High Diversity of Fusarium Species in Onychomycosis: Clinical Presentations, Molecular Identification, and Antifungal Susceptibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, Culture, and Microscopic/Histopathological Examination
2.2. Molecular Identification
2.3. Antifungal Susceptibility
3. Results
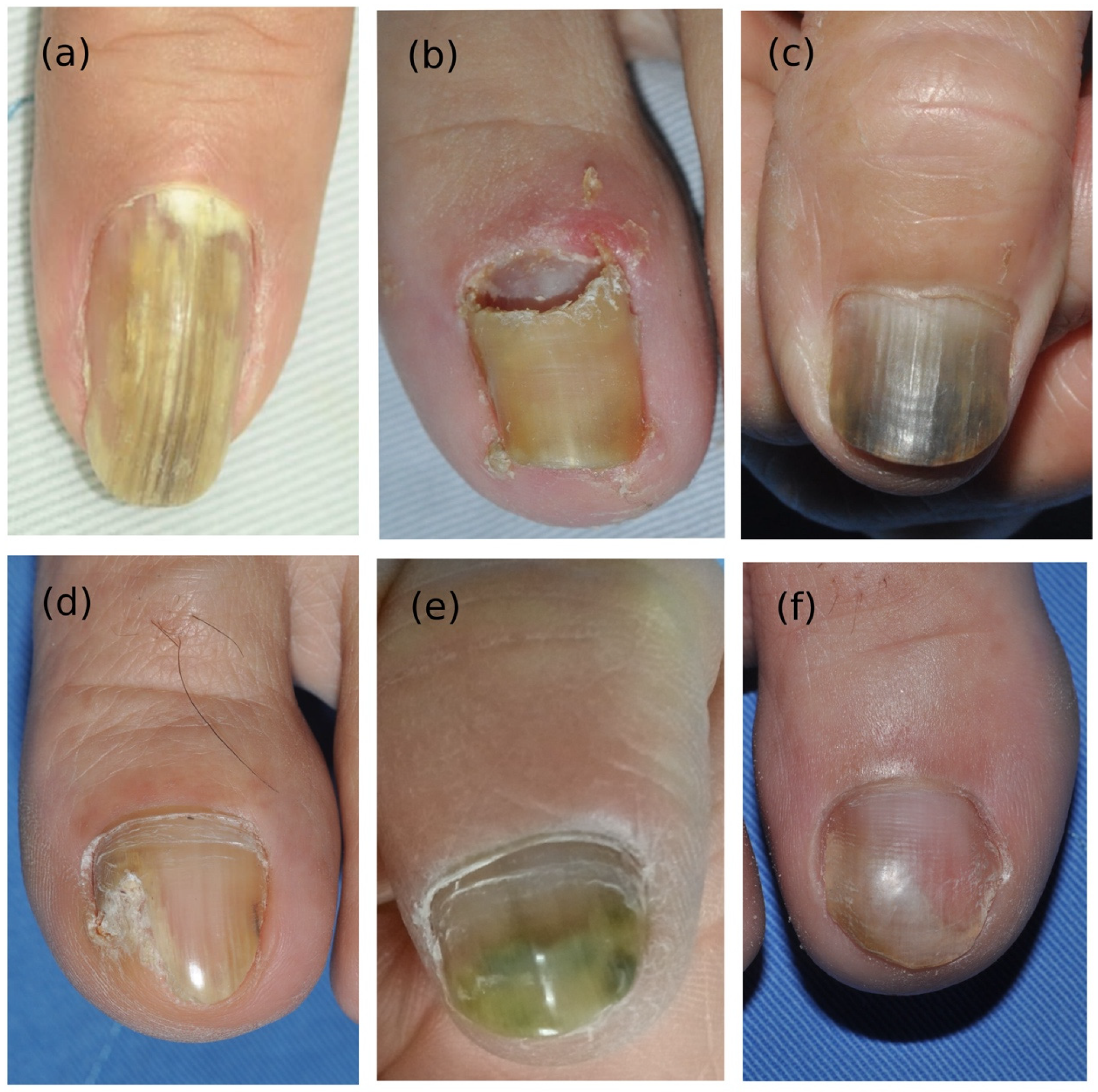
3.1. Demographic Data and Clinical Manifestations
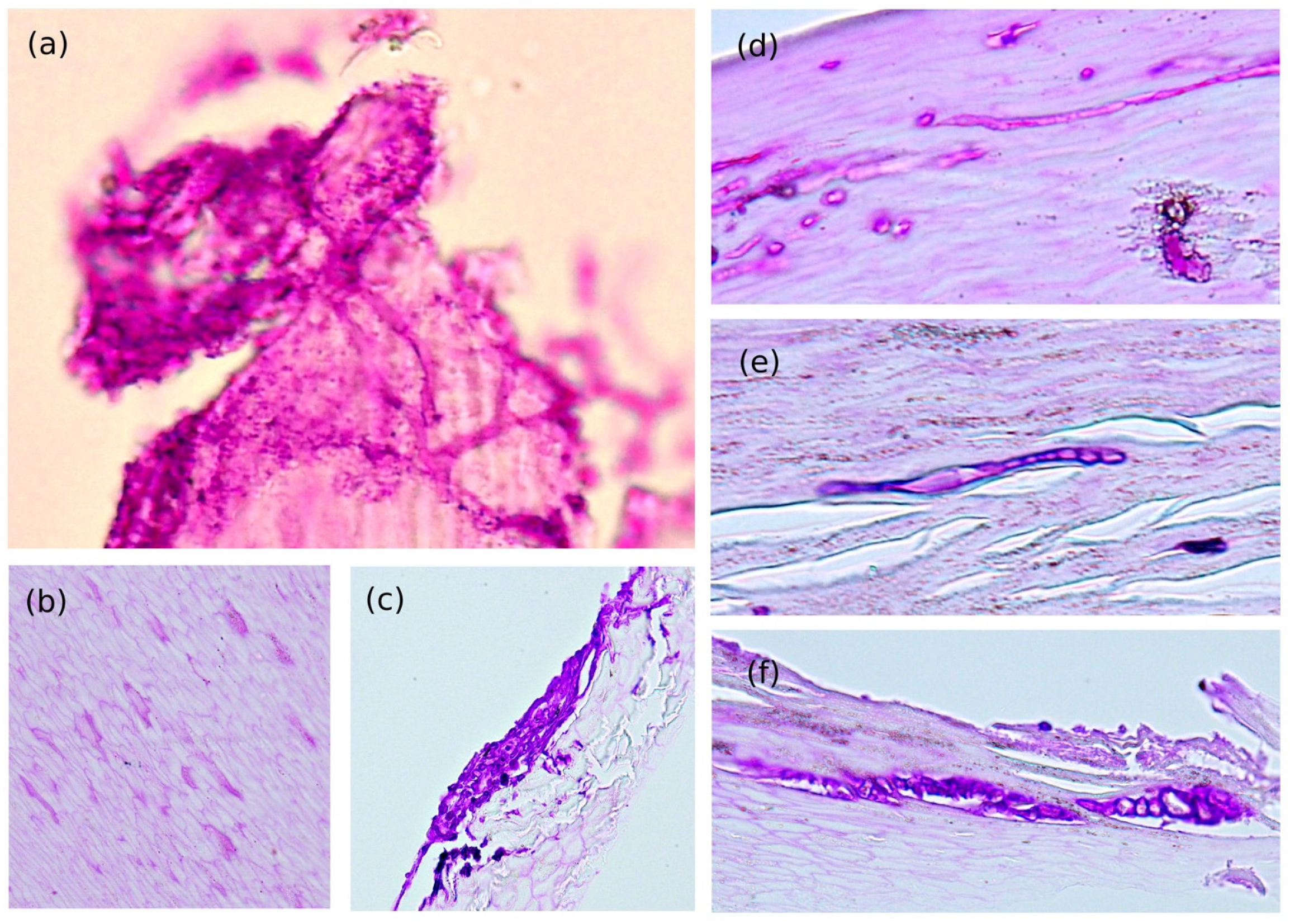
3.2. Direct Microscopic and Histological Findings
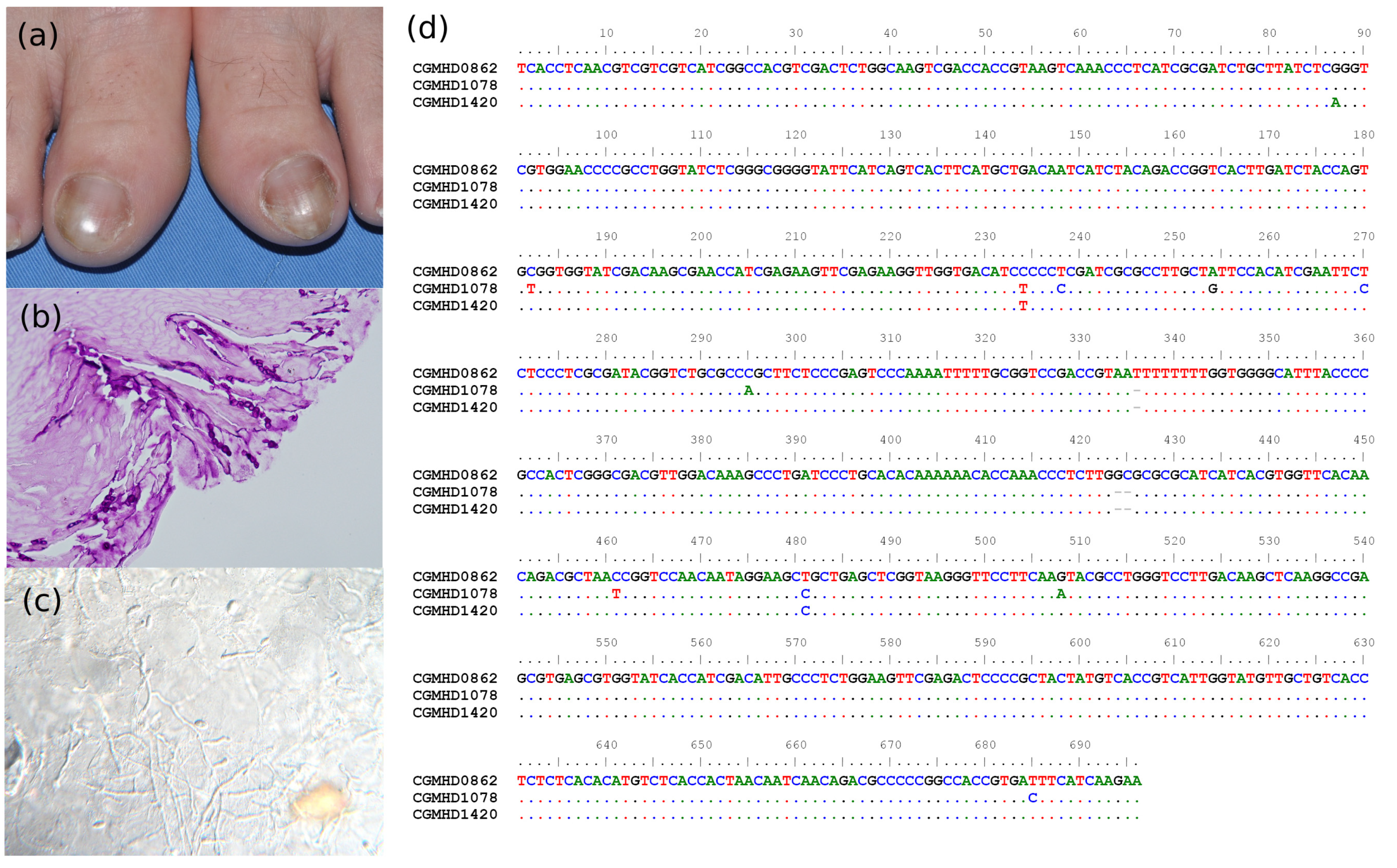
3.3. Molecular Identification
3.4. Antifungal Susceptibility Testing
3.5. Treatment Response and Prognosis
3.6. Presentation of Two Special Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geiser, D.M.; Al-Hatmi, A.; Aoki, T.; Arie, T.; Balmas, V.; Barnes, I.; Bergstrom, G.C.; Bhattacharyya, M.K.K.; Blomquist, C.L.; Bowden, R.; et al. Phylogenomic analysis of a 55.1 kb 19-gene dataset resolves a monophyletic Fusarium that includes the Fusarium solani Species Complex. Phytopathology 2020, 111, 1064–1079. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef]
- Nucci, F.; Nouer, S.A.; Capone, D.; Anaissie, E.; Nucci, M. Fusariosis. Semin. Respir. Crit. Care Med. 2015, 36, 706–714. [Google Scholar] [CrossRef]
- Guilhermetti, E.; Takahachi, G.; Shinobu, C.S.; Svidzinski, T.I. Fusarium spp. as agents of onychomycosis in immunocompetent hosts. Int. J. Dermatol. 2007, 46, 822–826. [Google Scholar] [CrossRef]
- Diongue, K.; Ndiaye, M.; Seck, M.C.; Diallo, M.A.; Badiane, A.S.; Ndiaye, D. Onychomycosis Caused by Fusarium spp. in Dakar, Senegal: Epidemiological, Clinical, and Mycological Study. Dermatol. Res. Pract. 2017, 2017, 1268130. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular Diversity and Intrinsic Drug Resistance. PLoS Pathog. 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Gupta, C.; Jongman, M.; Das, S.; Snehaa, K.; Bhattacharya, S.N.; Seyedmousavi, S.; van Diepeningen, A.D. Genotyping and In Vitro Antifungal Susceptibility Testing of Fusarium Isolates from Onychomycosis in India. Mycopathologia 2016, 181, 497–504. [Google Scholar] [CrossRef]
- Gupta, A.K.; Summerbell, R.C.; Venkataraman, M.; Quinlan, E.M. Nondermatophyte mould onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1628–1641. [Google Scholar] [CrossRef]
- Gupta, A.K.; Drummond-Main, C.; Cooper, E.A.; Brintnell, W.; Piraccini, B.M.; Tosti, A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012, 66, 494–502. [Google Scholar] [CrossRef]
- Mashima, J.; Kodama, Y.; Kosuge, T.; Fujisawa, T.; Katayama, T.; Nagasaki, H.; Okuda, Y.; Kaminuma, E.; Ogasawara, O.; Okubo, K.; et al. DNA data bank of Japan (DDBJ) progress report. Nucleic Acids Res. 2016, 44, D51–D57. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). M38: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; Approved Standard; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008. [Google Scholar]
- Bombace, F.; Iovene, M.R.; Galdiero, M.; Martora, F.; Nicoletti, G.F.; D’Andrea, M.; Della Pepa, M.E.; Vitiello, M. Non-dermatophytic onychomycosis diagnostic criteria: An unresolved question. Mycoses 2016, 59, 558–565. [Google Scholar] [CrossRef]
- Walshe, M.M.; English, M.P. Fungi in nails. Br. J. Dermatol. 1966, 78, 198–207. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cooper, E.A.; MacDonald, P.; Summerbell, R.C. Utility of inoculum counting (Walshe and English criteria) in clinical diagnosis of onychomycosis caused by nondermatophytic filamentous fungi. J. Clin. Microbiol. 2001, 39, 2115–2121. [Google Scholar] [CrossRef]
- Summerbell, R.C.; Cooper, E.; Bunn, U.; Jamieson, F.; Gupta, A.K. Onychomycosis: A critical study of techniques and criteria for confirming the etiologic significance of nondermatophytes. Med. Mycol. 2005, 43, 39–59. [Google Scholar] [CrossRef]
- Dignani, M.C.; Anaissie, E. Human fusariosis. Clin. Microbiol. Infect. 2004, 10, 67–75. [Google Scholar] [CrossRef]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A. Fusarium onychomycosis: Prevalence, clinical presentations, response to itraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int. J. Dermatol. 2015, 54, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Calado, N.B.; Sousa, F., Jr.; Gomes, N.O.; Cardoso, F.R.; Zaror, L.C.; Milan, E.P. Fusarium nail and skin infection: A report of eight cases from Natal, Brazil. Mycopathologia 2006, 161, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Matsumoto, T.; Kimura, U.; Hiruma, M.; Kano, R.; Yaguchi, T.; Ihn, H. Non-dermatophyte Mould Onychomycosis in Japan. Med. Mycol. J. 2020, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Uemura, E.V.G.; Barbosa, M.D.S.; Simionatto, S.; Al-Harrasi, A.; Al-Hatmi, A.M.S.; Rossato, L. Onychomycosis Caused by Fusarium Species. J. Fungi 2022, 8, 360. [Google Scholar] [CrossRef]
- Haghani, I.; Shams-Ghahfarokhi, M.; Dalimi Asl, A.; Shokohi, T.; Hedayati, M.T. Prevalence, genetic diversity and antifungal susceptibility profiles of F. fujikuroi, F. solani and Fusarium incarnatum-equiseti species complexes from onychomycosis in north of Iran. Mycoses 2022, 65, 1030–1039. [Google Scholar] [CrossRef]
- Baran, R.; Tosti, A.; Piraccini, B.M. Uncommon clinical patterns of Fusarium nail infection: Report of three cases. Br. J. Dermatol. 1997, 136, 424–427. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ryder, J.E.; Baran, R.; Summerbell, R.C. Non-dermatophyte onychomycosis. Dermatol. Clin. 2003, 21, 257–268. [Google Scholar] [CrossRef]
- Ranawaka, R.R.; de Silva, N.; Ragunathan, R.W. Onychomycosis caused by Fusarium sp in Sri Lanka: Prevalence, clinical features and response to itraconazole pulse therapy in six cases. J. Dermatol. Treat. 2008, 19, 308–312. [Google Scholar] [CrossRef]
- Rammlmair, A.; Muhlethaler, K.; Haneke, E. Fusarium onychomycoses in Switzerland-A mycological and histopathological study. Mycoses 2019, 62, 928–931. [Google Scholar] [CrossRef]
- Lavorato, F.G.; Guimarães, D.A.; Premazzi, M.G.; Piñeiro-Maceira, J.M.; Bernardes-Engemann, A.R.; Orofino-Costa, R. Performance of mycology and histopathology tests for the diagnosis of toenail onychomycosis due to filamentous fungi: Dermatophyte and non-dermatophyte moulds. Mycoses 2017, 60, 587–593. [Google Scholar] [CrossRef]
- Galletti, J.; Negri, M.; Grassi, F.L.; Kioshima-Cotica, E.S.; Svidzinski, T.I. Fusarium spp. is able to grow and invade healthy human nails as a single source of nutrients. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Gugnani, H.C. Nondermatophytes filamentous keratinophilic fungi and their role in human infection. Rev. Iberoam. Micol. 2000, 17, 109–114. [Google Scholar]
- Ito, T.; Meyer, K.C.; Ito, N.; Paus, R. Immune privilege and the skin. Curr. Dir. Autoimmun. 2008, 10, 27–52. [Google Scholar] [CrossRef] [PubMed]
- De Doncker, P.R.; Scher, R.K.; Baran, R.L.; Decroix, J.; Degreef, H.J.; Roseeuw, D.I.; Havu, V.; Rosen, T.; Gupta, A.K.; Piérard, G.E. Itraconazole therapy is effective for pedal onychomycosis caused by some nondermatophyte molds and in mixed infection with dermatophytes and molds: A multicenter study with 36 patients. J. Am. Acad. Dermatol. 1997, 36, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gregurek-Novak, T.; Konnikov, N.; Lynde, C.W.; Hofstader, S.; Summerbell, R.C. Itraconazole and terbinafine treatment of some nondermatophyte molds causing onychomycosis of the toes and a review of the literature. J. Cutan. Med. Surg. 2001, 5, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Bonifaz, A.; Ranque, S.; Sybren de Hoog, G.; Verweij, P.E.; Meis, J.F. Current antifungal treatment of fusariosis. Int. J. Antimicrob. Agents 2018, 51, 326–332. [Google Scholar] [CrossRef]
- Verrier, J.; Bontems, O.; Baudraz-Rosselet, F.; Monod, M. Oral terbinafine and itraconazole treatments against dermatophytes appear not to favor the establishment of Fusarium spp. in nail. Dermatology 2014, 228, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Bonifaz, A.; Calderón, L.; Curfs-Breuker, I.; Meis, J.F.; van Diepeningen, A.D.; de Hoog, G.S. Proximal subungual onychomycosis caused by Fusarium falciforme successfully cured with posaconazole. Br. J. Dermatol. 2015, 173, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Lurati, M.; Baudraz-Rosselet, F.; Vernez, M.; Spring, P.; Bontems, O.; Fratti, M.; Monod, M. Efficacious treatment of non-dermatophyte mould onychomycosis with topical amphotericin B. Dermatology 2011, 223, 289–292. [Google Scholar] [CrossRef]
- Khurana, A.; Chowdhary, A.; Sardana, K.; Gautam, R.K.; Sharma, P.K. Complete cure of Fusarium solani sp. complex onychomycosis with Qs NdYAG treatment. Dermatol. Ther. 2018, 31, e12580. [Google Scholar] [CrossRef]
- Espírito-Santo, G.A.D.; Leite, D.P., Jr.; Hoffmann-Santos, H.D.; Dias, L.B.; Hahn, R.C. 1340nm Laser Therapy For Onychomycosis: Negative Results of Prospective Treatment of 72 Toenails and a Literature Review. J. Clin. Aesthetic Dermatol. 2017, 10, 56–61. [Google Scholar]
- Bowornsathitchai, N.; Thammahong, A.; Shoosanglertwijit, J.; Kitsongsermthon, J.; Wititsuwannakul, J.; Asawanonda, P.; Boontaveeyuwat, E. Methylene blue-mediated photodynamic therapy may be superior to 5% amorolfine nail lacquer for non-dermatophyte onychomycosis. Photodermatol. Photoimmunol. Photomed. 2021, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Noguchi, H.; Matsumoto, T.; Kimura, U.; Hiruma, M.; Kano, R.; Yaguchi, T.; Fujimoto, N.; Satoh, T.; Ihn, H. Ungual hyalohyphomycosis caused by Fusarium cugenangense. Clin. Case Rep. 2020, 8, 3533–3538. [Google Scholar] [CrossRef] [PubMed]
- Badali, H.; Cañete-Gibas, C.; Patterson, H.; Sanders, C.; Mermella, B.; Garcia, V.; Mele, J.; Fan, H.; Wiederhold, N.P. In vitro activity of olorofim against clinical isolates of the Fusarium oxysporum and Fusarium solani species complexes. Mycoses 2021, 64, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.D.; Heidrich, D.; Correa, C.; Scroferneker, M.L.; Vettorato, G.; Fuentefria, A.M.; Goldani, L.Z. Genetic diversity and antifungal susceptibility of Fusarium isolates in onychomycosis. Mycoses 2017, 60, 616–622. [Google Scholar] [CrossRef]
- Castro Lopez, N.; Casas, C.; Sopo, L.; Rojas, A.; Del Portillo, P.; Cepero de Garcia, M.C.; Restrepo, S. Fusarium species detected in onychomycosis in Colombia. Mycoses 2009, 52, 350–356. [Google Scholar] [CrossRef]
- Motamedi, M.; Ghasemi, Z.; Shidfar, M.R.; Hosseinpour, L.; Khodadadi, H.; Zomorodian, K.; Mirhendi, H. Growing Incidence of Non-Dermatophyte Onychomycosis in Tehran, Iran. Jundishapur J. Microbiol. 2016, 9, e40543. [Google Scholar] [CrossRef]
- Bansal, Y.; Singla, N.; Kaistha, N.; Sood, S.; Chander, J. Molecular identification of Fusarium species complex isolated from clinical samples and its antifungal susceptibility patterns. Curr. Med. Mycol. 2019, 5, 43–49. [Google Scholar] [CrossRef]
- Godoy, P.; Nunes, E.; Silva, V.; Tomimori-Yamashita, J.; Zaror, L.; Fischman, O. Onychomycosis caused by Fusarium solani and Fusarium oxysporum in Sao Paulo, Brazil. Mycopathologia 2004, 157, 287–290. [Google Scholar] [CrossRef]
- Tosti, A.; Piraccini, B.M.; Lorenzi, S. Onychomycosis caused by nondermatophytic molds: Clinical features and response to treatment of 59 cases. J. Am. Acad. Dermatol. 2000, 42, 217–224. [Google Scholar] [CrossRef]
- Phaitoonwattanakij, S.; Leeyaphan, C.; Lertrujiwanit, K.; Bunyaratavej, S. Predisposing factors, clinical features and treatment outcomes of Fusarium onychomycosis and comparison of its characteristics with Neoscytalidium onychomycosis. J. Mycol. Med. 2021, 31, 101165. [Google Scholar] [CrossRef] [PubMed]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A.; Weerakoon, H.S.; de Silva, S.H. Randomized, double-blind, comparative study on efficacy and safety of itraconazole pulse therapy and terbinafine pulse therapy on nondermatophyte mold onychomycosis: A study with 90 patients. J. Dermatol. Treat. 2016, 27, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Van Diepeningen, A.D.; Feng, P.; Ahmed, S.; Sudhadham, M.; Bunyaratavej, S.; de Hoog, G.S. Spectrum of Fusarium infections in tropical dermatology evidenced by multilocus sequencing typing diagnostics. Mycoses 2015, 58, 48–57. [Google Scholar] [CrossRef] [PubMed]


| Sex/Age | Immune Status | Location | Duration (Month) | Species | Treatment | Prognosis | Contact to Soil | |
|---|---|---|---|---|---|---|---|---|
| FFSC | F/77 | IC | Right big toenail | 2 | Fusarium denticulatum | TAF | GR | Yes |
| F/61 | IC | Right middle finger | 1 | Fusarium annulatum | Surgery | GR | Yes | |
| F/84 | Lung cancer | Fingernails | 6 | Fusarium annulatum | ITC + TAF | GR | No | |
| FIESC | F/65 | Paraneoplastic pemphigus | Fingernails | 108 | Fusarium pernambucanum (FIESC 17) | ITC + TAF | PoR | No |
| M/46 | Tuberous sclerosis, left RCC | Toenails | 3 | Fusarium arcuatisporum (FIESC 7) | TAF | PaR | No | |
| FOSC | F/54 | IC | Right big toenail | 53 | Fusarium curvatum | TAF | Los | No |
| M/36 | IC | Bilateral big toenails | 4 | Fusarium sp. | ITC + TAF | GR | No | |
| FSSC | F/60 | SLE | Fingernails | 7 | Fusarium keratoplasticum (FSSC 2) Fusarium solani (FSSC 5) | ITC/TRB + TAF | GR | No |
| M/21 | IC | Left big toenail | 4 | Fusarium solani (FSSC 5) | TAF | PaR | No | |
| M/43 | IC | Bilateral big toenail | 1 | Fusarium falciforme (FSSC 3 + 4) | TAF | PoR | No | |
| F/58 | DM | Right big toenail | 32 | Fusarium falciforme (FSSC 3 + 4) | TRB + TAF | PoR | No | |
| F/45 | IC | Left big toenail | 4 | Fusarium keratoplasticum (FSSC 2) | TRB +TAF | GR | No | |
| F/67 | IC | Bilateral big toenail | 60 | Fusarium keratoplasticum (FSSC 2) | TRB + TAF | PoR | No | |
| M/77 | IC | Bilateral thumb | 48 | Fusarium keratoplasticum (FSSC 2) | TRB +TAF | PoR | Yes | |
| F/3 | IC | Fingernails + Toenails | 15 | Fusarium keratoplasticum (FSSC 2) | TAF | PoR | No | |
| F/31 | IC | Left big toenail | 32 | Fusarium keratoplasticum (FSSC 2) | ITC/TRB + TAF | GR | No | |
| M/34 | IC | Bilateral toenails | 4 | Fusarium keratoplasticum (FSSC 2) | TRB + TAF | GR | No | |
| F/79 | IC | Fingernails + Toenails | 25 | Fusarium keratoplasticum (FSSC 2) | TAF | PoR | No | |
| F/55 | IC | Bilateral big toenail | 41 | Fusarium keratoplasticum (FSSC 2) | Laser + Griseolfulbin + TAF | GR | No | |
| M/60 | IC | Fingernails + Toenails | 41 | Fusarium keratoplasticum (FSSC 2) | TRB + TAF | PaR | No | |
| M/45 | IC | Right big toenail | 2 | Fusarium keratoplasticum (FSSC 2) | TAF | PoR | No | |
| F/65 | IC | Right big toenail | 24 | Fusarium keratoplasticum (FSSC 2) | TRB + TAF | PoR | No | |
| M/48 | IC | Fingernails + Toenails | 9 | Fusarium keratoplasticum (FSSC 2) | TAF | PoR | No | |
| F/87 | Terminal ileal cancer | Bilateral big toenail | 36 | Fusarium keratoplasticum (FSSC 2) | TAF | PoR | No | |
| M/70 | DM | Left thumb | 2 | Fusarium suttonianum (FSSC 20) | TAF | GR | Yes | |
| M/77 | IC | Fingernails + Toenails | 6 | Fusarium lichenicola (FSSC 16) | TAF | PoR | Yes | |
| M/65 | IC | Right big toenail | 5 | Nectria bolbophylli | TRB + TAF | GR | No | |
| M/20 | IC | Left 2nd fingernail | 1 | Fusarium sp. | TAF | Los | No | |
| F/61 | IC | Fingernails + Toenails | 5 | Fusarium sp. | TRB + TAF | GR | Yes |
| (a) Frequently Branching Irregularly Septated Hyphae | (b) Arbitrarily Widening Hyphae | (c) Dermatophytoma Like Fungal Mass | (d) Thin Hyphae | (e) Moniliform Hyphae | (f) Hyphae with Terminal Swelling | |
|---|---|---|---|---|---|---|
| FSSC (N = 23) | ||||||
| F. keratoplasticum | 4 | 1 | 3 | 7 | 4 | |
| F. falciforme | 1 | 1 | ||||
| F. solani SC | 1 | |||||
| F. suttonianum | 1 | 1 | ||||
| F. lichenicola | 1 | |||||
| Nectria bolbophylli | 1 | |||||
| Fusarium species | 1 | 1 | 1 | |||
| FFSC (N = 3) | ||||||
| F. denticulatum | 1 | 1 | ||||
| F. annulatum | 2 | |||||
| FOSC (N = 2) | ||||||
| F. curvatum | 1 | |||||
| FIESC (N = 2) | ||||||
| F. pernambucanum | 1 | |||||
| F. arcuatisporum | 1 | |||||
| Total | 14 | 2 | 4 | 1 | 10 | 5 |
| Species | RLMM No. | Accession Number | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB | TRB | FLC | ITC | EFC | LNC | LLC | VRC | NAT | ITS | EF1a | |||
| FFSC | F. annulatum | CGMHD0248 | 1 | 4 | >64 | >32 | 0.25 | 0.063 | 0.031 | 4 | 4 | LC687503 | LC697741 |
| F. annulatum | CGMHD2913 | 2 | 4 | >64 | >32 | 0.5 | 0.125 | 0.063 | 4 | 8 | LC687504 | LC697742 | |
| F. denticulatum | CGMHD1101 | 1 | 2 | >64 | 4 | 0.125 | 0.031 | <0.008 | 1 | 4 | LC687507 | LC697745 | |
| FIESC | F. arcuatisporum | CGMHD0667 | ND | ND | ND | ND | ND | ND | ND | ND | ND | LC687505 | LC697743 |
| F. pernambucanum | CGMHD0550 | 1 | >32 | >64 | 32 | 0.5 | 0.063 | 0.063 | 2 | 4 | LC687537 | LC697775 | |
| FOSC | F. curvatum | CGMHD0436 | 1 | 8 | >64 | >32 | 0.5 | 0.125 | 0.063 | 4 | 4 | LC687506 | LC697744 |
| Fusarium sp. | CGMHD3594 | 2 | 4 | >64 | >32 | 0.5 | 0.125 | 0.063 | 8 | 8 | LC687546 | LC697784 | |
| Fusarium sp. | CGMHD3699 | 4 | 2 | >64 | >32 | 0.5 | 0.125 | 0.063 | 8 | 8 | LC687547 | LC697785 | |
| FSSC | F. falciforme | CGMHD0414 | 1 | >32 | >64 | >32 | 1 | 0.5 | 0.25 | 8 | 8 | LC687508 | LC697746 |
| F. falciforme | CGMHD2876 | 2 | >32 | >64 | >32 | 1 | 0.125 | 0.031 | 4 | 8 | LC687509 | LC697747 | |
| F. keratoplasticum | CGMHD0234 | 2 | >32 | >64 | >32 | 2 | 0.5 | 0.125 | 8 | 4 | LC687510 | LC697748 | |
| F. keratoplasticum | CGMHD0321 | 1 | >32 | >64 | >32 | 4 | 0.25 | 0.125 | 8 | 4 | LC687511 | LC697749 | |
| F. keratoplasticum | CGMHD0549 | 2 | >32 | >64 | >32 | 2 | 0.5 | 0.125 | 8 | 4 | LC687512 | LC697750 | |
| F. keratoplasticum | CGMHD0562 | >16 | >32 | >64 | >32 | >4 | >4 | >4 | >16 | >32 | LC687513 | LC697751 | |
| F. keratoplasticum | CGMHD0658 | 2 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687514 | LC697752 | |
| F. keratoplasticum | CGMHD0666 | 2 | >32 | >64 | >32 | 2 | 0.25 | 0.125 | 8 | 4 | LC687515 | LC697753 | |
| F. keratoplasticum | CGMHD0683 | 2 | >32 | >64 | >32 | 2 | 0.25 | 0.125 | 8 | 4 | LC687516 | LC697754 | |
| F. keratoplasticum | CGMHD0693 | 2 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687517 | LC697755 | |
| F. keratoplasticum | CGMHD0702 | 1 | >32 | >64 | >32 | 1 | 0.25 | 0.063 | 8 | 4 | LC687518 | LC697756 | |
| F. keratoplasticum | CGMHD0740 | 4 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687519 | LC697757 | |
| F. keratoplasticum | CGMHD0821 | 4 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687520 | LC697758 | |
| F. keratoplasticum | CGMHD0862 | 2 | >32 | >64 | >32 | 2 | 0.5 | 0.125 | 8 | 4 | LC687521 | LC697759 | |
| F. keratoplasticum | CGMHD0974 | >16 | >32 | >64 | >32 | >4 | 0.25 | >4 | >16 | >32 | LC687522 | LC697760 | |
| F. keratoplasticum | CGMHD1078 | 2 | >32 | >64 | >32 | 2 | 0.5 | 0.125 | 8 | 4 | LC687523 | LC697761 | |
| F. keratoplasticum | CGMHD1220 | 2 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687524 | LC697762 | |
| F. keratoplasticum | CGMHD1235 | 2 | >32 | >64 | >32 | 0.5 | 0.125 | 0.031 | 2 | 4 | LC687525 | LC697763 | |
| F. keratoplasticum | CGMHD1268 | 2 | >32 | >64 | >32 | 2 | 1 | 0.125 | 16 | 4 | LC687526 | LC697764 | |
| F. keratoplasticum | CGMHD1420 | 1 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687527 | LC697765 | |
| F. keratoplasticum | CGMHD1875 | 2 | >32 | >64 | >32 | 0.5 | 0.125 | 0.031 | 4 | 4 | LC687528 | LC697766 | |
| F. keratoplasticum | CGMHD1983 | 4 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 16 | 4 | LC687529 | LC697767 | |
| F. keratoplasticum | CGMHD2223 | 4 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687530 | LC697768 | |
| F. keratoplasticum | CGMHD2617 | 4 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687531 | LC697769 | |
| F. keratoplasticum | CGMHD3297 | 2 | >32 | >64 | >32 | 0.5 | 0.125 | 0.063 | 2 | 4 | LC687532 | LC697770 | |
| F. keratoplasticum | CGMHD3335 | 2 | >32 | >64 | >32 | 1 | 0.25 | 0.063 | 4 | 4 | LC687533 | LC697771 | |
| F. keratoplasticum | CGMHD3978 | 4 | >32 | >64 | >32 | 2 | 0.25 | 0.063 | 8 | 4 | LC687534 | LC697772 | |
| F. keratoplasticum | CGMHD4568 | 2 | >32 | >64 | >32 | 1 | 0.25 | 0.063 | 8 | 4 | LC687535 | LC697773 | |
| F. lichenicola | CGMHD2213 | 1 | >32 | >64 | >32 | 0.5 | 0.125 | 0.063 | 4 | 16 | LC687536 | LC697774 | |
| F. solani | CGMHD0530 | 0.5 | >32 | >64 | >32 | 1 | 0.25 | 0.063 | 8 | 8 | LC687538 | LC697776 | |
| F. solani | CGMHD0975 | 1 | >32 | >64 | >32 | 2 | 0.5 | 0.063 | 8 | 4 | LC687539 | LC697777 | |
| F. solani | CGMHD0976 | 1 | >32 | >64 | >32 | 2 | 0.5 | 0.063 | 8 | 4 | LC687540 | LC697778 | |
| F. solani | CGMHD0977 | 1 | >32 | >64 | >32 | 2 | 0.5 | 0.063 | 8 | 4 | LC687541 | LC697779 | |
| F. solani | CGMHD1080 | 1 | >32 | >64 | >32 | 2 | 0.5 | 0.063 | 8 | 4 | LC687542 | LC697780 | |
| F. solani | CGMHD1329 | 0.5 | >32 | >64 | >32 | 1 | 0.5 | 0.125 | 8 | 4 | LC687543 | LC697781 | |
| Fusarium sp. | CGMHD0943 | >16 | >32 | >64 | >32 | >4 | 0.5 | >4 | >16 | >32 | LC687544 | LC697782 | |
| Fusarium sp. | CGMHD0944 | 4 | 32 | >64 | >32 | 0.5 | 0.125 | 0.031 | 4 | 8 | LC687545 | LC697783 | |
| F. suttonianum | CGMHD1911 | 0.5 | >32 | >64 | >32 | 1 | 0.5 | 0.25 | 4 | 8 | LC687548 | LC697786 | |
| Nectria bolbophylli | CGMHD0225 | 4 | >32 | >64 | >32 | 2 | 0.5 | 0.125 | 16 | 4 | LC687549 | LC697787 | |
| Treatment Methods | GR | PaR | PoR | Los |
|---|---|---|---|---|
| Itraconazole + topical antifungals (N = 2) | 1 | 0 | 1 | 0 |
| Terbinafine + topical antifungals (N = 9) | 3 | 1 | 5 | 0 |
| Itraconazole/Terbinafine + topical antifungals (N = 3) | 3 | 0 | 0 | 0 |
| Topical antifungals only (N = 13) | 2 | 2 | 7 | 2 |
| Laser + Griseofulvin + topical antifungals (N = 1) | 1 | 0 | 0 | 0 |
| Surgery (N = 1) | 1 | 0 | 0 | 0 |
| Total number (N = 29) | 11 | 3 | 13 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.-Y.; Ou, J.-H.; Hui, R.C.-Y.; Chuang, Y.-H.; Fan, Y.-C.; Sun, P.-L. High Diversity of Fusarium Species in Onychomycosis: Clinical Presentations, Molecular Identification, and Antifungal Susceptibility. J. Fungi 2023, 9, 534. https://doi.org/10.3390/jof9050534
Lu L-Y, Ou J-H, Hui RC-Y, Chuang Y-H, Fan Y-C, Sun P-L. High Diversity of Fusarium Species in Onychomycosis: Clinical Presentations, Molecular Identification, and Antifungal Susceptibility. Journal of Fungi. 2023; 9(5):534. https://doi.org/10.3390/jof9050534
Chicago/Turabian StyleLu, Lai-Ying, Jie-Hao Ou, Rosaline Chung-Yee Hui, Ya-Hui Chuang, Yun-Chen Fan, and Pei-Lun Sun. 2023. "High Diversity of Fusarium Species in Onychomycosis: Clinical Presentations, Molecular Identification, and Antifungal Susceptibility" Journal of Fungi 9, no. 5: 534. https://doi.org/10.3390/jof9050534
APA StyleLu, L.-Y., Ou, J.-H., Hui, R. C.-Y., Chuang, Y.-H., Fan, Y.-C., & Sun, P.-L. (2023). High Diversity of Fusarium Species in Onychomycosis: Clinical Presentations, Molecular Identification, and Antifungal Susceptibility. Journal of Fungi, 9(5), 534. https://doi.org/10.3390/jof9050534






