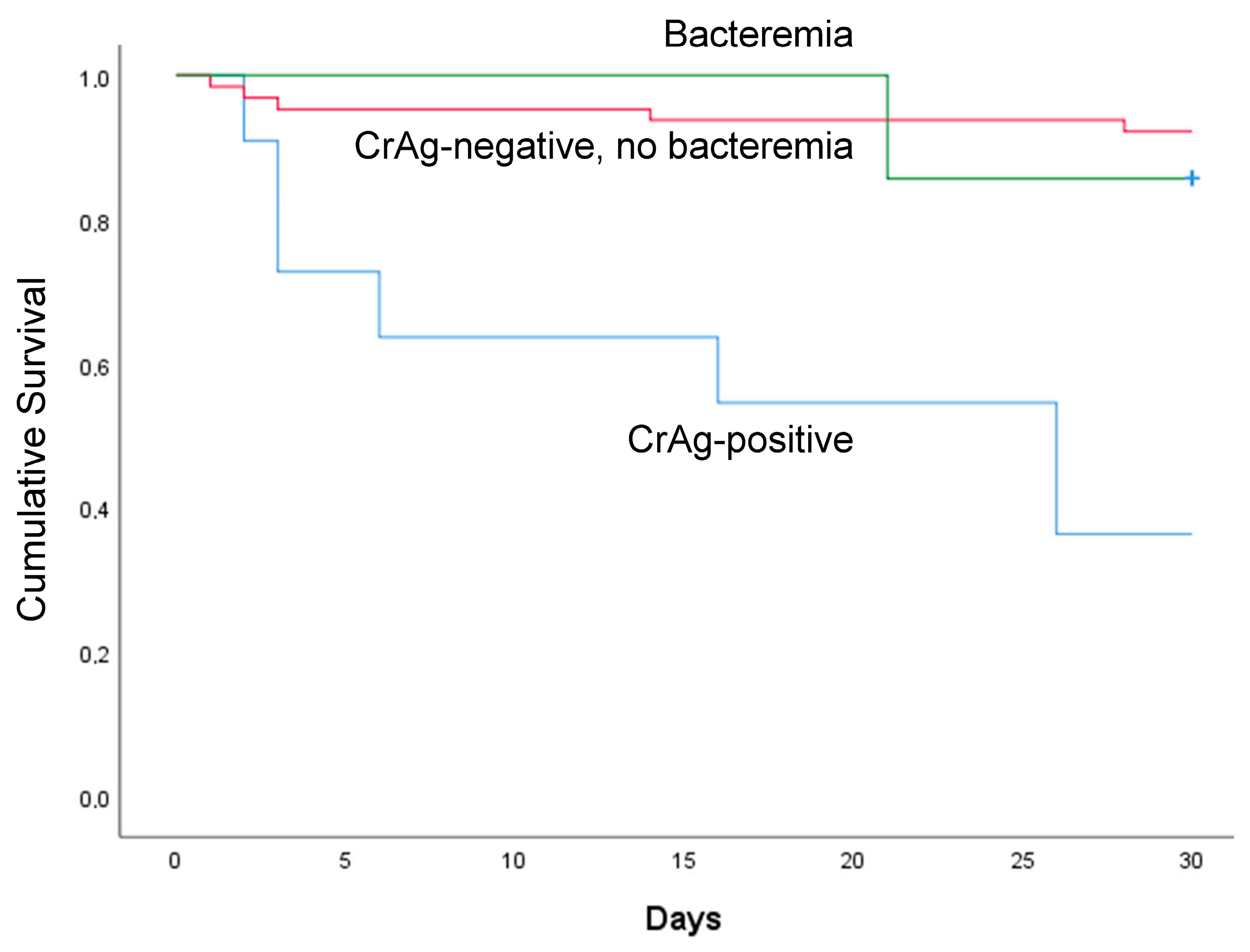
3.5. Participants’ Follow-up Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaka, W.; Berger, C.; Huo, S.; Robertson, V.; Tachiona, C.; Magwenzi, M.; Magombei, T.; Mpamhanga, C.; Katzenstein, D.; Metcalfe, J. Presentation and outcome of suspected sepsis in a high-HIV burden, high antiretroviral coverage setting. Int. J. Infect. Dis. 2020, 96, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Rajasingham, R.; Govender, N.P.; Jordan, A.; Loyse, A.; Shroufi, A.; Denning, D.W.; Meya, D.B.; Chiller, T.M.; Boulware, D.R. The global burden of HIV-associated cryptococcal infection in adults in 2020: A modelling analysis. Lancet Infect. Dis. 2022, 22, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Muzazu, S.G.Y.; Assefa, D.G.; Phiri, C.; Getinet, T.; Solomon, S.; Yismaw, G.; Manyazewal, T. Prevalence of cryptococcal meningitis among people living with human immuno-deficiency virus and predictors of mortality in adults on induction therapy in Africa: A systematic review and meta-analysis. Front. Med. (Lausanne) 2022, 9, 989265. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease among Adults, Adolescents and Children Living with HIV. World Health Organization, 2022. Available online: https://apps.who.int/iris/handle/10665/357088 (accessed on 21 May 2023).
- Rajasingham, R.; Wake, R.M.; Beyene, T.; Katende, A.; Letang, E.; Boulware, D.R. Cryptococcal Meningitis Diagnostics and Screening in the Era of Point-of-Care Laboratory Testing. J. Clin. Microbiol. 2019, 57, e01238-18. [Google Scholar] [CrossRef] [PubMed]
- Meda, J.; Kalluvya, S.; Downs, J.A.; Chofle, A.A.; Seni, J.; Kidenya, B.; Fitzgerald, D.W.; Peck, R.N. Cryptococcal meningitis management in Tanzania with strict schedule of serial lumber punctures using intravenous tubing sets: An operational research study. J. Acquir. Immune Defic. Syndr. 2014, 66, e31-6. [Google Scholar] [CrossRef]
- Rhein, J.; Hullsiek, K.H.; Evans, E.E.; Tugume, L.; Nuwagira, E.; Ssebambulidde, K.; Kiggundu, R.; Mpoza, E.; Musubire, A.K.; Bangdiwala, A.S.; et al. Detrimental Outcomes of Unmasking Cryptococcal Meningitis with Recent ART Initiation. Open Forum. Infect. Dis. 2018, 5, ofy122. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Tufa, T.B.; Hörner, J.; Hurissa, Z.; Nordmann, T.; Bosselmann, M.; Abdissa, S.; Sorsa, A.; Orth, H.M.; Jensen, B.O.; et al. Clinical and microbiological characterization of sepsis and evaluation of sepsis scores. PLoS ONE 2021, 16, e0247646. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third Inter-national Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Marik, P.E.; Taeb, A.M. SIRS, qSOFA and new sepsis definition. J. Thorac. Dis. 2017, 9, 943–945. [Google Scholar] [CrossRef]
- Letang, E.; Rakislova, N.; Martinez, M.J.; Carlos Hurtado, J.; Carrilho, C.; Bene, R.; Mandomando, I.; Quintó, L.; Nhampossa, T.; Chicamba, V.; et al. Minimally Invasive Tissue Sampling: A Tool to Guide Efforts to Reduce AIDS-Related Mortality in Resource-Limited Settings. Clin. Infect. Dis. 2021, 73, S343–S350. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Rolfes, M.A.; Rajasingham, R.; von Hohenberg, M.; Qin, Z.; Taseera, K.; Schutz, C.; Kwizera, R.; Butler, E.K.; Meintjes, G.; et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerg. Infect. Dis. 2014, 20, 45–53. [Google Scholar] [CrossRef] [PubMed]
- WHO Fungal Priority Pathogens List to Guide Research Daphag; World Health Organization: Geneva, Switzerland, 2022.
- Tufa, T.B.; Mackenzie, C.R.; Orth, H.M.; Wienemann, T.; Nordmann, T.; Abdissa, S.; Hurissa, Z.; Schönfeld, A.; Bosselmann, M.; Häussinger, D.; et al. Prevalence and characterization of antimicrobial resistance among gram-negative bacteria isolated from febrile hospitalized patients in central Ethiopia. Antimicrob. Resist. Infect. Control 2022, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, L.; Tatarelli, P.; Di Biagio, A. Bloodstream infections in HIV-infected patients. Virulence 2016, 7, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Kiertiburanakul, S.; Watcharatipagorn, S.; Chongtrakool, P.; Santanirand, P. Epidemiology of bloodstream infections and predictive factors of mortality among HIV-infected adult patients in Thailand in the era of highly active antiretroviral therapy. Jpn. J. Infect. Dis. 2012, 65, 28–32. [Google Scholar] [CrossRef]
- Beyene, T.; Zewde, A.G.; Balcha, A.; Hirpo, B.; Yitbarik, T.; Gebissa, T.; Rajasingham, R.; Boulware, D.R. Inadequacy of High-Dose Fluconazole Monotherapy Among Cerebrospinal Fluid Cryptococcal Antigen (CrAg)-Positive Human Immunodeficiency Virus-Infected Persons in an Ethiopian CrAg Screening Program. Clin. Infect. Dis. 2017, 65, 2126–2129. [Google Scholar] [CrossRef]
- Lakoh, S.; Rickman, H.; Sesay, M.; Kenneh, S.; Burke, R.; Baldeh, M.; Jiba, D.F.; Tejan, Y.S.; Boyle, S.; Koroma, C.; et al. Prevalence and mortality of cryptococcal disease in adults with advanced HIV in an urban tertiary hospital in Sierra Leone: A prospective study. BMC Infect. Dis. 2020, 20, 141. [Google Scholar] [CrossRef]
- Faini, D.; Kalinjuma, A.V.; Katende, A.; Mbwaji, G.; Mnzava, D.; Nyuri, A.; Glass, T.R.; Furrer, H.; Hatz, C.; Boulware, D.R.; et al. Laboratory-Reflex Cryptococcal Antigen Screening Is Associated with a Survival Benefit in Tanzania. J. Acquir. Immune Defic. Syndr. 2019, 80, 205–213. [Google Scholar] [CrossRef]
- Wake, R.M.; Molloy, S.F.; Jarvis, J.N.; Harrison, T.S.; Govender, N.P. Cryptococcal Antigenemia in Advanced Human Immunodefi-ciency Virus Disease: Pathophysiology, Epidemiology, and Clinical Implications. Clin. Infect. Dis. 2023, 76, 764–770. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, X.L.; Nie, J.M.; Chen, X.H.; Jiang, Z.S.; Liu, S.Q.; Yang, T.T.; Yang, X.; Sun, F.; Lu, Y.Q.; et al. Establishment of a novel scoring model for mortality risk prediction in HIV-infected patients with cryptococcal meningitis. BMC Infect. Dis. 2021, 21, 786. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Shen, Y.; Liu, L.; Qi, T.; Wang, Z.; Mehraj, V.; Routy, J.P.; Lu, H. Serum cryptococcal antigen titre as a diagnostic tool and a predictor of mortality in HIV-infected patients with cryptococcal meningitis. HIV Med. 2019, 20, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mfinanga, S.; Chanda, D.; Kivuyo, S.L.; Guinness, L.; Bottomley, C.; Simms, V.; Chijoka, C.; Masasi, A.; Kimaro, G.; Ngowi, B.; et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: An open-label, randomised controlled trial. Lancet 2015, 385, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Longley, N.; Jarvis, J.N.; Meintjes, G.; Boulle, A.; Cross, A.; Kelly, N.; Govender, N.P.; Bekker, L.G.; Wood, R.; Harrison, T.S. Cryptococcal Antigen Screening in Patients Initiating ART in South Africa: A Prospective Cohort Study. Clin. Infect. Dis. 2016, 62, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, D.S.; Muthoga, C.; Meya, D.B.; Tugume, L.; Williams, D.; Rajasingham, R.; Boulware, D.R.; Mwandumba, H.C.; Moyo, M.; Dziwani, E.N.; et al. Cost-effectiveness of single, high-dose, liposomal amphotericin regimen for HIV-associated cryptococcal meningitis in five countries in sub-Saharan Africa: An economic analysis of the AMBITION-cm trial. Lancet Glob. Health 2022, 10, e1845–e1854. [Google Scholar] [CrossRef]
- National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. In Ethiopian HIV Prevention, Care and Treatment Guideline; Ethiopian Federal Ministry of Health: Addis Ababa, Ethiopia, 2018; p. 113. Available online: https://www.afro.who.int/publications/national-consolidated-guidelines-comprehensive-hiv-prevention-care-and-treatment (accessed on 21 May 2023).
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the emerging threat of antifungal resistance to human health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef]
- Gerlach, E.S.; Altamirano, S.; Yoder, J.M.; Luggya, T.S.; Akampurira, A.; Meya, D.B.; Boulware, D.R.; Rhein, J.; Nielsen, K. ATI-2307 Exhibits Equivalent Antifungal Activity in Cryptococcus neoformans Clinical Isolates with High and Low Fluconazole IC (50). Front. Cell Infect. Microbiol. 2021, 11, 695240. [Google Scholar] [CrossRef]
| Variables | Frequency |
|---|---|
| Age in years, median (IQR) | 35 (27–40) |
| Study site | |
| Asella Hospital | 54 (66%) |
| Adama Hospital | 28 (34%) |
| Sex | |
| Female | 52 (63%) |
| Male | 30 (37%) |
| HIV Therapy status | |
| ART-naïve | 36 (44%) |
| Receiving ART | 46 (56%) |
| qSOFA score | |
| ≥2 at baseline | 64 (78%) |
| ≥2 after 24 h | 53 (65%) |
| Variables | Positive n (%) | Negative n (%) | Total n (%) | p Value |
|---|---|---|---|---|
| HIV Therapy status | ||||
| ART naïve | 4 (11%) | 32 (89%) | 36 (44%) | |
| Receiving ART | 7 (15%) | 39 (85%) | 46 (56%) | 0.419 |
| ≥2 qSOFA score at baseline (n = 64) | 9 (14%) | 55 (86%) | 64 (78%) | 0.549 |
| ≥2 qSOFA score after 24 h (n = 53) | 8 (15%) | 45 (85%) | 53 (65%) | 0.406 |
| Total | 11 (13%) | 71 (87%) | 82 (100%) |
| Name of the Microorganisms | Frequency of Isolates |
|---|---|
| Cryptococcus neoformans | 5 |
| Escherichia coli | 3 |
| Salmonella typhi | 1 |
| Staphylococcus aureus | 1 |
| Streptococcus pneumoniae | 1 |
| Enterococcus faecalis | 1 |
| Total | 12 |
| Minimum Inhibitory of Concentration (mg/L) | Number Yeasts | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antifungal Agents | ≤0.015 | 0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | >32 | Susceptible | Resistant |
| Amphotericin B | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| 5-Flucytosine | 0 | 0 | 0 | 0 | 1 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Fluconazole | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 4 | 1 |
| Voriconazole | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Posaconazole | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Micafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 5 |
| Anidulafungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 |
| Caspofungin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufa, T.B.; Orth, H.M.; Wienemann, T.; Jensen, B.-E.O.; Mackenzie, C.R.; Boulware, D.R.; Luedde, T.; Feldt, T. Disseminated Cryptococcosis Is a Common Finding among Human Immunodeficiency Virus-Infected Patients with Suspected Sepsis and Is Associated with Higher Mortality Rates. J. Fungi 2023, 9, 836. https://doi.org/10.3390/jof9080836
Tufa TB, Orth HM, Wienemann T, Jensen B-EO, Mackenzie CR, Boulware DR, Luedde T, Feldt T. Disseminated Cryptococcosis Is a Common Finding among Human Immunodeficiency Virus-Infected Patients with Suspected Sepsis and Is Associated with Higher Mortality Rates. Journal of Fungi. 2023; 9(8):836. https://doi.org/10.3390/jof9080836
Chicago/Turabian StyleTufa, Tafese Beyene, Hans Martin Orth, Tobias Wienemann, Bjoern-Erik Ole Jensen, Colin R. Mackenzie, David R. Boulware, Tom Luedde, and Torsten Feldt. 2023. "Disseminated Cryptococcosis Is a Common Finding among Human Immunodeficiency Virus-Infected Patients with Suspected Sepsis and Is Associated with Higher Mortality Rates" Journal of Fungi 9, no. 8: 836. https://doi.org/10.3390/jof9080836
APA StyleTufa, T. B., Orth, H. M., Wienemann, T., Jensen, B.-E. O., Mackenzie, C. R., Boulware, D. R., Luedde, T., & Feldt, T. (2023). Disseminated Cryptococcosis Is a Common Finding among Human Immunodeficiency Virus-Infected Patients with Suspected Sepsis and Is Associated with Higher Mortality Rates. Journal of Fungi, 9(8), 836. https://doi.org/10.3390/jof9080836







