Neuroimaging of Cryptococcal Meningitis in Patients without Human Immunodeficiency Virus: Data from a Multi-Center Cohort Study
Abstract
1. Introduction
| HIV Status | Non-HIV | HIV | ||||||
|---|---|---|---|---|---|---|---|---|
| Feature | Reference | # | Total | % | Reference | # | Total | % |
| Normal CT | ||||||||
| Tjia [6] | 13 | 25 | 52% | Charlier [4] | 26 | 55 | 47% | |
| Tan [7] | 10 | 20 | 50% | Tien [8] | 9 | 29 | 31% | |
| Normal MRI | ||||||||
| Tan [9] | 2 | 18 | 11% | Loyse [10] | 2 | 87 | 2% | |
| Miszkiel [11] | 4 | 25 | 16% | |||||
| Charlier [4] | 2 | 24 | 8% | |||||
| Meningeal enhancement | ||||||||
| Lu [12] | 8 | 15 | 53% | Miszkiel [11] | 4 | 25 | 16% | |
| Tsai [13] | 29 | 65 | 45% | Loyse [10] | 24 | 87 | 28% | |
| Tan [9] | 8 | 17 | 47% | Mathews [14] | 1 | 5 | 20% | |
| Zhong [15] | 71 | 114 | 62% | Xia [16] | 21 | 55 | 38% | |
| Singh [17] | 8 | 16 | 50% | Hammoud [3] | 9 | 11 | 81% | |
| Hammoud [3] | 32 | 45 | 71% | |||||
| Infarcts | ||||||||
| Chen [18] | 7 | 37 | 19% | Loyse [10] | 12 | 87 | 14% | |
| Chang | 7 | 12 | 58% | Charlier [4] | 2 | 55 | 4% | |
| Tsai [13] | 14 | 65 | 22% | Nguyen [19] | 1 | 36 | 3% | |
| Nguyen [19] | 3 | 24 | 13% | |||||
| Zhong [15] | 5 | 114 | 4% | |||||
| Hydrocephalus | ||||||||
| Lu [12] | 5 | 15 | 33% | Loyse [10] | 2 | 87 | 2% | |
| Tsai [13] | 21 | 65 | 32% | Charlier [4] | 2 | 55 | 4% | |
| Tjia [6] | 5 | 25 | 20% | Hammoud [3] | 1 | 11 | 9% | |
| Lee [20] | 10 | 76 | 13% | |||||
| Zhong [15] | 38 | 114 | 33% | |||||
| Singh [17] | 2 | 16 | 13% | |||||
| Hammoud [3] | 23 | 45 | 51% | |||||
| Cryptococcoma (other parenchymal lesions) | ||||||||
| Tan [9] | 11 | 18 | 61% | Tien [8] | 3 | 10 | 30% | |
| Lee [20] | 12 | 76 | 16% | Loyse [10] | 23 | 87 | 26% | |
| Zhong [15] | 35 | 114 | 31% | Miszkiel [11] | 9 | 25 | 36% | |
| Singh [17] | 6 | 16 | 38% | Charlier [4] | 4 | 24 | 17% | |
| Hammoud [3] | 22 | 45 | 49% | Hammoud [3] | 3 | 11 | 27% | |
| Dilated V-R spaces | ||||||||
| Zhong [15] | 45 | 114 | 39% | Mizskiel [11] | 4 | 25 | 16% | |
| Tsai [13] | 28 | 65 | 43% | Andreula [21] | 5 | 9 | 56% | |
| Tan [9] | 5 | 18 | 28% | Wehn [22] | 2 | 2 | 100% | |
| Hammoud [3] | 25 | 45 | 56% | Charlier [4] | 11 | 24 | 46% | |
| Mathews [14] | 5 | 15 | 33% | |||||
| Loyse [10] | 31 | 87 | 36% | |||||
| Xia [16] | 28 | 55 | 51% | |||||
| Hammoud [3] | 5 | 11 | 45% | |||||
| Atrophy | ||||||||
| Zhong [15] | 7 | 114 | 6% | Loyse [10] | 30 | 87 | 34% | |
| Charlier [4] | 4 | 24 | 17% | |||||
| Tien [8] | 13 | 30 | 43% | |||||
2. Materials and Methods
Available Imaging Studies
3. Results
3.1. Demographics
3.2. MRI Findings
4. Discussion
4.1. Mass Lesions and Dilated VR Spaces
4.2. Infarcts and Atrophy
4.3. Clinical Correlates
4.4. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G. Cryptococcal infections in non-HIV-infected patients. Trans. Am. Clin. Clim. Assoc. 2013, 124, 61–79. [Google Scholar]
- Katchanov, J.; Branding, G.; Jefferys, L.; Arastéh, K.; Stocker, H.; Siebert, E. Neuroimaging of HIV-associated cryptococcal meningitis: Comparison of magnetic resonance imaging findings in patients with and without immune reconstitution. Int. J. STD AIDS 2015, 27, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, D.A.; Mahdi, E.; Panackal, A.A.; Wakim, P.; Sheikh, V.; Sereti, I.; Bielakova, B.; Bennett, J.E.; Williamson, P.R. Choroid Plexitis and Ependymitis by Magnetic Resonance Imaging are Biomarkers of Neuronal Damage and Inflammation in HIV-negative Cryptococcal Meningoencephalitis. Sci. Rep. 2017, 7, 9184. [Google Scholar] [CrossRef] [PubMed]
- Charlier, C.; Dromer, F.; Lévêque, C.; Chartier, L.; Cordoliani, Y.-S.; Fontanet, A.; Launay, O.; Lortholary, O. For the French Cryptococcosis Study Group Cryptococcal Neuroradiological Lesions Correlate with Severity during Cryptococcal Meningoencephalitis in HIV-Positive Patients in the HAART Era. PLoS ONE 2008, 3, e1950. [Google Scholar] [CrossRef]
- Marr, K.A.; Sun, Y.; Spec, A.; Lu, N.; Panackal, A.; Bennett, J.; Pappas, P.; Ostrander, D.; Datta, K.; Zhang, S.X.; et al. A Multicenter, Longitudinal Cohort Study of Cryptococcosis in Human Immunodeficiency Virus–negative People in the United States. Clin. Infect. Dis. 2019, 70, 252–261. [Google Scholar] [CrossRef]
- Tjia, T.L.; Yeow, Y.K.; Tan, C.B. Cryptococcal meningitis. J. Neurol. Neurosurg. Psychiatry 1985, 48, 853858. [Google Scholar] [CrossRef]
- Tan, C.T.; Kuan, B.B. Cryptococcus meningitis, clinical—CT scan considerations. Neuroradiology 1987, 29, 43–46. [Google Scholar] [CrossRef]
- Tien, R.D.; Chu, P.K.; Hesselink, J.R.; Duberg, A.; Wiley, C. Intracranial cryptococcosis in immunocompromised patients: CT and MR findings in 29 cases. AJNR Am. J. Neuroradiol. 1991, 12, 283–289. [Google Scholar]
- Tan, Z.-R.; Long, X.-Y.; Li, G.-L.; Zhou, J.-X.; Long, L. Spectrum of neuroimaging findings in cryptococcal meningitis in immunocompetent patients in China—A series of 18 cases. J. Neurol. Sci. 2016, 368, 132–137. [Google Scholar] [CrossRef]
- Loyse, A.; Moodley, A.; Rich, P.; Molloy, S.; Bicanic, T.; Bishop, L.; Rae, W.; Bhigjee, A.; Loubser, N.; Michowicz, A.; et al. Neurological, visual, and MRI brain scan findings in 87 South African patients with HIV-associated cryptococcal meningoencephalitis. J. Infect. 2014, 70, 668–675. [Google Scholar] [CrossRef]
- Miszkiel, K.; Hall-Craggs, M.; Miller, R.; Kendall, B.; Wilkinson, I.; Paley, M.; Harrison, M. The spectrum of MRI findings in CNS cryptococcosis in AIDS. Clin. Radiol. 1996, 51, 842–850. [Google Scholar] [CrossRef]
- Lu, C.-H.; Chen, H.-L.; Chang, W.-N.; Tsai, N.-W.; Wang, H.-C.; Yang, T.-M.; Lin, Y.-J.; Lin, C.-P.; Chen, C.-C.; Cheng, B.-C.; et al. Assessing the Chronic Neuropsychologic Sequelae of Human Immunodeficiency Virus–Negative Cryptococcal Meningitis by Using Diffusion Tensor Imaging. Am. J. Neuroradiol. 2011, 32, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Lien, C.-Y.; Lee, J.-J.; Lin, W.-C.; Hsu, C.-W.; Huang, C.-R.; Tsai, N.-W.; Chang, C.-C.; Lu, C.-H.; Chang, W.-N. The prognostic factors of HIV-negative adult cryptococcal meningitis with a focus on cranial MRI-based neuroimaging findings. J. Clin. Neurosci. 2018, 55, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mathews, V.P.; Alo, P.L.; Glass, J.D.; Kumar, A.J.; McArthur, J.C. AIDS-related CNS cryptococcosis: Radiologic-pathologic correlation. Am. J. Neuroradiol. 1992, 13, 1477–1486. [Google Scholar] [PubMed]
- Zhong, Y.; Zhou, Z.; Fang, X.; Peng, F.; Zhang, W. Magnetic resonance imaging study of cryptococcal neuroradiological lesions in HIV-negative cryptococcal meningitis. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1367–1372. [Google Scholar] [CrossRef]
- Xia, S.; Li, X.; Shi, Y.; Liu, J.; Zhang, M.; Gu, T.; Pan, S.; Song, L.; Xu, J.; Sun, Y.; et al. A Retrospective Cohort Study of Lesion Distribution of HIV-1 Infection Patients With Cryptococcal Meningoencephalitis on MRI: Correlation With Immunity and Immune Reconstitution. Medicine 2016, 95, e2654. [Google Scholar] [CrossRef]
- Singh, N.; Lortholary, O.; Dromer, F.; Alexander, B.D.; Gupta, K.L.; John, G.T.; Del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Central Nervous System Cryptococcosis in Solid Organ Transplant Recipients: Clinical Relevance of Abnormal Neuroimaging Findings. Transplantation 2008, 86, 647–651. [Google Scholar] [CrossRef]
- Chen, S.-F.; Lu, C.-H.; Lui, C.-C.; Huang, C.-R.; Chuang, Y.-C.; Tan, T.-Y.; Tsai, N.-W.; Chang, C.-C.; Tsai, W.-C.; Chang, W.-N. Acute/subacute cerebral infarction (ASCI) in HIV-negative adults with cryptococcal meningoencephalitis (CM): A MRI-based follow-up study and a clinical comparison to HIV-negative CM adults without ASCI. BMC Neurol. 2011, 11, 12. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Husain, S.; Clancy, C.J.; Peacock, J.E.; Hung, C.-C.; Kontoyiannis, D.P.; Morris, A.J.; Heath, C.H.; Wagener, M.; Yu, V.L. Outcomes of central nervous system cryptococcosis vary with host immune function: Results from a multi-center, prospective study. J. Infect. 2010, 61, 419–426. [Google Scholar] [CrossRef]
- Lee, W.J.; Ryu, Y.J.; Moon, J.; Lee, S.T.; Jung, K.H.; Park, K.I.; Kim, M.; Lee, S.K.; Chu, K. Enlarged periventricular space and periventricular lesion extension on baseline brain MRI predicts poor neurological outcomes in cryptococcus meningoencephalitis. Sci Rep. 2021, 11, 6446. [Google Scholar] [CrossRef]
- Andreula, C.F.; Burdi, N.; Carella, A. CNS cryptococcosis in AIDS: Spectrum of MR findings. J. Comput. Assist. Tomogr. 1993, 17, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Wehn, S.M.; Heinz, E.R.; Burger, P.C.; Boyko, O.B. Dilated Virchow-Robin spaces in cryptococcal meningitis associated with AIDS: CT and MR findings. J. Comput. Assist. Tomogr. 1989, 13, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Lee, H.; Lee, K.; Chen, W. Diffusion-weighted and conventional magnetic resonance imaging in cerebral cryptococcoma. Acta Radiol. 2005, 46, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Pyrgos, V.; Seitz, A.E.; Steiner, C.A.; Prevots, D.R.; Williamson, P.R. Epidemiology of Cryptococcal Meningitis in the US: 1997–2009. PLoS ONE 2013, 8, e56269. [Google Scholar] [CrossRef] [PubMed]
- Kaufman-Francis, K.; Djordjevic, J.T.; Juillard, P.-G.; Lev, S.; Desmarini, D.; Grau, G.E.; Sorrell, T.C. The Early Innate Immune Response to, and Phagocyte-Dependent Entry of, Cryptococcus neoformans Map to the Perivascular Space of Cortical Post-Capillary Venules in Neurocryptococcosis. Am. J. Pathol. 2018, 188, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Virchow-Robin Spaces at MR Imaging. RadioGraphics 2007, 27, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Puglielli, E.; De Amicis, R.; Necozione, S.; Masciocchi, C.; Gallucci, M. Contrast-enhanced FLAIR in the early diagnosis of infectious meningitis. Neuroradiology 2005, 47, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, B.; Mehkri, Y.; De Prey, J.; Hu, C.; Tuna, I.S.; Shuhaiber, H. Cryptococcosis Presenting as Cerebrovascular Disease. Cureus 2021, 13, e19442. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, D.-N.; Chen, Z.-T.; Zhao, Z.-H.; Lin, Y.; Wang, H.-Y.; Wang, N. Risk factors associated with acute/subacute cerebral infarction in HIV-negative patients with cryptococcal meningitis. J. Neurol. Sci. 2016, 364, 19–23. [Google Scholar] [CrossRef]
- Klock, C.; Cerski, M.; Goldani, L.Z. Histopathological Aspects of Neurocryptococcosis in HIV-Infected Patients: Autopsy Report of 45 Patients. Int. J. Surg. Pathol. 2008, 17, 444–448. [Google Scholar] [CrossRef]
- Lee, S.C.; Dickson, D.W.; Casadevall, A. Pathology of cryptococcal meningoencephalitis: Analysis of 27 patients with pathogenetic implications. Hum. Pathol. 1996, 27, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, Y.; Ohtomo, S.; Arai, H.; Ohtoh, T.; Tominaga, T. Subarachnoid small vein occlusion due to inflammatory fibrosis—A possible mechanism for cerebellar infarction in cryptococcal meningoencephalitis: A case report. BMC Neurol. 2017, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Haynor, D. MR angiography of large-vessel intracranial stenosis after cryptococcal meningitis. Radiol. Case Rep. 2011, 6, 528. [Google Scholar] [CrossRef]
- Abu-Rumeileh, S.; Garibashvili, T.; Hagemann, J.B.; Still, V.; Bachhuber, F.; Otto, M.; Tumani, H.; Senel, M. A one-year longitudinal evaluation of cerebrospinal fluid and blood neurochemical markers in a patient with cryptococcal meningitis complicated by ischemic stroke. J. Neurol. Sci. 2021, 432, 120090. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.D.P.S.C.; Severo, L.C.; Oliveira, F.D.M.; Irion, K.; Londero, A.T. The spectrum of computerized tomography (CT) findings in central nervous system (CNS) infection due to Cryptococcus neoformans var. gattii in immunocompetent children. Rev. Inst. Med. Trop. São Paulo 2002, 44, 283–287. [Google Scholar] [CrossRef]
- Moodley, A.; Rae, W.; Bhigjee, A.; Connolly, C.; Devparsad, N.; Michowicz, A.; Harrison, T.; Loyse, A. Early clinical and subclinical visual evoked potential and Humphrey’s visual field defects in cryptococcal meningitis. PLoS ONE 2012, 7, e52895. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-M.; Hao, D.-P.; Tang, G.-Z.; Zhou, R.-Z.; Pang, J.; Dong, C. High-resolution MRI assessment of optic nerve sheath diameter in adults: Optic nerve sheath variation and a new diagnostic tool for intracranial hypertension. Acta Radiol. 2020, 62, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- King, K.A.; Ansari, G.; Panackal, A.A.; Zalewski, C.; Anjum, S.; Bennett, J.E.; Beri, A.; Kim, H.J.; Hammoud, D.; Brewer, C.C.; et al. Audiologic and Otologic Complications of Cryptococcal Meningoencephalitis in Non-HIV Previously Healthy Patients. Otol. Neurotol. 2019, 40, e657–e664. [Google Scholar] [CrossRef]
- Lin, W.-C.; Chen, P.-C.; Wang, H.-C.; Tsai, N.-W.; Chou, K.-H.; Chen, H.-L.; Su, Y.-J.; Lin, C.-P.; Li, S.-H.; Chang, W.-N.; et al. Diffusion Tensor Imaging Study of White Matter Damage in Chronic Meningitis. PLoS ONE 2014, 9, e98210. [Google Scholar] [CrossRef]
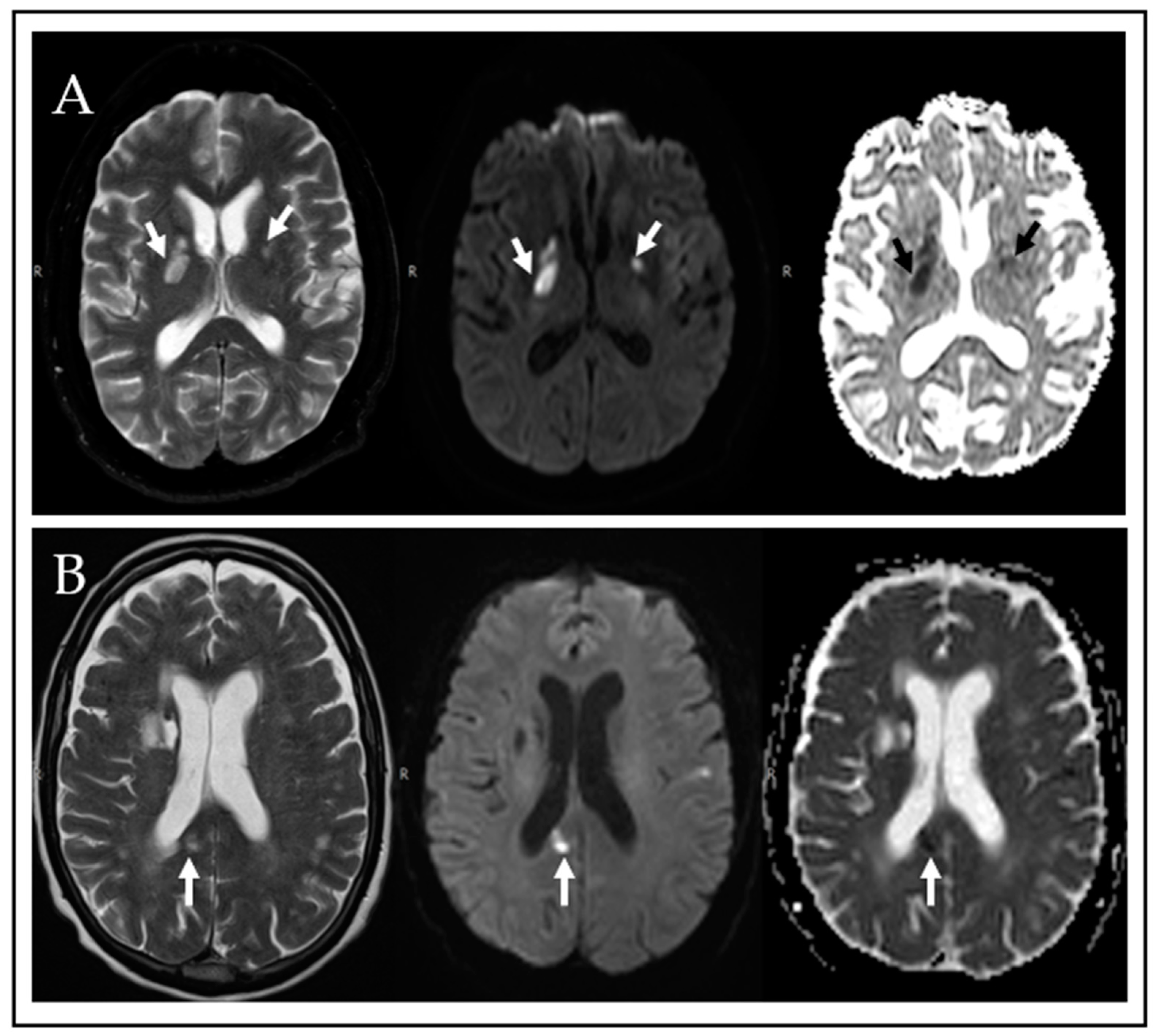
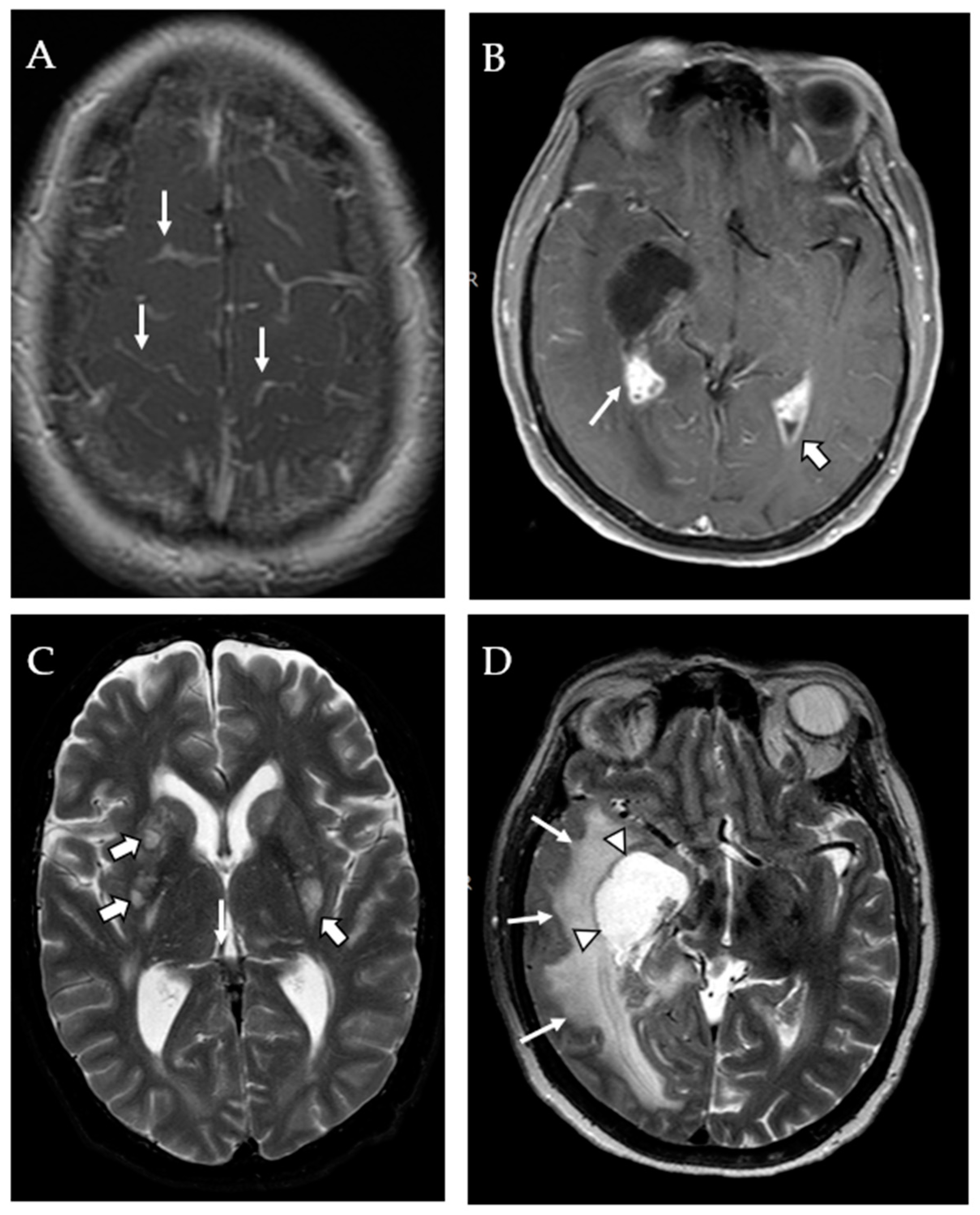
| Age, Median Years (IQR) | 61 (53–70) |
|---|---|
| Sex, female | 11 (38%) |
| Male | 18(62%) |
| Race, | |
| Caucasian | 23(79%) |
| African American | 4 (14%) |
| Asian | 2 (7%) |
| Documented cryptococcal lung infection | 9 (31%) |
| Underlying Disease | |
| Solid organ transplantation | 6 (21%) |
| Solid Tumor | 2(7%) |
| Hematologic Malignancy * | 3 (11%) |
| Liver Disease | 5(17%) |
| Liver Cirrhosis | 4(14%) |
| Hepatitis | 2 (7%) |
| Autoimmune Syndromes ^ | 8 (28%) |
| Primary immunodeficiency + | 3 (10%) |
| Diabetes mellitus | 10(34%) |
| None | 7(24%) |
| Immunosuppressive medications | |
| Glucocorticoids | 16 (55%) |
| Cytotoxic chemotherapy | 9 (31%) |
| Calcineurin/mTOR inhibitors ** | 5 (19%) |
| Antimetabolites ** | 6 (22%) |
| Targeted antibodies ** | 1(4%) |
| Study | Result | # Cases | Evaluable | % |
|---|---|---|---|---|
| CT | Normal | 24 | 51 | 47% |
| MRI | Normal | 3 | 29 | 10% |
| Hydrocephalus | 5 | 28 | 18% | |
| transependymal flow | 2 | 28 | 7% | |
| lesions with restricted diffusion (infarcts) | ||||
| basal ganglia | 11 | 29 | 38% | |
| elsewhere * | 3 | 28 | 11% | |
| pseudocysts in basal ganglia | 7 | 29 | 24% | |
| gadolinium contrast | 18 | 29 | 62% | |
| parenchymal enhancing lesions (“cryptococcomas”) | ||||
| basal ganglia | 4 | 18 | 22% | |
| elsewhere | 4 | 18 | 22% | |
| meningeal enhancement | 10 | 18 | 56% | |
| choroid plexus enhancement | 2 | 18 | 11% | |
| ependymal enhancement | 4 | 17 | 24% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjum, S.H.; Bennett, J.E.; Dean, O.; Marr, K.A.; Hammoud, D.A.; Williamson, P.R. Neuroimaging of Cryptococcal Meningitis in Patients without Human Immunodeficiency Virus: Data from a Multi-Center Cohort Study. J. Fungi 2023, 9, 594. https://doi.org/10.3390/jof9050594
Anjum SH, Bennett JE, Dean O, Marr KA, Hammoud DA, Williamson PR. Neuroimaging of Cryptococcal Meningitis in Patients without Human Immunodeficiency Virus: Data from a Multi-Center Cohort Study. Journal of Fungi. 2023; 9(5):594. https://doi.org/10.3390/jof9050594
Chicago/Turabian StyleAnjum, Seher H., John E. Bennett, Owen Dean, Kieren A. Marr, Dima A. Hammoud, and Peter R. Williamson. 2023. "Neuroimaging of Cryptococcal Meningitis in Patients without Human Immunodeficiency Virus: Data from a Multi-Center Cohort Study" Journal of Fungi 9, no. 5: 594. https://doi.org/10.3390/jof9050594
APA StyleAnjum, S. H., Bennett, J. E., Dean, O., Marr, K. A., Hammoud, D. A., & Williamson, P. R. (2023). Neuroimaging of Cryptococcal Meningitis in Patients without Human Immunodeficiency Virus: Data from a Multi-Center Cohort Study. Journal of Fungi, 9(5), 594. https://doi.org/10.3390/jof9050594





