The Distinction between Dematiaceous Molds and Non-Dematiaceous Fungi in Clinical and Spiked Samples Treated with Hydrogen Peroxide Using Direct Fluorescence Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolates
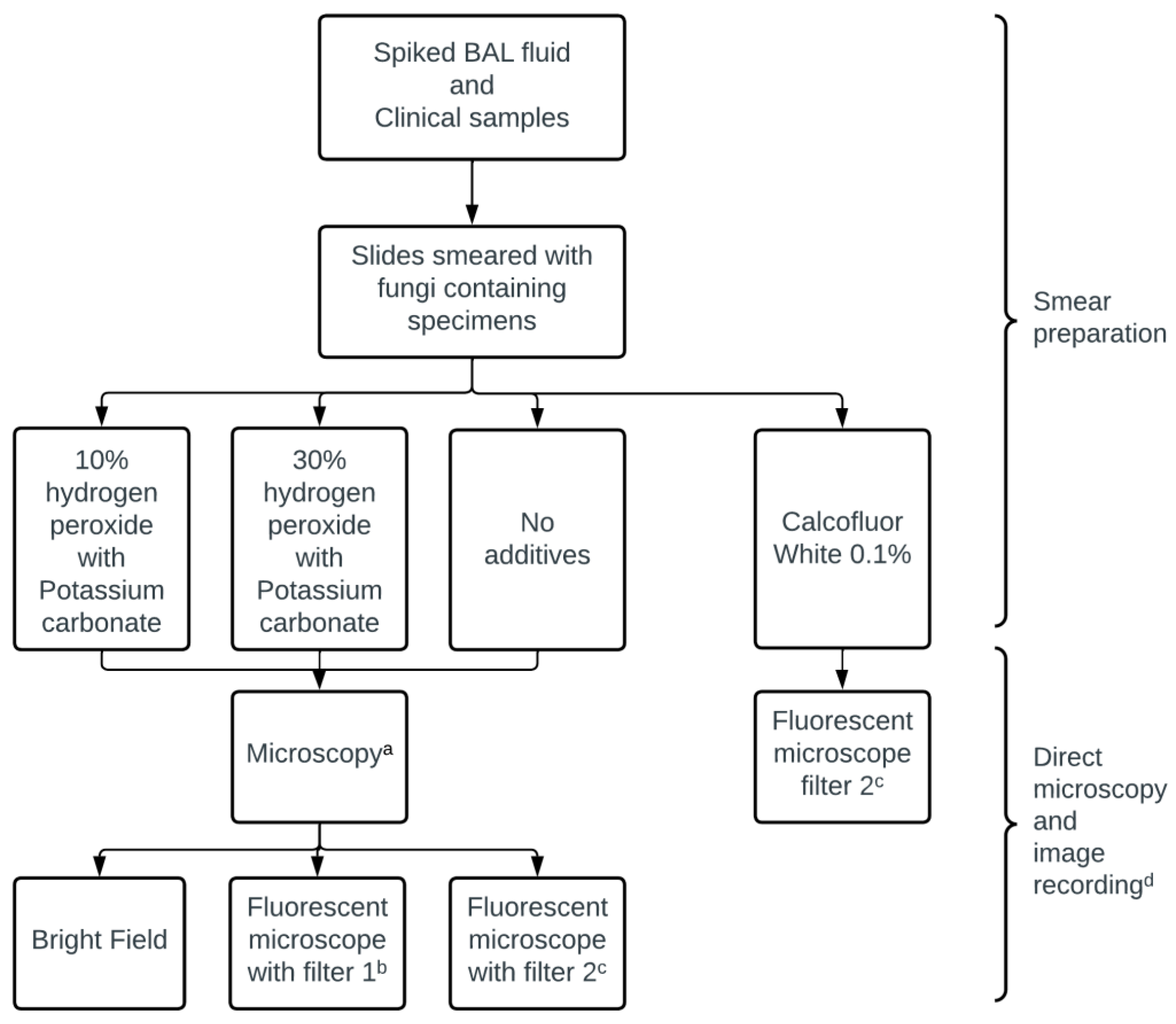
2.2. Smear Preparation
2.3. Fluorescence Microscopy Staining
2.4. Microscopic Examination
2.5. Image Recording
2.6. Statistical Analysis
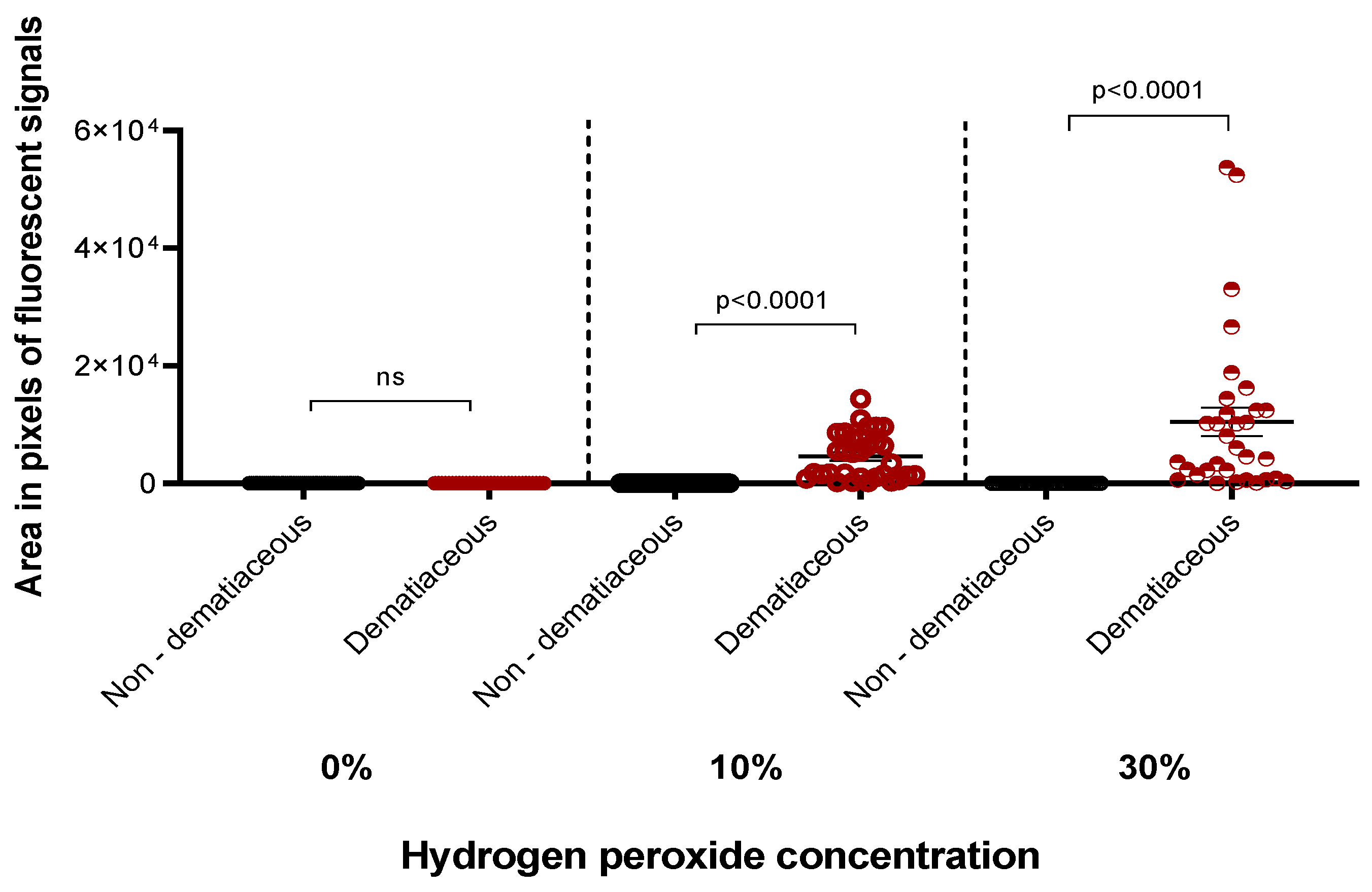
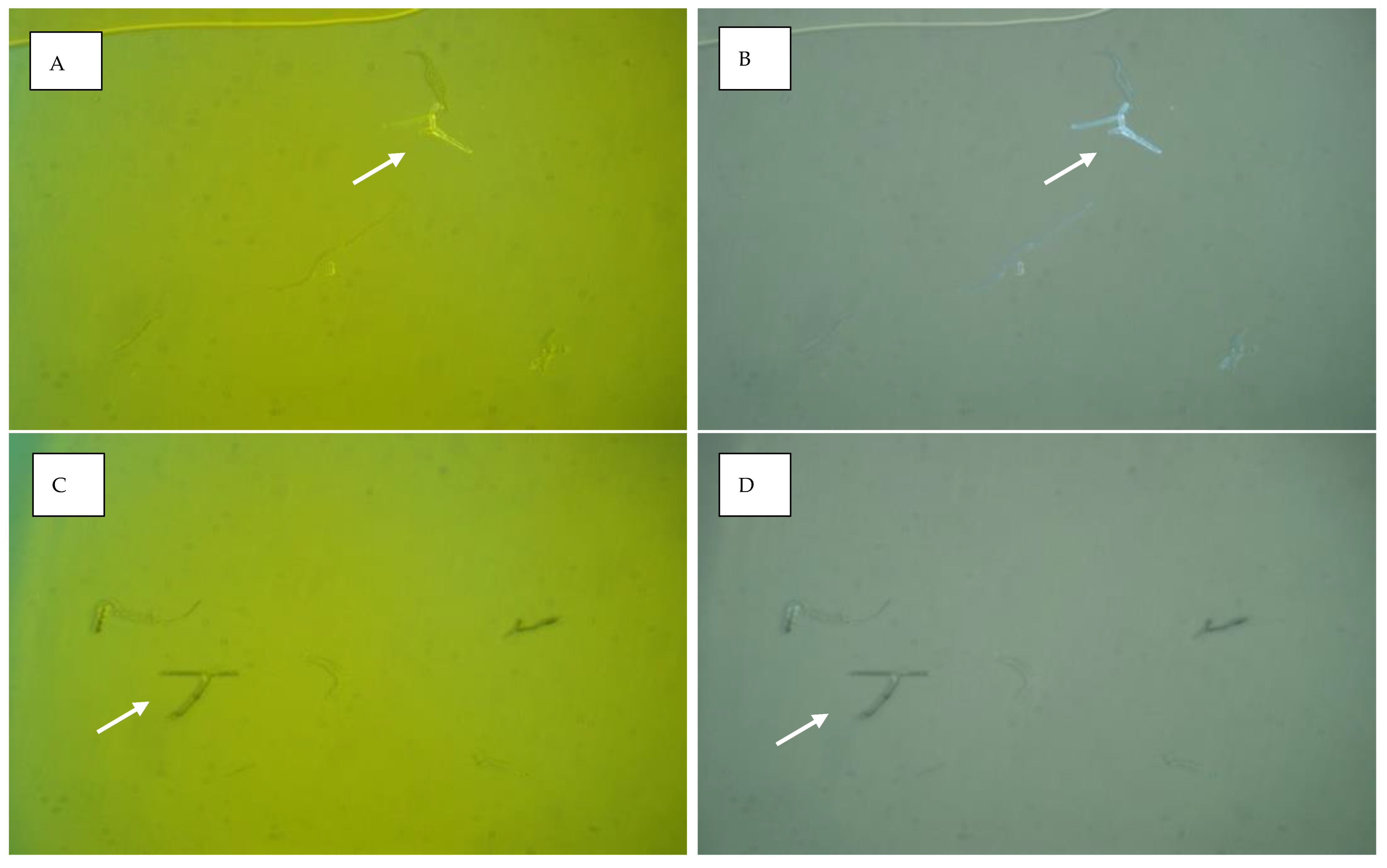
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Revankar, S.G. Phaeohyphomycosis in Transplant Patients. J. Fungi 2015, 2, 2. [Google Scholar] [CrossRef]
- Revankar, S.G.; Patterson, J.E.; Sutton, D.A.; Pullen, R.; Rinaldi, M.G. Disseminated phaeohyphomycosis: Review of an emerging mycosis. Clin. Infect. Dis. 2002, 34, 467–476. [Google Scholar] [CrossRef]
- Velasco, J.; Revankar, S. CNS Infections Caused by Brown-Black Fungi. J. Fungi 2019, 5, 60. [Google Scholar] [CrossRef]
- Revankar, S.G.; Sutton, D.A. Melanized fungi in human disease. Clin. Microbiol. Rev. 2010, 23, 884–928. [Google Scholar] [CrossRef]
- Polacheck, I.; Kwon-Chung, K.J. Melanogenesis in Cryptococcus neoformans. J. Gen. Microbiol. 1988, 134, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.P.; Chrissian, C.; Stark, R.E.; Casadevall, A. Cryptococcus neoformans melanization incorporates multiple catecholamines to produce polytypic melanin. J. Biol. Chem. 2022, 298, 101519. [Google Scholar] [CrossRef] [PubMed]
- Arcobello, J.T.; Revankar, S.G. Phaeohyphomycosis. Semin. Respir. Crit. Care Med. 2020, 41, 131–140. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Bille, J.; Dannaoui, E.; Ruhnke, M.; Heussel, C.P.; Kibbler, C. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow. Transplant. 2012, 47, 1030–1045. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Huang, Y.; Feng, S.; Zheng, Z.; Liu, X.; Liu, M. Label-free detection of hydrogen peroxide-induced oxidative stress in human retinal pigment epithelium cells via laser tweezers Raman spectroscopy. Biomed. Opt. Express. 2019, 10, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Kayatz, P.; Thumann, G.; Luther, T.T.; Jordan, J.F.; Bartz-Schmidt, K.U.; Esser, P.J.; Schraermeyer, U. Oxidation causes melanin fluorescence. Investig. Ophthalmol. Vis. Sci. 2001, 42, 241–246. [Google Scholar]
- Elleder, M.; Borovanský, J. Autofluorescence of melanins induced by ultraviolet radiation and near ultraviolet light. A histochemical and biochemical study. Histochem. J. 2001, 33, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Dontsov, A.E.; Sakina, N.L.; Koromyslova, A.D.; Ostrovsky, M.A. Effect of UV radiation and hydrogen peroxide on the antiradical and antioxidant activities of DOPA-melanin and melanosomes from retinal pigment epithelial cells. Russ. Chem. Bull. Int. Ed. 2015, 64, 1623–1628. [Google Scholar] [CrossRef]
- Aberkane, A.; Cuenca-Estrella, M.; Gomez-Lopez, A.; Petrikkou, E.; Mellado, E.; Monzón, A.; Rodriguez-Tudela, J.L. Comparative evaluation of two different methods of inoculum preparation for antifungal susceptibility testing of filamentous fungi. J. Antimicrob. Chemother. 2002, 50, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.W.; Garrett, B.; Naqvi, K.R.; Fülöp, A.; Godfrey, S.P.; Marsh, J.M.; Chechik, V. Mechanistic insights into the bleaching of melanin by alkaline hydrogen peroxide. Free Radic. Biol. Med. 2017, 108, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Grossman, N.T.; Casadevall, A. Physiological Differences in Cryptococcus neoformans Strains In Vitro versus In Vivo and Their Effects on Antifungal Susceptibility. Antimicrob. Agents Chemother. 2017, 61, e02108-16. [Google Scholar] [CrossRef] [PubMed]
| Fungal Strains | Source of Fungal Strains | Source of Medium Used for Preparation of Slide-Smears | |
|---|---|---|---|
| Dematiaceous fungi | Exserohilum rostratum | Clinical specimen b | Nasal biopsy |
| Alternaria species | Laboratory collection | BAL | |
| Neoscytalidium dimidiatum | Laboratory collection | BAL | |
| Cladosporium species | Laboratory collection | BAL | |
| Alternaria alternata | Laboratory collection | BAL | |
| Alternaria alternata | Laboratory collection | BAL | |
| Ulocladium species | Laboratory collection | BAL | |
| Alternaria alternata | A reference strain, NRRL 54028 | BAL | |
| Aspergillus | Aspergillus species | Clinical specimen b | Ear swab |
| Aspergillus fumigatus | Laboratory collection | BAL | |
| Aspergillus terreus | Laboratory collection | BAL | |
| Aspergillus sydowii | Laboratory collection | BAL | |
| Aspergillus fumigatus | A reference strain, NRRL 2427 | BAL | |
| Aspergillus terreus | A reference strain, NRRL 269 | BAL | |
| Aspergillus flavus | A reference strain, NRRL 3518 | BAL | |
| Fusarium | Fusarium solani | Laboratory collection | BAL |
| Lichtheimia (Mucorales order) | Lichtheimia corymbifera | Laboratory collection | BAL |
| Candida | Candida species c | Clinical specimen b | Vaginal swab |
| Candida species c | Laboratory collection | BAL | |
| Candida parapsilosis | A reference strain, ATTC 22019 | BAL | |
| Candida albicans | A reference strain, ATTC 90028 | BAL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juravel, E.; Polacheck, I.; Isaacson, B.; Dagan, A.; Korem, M. The Distinction between Dematiaceous Molds and Non-Dematiaceous Fungi in Clinical and Spiked Samples Treated with Hydrogen Peroxide Using Direct Fluorescence Microscopy. J. Fungi 2023, 9, 227. https://doi.org/10.3390/jof9020227
Juravel E, Polacheck I, Isaacson B, Dagan A, Korem M. The Distinction between Dematiaceous Molds and Non-Dematiaceous Fungi in Clinical and Spiked Samples Treated with Hydrogen Peroxide Using Direct Fluorescence Microscopy. Journal of Fungi. 2023; 9(2):227. https://doi.org/10.3390/jof9020227
Chicago/Turabian StyleJuravel, Elchanan, Itzhack Polacheck, Batya Isaacson, Arie Dagan, and Maya Korem. 2023. "The Distinction between Dematiaceous Molds and Non-Dematiaceous Fungi in Clinical and Spiked Samples Treated with Hydrogen Peroxide Using Direct Fluorescence Microscopy" Journal of Fungi 9, no. 2: 227. https://doi.org/10.3390/jof9020227
APA StyleJuravel, E., Polacheck, I., Isaacson, B., Dagan, A., & Korem, M. (2023). The Distinction between Dematiaceous Molds and Non-Dematiaceous Fungi in Clinical and Spiked Samples Treated with Hydrogen Peroxide Using Direct Fluorescence Microscopy. Journal of Fungi, 9(2), 227. https://doi.org/10.3390/jof9020227





