Development of a Multiplex qPCR Assay for Fast Detection and Differentiation of Paracoccidioidomycosis Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Fungal Isolates and Culture Conditions
2.3. DNA Extraction
2.4. Primer and Probe Design and In Silico PCR
2.5. In Silico Validation of Primers and Probe Sequences
2.6. qPCR Optimization
2.7. Determining Assay Specificity In Vitro
2.8. Determining Assay Sensitivity
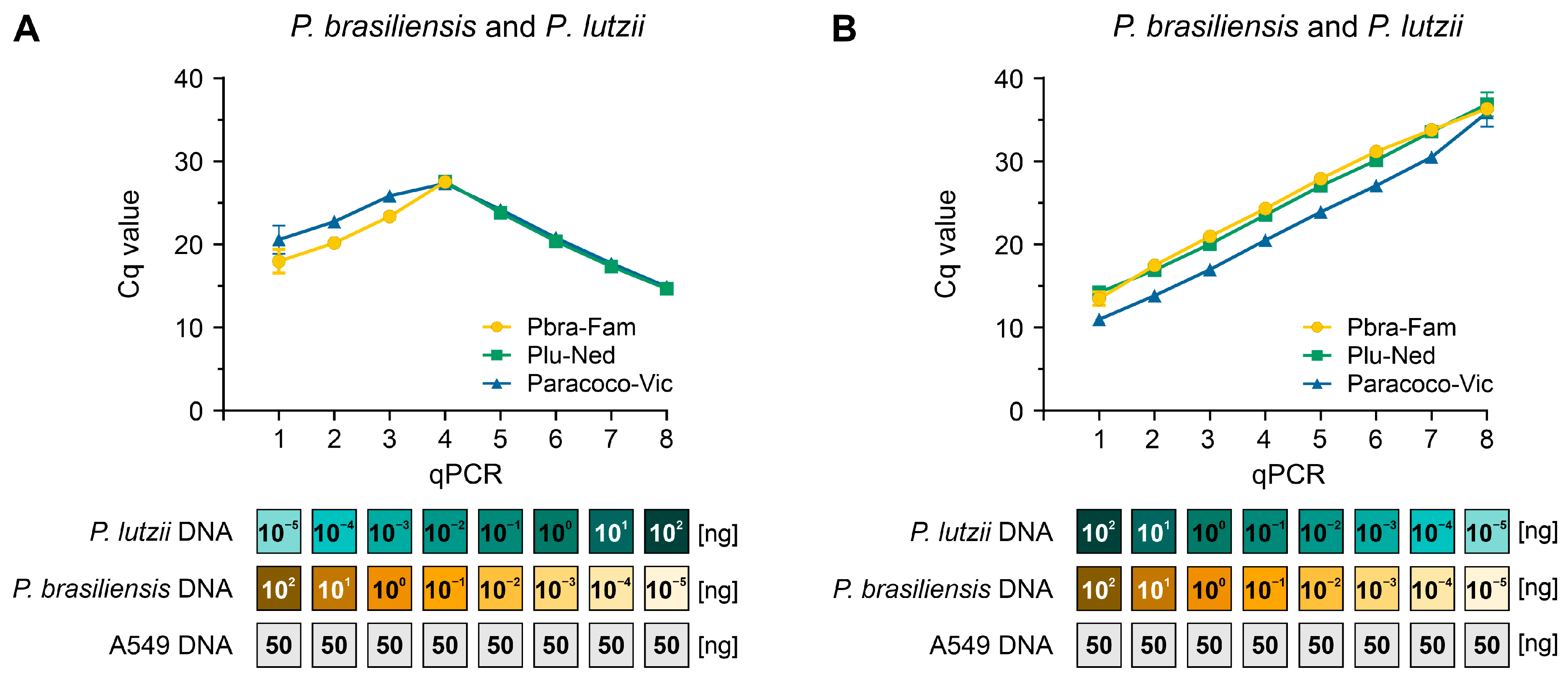
2.9. Target Template Competition
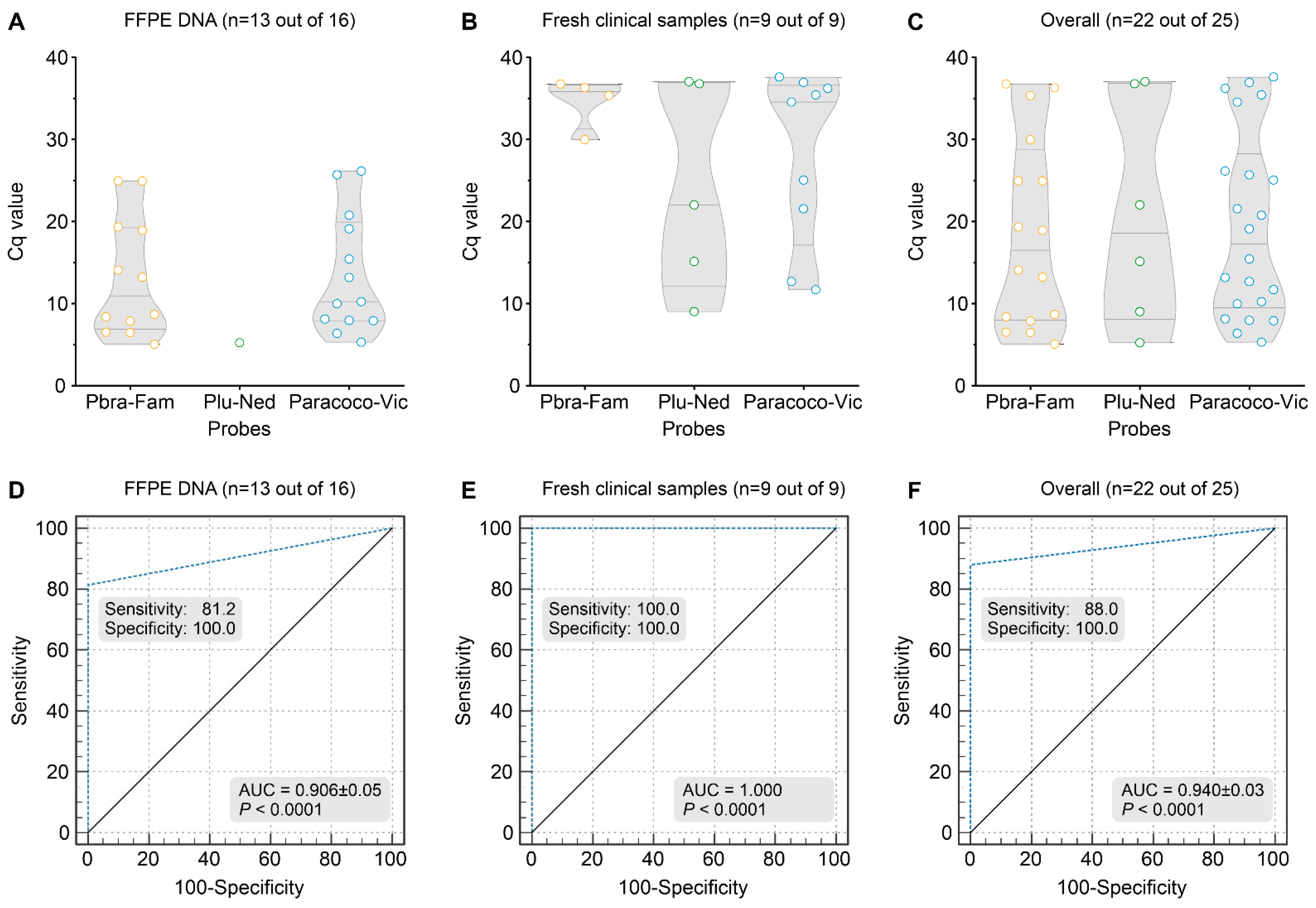
2.10. Detection of Paracoccidioides DNA from Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue and Other Biological Samples
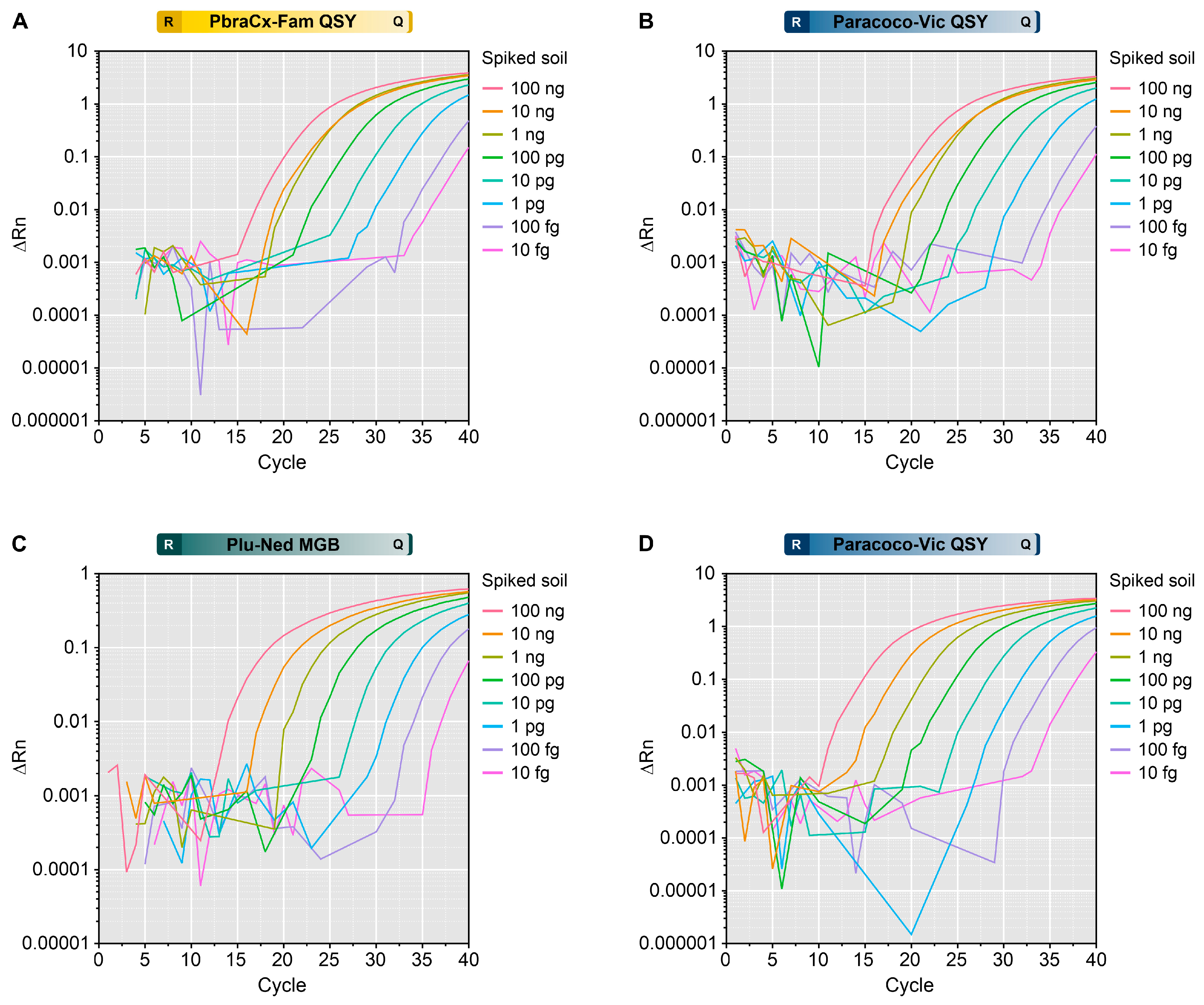
2.11. Detection of Paracoccidioides DNA from Spiked Soil Samples
2.12. Statistical Analysis
3. Results
3.1. Primers and Probes Design
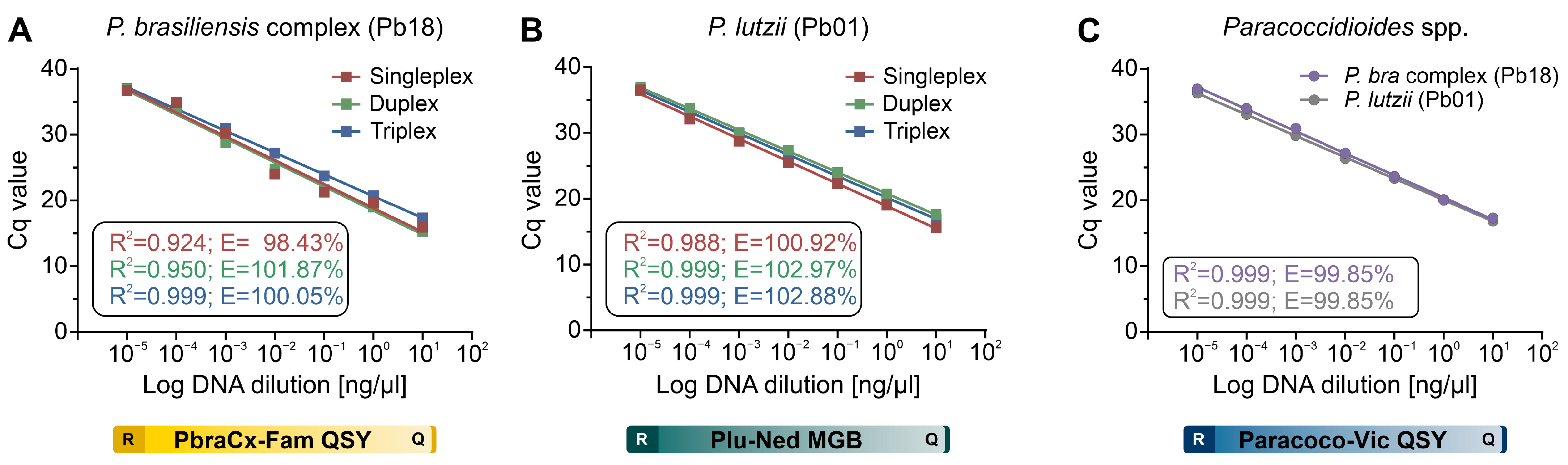
3.2. qPCR Standardization
3.3. qPCR Efficiency
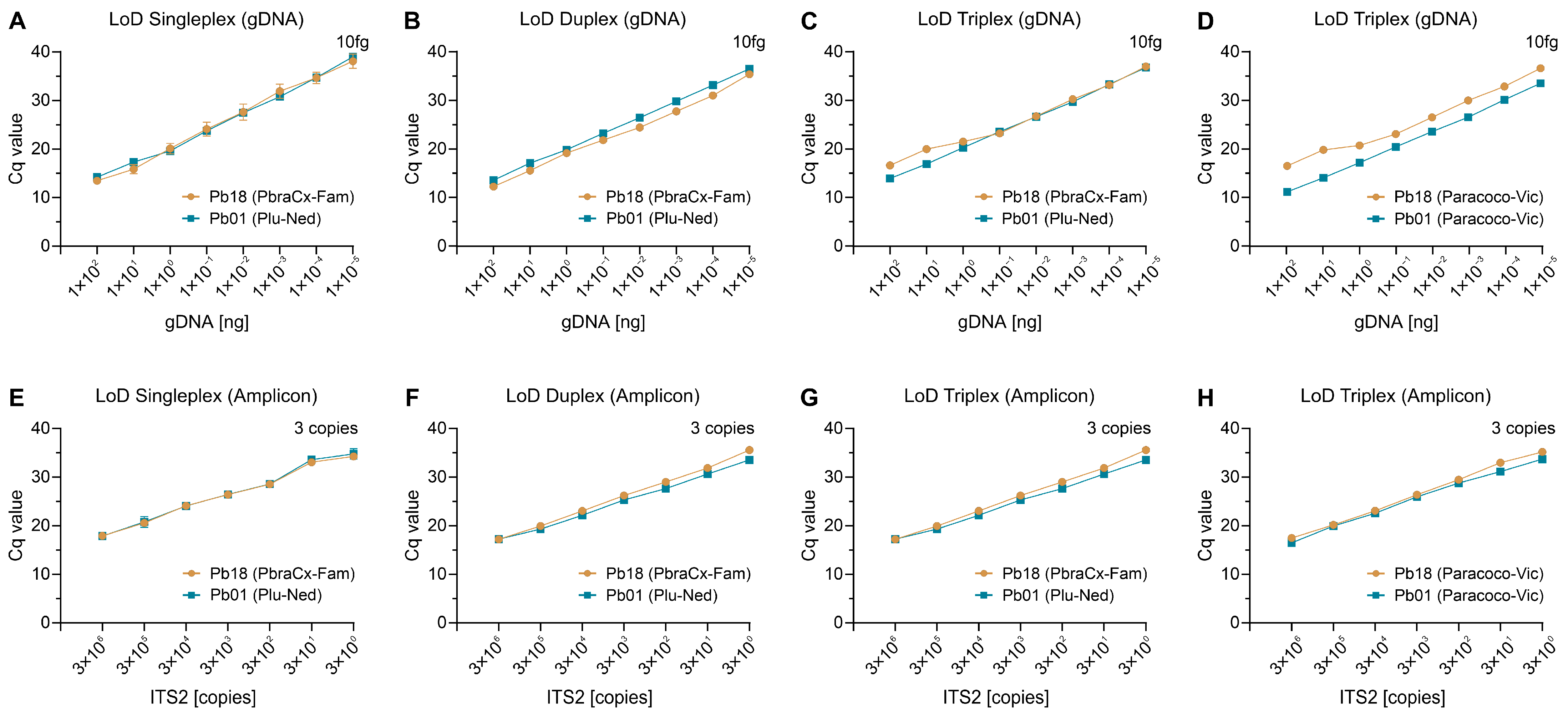
3.4. Analytical Sensitivity (LoD)
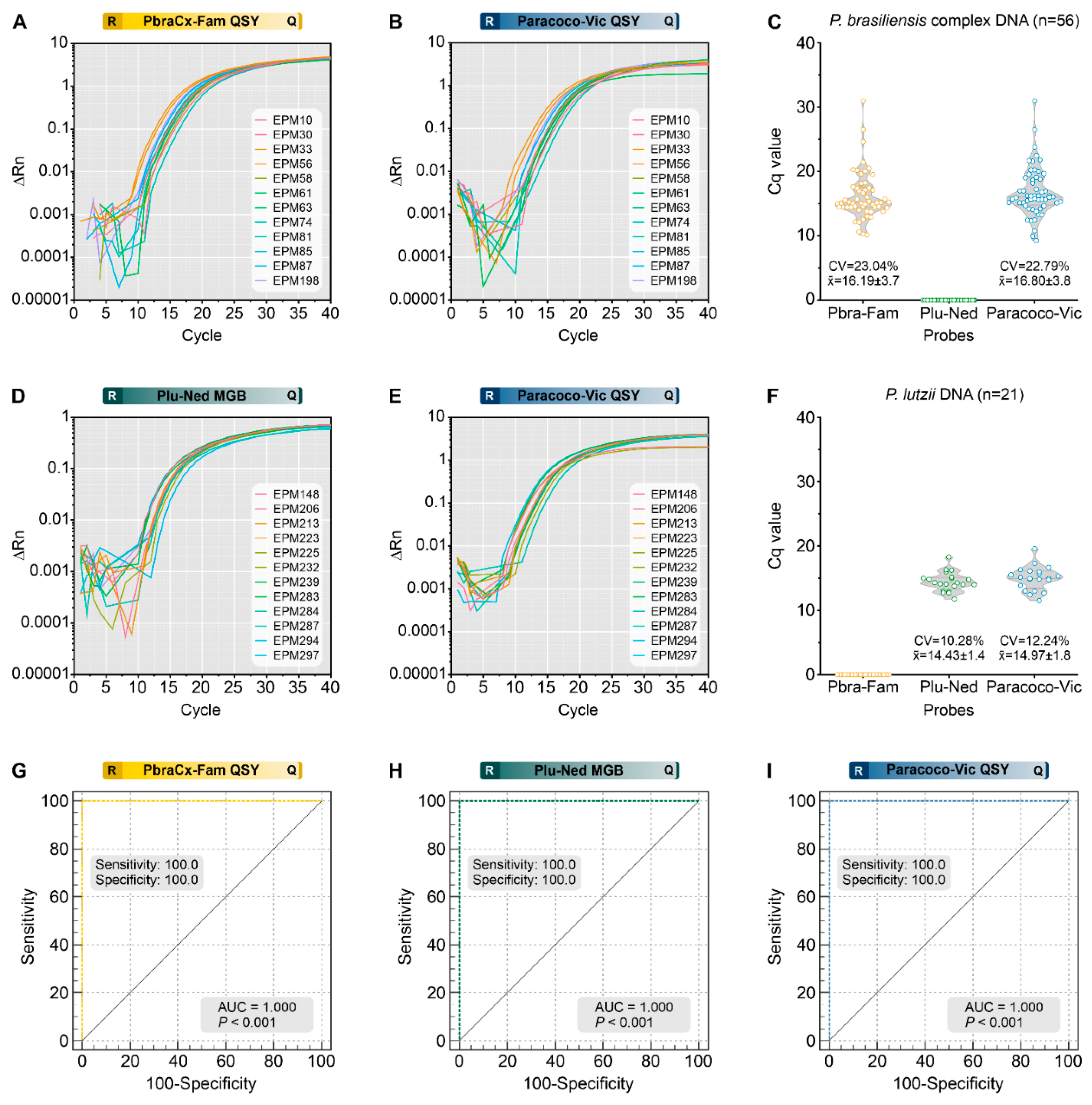
3.5. Analytical Specificity
3.6. Target Template Competition
3.7. Detection of Paracoccidioides DNA from Clinical Samples
3.8. Detection of Paracoccidioides DNA from Spiked Soil Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, A.M.; Hagen, F.; Puccia, R.; Hahn, R.C.; de Camargo, Z.P. Paracoccidioides and paracoccidioidomycosis in the 21st century. Mycopathologia, 2023, in press. [CrossRef]
- Kidd, S.E.; Abdolrasouli, A.; Hagen, F. Fungal nomenclature: Managing change is the name of the game. Open Forum Infect. Dis. 2023, 10, ofac559. [Google Scholar] [CrossRef] [PubMed]
- Carrero, L.L.; Nino-Vega, G.; Teixeira, M.M.; Carvalho, M.J.; Soares, C.M.; Pereira, M.; Jesuino, R.S.; McEwen, J.G.; Mendoza, L.; Taylor, J.W.; et al. New Paracoccidioides brasiliensis isolate reveals unexpected genomic variability in this human pathogen. Fungal Genet. Biol. 2008, 45, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Theodoro, R.C.; de Carvalho, M.J.A.; Fernandes, L.; Paes, H.C.; Hahn, R.C.; Mendoza, L.; Bagagli, E.; San-Blas, G.; Felipe, M.S.S. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol. Phylogenet. Evol. 2009, 52, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.C.; Rodrigues, A.M.; Della Terra, P.P.; Nery, A.F.; Hoffmann-Santos, H.D.; Góis, H.M.; Fontes, C.J.; de Camargo, Z.P. Clinical and epidemiological features of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2019, 13, e0007437. [Google Scholar] [CrossRef] [PubMed]
- de Macedo, P.M.; Teixeira, M.D.M.; Barker, B.M.; Zancopé-Oliveira, R.M.; Almeida-Paes, R.; Francesconi do Valle, A.C. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl. Trop. Dis. 2019, 13, e0007309. [Google Scholar] [CrossRef]
- Vilela, R.; Huebner, M.; Vilela, C.; Vilela, G.; Pettersen, B.; Oliveira, C.; Mendoza, L. The taxonomy of two uncultivated fungal mammalian pathogens is revealed through phylogeny and population genetic analyses. Sci. Rep. 2021, 11, 18119. [Google Scholar] [CrossRef]
- Hahn, R.C.; Hagen, F.; Mendes, R.P.; Burger, E.; Nery, A.F.; Siqueira, N.P.; Guevara, A.; Rodrigues, A.M.; de Camargo, Z.P. Paracoccidioidomycosis: Current status and future trends. Clin. Microbiol. Rev. 2022, 35, e0023321. [Google Scholar] [CrossRef]
- Roberto, T.N.; De Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Exploring genetic diversity, population structure, and phylogeography in Paracoccidioides species using AFLP markers. Stud. Mycol. 2021, 100, 100131. [Google Scholar] [CrossRef]
- Theodoro, R.C.; Teixeira, M.D.M.; Felipe, M.S.S.; Paduan, K.D.S.; Ribolla, P.M.; San-Blas, G.; Bagagli, E. Genus Paracoccidioides: Species recognition and biogeographic aspects. PLoS ONE 2012, 7, e37694. [Google Scholar] [CrossRef] [PubMed]
- Theodoro, R.C.; Bagagli, E.; Oliveira, C. Phylogenetic analysis of PRP8 intein in Paracoccidioides brasiliensis species complex. Fungal Genet. Biol. 2008, 45, 1284–1291. [Google Scholar] [CrossRef]
- Gonçalves, F.G.; Rosa, P.S.; Belone, A.F.F.; Carneiro, L.B.; de Barros, V.L.Q.; Bispo, R.F.; Sbardelott, Y.; Neves, S.; Vittor, A.Y.; Woods, W.J.; et al. Lobomycosis epidemiology and management: The quest for a cure for the most neglected of neglected tropical diseases. J. Fungi 2022, 8, 494. [Google Scholar] [CrossRef]
- Camargo, Z.P.; Rodrigues, A.M. Paracoccidioides complex. In Pocket Guide to Mycological Diagnosis; Cordeiro, R.D.A., Yuan, L., Eds.; Pocket Guides to Biomedical Sciences; CRC Press: Boca Raton, FL, USA, 2019; Volume 78, pp. 125–134. [Google Scholar]
- Gomes, G.M.; Cisalpino, P.S.; Taborda, C.P.; de Camargo, Z.P. PCR for diagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 2000, 38, 3478–3480. [Google Scholar] [CrossRef] [PubMed]
- Roberto, T.N.; Rodrigues, A.M.; Hahn, R.C.; de Camargo, Z.P. Identifying Paracoccidioides phylogenetic species by PCR-RFLP of the alpha-tubulin gene. Med. Mycol. 2016, 54, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Matute, D.R.; Sepulveda, V.E.; Quesada, L.M.; Goldman, G.H.; Taylor, J.W.; Restrepo, A.; McEwen, J.G. Microsatellite analysis of three phylogenetic species of Paracoccidioides brasiliensis. J. Clin. Microbiol. 2006, 44, 2153–2157. [Google Scholar] [CrossRef]
- Matute, D.R.; McEwen, J.G.; Puccia, R.; Montes, B.A.; San-Blas, G.; Bagagli, E.; Rauscher, J.T.; Restrepo, A.; Morais, F.; Niño-Vega, G.; et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol. Biol. Evol. 2006, 23, 65–73. [Google Scholar] [CrossRef]
- Turissini, D.A.; Gomez, O.M.; Teixeira, M.M.; McEwen, J.G.; Matute, D.R. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet. Biol. 2017, 106, 9–25. [Google Scholar] [CrossRef]
- Teixeira, M.D.M.; Cattana, M.E.; Matute, D.R.; Muñoz, J.F.; Arechavala, A.; Isbell, K.; Schipper, R.; Santiso, G.; Tracogna, F.; Sosa, M.D.L.Á.; et al. Genomic diversity of the human pathogen Paracoccidioides across the South American continent. Fungal Genet. Biol. 2020, 140, 103395. [Google Scholar] [CrossRef]
- Muñoz, J.F.; Farrer, R.A.; Desjardins, C.A.; Gallo, J.E.; Sykes, S.; Sakthikumar, S.; Misas, E.; Whiston, E.A.; Bagagli, E.; Soares, C.M.A.; et al. Genome diversity, recombination, and virulence across the major lineages of Paracoccidioides. mSphere 2016, 1, e00213-16. [Google Scholar] [CrossRef] [PubMed]
- Fava-Netto, C. Contribuição para o estudo imunológico da blastomicose de Lutz (blastomicose sul-americana). Rev. Inst. Adolfo Lutz 1961, 21, 99–194. [Google Scholar]
- Fava-Netto, C.; Vegas, V.S.; Sciannamea, I.M.; Guarnieri, D.B. The polysaccharidic antigen from Paracoccidioides brasiliensis. Study of the time of cultivation necessary for the preparation of the antigen. Rev. Inst. Med. Trop. São Paulo 1969, 11, 177–181. [Google Scholar]
- Rodrigues, A.M.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Terra, P.P.D.; de Hoog, S.; Brilhante, R.S.N.; de Aguiar Cordeiro, R.; de Souza Collares Maia Castelo-Branco, D.; Rocha, M.F.G.; et al. The global epidemiology of emerging Histoplasma species in recent years. Stud. Mycol. 2020, 97, 100095. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M., Gelfand, D., Shinsky, J., White, T., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Morais, F.V.; Barros, T.F.; Fukada, M.K.; Cisalpino, P.S.; Puccia, R. Polymorphism in the gene coding for the immunodominant antigen gp43 from the pathogenic fungus Paracoccidioides brasiliensis. J. Clin. Microbiol. 2000, 38, 3960. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; Rosa, P.S.; Belone, A.F.; Taylor, J.W.; Diorio, S.M.; Mendoza, L. Molecular phylogeny of animal pathogen Lacazia loboi inferred from rDNA and DNA coding sequences. Mycol. Res. 2009, 113, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef]
- Pinheiro, B.G.; Pôssa, A.P.; Della Terra, P.P.D.; de Carvalho, J.A.D.; Ricci, G.; Nishikaku, A.S.; Hahn, R.C.; Camargo, Z.P.D.; Rodrigues, A.M. A new duplex PCR-assay for the detection and identification of Paracoccidioides species. J. Fungi 2021, 7, 169. [Google Scholar] [CrossRef]
- van Beers, E.H.; Joosse, S.A.; Ligtenberg, M.J.; Fles, R.; Hogervorst, F.B.L.; Verhoef, S.; Nederlof, P.M. A multiplex PCR predictor for aCGH success of FFPE samples. Br. J. Cancer 2006, 94, 333–337. [Google Scholar] [CrossRef]
- Pahl, A.; Kuhlbrandt, U.; Brune, K.; Rollinghoff, M.; Gessner, A. Quantitative detection of Borrelia burgdorferi by real-time PCR. J. Clin. Microbiol. 1999, 37, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Della Terra, P.P.; Gonsales, F.F.; de Carvalho, J.A.; Hagen, F.; Kano, R.; Bonifaz, A.; Camargo, Z.P.; Rodrigues, A.M. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound. Emerg. Dis. 2022, 69, e704–e716. [Google Scholar] [CrossRef] [PubMed]
- Bialek, R.; Feucht, A.; Aepinus, C.; Just-Nübling, G.; Robertson, V.J.; Knobloch, J.; Hohle, R. Evaluation of two nested PCR assays for detection of Histoplasma capsulatum DNA in human tissue. J. Clin. Microbiol. 2002, 40, 1644–1647. [Google Scholar] [CrossRef]
- Ricci, G.; Zelck, U.; Mota, F.; Lass-Florl, C.; Franco, M.F.; Bialek, R. Genotyping of Paracoccidioides brasiliensis directly from paraffin embedded tissue. Med. Mycol. 2008, 46, 31–34. [Google Scholar] [CrossRef]
- Cho, M.; Ahn, S.; Hong, M.; Bang, H.; Van Vrancken, M.; Kim, S.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; et al. Tissue recommendations for precision cancer therapy using next generation sequencing: A comprehensive single cancer center’s experiences. Oncotarget 2017, 8, 42478–42486. [Google Scholar] [CrossRef]
- Blow, N. Tissue preparation: Tissue issues. Nature 2007, 448, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Rickerts, V. Identification of fungal pathogens in Formalin-fixed, Paraffin-embedded tissue samples by molecular methods. Fungal Biol. 2016, 120, 279–287. [Google Scholar] [CrossRef]
- Ricci, G.; Campanini, E.B.; Nishikaku, A.S.; Puccia, R.; Marques, M.; Bialek, R.; Rodrigues, A.M.; Batista, W.L. PbGP43 genotyping using paraffin-embedded biopsies of human paracoccidioidomycosis reveals a genetically distinct lineage in the Paracoccidioides brasiliensis complex. Mycopathologia 2022, 187, 157–168. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Najafzadeh, M.J.; de Hoog, G.S.; de Camargo, Z.P. Rapid identification of emerging human-pathogenic Sporothrix species with rolling circle amplification. Front. Microbiol. 2015, 6, 1385. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G. Practical Statistics for Medical Research; Chapman and Hall: London, UK, 1991; p. 624. [Google Scholar]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.; Denning, D. Global and multi-national prevalence of fungal diseases—Estimate precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Coutinho, Z.F.; Wanke, B.; Travassos, C.; Oliveira, R.M.; Xavier, D.R.; Coimbra, C.E., Jr. Hospital morbidity due to paracoccidioidomycosis in Brazil (1998–2006). Trop. Med. Int. Health 2015, 20, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.G.; Hahn, R.C.; Camargo, Z.P.; Rodrigues, A.M. Molecular tools for detection and identification of Paracoccidioides species: Current status and future perspectives. J. Fungi 2020, 6, 293. [Google Scholar] [CrossRef] [PubMed]
- Buitrago, M.J.; Merino, P.; Puente, S.; Gomez-Lopez, A.; Arribi, A.; Zancope-Oliveira, R.M.; Gutierrez, M.C.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M. Utility of real-time PCR for the detection of Paracoccidioides brasiliensis DNA in the diagnosis of imported paracoccidioidomycosis. Med. Mycol. 2009, 47, 879–882. [Google Scholar] [CrossRef]
- Theodoro, R.C.; Candeias, J.M.; Araujo, J.P., Jr.; Bosco Sde, M.; Macoris, S.A.; Padula, L.O.; Franco, M.; Bagagli, E. Molecular detection of Paracoccidioides brasiliensis in soil. Med. Mycol. 2005, 43, 725–729. [Google Scholar] [CrossRef]
- Arantes, T.D.; Theodoro, R.C.; Teixeira, M.M.; Bagagli, E. Use of fluorescent oligonucleotide probes for differentiation between Paracoccidioides brasiliensis and Paracoccidioides lutzii in yeast and mycelial phase. Mem. Inst. Oswaldo Cruz 2017, 112, 140–145. [Google Scholar] [CrossRef]
- Arantes, T.D.; Theodoro, R.C.; Da Graça Macoris, S.A.; Bagagli, E. Detection of Paracoccidioides spp. in environmental aerosol samples. Med. Mycol. 2013, 51, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.M.; Theodoro, R.C.; Oliveira, F.F.; Machado, G.C.; Hahn, R.C.; Bagagli, E.; San-Blas, G.; Felipe, M.S. Paracoccidioides lutzii sp. nov.: Biological and clinical implications. Med. Mycol. 2014, 52, 19–28. [Google Scholar] [CrossRef]
- Munoz, J.F.; Gallo, J.E.; Misas, E.; Priest, M.; Imamovic, A.; Young, S.; Zeng, Q.; Clay, O.K.; McEwen, J.G.; Cuomo, C.A. Genome update of the dimorphic human pathogenic fungi causing paracoccidioidomycosis. PLoS Negl. Trop. Dis. 2014, 8, e3348. [Google Scholar] [CrossRef]
- Griffiths, J.; Lopes Colombo, A.; Denning, D.W. The case for paracoccidioidomycosis to be accepted as a neglected tropical (fungal) disease. PLoS Negl. Trop. Dis. 2019, 13, e0007195. [Google Scholar] [CrossRef] [PubMed]
- Maifrede, S.B.; Kruschewsky, W.L.L.; Patrício, S.A.; Falqueto, A.; Peçanha, P.M.; Malaquias, L.C.C.; Pôssa, A.P.; de Camargo, Z.P.; Rodrigues, A.M.; Gonçalves, S.S.; et al. Screening paracoccidioidomycosis by double immunodiffusion test in a referred diagnostic center in Brazilian southeastern: An accessible tool. Infection 2021, 49, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Kubitschek-Barreira, P.H.; Pinheiro, B.G.; Teixeira-Ferreira, A.; Hahn, R.C.; de Camargo, Z.P. Immunoproteomic analysis reveals novel candidate antigens for the diagnosis of paracoccidioidomycosis due to Paracoccidioides lutzii. J. Fungi 2020, 6, 357. [Google Scholar] [CrossRef]
- Dos Santos, P.O.; Rodrigues, A.M.; Fernandes, G.F.; da Silva, S.H.; Burger, E.; de Camargo, Z.P. Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: Detection of specific antibody anti-gp43 and specific antigen gp43. PLoS Negl. Trop. Dis. 2015, 9, e0003516. [Google Scholar] [CrossRef]
- Queiroz Junior, L.P.; de Camargo, Z.P.; Tadano, T.; Rodrigues, A.M.; Takarara, D.T.; Gegembauer, G.; Araujo, L.M.; Hahn, R.C. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from Central Western Brazil. Mycoses 2014, 57, 466–472. [Google Scholar] [CrossRef]
- Gegembauer, G.; Araujo, L.M.; Pereira, E.F.; Rodrigues, A.M.; Paniago, A.M.; Hahn, R.C.; de Camargo, Z.P. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2014, 8, e2986. [Google Scholar] [CrossRef] [PubMed]
- Nery, A.F.; de Camargo, Z.P.; Rodrigues, A.M.; Portela, T.F.; Hoffmann-Santos, H.D.; Dambros, P.V.K.; de Souza, J.F.R.; Garcia, A.C.; Santos, C.A.D.; Hagen, F.; et al. Paracoccidioidomycosis due to P. lutzii: The importance of neutrophil/lymphocyte ratio in the symptomatic and asymptomatic phases in severe cases. Mycoses 2021, 64, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Nery, A.F.; de Camargo, Z.P.; Rodrigues, A.M.; Portela, T.F.; Hoffmann-Santos, H.D.; Pinheiro, B.G.; Possa, A.P.; Cavalcante, L.; Hagen, F.; Hahn, R.C. Puzzling paracoccidioidomycosis: Factors associated with the severity of Paracoccidioides lutzii infections. Int. J. Infect. Dis. 2021, 107, 284–290. [Google Scholar] [CrossRef]
- Pereira, E.F.; Gegembauer, G.; Chang, M.R.; Camargo, Z.P.D.; Nunes, T.F.; Ribeiro, S.M.; Carvalho, L.R.D.; Maldonado, B.M.; Mendes, R.P.; Paniago, A.M.M. Comparison of clinico-epidemiological and radiological features in paracoccidioidomycosis patients regarding serological classification using antigens from Paracoccidioides brasiliensis complex and Paracoccidioides lutzii. PLoS Negl. Trop. Dis. 2020, 14, e0008485. [Google Scholar] [CrossRef]
- Imai, T.; Sano, A.; Mikami, Y.; Watanabe, K.; Aoki, F.H.; Branchini, M.L.; Negroni, R.; Nishimura, K.; Miyaji, M. A new PCR primer for the identification of Paracoccidioides brasiliensis based on rRNA sequences coding the internal transcribed spacers (ITS) and 5.8S regions. Med. Mycol. 2000, 38, 323–326. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum Information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Rocha-Silva, F.; Gomes, L.I.; Gracielle-Melo, C.; Goes, A.M.; Caligiorne, R.B. Real Time Polymerase Chain Reaction (rt-PCR): A New Patent to Diagnostic Purposes for Paracoccidioidomycosis. Recent Pat. Endocr. Metab. Immune Drug Discov. 2017, 10, 143–149. [Google Scholar] [CrossRef]
- Cocio, T.A.; Nascimento, E.; von Zeska Kress, M.R.; Bagagli, E.; Martinez, R. Phylogenetic species of Paracoccidioides spp. isolated from clinical and environmental samples in a hyperendemic area of paracoccidioidomycosis in Southeastern Brazil. J. Fungi 2020, 6, 132. [Google Scholar] [CrossRef]
- Cocio, T.A.; Nascimento, E.; Kress, M.R.; Bagagli, E.; Martinez, R. Characterization of a Paracoccidioides spp. strain from southeastern Brazil genotyped as Paracoccidioides restrepiensis (PS3) and review of this phylogenetic species. Genet. Mol. Biol. 2020, 43, e20190201. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R. New trends in paracoccidioidomycosis epidemiology. J. Fungi 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Vilela, R.; Mendoza, L.; Rosa, P.S.; Belone, A.F.F.; Madeira, S.; Opromolla, D.V.A.; de Resende, M.A. Molecular model for studying the uncultivated fungal pathogen Lacazia loboi. J. Clin. Microbiol. 2005, 43, 3657–3661. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.; Bagagli, E.; Scapolio, S.; Da Silva Lacaz, C. A critical analysis of isolation of Paracoccidioides brasiliensis from soil. Med. Mycol. 2000, 38, 185–191. [Google Scholar] [CrossRef]
- Bagagli, E.; Sano, A.; Coelho, K.I.; Alquati, S.; Miyaji, M.; de Camargo, Z.P.; Gomes, G.M.; Franco, M.; Montenegro, M.R. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus noveminctus) captured in an endemic area of paracoccidioidomycosis. Am. J. Trop. Med. Hyg. 1998, 58, 505–512. [Google Scholar] [CrossRef]
- Bagagli, E.; Theodoro, R.C.; Bosco, S.M.; McEwen, J.G. Paracoccidioides brasiliensis: Phylogenetic and ecological aspects. Mycopathologia 2008, 165, 197–207. [Google Scholar] [CrossRef]
- Richini-Pereira, V.B.; Bosco Sde, M.; Theodoro, R.C.; Macoris, S.A.; Bagagli, E. Molecular approaches for eco-epidemiological studies of Paracoccidioides brasiliensis. Mem. Inst. Oswaldo Cruz 2009, 104, 636–643. [Google Scholar] [CrossRef]
- Silva-Vergara, M.L.; Martinez, R.; Camargo, Z.P.; Malta, M.H.; Maffei, C.M.; Chadu, J.B. Isolation of Paracoccidioides brasiliensis from armadillos (Dasypus novemcinctus) in an area where the fungus was recently isolated from soil. Med. Mycol. 2000, 38, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Silva-Vergara, M.L.; Martinez, R.; Chadu, A.; Madeira, M.; Freitas-Silva, G.; Leite Maffei, C.M. Isolation of a Paracoccidioides brasiliensis strain from the soil of a coffee plantation in Ibia, State of Minas Gerais, Brazil. Med. Mycol. 1998, 36, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Arantes, T.D.; Theodoro, R.C.; Teixeira, M.D.M.; Bosco, S.D.M.G.; Bagagli, E. Environmental mapping of Paracoccidioides spp. in Brazil reveals new clues into genetic diversity, biogeography and wild host association. PLoS Negl. Trop. Dis. 2016, 10, e0004606. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Freitas, L.H.; Lacaz Cda, S.; del Negro, G.M.; de Melo, N.T.; Garcia, N.M.; de Assis, C.M.; Salebian, A.; Heins-Vaccari, E.M. Isolation and characterization of a Paracoccidioides brasiliensis strain from a dogfood probably contaminated with soil in Uberlandia, Brazil. J. Med. Vet. Mycol. 1990, 28, 253–256. [Google Scholar] [CrossRef]
- De Albornoz, M.B. Isolation of Paracoccidioides brasiliensis from rural soil in Venezuela. Sabouraudia 1971, 9, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Guarro, J.; Gene, J.; Stchigel, A.M.; Figueras, M.J. Atlas of Soil Ascomycetes; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012; p. 486. [Google Scholar]
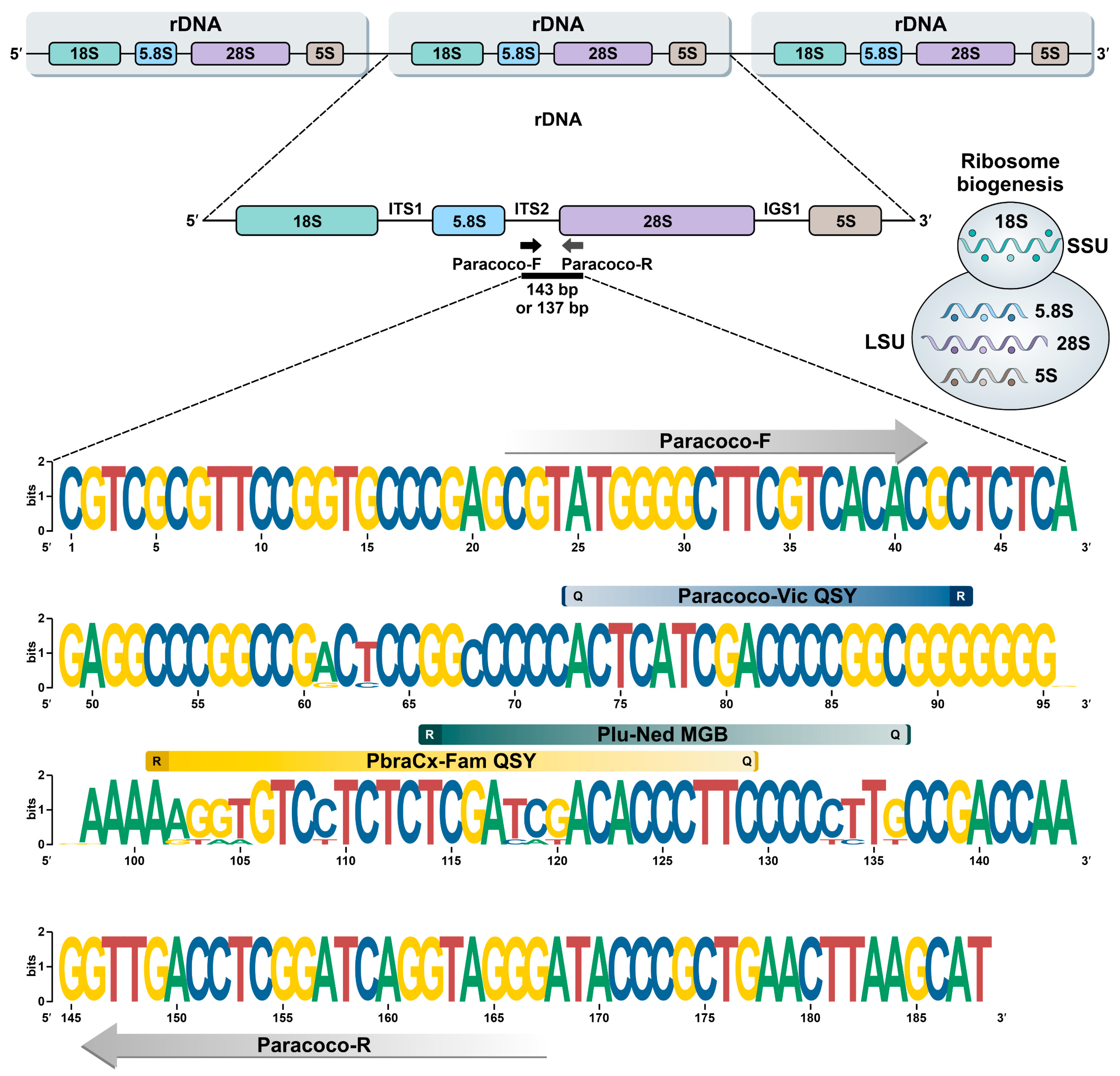
| Target | Primer and Probe | Primer and Probe Sequences (5′–3′) | Tm (°C) | Final Concentration | Reporter (5′)/Quencher (3′) 1 |
|---|---|---|---|---|---|
| Genus specific | Paracoco-F | CGTATGGGGCTTCGTCACAC | 60 | 900 nM | -/- |
| Paracoco-R | CCCTACCTGATCCGAGGTCAAC | 60 | 300 nM | -/- | |
| P. brasiliensis complex 2 | PbraCx-Fam | AAGGTGTCCTCTCTCGATCGACACCCTTC | 70 | 250 nM | FAM/QSY |
| P. lutzii | Plu-Ned | TCGACATCTTCCCCTCTT | 70 | 250 nM | NED/MGB-NFQ |
| Genus specific | Paracoco-Vic | CCCGCCGGGGTCGATGAGT | 70 | 250 nM | VIC/QSY |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, B.G.; Pôssa, A.P.; Ricci, G.; Nishikaku, A.S.; Hagen, F.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Development of a Multiplex qPCR Assay for Fast Detection and Differentiation of Paracoccidioidomycosis Agents. J. Fungi 2023, 9, 358. https://doi.org/10.3390/jof9030358
Pinheiro BG, Pôssa AP, Ricci G, Nishikaku AS, Hagen F, Hahn RC, de Camargo ZP, Rodrigues AM. Development of a Multiplex qPCR Assay for Fast Detection and Differentiation of Paracoccidioidomycosis Agents. Journal of Fungi. 2023; 9(3):358. https://doi.org/10.3390/jof9030358
Chicago/Turabian StylePinheiro, Breno Gonçalves, Ana Paula Pôssa, Giannina Ricci, Angela Satie Nishikaku, Ferry Hagen, Rosane Christine Hahn, Zoilo Pires de Camargo, and Anderson Messias Rodrigues. 2023. "Development of a Multiplex qPCR Assay for Fast Detection and Differentiation of Paracoccidioidomycosis Agents" Journal of Fungi 9, no. 3: 358. https://doi.org/10.3390/jof9030358
APA StylePinheiro, B. G., Pôssa, A. P., Ricci, G., Nishikaku, A. S., Hagen, F., Hahn, R. C., de Camargo, Z. P., & Rodrigues, A. M. (2023). Development of a Multiplex qPCR Assay for Fast Detection and Differentiation of Paracoccidioidomycosis Agents. Journal of Fungi, 9(3), 358. https://doi.org/10.3390/jof9030358







