The Role of the Mycobiome in Women’s Health
Abstract
1. Introduction
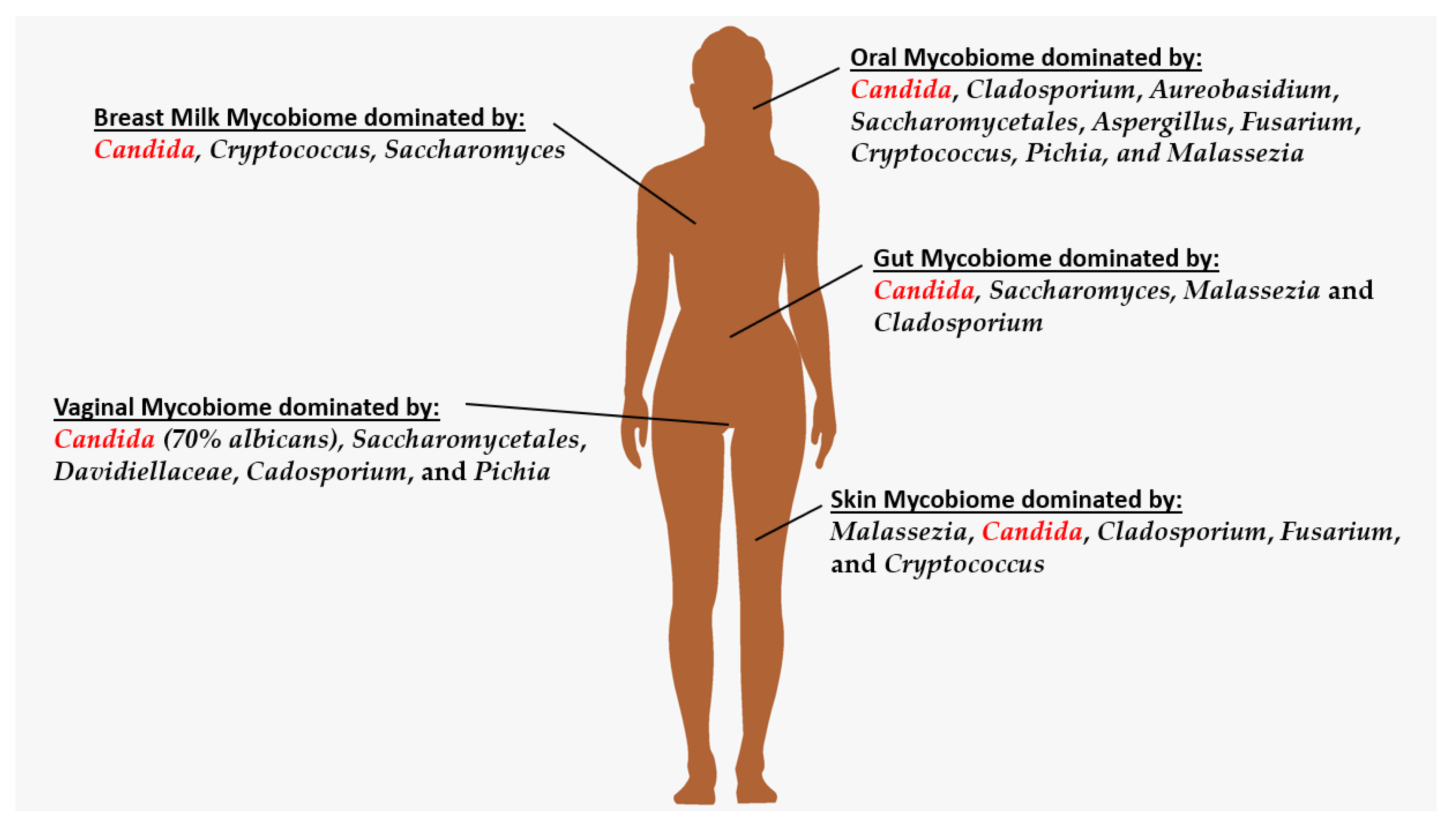
2. Oral Mycobiome
3. Breastmilk Mycobiome
4. Skin Mycobiome
5. Gut Mycobiome
6. Vaginal Mycobiome
7. Mycobiome Dysbiosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seed, P.C. The Human Mycobiome. Cold Spring Harb. Perspect. Med. 2015, 5, a019810. [Google Scholar] [CrossRef]
- Belvoncikova, P.; Splichalova, P.; Videnska, P.; Gardlik, R. The Human Mycobiome: Colonization, Composition and the Role in Health and Disease. J. Fungi 2022, 8, 1046. [Google Scholar] [CrossRef] [PubMed]
- Development of the Human Mycobiome over the First Month of Life and across Body Sites|MSystems. Available online: https://journals.asm.org/doi/full/10.1128/mSystems.00140-17 (accessed on 26 November 2022).
- Tarracchini, C.; Fontana, F.; Lugli, G.A.; Mancabelli, L.; Alessandri, G.; Turroni, F.; Ventura, M.; Milani, C. Investigation of the Ecological Link between Recurrent Microbial Human Gut Communities and Physical Activity. Microbiol. Spectr. 2022, 10, e00420-22. [Google Scholar] [CrossRef]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary Defense Proteins: Their Network and Role in Innate and Acquired Oral Immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chiș, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral Microbiome: Getting to Know and Befriend Neighbors, a Biological Approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the Human Oral Mycobiome with Improved Practices in Amplicon-Based Taxonomy: Discovery of Malassezia as a Prominent Commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Retuerto, M.; Sikaroodi, M.; Brown, R.E.; Jurevic, R.; Salata, R.A.; Lederman, M.M.; Gillevet, P.M.; Ghannoum, M.A. Oral Mycobiome Analysis of HIV-Infected Patients: Identification of Pichia as an Antagonist of Opportunistic Fungi. PLoS Pathog. 2014, 10, e1003996. [Google Scholar] [CrossRef]
- Sajid, M.; Sharma, P.; Srivastava, S.; Hariprasad, R.; Singh, H.; Bharadwaj, M. Smokeless Tobacco Consumption Induces Dysbiosis of Oral Mycobiome: A Pilot Study. Appl. Microbiol. Biotechnol. 2022, 106, 5643–5657. [Google Scholar] [CrossRef]
- Diaz, P.I.; Hong, B.-Y.; Dupuy, A.K.; Strausbaugh, L.D. Mining the Oral Mycobiome: Methods, Components, and Meaning. Virulence 2017, 8, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.; Furuta, M.; Takeshita, T.; Shibata, Y.; Sundari, R.; Eshima, N.; Ninomiya, T.; Yamashita, Y. Oral Mycobiome in Community-Dwelling Elderly and Its Relation to Oral and General Health Conditions. Oral Dis. 2017, 23, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Khadija, B.; Imran, M.; Faryal, R. Keystone Salivary Mycobiome in Postpartum Period in Health and Disease Conditions. J. Med. Mycol. 2021, 31, 101101. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.K.; Chan, J.Y.K.; Wong, M.C.S.; Wong, P.Y.; Lei, P.; Cai, L.; Lan, L.; Ho, W.C.S.; Yeung, A.C.M.; Chan, P.K.S.; et al. Determinants and Interactions of Oral Bacterial and Fungal Microbiota in Healthy Chinese Adults. Microbiol. Spectr. 2022, 10, e02410-21. [Google Scholar] [CrossRef] [PubMed]
- Pitarch, A.; Gil, C.; Blanco, G. Vultures from Different Trophic Guilds Show Distinct Oral Pathogenic Yeast Signatures and Co-Occurrence Networks. Sci. Total Environ. 2020, 723, 138166. [Google Scholar] [CrossRef]
- Trofa, D.; Gácser, A.; Nosanchuk, J.D. Candida Parapsilosis, an Emerging Fungal Pathogen. Clin. Microbiol. Rev. 2008, 21, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Tramice, A.; Paris, D.; Manca, A.; Guevara Agudelo, F.A.; Petrosino, S.; Siracusa, L.; Carbone, M.; Melck, D.; Raymond, F.; Piscitelli, F. Analysis of the Oral Microbiome during Hormonal Cycle and Its Alterations in Menopausal Women: The “AMICA” Project. Sci. Rep. 2022, 12, 22086. [Google Scholar] [CrossRef]
- Caixeta, C.A.; de Carli, M.L.; Ribeiro Júnior, N.V.; Sperandio, F.F.; Nonogaki, S.; Nogueira, D.A.; Pereira, A.A.C.; Hanemann, J.A.C. Estrogen Receptor-α Correlates with Higher Fungal Cell Number in Oral Paracoccidioidomycosis in Women. Mycopathologia 2018, 183, 785–791. [Google Scholar] [CrossRef]
- Heisel, T.; Nyaribo, L.; Sadowsky, M.J.; Gale, C.A. Breastmilk and NICU Surfaces Are Potential Sources of Fungi for Infant Mycobiomes. Fungal Genet. Biol. 2019, 128, 29–35. [Google Scholar] [CrossRef]
- Warth, B.; Braun, D.; Ezekiel, C.N.; Turner, P.C.; Degen, G.H.; Marko, D. Biomonitoring of Mycotoxins in Human Breast Milk: Current State and Future Perspectives|Chemical Research in Toxicology. Chem. Res. Toxicol. 2016, 29, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Saad, A.M.; Taha, T.F.; Najjar, A.A.; Zabermawi, N.M.; Nader, M.M.; AbuQamar, S.F.; El-Tarabily, K.A.; Salama, A. Selenium Nanoparticles from Lactobacillus Paracasei HM1 Capable of Antagonizing Animal Pathogenic Fungi as a New Source from Human Breast Milk. Saudi J. Biol. Sci. 2021, 28, 6782–6794. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Kalan, L.R. Forgotten Fungi: The Importance of the Skin Mycobiome. Curr. Opin. Microbiol. 2022, 70, 102235. [Google Scholar] [CrossRef] [PubMed]
- Keum, H.L.; Kim, H.; Kim, H.-J.; Park, T.; Kim, S.; An, S.; Sul, W.J. Structures of the Skin Microbiome and Mycobiome Depending on Skin Sensitivity. Microorganisms 2020, 8, 1032. [Google Scholar] [CrossRef] [PubMed]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The Role of Lactic Acid Production by Probiotic Lactobacillus Species in Vaginal Health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Zhu, T.; Kong, F.-Q.; Duan, Y.-Y.; Galzote, C.; Quan, Z.-X. Infant Mode of Delivery Shapes the Skin Mycobiome of Prepubescent Children. Microbiol. Spectr. 2022, 10, e02267-22. [Google Scholar] [CrossRef]
- Shah, P.A.; Govindarajan, V.; Diggikar, S.; Rangaiah, A.; Devadas, S.; Kariyappa, M. Exploring the Skin Mycobiome in Very Preterm Babies during the Early Neonatal Period in a Neonatal Intensive Care Unit of India. Trop. Doct. 2022, 52, 362–364. [Google Scholar] [CrossRef]
- Jovel, J.; Dieleman, L.A.; Kao, D.; Mason, A.L.; Wine, E. Chapter 10—The Human Gut Microbiome in Health and Disease. In Metagenomics; Nagarajan, M., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 197–213. ISBN 978-0-08-102268-9. [Google Scholar]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef]
- Niccolai, E.; Boem, F.; Emmi, G.; Amedei, A. The Link “Cancer and Autoimmune Diseases” in the Light of Microbiota: Evidence of a Potential Culprit. Immunol. Lett. 2020, 222, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Dvořák, A.; Folwarski, M.; Daca, A.; Przewłócka, K.; Makarewicz, W. Fungal Gut Microbiota Dysbiosis and Its Role in Colorectal, Oral, and Pancreatic Carcinogenesis. Cancers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A.; et al. The Gut Mycobiome of the Human Microbiome Project Healthy Cohort. Microbiome 2017, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.L.; Sokol, H. The Gut Mycobiota: Insights into Analysis, Environmental Interactions and Role in Gastrointestinal Diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Reynoso-García, J.; Narganes-Storde, Y.; Santiago-Rodriguez, T.M.; Toranzos, G.A. Mycobiome-Host Coevolution? The Mycobiome of Ancestral Human Populations Seems to Be Different and Less Diverse Than Those of Extant Native and Urban-Industrialized Populations. Microorganisms 2022, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Ghannoum, M.; Cominelli, F.; Martino, L.D. Mycobiome and Inflammatory Bowel Disease: Role in Disease Pathogenesis, Current Approaches and Novel Nutritional-Based Therapies. Inflamm. Bowel Dis. 2022, 29, izac156. [Google Scholar] [CrossRef]
- Zeng, S.; Schnabl, B. Roles for the Mycobiome in Liver Disease. Liver Int. 2022, 42, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, X.; Moog, C.; Wu, H.; Su, B.; Zhang, T. Neglected Mycobiome in HIV Infection: Alterations, Common Fungal Diseases and Antifungal Immunity. Front. Immunol. 2022, 13, 1015775. [Google Scholar] [CrossRef] [PubMed]
- Limon, J.J.; Tang, J.; Li, D.; Wolf, A.J.; Michelsen, K.S.; Funari, V.; Gargus, M.; Nguyen, C.; Sharma, P.; Maymi, V.I.; et al. Malassezia Is Associated with Crohn’s Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe 2019, 25, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Zuo, T.; Ho, M.; Chan, F.K.L.; Chan, P.K.S.; Ng, S.C. Review Article: Fungal Alterations in Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2019, 50, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The Gut Mycobiome in Health, Disease, and Clinical Applications in Association with the Gut Bacterial Microbiome Assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Bischoff, A.; Galley, J.D.; Peck, L.; Bailey, M.T.; Gur, T.L. Discrete Role for Maternal Stress and Gut Microbes in Shaping Maternal and Offspring Immunity. Neurobiol. Stress 2022, 21, 100480. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Mu, A.; Haslam, N.; Schwartz, O.S.; Simmons, J.G. Feeling down? A Systematic Review of the Gut Microbiota in Anxiety/Depression and Irritable Bowel Syndrome. J. Affect. Disord. 2020, 266, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Bradford, L.L.; Ravel, J. The Vaginal Mycobiome: A Contemporary Perspective on Fungi in Women’s Health and Diseases. Virulence 2017, 8, 342–351. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.G.G.; Metsis, M. Characterization of the Vaginal Micro- and Mycobiome in Asymptomatic Reproductive-Age Estonian Women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Bukavina, L.; Ghannoum, M. Symbiosis and Dysbiosis of the Human Mycobiome. Front. Microbiol. 2021, 12, 636131. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Genital Candidosis*. Clin. Exp. Dermatol. 1982, 7, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Chatzivasileiou, P.; Vyzantiadis, T.-A. Vaginal Yeast Colonisation: From a Potential Harmless Condition to Clinical Implications and Management Approaches—A Literature Review. Mycoses 2019, 62, 638–650. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, N.; Lu, H.; Yin, H.; Yao, J.; Chen, Y. Increased Diversity of Fungal Flora in the Vagina of Patients with Recurrent Vaginal Candidiasis and Allergic Rhinitis. Microb. Ecol. 2012, 64, 918–927. [Google Scholar] [CrossRef]
- Ackerman, A.L.; Underhill, D.M. The Mycobiome of the Human Urinary Tract: Potential Roles for Fungi in Urology. Ann. Transl. Med. 2017, 5, 31. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Sobel, J.D. Vulvovaginal Candidiasis Caused by Non-Albicans Candida Species: New Insights. Curr. Infect. Dis. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef]
- Singh, S.; Sobel, J.D.; Bhargava, P.; Boikov, D.; Vazquez, J.A. Vaginitis Due to Candida Krusei: Epidemiology, Clinical Aspects, and Therapy. Clin. Infect. Dis. 2002, 35, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Ipci, K.; Altıntoprak, N.; Bayar Muluk, N.; Senturk, M.; Cingi, C. The Possible Mechanisms of the Human Microbiome in Allergic Diseases. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-N.; Zhao, X.; Tan, J.-C.; Liu, S.; Li, B.-W.; Xu, W.-X.; Peng, L.; Gu, P.; Li, W.; Shapiro, R.; et al. Mycobiome Dysbiosis in Women with Intrauterine Adhesions. Microbiol. Spectr. 2022, 10, e01324-22. [Google Scholar] [CrossRef]
- Begum, N.; Harzandi, A.; Lee, S.; Uhlen, M.; Moyes, D.L.; Shoaie, S. Host-Mycobiome Metabolic Interactions in Health and Disease. Gut Microbes 2022, 14, 2121576. [Google Scholar] [CrossRef] [PubMed]
- Gonia, S.; Archambault, L.; Shevik, M.; Altendahl, M.; Fellows, E.; Bliss, J.M.; Wheeler, R.T.; Gale, C.A. Candida Parapsilosis Protects Premature Intestinal Epithelial Cells from Invasion and Damage by Candida Albicans. Front. Pediatr. 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Lu, N.; Huang, S.; Chai, X.; Wang, S.; Peng, L.; Wang, J. Phenotype and Biological Characteristics of Endometrial Mesenchymal Stem/Stromal Cells: A Comparison between Intrauterine Adhesion Patients and Healthy Women. Am. J. Reprod. Immunol. 2021, 85, e13379. [Google Scholar] [CrossRef]
- Cools, P.; Jespers, V.; Hardy, L.; Crucitti, T.; Delany-Moretlwe, S.; Mwaura, M.; Ndayisaba, G.F.; van de Wijgert, J.H.; Vaneechoutte, M. A Multi-Country Cross-Sectional Study of Vaginal Carriage of Group B Streptococci (GBS) and Escherichia Coli in Resource-Poor Settings: Prevalences and Risk Factors. PLoS ONE 2016, 11, e0148052. [Google Scholar] [CrossRef]
- van de Wijgert, J.; Verwijs, M. Lactobacilli-Containing Vaginal Probiotics to Cure or Prevent Bacterial or Fungal Vaginal Dysbiosis: A Systematic Review and Recommendations for Future Trial Designs. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 287–299. [Google Scholar] [CrossRef]
- Szczepańska, M.; Blicharz, L.; Nowaczyk, J.; Makowska, K.; Goldust, M.; Waśkiel-Burnat, A.; Czuwara, J.; Samochocki, Z.; Rudnicka, L. The Role of the Cutaneous Mycobiome in Atopic Dermatitis. J. Fungi 2022, 8, 1153. [Google Scholar] [CrossRef]
- Iliev, I.D.; Leonardi, I. Fungal Dysbiosis: Immunity and Interactions at Mucosal Barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Tao, R.; Li, R.; Wang, R. Dysbiosis of Skin Mycobiome in Atopic Dermatitis. Mycoses 2022, 65, 285–293. [Google Scholar] [CrossRef]
- Vallianou, N.; Kounatidis, D.; Christodoulatos, G.S.; Panagopoulos, F.; Karampela, I.; Dalamaga, M. Mycobiome and Cancer: What Is the Evidence? Cancers 2021, 13, 3149. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, N.; Okubo, S.; Kikumoto, S.; Kimura, K.; Saito, G.; Sakai, S. Host-mediated antitumor action of schizophyllan, a glucan produced by Schizophyllum commune. GANN Jpn. J. Cancer Res. 1969, 60, 137–144. [Google Scholar] [CrossRef]
- Gökşen Tosun, N.; Kaplan, Ö.; Türkekul, İ.; Gökçe, İ.; Özgür, A. Green Synthesis of Silver Nanoparticles Using Schizophyllum Commune and Geopora Sumneriana Extracts and Evaluation of Their Anticancer and Antimicrobial Activities. Part. Sci. Technol. 2022, 40, 801–811. [Google Scholar] [CrossRef]
- Lee, J.-S.; Lee, S.-H.; Jang, Y.-M.; Lee, J.-D.; Lee, B.-H.; Jung, J.-Y. Macrophage and Anticancer Activities of Feed Additives on β-Glucan from Schizophyllum commune in Breast Cancer Cells. J. Korean Soc. Food Sci. Nutr. 2011, 40, 949–955. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Bar, A.S.; Leal, C.A.; Gargus, M.; Tang, J.; Brown, J.; Funari, V.A.; Wang, H.L.; Crother, T.R.; et al. Immunological Consequences of Intestinal Fungal Dysbiosis. Cell Host Microbe 2016, 19, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Liu, E.J.; Kheradman, R.; Fagan, A.; Heuman, D.M.; White, M.; Gavis, E.A.; Hylemon, P.; Sikaroodi, M.; Gillevet, P.M. Fungal Dysbiosis in Cirrhosis. Gut 2018, 67, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Lamas, B.; Richard, M.L.; Brandi, G.; da Costa, G.; Hoffmann, T.W.; Di Simone, M.P.; Calabrese, C.; Poggioli, G.; Langella, P.; et al. Fungal Dysbiosis in Mucosa-Associated Microbiota of Crohn’s Disease Patients. J. Crohn’s Colitis 2016, 10, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Schnabl, B. Fungal Infections and the Fungal Microbiome in Hepatobiliary Disorders. J. Hepatol. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.-N.; Ma, Q.; Ge, Y.; Yi, C.-X.; Wei, L.-Q.; Tan, J.-C.; Chu, Q.; Li, J.-Q.; Zhang, P.; Wang, H. Microbiome Dysbiosis in Lung Cancer: From Composition to Therapy. NPJ Precis. Oncol. 2020, 4, 33. [Google Scholar] [CrossRef]
- Menakongka, A.; Ruaengsritanyakij, S.; Sripayak, S.; Suthiphongchai, T. Anti-Proliferation and Anti-Migration Effect of a Medicinal Mushroom, Schizophyllum Commune, on Human Cholangiocarcinoma Cell Line. Vajira Med. J. J. Urban Med. 2019, 63, 325–336. [Google Scholar] [CrossRef]
- Gutierrez, M.W.; van Tilburg Bernardes, E.; Changirwa, D.; McDonald, B.; Arrieta, M.-C. “Molding” Immunity—Modulation of Mucosal and Systemic Immunity by the Intestinal Mycobiome in Health and Disease. Mucosal Immunol. 2022, 15, 573–583. [Google Scholar] [CrossRef]
- Gradisteanu Pircalabioru, G.; Ilie, I.; Oprea, L.; Picu, A.; Petcu, L.M.; Burlibasa, L.; Chifiriuc, M.-C.; Musat, M. Microbiome, Mycobiome and Related Metabolites Alterations in Patients with Metabolic Syndrome—A Pilot Study. Metabolites 2022, 12, 218. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the Vaginal Microbiome and Candida Colonization in Women of Reproductive Age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef]
- Douglas, P. Overdiagnosis and Overtreatment of Nipple and Breast Candidiasis: A Review of the Relationship between Diagnoses of Mammary Candidiasis and Candida Albicans in Breastfeeding Women. Womens Health 2021, 17, 17455065211031480. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Chang, M.W.; Chai, L.Y.A. The Fungal Mycobiome and Its Interaction with Gut Bacteria in the Host. Int. J. Mol. Sci. 2017, 18, 330. [Google Scholar] [CrossRef] [PubMed]
- Oever, J.t.; Netea, M.G. The Bacteriome–Mycobiome Interaction and Antifungal Host Defense. Eur. J. Immunol. 2014, 44, 3182–3191. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Ghedin, E. The Human Mycobiome in Health and Disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef] [PubMed]
| Region of Dysbiosis | Significance |
|---|---|
| Vaginal |
|
| Gut |
|
| Skin |
|
| Overall Mycobiome |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, M.M.; Patsakos, S.; Borruso, L. The Role of the Mycobiome in Women’s Health. J. Fungi 2023, 9, 348. https://doi.org/10.3390/jof9030348
Esposito MM, Patsakos S, Borruso L. The Role of the Mycobiome in Women’s Health. Journal of Fungi. 2023; 9(3):348. https://doi.org/10.3390/jof9030348
Chicago/Turabian StyleEsposito, Michelle Marie, Savannah Patsakos, and Larisa Borruso. 2023. "The Role of the Mycobiome in Women’s Health" Journal of Fungi 9, no. 3: 348. https://doi.org/10.3390/jof9030348
APA StyleEsposito, M. M., Patsakos, S., & Borruso, L. (2023). The Role of the Mycobiome in Women’s Health. Journal of Fungi, 9(3), 348. https://doi.org/10.3390/jof9030348








