Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Identification
2.2. General Experimental Procedure
2.3. Fungal Materials, Cultivation, Fermentation, and Isolation
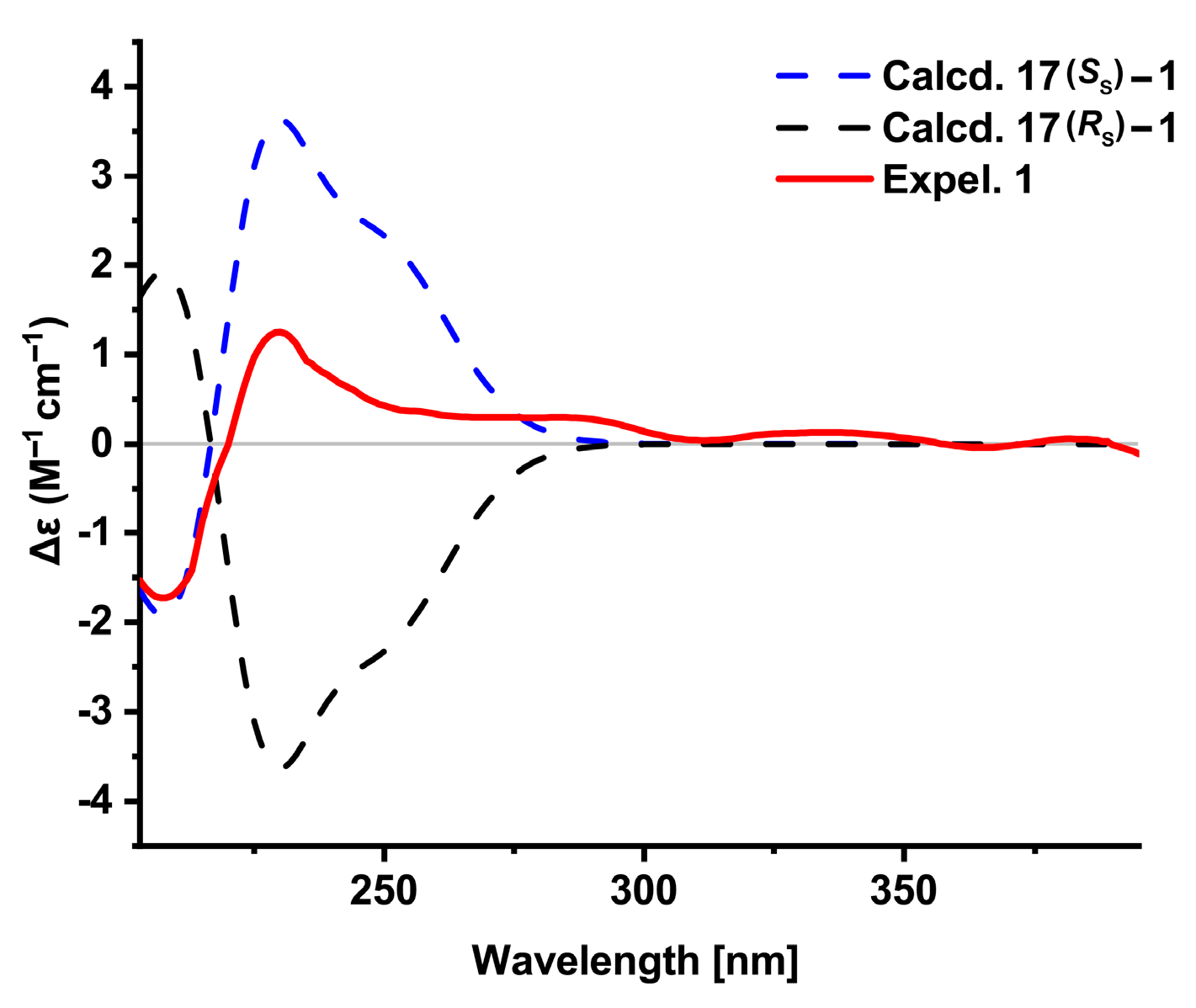
2.4. ECD Calculation Methods
2.5. The Antibacterial Assay
2.6. The Inhibition of Spore Germination Assay
2.7. The Nematode Toxic Assay
2.8. Data Analysis and Process
3. Results
3.1. Phylogenetic Analysis
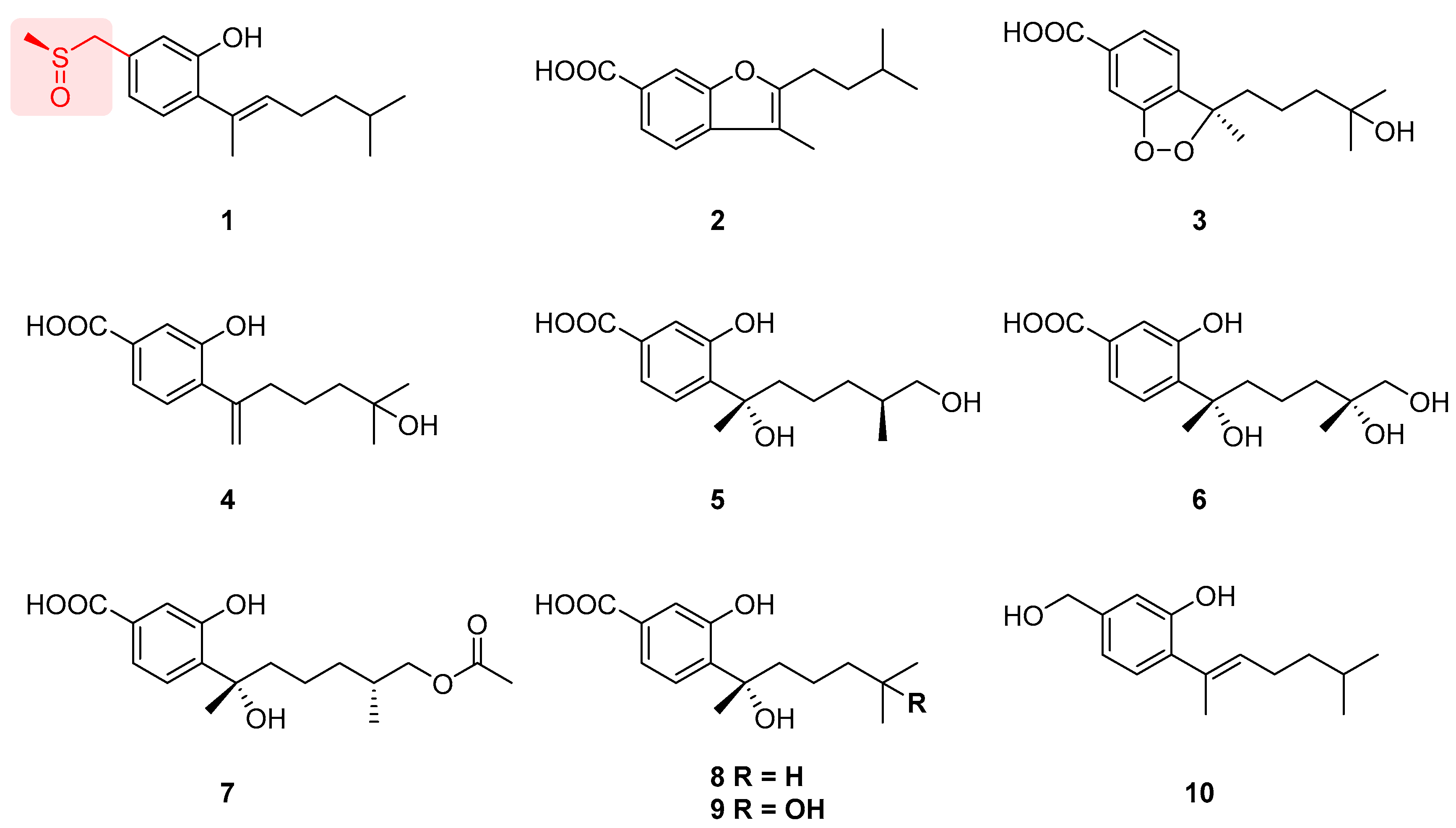
3.2. Structure Elucidation
3.3. Antibacterial Activities of the Isolated Compounds
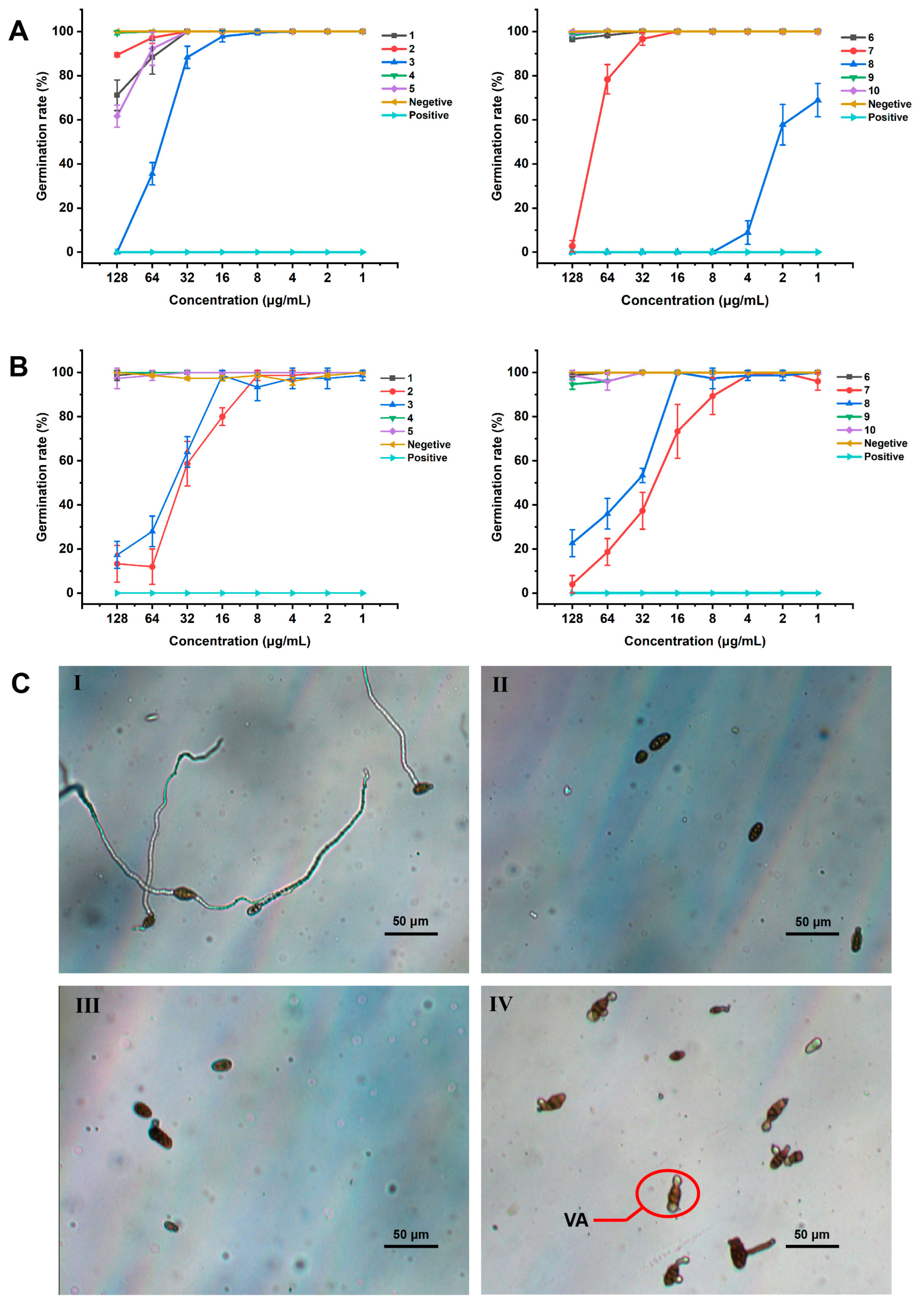
3.4. Inhibition of Spore Germination of the Isolated Compounds
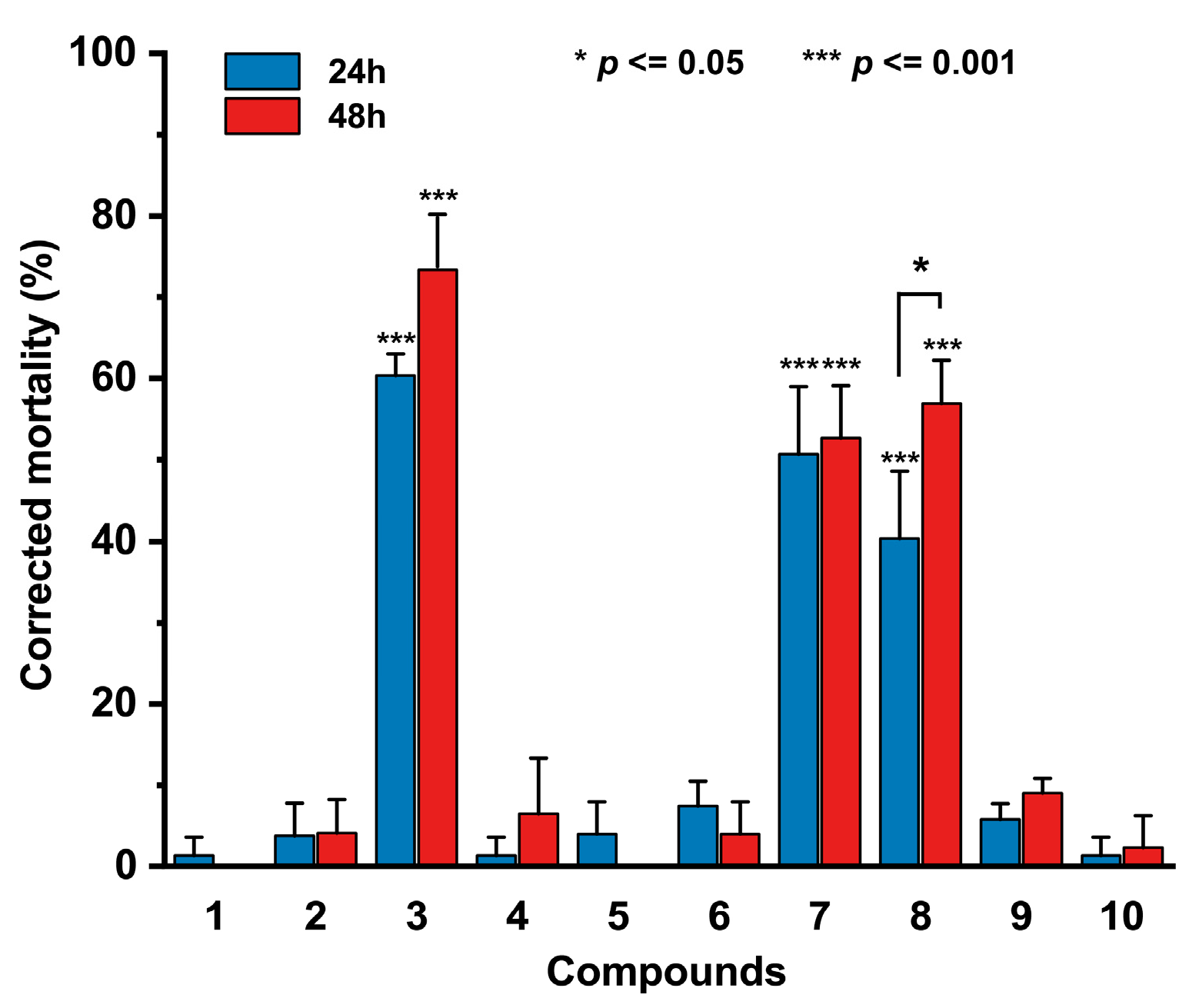
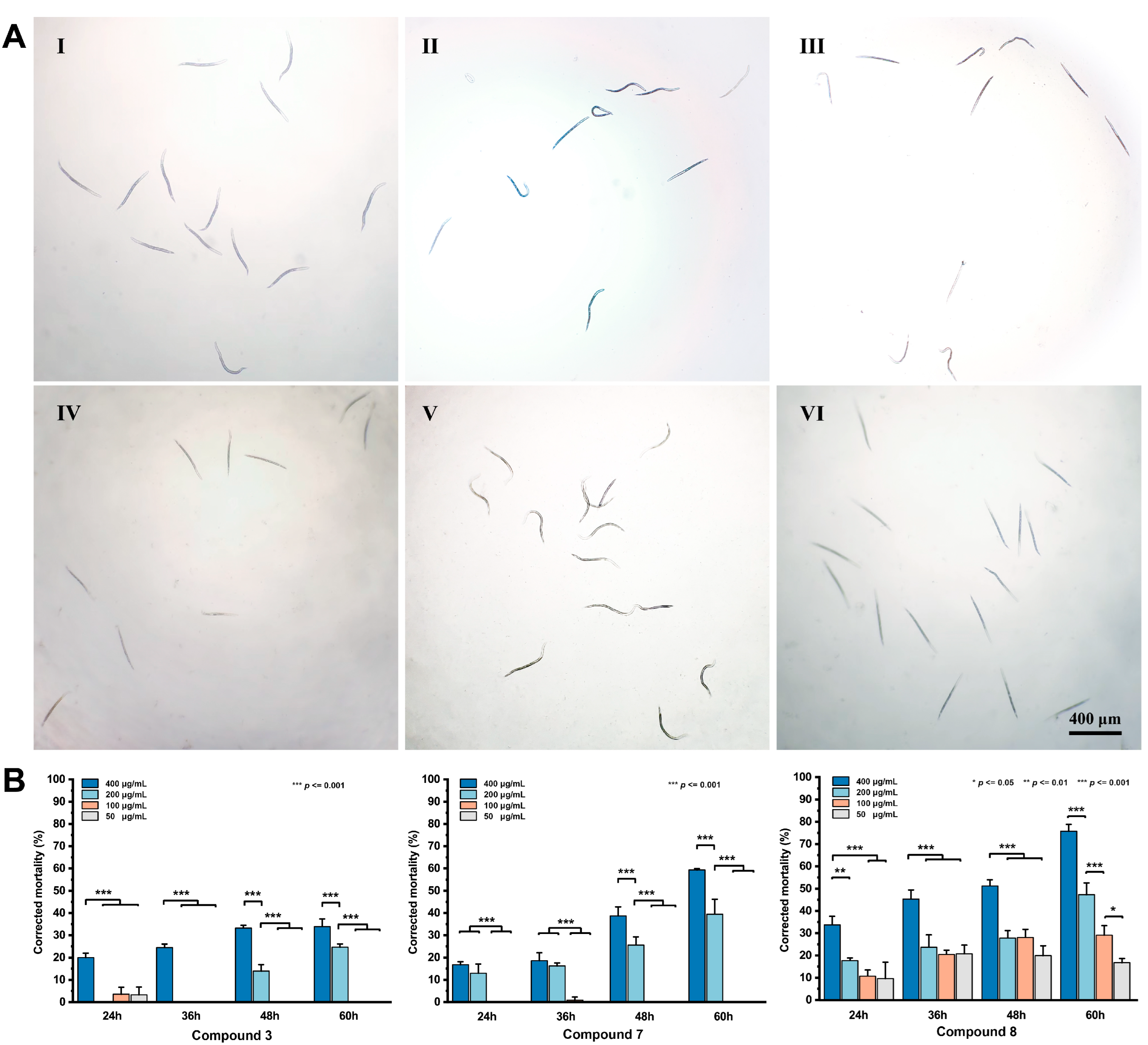
3.5. Nematicidal Activity of the Isolated Compounds
3.6. Plausible Biosynthetic Pathways of the Phenolic Bisabolane Sesquiterpenoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.K.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43, 83–116. [Google Scholar] [CrossRef]
- Kaushik, G.; Satya, S.; Naik, S.N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Leroch, M.; Kretschmer, M.; Hahn, M. Fungicide resistance phenotypes of Botrytis cinerea isolates from commercial vineyards in South West Germany. J. Phytopathol. 2011, 159, 63–65. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Rodrigo, S.; García-Latorre, C.; Santamaria, O. Metabolites produced by fungi against fungal phytopathogens: Review, implementation and perspectives. Plants 2022, 11, 81. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.S. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar] [CrossRef]
- Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E.; Raya-Ortegaa, M.C.; Trapero-Casas, A. Metarhizium brunneum and Beauveria bassiana release secondary metabolites with antagonistic activity against Verticillium dahliae and Phytophthora megasperma olive pathogens. Crop Prot. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W. Chemical ecology-driven discovery of bioactive marine natural products as potential drug leads. Chin. J. Nat. Med. 2020, 18, 837–838. [Google Scholar] [CrossRef]
- Rateb, M.E.; Ebel, R. Secondary metabolites of fungi from marine habitats. Nat. Prod. Rep. 2011, 28, 290–344. [Google Scholar] [CrossRef]
- Orfali, R.; Aboseada, M.A.; Abdel-Wahab, N.M.; Hassan, H.M.; Perveen, S.; Ameen, F.; Alturki, E.; Abdelmohsen, U.R. Recent updates on the bioactive compounds of the marine-derived genus Aspergillus. RSC Adv. 2021, 11, 17116–17150. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Huo, R.; Liu, G.; Liu, L. New Andrastin-Type Meroterpenoids from the Marine-Derived Fungus Penicillium sp. Mar. Drugs 2021, 19, 189. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Liu, S.; Huo, R.; Li, Y.; Ren, J.; Wang, W.; Wei, T.; Jiang, X.; Yin, W.; Liu, H.; et al. New Diterpenoids and Isocoumarin Derivatives from the Mangrove-Derived Fungus Hypoxylon sp. Mar. Drugs 2021, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huo, R.Y.; Niu, S.B.; Liu, X.Z.; Liu, L. Alternariasin A, new pentacyclic cytochalasin from the fungus Alternaria alternate. J. Antibiot. 2021, 7, 596–600. [Google Scholar] [CrossRef]
- Liu, G.R.; Huo, R.Y.; Niu, S.B.; Song, F.H.; Liu, L. Two new cytotoxic decalin derivatives from marine-derived fungus Talaromyces sp. Chem. Biodivers. 2022, 19, e202100990. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.B.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.S.; Seifert, K.A.; Samson, R.A. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural products for drug discovery: Discovery of gramines as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef]
- Li, Z.; Guo, B.K.; Wan, K.; Ge, Y.Y. Effects of bacteria-free filtrate from Bacillus megaterium strain L2 on the mycelium growth and spore germination of Alternaria alternata. Biotechnol. Biotechnol. Equip. 2015, 29, 1062–1068. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Lu, F.; Guo, K.; Huang, L.; Su, X.; Chen, Y. Nematotoxicity of a Cyt-like protein toxin from Conidiobolus obscurus (Entomophthoromycotina) on the pine wood nematode Bursaphelenchus xylophilus. Pest. Manag. Sci. 2021, 77, 686–692. [Google Scholar] [CrossRef]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Zhou, Y.H.; Zhu, R.X.; Chang, W.Q.; Yuan, H.Q.; Gao, W.; Zhang, L.L.; Zhao, Z.T.; Lou, H.X. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem. Biodivers. 2015, 12, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, J.; Huang, S.H.; He, J.L.; Hong, B.H.; Yu, M.; Niu, S.W. Bisabolane sesquiterpenes with anti-inflammatory activities from the endophytic fungus Penicillium citrinum DF47. Chem. Biodivers. 2022, 19, e202200178. [Google Scholar] [CrossRef]
- Chung, Y.M.; Wei, C.K.; Chuang, D.W.; El-Shazly, M.; Hsieh, C.T.; Asai, T.; Oshima, Y.; Hsieh, T.J.; Hwang, T.L.; Wu, Y.C.; et al. An epigenetic modifier enhances the production of anti-diabetic and anti-inflammatory sesquiterpenoids from Aspergillus sydowii. Bioorg. Med. Chem. 2013, 21, 3866–3872. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, M.S.; Ebada, S.S.; Ashour, M.L.; Ebrahim, W.; Müller, W.E.; Mándi, A.; Kurtán, T.; Singab, A.; Lin, W.; Liu, Z.; et al. Xanthones and sesquiterpene derivatives from a marine-derived fungus Scopulariopsis sp. Tetrahedron 2016, 72, 2411–2419. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Xu, Y.; Wang, C.Y.; Cao, F. Alkaloids and sesquiterpenoids from the marine-derived fungus Aspergillus versicolor. Chem. Nat. Compd. 2020, 56, 971–973. [Google Scholar] [CrossRef]
- Hamasaki, T.; Nagayama, K.; Hatsuda, Y. Two new metabolites, sydonic acid and hydroxysydonic acid, from Aspergillus sydowi. Agric. Biol. Chem. 1978, 42, 37–40. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Yin, X.; Li, X.; Wang, B. Antimicrobial sesquiterpenoid derivatives and monoterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Mar. Drugs 2019, 17, 563. [Google Scholar] [CrossRef]
- Huang, Y.; Hang, X.; Jiang, X.; Zeng, L.; Jia, J.; Xie, Y.; Li, F.; Bi, H. In vitro and in vivo activities of zinc linolenate, a selective antibacterial agent against Helicobacter pylori. Antimicrob. Agents Chemother. 2019, 63, e00004-19. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, X.L.; Du, Y.M.; Zhang, H.B.; Shen, G.M.; Zhang, Z.F.; Liang, Y.J.; Zhao, D.L.; Xu, K. Angularly prenylated indole alkaloids with antimicrobial and insecticidal activities from an endophytic fungus Fusarium sambucinum TE-6L. J. Agric. Food Chem. 2019, 67, 11994–12001. [Google Scholar] [CrossRef]
- Chaerani, R.; Voorrips, R.E. Tomato early blight (Alternaria solani): The pathogen, genetics, and breeding for resistance. J. Gen. Plant. Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Fravel, D.; Olivain, C.; Alabouvette, C. Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A.; Cimmino, A.; Andolfi, A.; Lefranc, F.; Mathieu, V.; Kiss, R. Fungal metabolites with anticancer activity. Nat. Prod. Rep. 2014, 31, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.A.L.A.; Haggag, W.M. Biocontrol potential of salinity tolerant mutants of Trichoderma harzianum against Fusarium oxysporum. Braz. J. Microbiol. 2006, 37, 181–191. [Google Scholar] [CrossRef]
- Caloni, F.; Fossati, P.; Anadón, A.; Bertero, A. Beauvericin: The beauty and the beast. Environ. Toxicol. Pharmacol. 2020, 75, 103349. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, H.Y.; Xu, L.C.; Wang, S.P.; Liu, S.; Liu, G.D.; Luo, W.H.; Cao, G.Y.; Zhang, Z.X. Antimicrobial and cytotoxic phenolic bisabolane sesquiterpenoids from the fungus Aspergillus flavipes 297. Fitoterapia 2021, 155, 105038. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Tang, W.L.; Huang, Q.R.; Wei, M.L.; Li, Y.Z.; Jiang, L.L.; Li, C.L.; Yu, X.; Zhu, H.W.; Chen, G.Z.; et al. New Enantiomers of a nor-bisabolane derivative and two new phthalides produced by the marine-derived fungus Penicillium chrysogenum LD-201810. Front. Microbiol. 2021, 12, 727670. [Google Scholar] [CrossRef] [PubMed]
- D’Enfert, C. Fungal spore germination: Insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 1997, 21, 163–172. [Google Scholar] [CrossRef]
- Castagnone-Sereno, P.; Danchin, E.G.J.; Perfus-Barbeoch, L.; Abad, P. Diversity and evolution of root-knot nematodes, genus Meloidogyne: New insights from the genomic era. Annu. Rev. Phytopathol. 2013, 51, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef]
- Stadler, M.; Anke, H.; Sterner, O. New nematicidal and antimicrobial compounds from the basidiomycete Cheimonophyllum candidissimum (Berk & Curt.) sing. 1. Producing organism, fermentation, isolation, and biological activities. J. Antibiot. 1994, 47, 1284–1289. [Google Scholar]

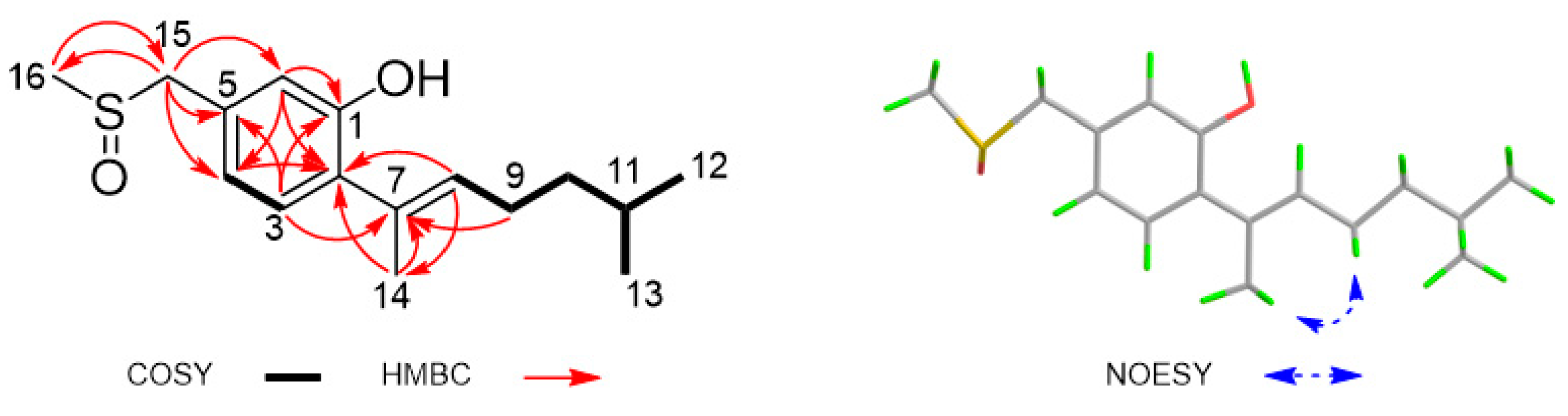

| Position | δH J (Hz) | δC, Type | HMBC |
|---|---|---|---|
| 1 | 155.0, C | ||
| 2 | 133.3, C | ||
| 3 | 7.05, d (7.7) | 130.3, CH | C-7, C-5, C-4, C-2, C-1 |
| 4 | 6.77, dd (7.7, 1.4) | 122.2, CH | C-15, C-6, C-5, C-3, C-2 |
| 5 | 131.7, C | ||
| 6 | 6.84, s | 118.1, CH | C-15, C-4, C-2, C-1 |
| 7 | 134.7, C | ||
| 8 | 5.46, tq (7.4, 1.2) | 130.9, CH | C-14, C-10, C-9, C-2 |
| 9 | 2.18, q (7.4) | 26.9, CH2 | C-11, C-10, C-8, C-7 |
| 10 | 1.33, m | 39.5, CH2 | C-13, C-12, C-11, C-9, C-8 |
| 11 | 1.63, m | 28.4, CH | C-13, C-12, C-10, C-9 |
| 12 | 0.92, d (6.5) | 22.9, CH3 | C-13, C-11, C-10 |
| 13 | 0.92, d (6.5) | 22.9, CH3 | C-12, C-11, C-10 |
| 14 | 1.99, s | 17.1, CH3 | C-8, C-7, C-2 |
| 15 | 3.85, d (13.0) 3.94, d (13.0) | 60.3, CH2 | C-16, C-6, C-5, C-4 |
| 16 | 2.46, s | 38.1, CH3 | C-15 |
| 1-OH | 8.16, br s |
| Organisms | MIC b Values (μg/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Positive a | |
| P. syringae | >64 | >64 | >64 | >64 | 32 | >64 | >64 | >64 | >64 | >64 | 1 |
| R. solanacarum | >64 | 32 | >64 | >64 | >64 | >64 | 32 | 32 | >64 | >64 | 0.125 |
| Compounds | F. oxysporum | A. alternata |
|---|---|---|
| EC50 (μg/mL) a | ||
| 1 | >128 | >128 |
| 2 | >128 | 34.04 ± 1.06 |
| 3 | 54.55 ± 1.02 | 44.44 ± 1.06 |
| 4 | >128 | >128 |
| 5 | >128 | >128 |
| 6 | >128 | >128 |
| 7 | 77.16 ± 1.02 | 26.02 ± 1.06 |
| 8 | 1.85 ± 1.07 | 46.15 ± 1.10 |
| 9 | >128 | >128 |
| 10 | >128 | >128 |
| Chlorothalonil | <1 | <1 |
| Compounds | LC50 (μg/mL) a | |||
|---|---|---|---|---|
| 24 h | 36 h | 48 h | 60 h | |
| 3 | >400 | >400 | >400 | >400 |
| 7 | >400 | >400 | >400 | 302.8 ± 1.08 |
| 8 | >400 | >400 | 367.60 ± 1.06 | 192.40 ± 1.05 |
| Ivermectin | >400 | 311.00 ± 1.21 | 273.80 ± 1.07 | 206.90 ± 1.07 |
| Abamectin | 375.40 ± 1.05 | 220.00 ± 1.05 | 192.80 ± 1.04 | 146.10 ± 1.07 |
| Observation Time (h) | Treated Concentration (μg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 8 | p | ||||||||||
| 400 | 200 | 100 | 50 | 400 | 200 | 100 | 50 | 400 | 200 | 100 | 50 | 50 | |
| 24 | – | + | ++ | ++ | – | – | + | + | – | – | – | – | – |
| 36 | – | ++ | ++ | ++ | – | – | ++ | ++ | – | + | + | + | – |
| 48 | – | ++ | ++ | ++ | – | + | ++ | ++ | – | + | + | + | – |
| 60 | – | + | ++ | ++ | – | – | + | ++ | – | – | + | + | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yu, H.; Ren, J.; Cai, L.; Xu, L.; Liu, L. Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09. J. Fungi 2023, 9, 347. https://doi.org/10.3390/jof9030347
Yang X, Yu H, Ren J, Cai L, Xu L, Liu L. Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09. Journal of Fungi. 2023; 9(3):347. https://doi.org/10.3390/jof9030347
Chicago/Turabian StyleYang, Xiao, Hongjia Yu, Jinwei Ren, Lei Cai, Lijian Xu, and Ling Liu. 2023. "Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09" Journal of Fungi 9, no. 3: 347. https://doi.org/10.3390/jof9030347
APA StyleYang, X., Yu, H., Ren, J., Cai, L., Xu, L., & Liu, L. (2023). Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09. Journal of Fungi, 9(3), 347. https://doi.org/10.3390/jof9030347








