Copper Ion Mediates Yeast-to-Hypha Transition in Yarrowia lipolytica
Abstract
1. Introduction
2. Results
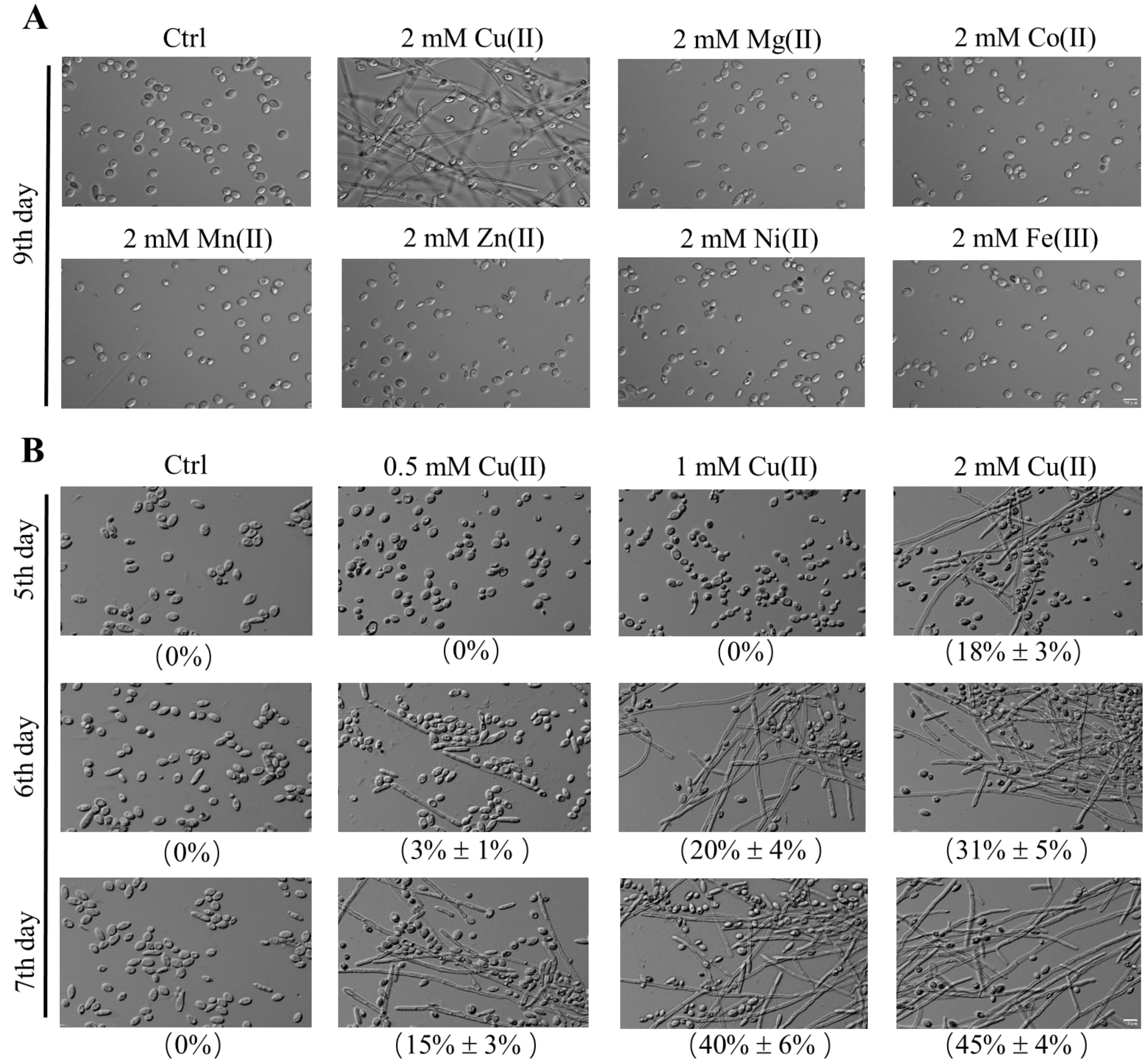
2.1. Cu(II)-Induced Yeast-to-Hypha Transition in Y. lipolytica in the Stable Growth Period
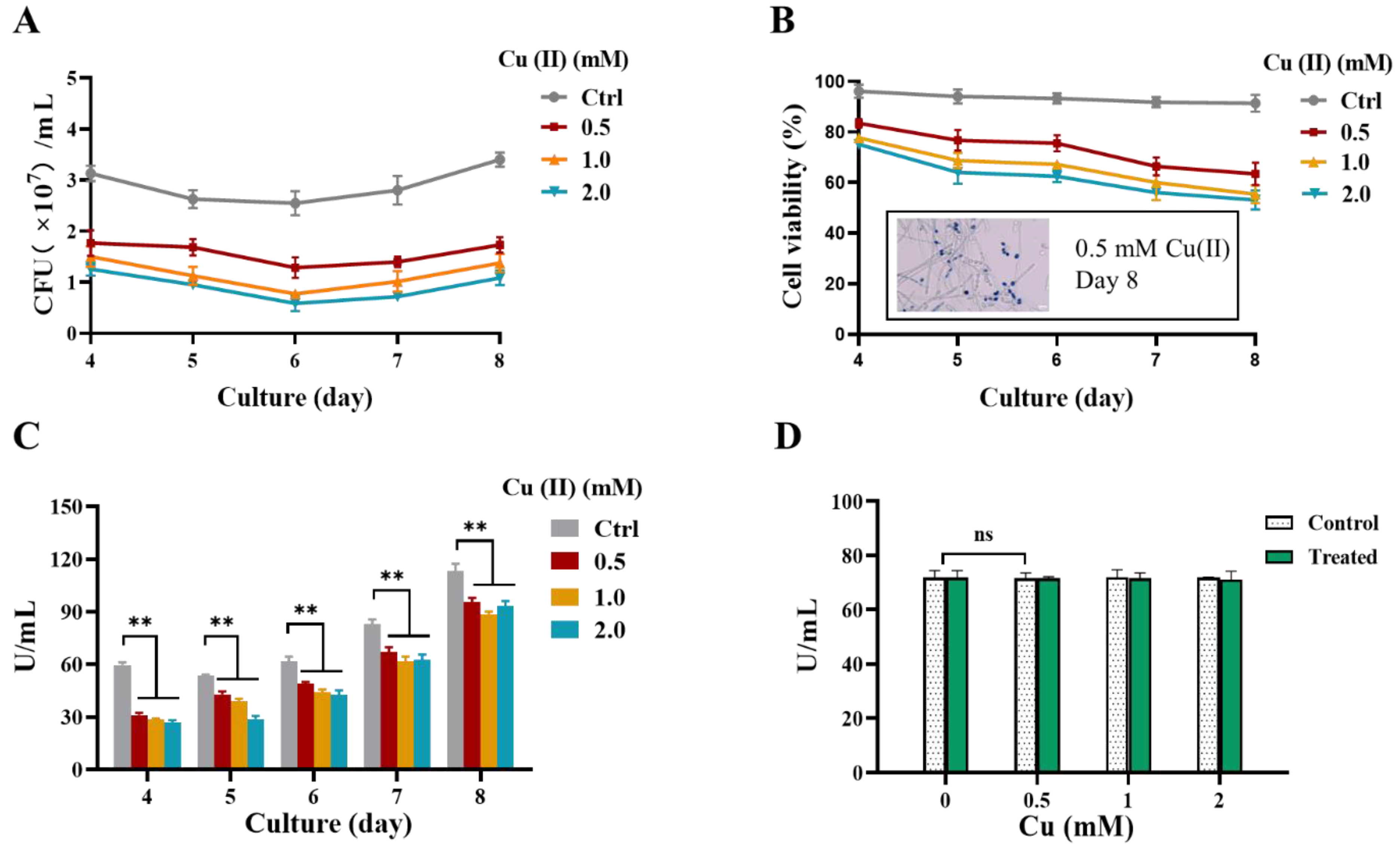
2.2. Cu(II) Accumulation of Y. lipolytica Cells Decreased after Hyphae Formation
2.3. Cu(II) Reduces the Viability and the Thermomyces Lanuginosus Lipase (TLL) Activity of Y. lipolytica
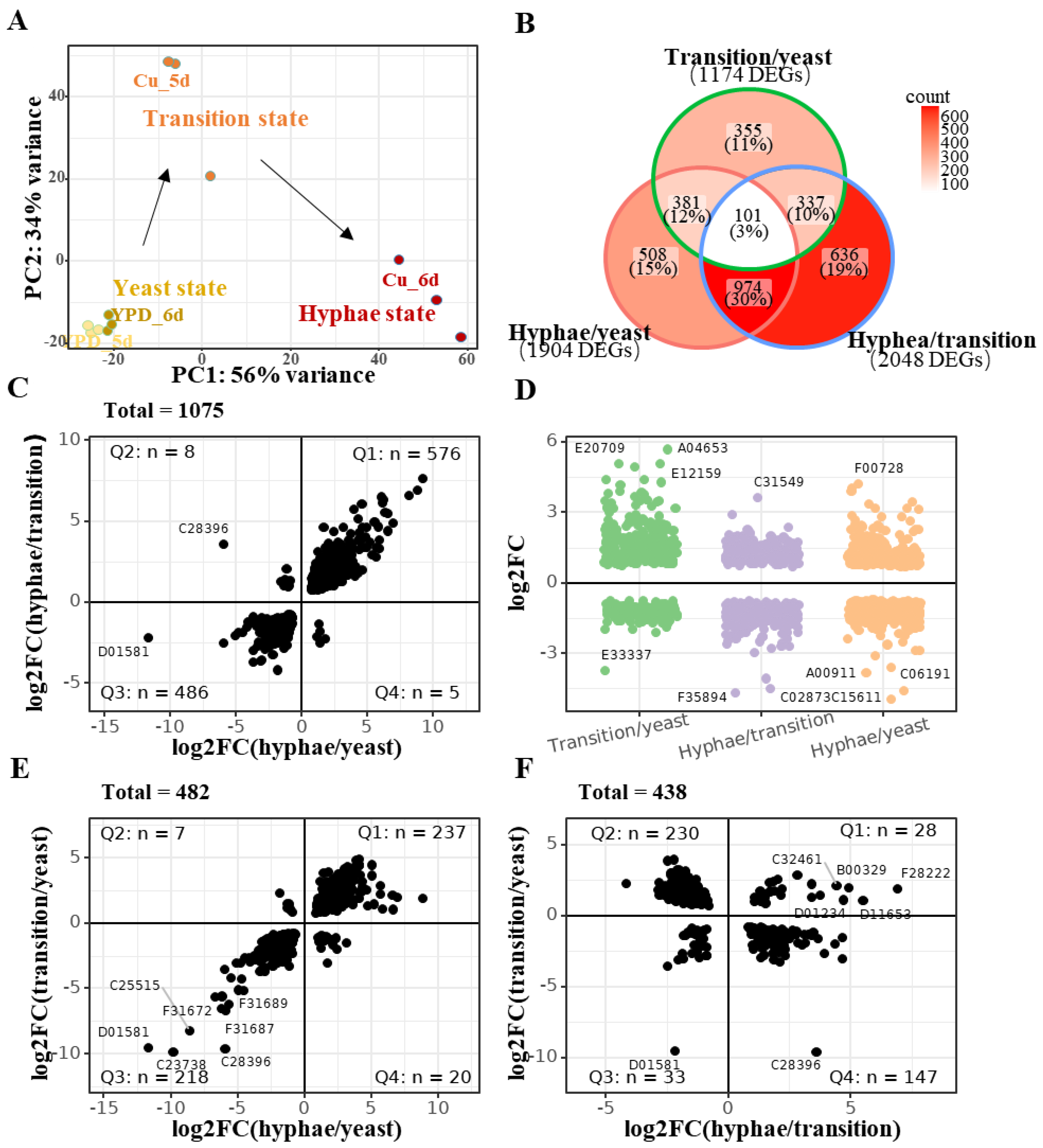
2.4. Overall Gene Expression Changes in Cu-Induced Yeast-to-Hypha Transition
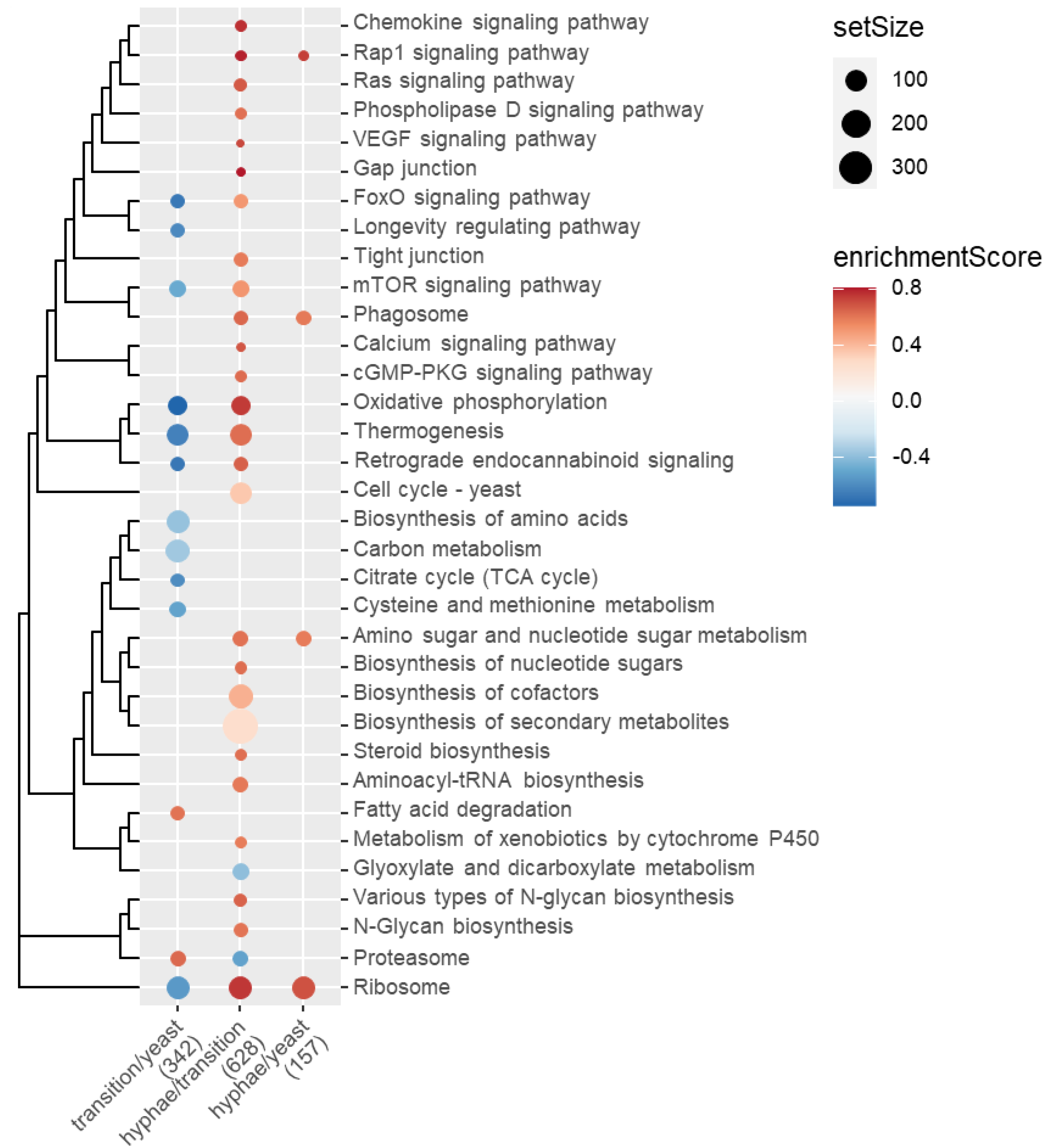
2.5. Functional Enrichment of Gene Expression Changes
2.6. Novel Genes Involved in Cu-Induced Dimorphic Transition
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains, Plasmids, and Culture Conditions
5.2. Construction of Overexpressing Strain
5.3. Construction of MHY1 Knockout Strain
5.4. Measurement of Cell Morphologies, Hyphal Quantification, and Dry Cell Weight
5.5. Assay of Copper Ion Accumulation
5.6. Quantification of Cell Viability
5.7. Determination of Thermomyces Lanuginosus Lipase (TLL) Activity
5.8. RNA Extraction and RNA-Seq Analysis
5.9. qRT-PCR Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, N. Metal Ion Transporters and Homeostasis. EMBO J. 1999, 18, 4361–4371. [Google Scholar] [CrossRef]
- Karlin, K.D. Metalloenzymes, Structural Motifs, and Inorganic Models. Science 1993, 261, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.D.; Wintz, H.; Kim, J.H.; Poynton, H.; Fox, T.; Vulpe, C. Yeast, a Model Organism for Iron and Copper Metabolism Studies. Biometals 2003, 16, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorption of Heavy Metals by Saccharomyces Cerevisiae: A Review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Stoll, A.D.; Starke, L.; Duncan, J.R. Chemical and Enzymatic Extraction of Heavy Metal Binding Polymers from Isolated Cell Walls of Saccharomyces Cerevisiae. Biotechnol. Bioeng. 1994, 44, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, R.; Tamás, M.J. How Saccharomyces Cerevisiae Copes with Toxic Metals and Metalloids. FEMS Microbiol. Rev. 2010, 34, 925–951. [Google Scholar] [CrossRef]
- Presta, A.; Stillman, M.J. Incorporation of Copper ELSEVIER into the Yeast Saccharomycescerevisiae. Identification of Cu(I)-Metallothionein in Intact Yeast Cells. J. Inorg. Biochem. 1997, 66, 231–240. [Google Scholar] [CrossRef]
- Volesky, B. Advances in Biosorption of Metals: Selection of Biomass Types. FEMS Microbiol. Rev. 1994, 14, 291–302. [Google Scholar] [CrossRef]
- Bahafid, W.; Joutey, N.T.; Asri, M.; Sayel, H.; Tirry, N.; Ghachtouli, N.E. Yeast Biomass: An Alternative for Bioremediation of Heavy Metals; IntechOpen: London, UK, 2017; ISBN 978-953-51-3600-2. [Google Scholar]
- Bankar, A.; Winey, M.; Prakash, D.; Kumar, A.R.; Gosavi, S.; Kapadnis, B.; Zinjarde, S. Bioleaching of Fly Ash by the Tropical Marine Yeast, Yarrowia Lipolytica NCIM 3589. Appl. Biochem. Biotechnol. 2012, 168, 2205–2217. [Google Scholar] [CrossRef]
- Bankar, A.; Zinjarde, S.; Telmore, A.; Walke, A.; Ravikumar, A. Morphological Response of Yarrowia Lipolytica under Stress of Heavy Metals. Can. J. Microbiol. 2018, 64, 559–566. [Google Scholar] [CrossRef]
- Lengeler, K.B.; Davidson, R.C.; D’souza, C.; Harashima, T.; Shen, W.-C.; Wang, P.; Pan, X.; Waugh, M.; Heitman, J. Signal Transduction Cascades Regulating Fungal Development and Virulence. Microbiol. Mol. Biol. Rev. 2000, 64, 746–785. [Google Scholar] [CrossRef]
- Domínguez, A.; Fermiñán, E.; Gaillardin, C. Yarrowia Lipolytica: An Organism Amenable to Genetic Manipulation as a Model for Analyzing Dimorphism in Fungi. In Contributions to Microbiology; Ernst, J.F., Schmidt, A., Eds.; KARGER: Basel, Switzerland, 2000; Volume 5, pp. 151–172. ISBN 978-3-8055-6986-6. [Google Scholar]
- Moseley, J.B.; Nurse, P. Cdk1 and Cell Morphology: Connections and Directions. Curr. Opin. Cell Biol. 2009, 21, 82–88. [Google Scholar] [CrossRef]
- Kijpornyongpan, T.; Aime, M.C. Investigating the Smuts: Common Cues, Signaling Pathways, and the Role of MAT in Dimorphic Switching and Pathogenesis. J. Fungi 2020, 6, 368. [Google Scholar] [CrossRef]
- Gimeno, C.J.; Ljungdahl, P.O.; Styles, C.A.; Fink, G.R. Unipolar Cell Divisions in the Yeast S. Cerevisiae Lead to Filamentous Growth: Regulation by Starvation and RAS. Cell 1992, 68, 1077–1090. [Google Scholar] [CrossRef]
- Kadosh, D. Shaping Up for Battle: Morphological Control Mechanisms in Human Fungal Pathogens. PLOS Pathog. 2013, 9, e1003795. [Google Scholar] [CrossRef]
- Wang, J.; Ledesma-Amaro, R.; Wei, Y.; Ji, B.; Ji, X.-J. Metabolic Engineering for Increased Lipid Accumulation in Yarrowia Lipolytica—A Review. Bioresour. Technol. 2020, 313, 123707. [Google Scholar] [CrossRef]
- Miller, K.K.; Alper, H.S. Yarrowia Lipolytica: More than an Oleaginous Workhorse. Appl. Microbiol. Biotechnol. 2019, 103, 9251–9262. [Google Scholar] [CrossRef]
- Medina, A.; Vassileva, M.; Caravaca, F.; Roldán, A.; Azcón, R. Improvement of Soil Characteristics and Growth of Dorycnium Pentaphyllum by Amendment with Agrowastes and Inoculation with AM Fungi and/or the Yeast Yarowia Lipolytica. Chemosphere 2004, 56, 449–456. [Google Scholar] [CrossRef]
- Zinjarde, S.; Apte, M.; Mohite, P.; Kumar, A.R. Yarrowia Lipolytica and Pollutants: Interactions and Applications. Biotechnol. Adv. 2014, 32, 920–933. [Google Scholar] [CrossRef]
- Mou, J.-H.; Tahar, I.B.; Wang, Z.-Y.; Ong, K.L.; Li, C.; Qin, Z.-H.; Wang, X.; Lin, C.S.K.; Fickers, P. Enhancing the Recombinant Protein Productivity of Yarrowia Lipolytica Using Insitu Fibrous Bed Bioreactor. Bioresour. Technol. 2021, 340, 125672. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, N.; Greisen, P.; Li, J.; Qiao, K.; Huang, S.; Stephanopoulos, G. Removal of Lycopene Substrate Inhibition Enables High Carotenoid Productivity in Yarrowia Lipolytica. Nat. Commun. 2022, 13, 572. [Google Scholar] [CrossRef] [PubMed]
- Timoumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of Environmental Conditions on Product Formation and Morphology of Yarrowia Lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Ye, J.; Qi, Q.; Hou, J. Morphological and Metabolic Engineering of Yarrowia Lipolytica to Increase β-Carotene Production. ACS Synth. Biol. 2021, 10, 3551–3560. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Zeng, S.-Y.; Shi, T.-Q.; Ding, Y.; Ren, L.-J.; Song, P.; Huang, H.; Madzak, C.; Ji, X.-J. A Yarrowia Lipolytica Strain Engineered for Arachidonic Acid Production Counteracts Metabolic Burden by Redirecting Carbon Flux towards Intracellular Fatty Acid Accumulation at the Expense of Organic Acids Secretion. Biochem. Eng. J. 2017, 128, 201–209. [Google Scholar] [CrossRef]
- Braga, A.; Mesquita, D.P.; Amaral, A.L.; Ferreira, E.C.; Belo, I. Quantitative Image Analysis as a Tool for Yarrowia Lipolytica Dimorphic Growth Evaluation in Different Culture Media. J. Biotechnol. 2016, 217, 22–30. [Google Scholar] [CrossRef]
- Linder, M.C. Copper in Growth and Development. Biochem. Copp. 1991, 10, 301–330. [Google Scholar] [CrossRef]
- Bannister, W. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life: By J J R Fraústo Da Silva and R J P Williams. Pp 561. Clarendon Press, Oxford. 1991. £60. Biochem. Educ. 1992, 20, 62–63. [Google Scholar] [CrossRef]
- Rae, T.D.; Schmidt, P.J.; Pufahl, R.A.; Culotta, V.C.; O’Halloran, T.V. Undetectable Intracellular Free Copper: The Requirement of a Copper Chaperone for Superoxide Dismutase. Science 1999, 284, 805–808. [Google Scholar] [CrossRef]
- Miskowski, V.; Tang, S.P.W.; Spiro, T.G.; Shapiro, E.; Moss, T.H. Copper Coordination Group in Blue Copper Proteins. Evidence from Resonance Raman Spectra. Biochemistry 1975, 14, 1244–1250. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper Induces Cell Death by Targeting Lipoylated TCA Cycle Proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Wierzba, S. Biosorption of Cu(II) by Live and Dead Cells of Yarrowia Lipolytica. Proc. ECOpole 2014, 8, 103–108. [Google Scholar]
- Garcȷa, S.; Prado, M.; Dégano, R.; Domȷnguez, A. A Copper-Responsive Transcription Factor, CRF1, Mediates Copper and Cadmium Resistance in Yarrowia Lipolytica. J. Biol. Chem. 2002, 277, 37359–37368. [Google Scholar] [CrossRef]
- Ito, H.; Inouhe, M.; Tohoyama, H.; Joho, M. Characteristics of Copper Tolerance in Yarrowia Lipolytica. Biometals 2007, 20, 773–780. [Google Scholar] [CrossRef]
- Ito, H.; Inouhe, M.; Tohoyama, H.; Joho, M. Effect of Copper on Acid Phosphatase Activity in Yeast Yarrowia Lipolytica. Z. Für Nat. C 2007, 62, 70–76. [Google Scholar] [CrossRef]
- Aloulou, A.; Puccinelli, D.; De Caro, A.; Leblond, Y.; Carrière, F. A Comparative Study on Two Fungal Lipases from Thermomyces Lanuginosus and Yarrowia Lipolytica Shows the Combined Effects of Detergents and PH on Lipase Adsorption and Activity. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2007, 1771, 1446–1456. [Google Scholar] [CrossRef]
- Schäfer, T.; Borchert, T.W.; Nielsen, V.S.; Skagerlind, P.; Gibson, K.; Wenger, K.; Hatzack, F.; Nilsson, L.D.; Salmon, S.; Pedersen, S.; et al. Industrial Enzymes. White Biotechnol. 2006, 105, 59–131. [Google Scholar] [CrossRef]
- Shu, T.; He, X.-Y.; Chen, J.-W.; Mao, Y.-S.; Gao, X.-D. The PH-Responsive Transcription Factors YlRim101 and Mhy1 Regulate Alkaline PH-Induced Filamentation in the Dimorphic Yeast Yarrowia Lipolytica. mSphere 2021, 6, e00179-21. [Google Scholar] [CrossRef]
- Wu, H.; Shu, T.; Mao, Y.-S.; Gao, X.-D. Characterization of the Promoter, Downstream Target Genes and Recognition DNA Sequence of Mhy1, a Key Filamentation-Promoting Transcription Factor in the Dimorphic Yeast Yarrowia Lipolytica. Curr. Genet. 2020, 66, 245–261. [Google Scholar] [CrossRef]
- Chen, J.-W.; Mao, Y.-S.; Yan, L.-Q.; Gao, X.-D. The Zinc Finger Transcription Factor Fts2 Represses the Yeast-to-Filament Transition in the Dimorphic Yeast Yarrowia Lipolytica. mSphere 2022, 7, e0045022. [Google Scholar] [CrossRef]
- Hurtado, C.A.R.; Rachubinski, R.A. MHY1 Encodes a C2H2-Type Zinc Finger Protein That Promotes Dimorphic Transition in the Yeast Yarrowia Lipolytica. J. Bacteriol. 1999, 181, 3051–3057. [Google Scholar] [CrossRef]
- Richard, M.; Quijano, R.R.; Bezzate, S.; Bordon-Pallier, F.; Gaillardin, C. Tagging Morphogenetic Genes by Insertional Mutagenesis in the Yeast Yarrowia Lipolytica. J. Bacteriol. 2001, 183, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.A.R.; Beckerich, J.-M.; Gaillardin, C.; Rachubinski, R.A. A Rac Homolog Is Required for Induction of Hyphal Growth in the Dimorphic Yeast Yarrowia Lipolytica. J. Bacteriol. 2000, 182, 2376–2386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-F.; Li, M.; Li, Y.-Q.; Chen, X.-D.; Gao, X.-D. The TEA/ATTS Transcription Factor YlTec1p Represses the Yeast-to-Hypha Transition in the Dimorphic Yeast Yarrowia Lipolytica. FEMS Yeast Res. 2013, 13, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Li, M.; Zhao, X.-F.; Gao, X.-D. A Role for the Rap GTPase YlRsr1 in Cellular Morphogenesis and the Involvement of YlRsr1 and the Ras GTPase YlRas2 in Bud Site Selection in the Dimorphic Yeast Yarrowia Lipolytica. Eukaryot. Cell 2014, 13, 580–590. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Dimou, A.; Fakas, S.; Diamantopoulou, P.; Philippoussis, A.; Galiotou-Panayotou, M.; Aggelis, G. Biotechnological Conversion of Waste Cooking Olive Oil into Lipid-Rich Biomass Using Aspergillus and Penicillium Strains. J. Appl. Microbiol. 2011, 110, 1138–1150. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Liu, N.; Eser, B.E.; Guo, Z.; Jensen, P.R.; Stephanopoulos, G. Synthesis of High-Titer Alka(e)Nes in Yarrowia Lipolytica Is Enabled by a Discovered Mechanism. Nat. Commun. 2020, 11, 6198. [Google Scholar] [CrossRef]
- Schröter, C.; Hipler, U.C.; Wilmer, A.; Künkel, W.; Wollina, U. Generation of Reactive Oxygen Species by Candida Albicans in Relation to Morphogenesis. Arch. Derm. Res. 2000, 292, 260–264. [Google Scholar] [CrossRef]
- Basso, V.; Znaidi, S.; Lagage, V.; Cabral, V.; Schoenherr, F.; LeibundGut-Landmann, S.; d’Enfert, C.; Bachellier-Bassi, S. The Two-Component Response Regulator Skn7 Belongs to a Network of Transcription Factors Regulating Morphogenesis in Candida Albicans and Independently Limits Morphogenesis-Induced ROS Accumulation. Mol. Microbiol. 2017, 106, 157–182. [Google Scholar] [CrossRef]
- Weber, M.; Basu, S.; González, B.; Greslehner, G.P.; Singer, S.; Haskova, D.; Hasek, J.; Breitenbach, M.; W Gourlay, C.; Cullen, P.J.; et al. Actin Cytoskeleton Regulation by the Yeast NADPH Oxidase Yno1p Impacts Processes Controlled by MAPK Pathways. Antioxidants 2021, 10, 322. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-Q.; Zhao, X.-F.; Gao, X.-D. Roles of the Three Ras Proteins in the Regulation of Dimorphic Transition in the Yeast Yarrowia Lipolytica. FEMS Yeast Res. 2014, 14, 451–463. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Day, A.M.; Quinn, J.; Mirończuk, A.M. Influence of YlHog1 MAPK Kinase on Yarrowia Lipolytica Stress Response and Erythritol Production. Sci. Rep. 2018, 8, 14735. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, L.J.; Peng, X.; Zhang, Z.B.; Yang, H.L.; Yan, R.M.; Zhu, D. Transcriptome Analysis of the Dimorphic Transition Induced by PH Change and Lipid Biosynthesis in Trichosporon Cutaneum. J. Ind. Microbiol. Biotechnol. 2020, 47, 49–61. [Google Scholar] [CrossRef]
- Jiao, L.; Li, W.; Li, Y.; Zhou, Q.; Zhu, M.; Zhao, G.; Zhang, H.; Yan, Y. Employing Engineered Enolase Promoter for Efficient Expression of Thermomyces Lanuginosus Lipase in Yarrowia Lipolytica via a Self-Excisable Vector. Int. J. Mol. Sci. 2022, 24, 719. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiao, L.; Li, W.; Hu, Z.; Li, Y.; Zhang, H.; Yang, M.; Xu, L.; Yan, Y. A Novel Cre/Lox-Based Genetic Tool for Repeated, Targeted and Markerless Gene Integration in Yarrowia Lipolytica. Int. J. Mol. Sci. 2021, 22, 739. [Google Scholar] [CrossRef]
- Yu, M.; Lange, S.; Richter, S.; Tan, T.; Schmid, R.D. High-Level Expression of Extracellular Lipase Lip2 from Yarrowia Lipolytica in Pichia Pastoris and Its Purification and Characterization. Protein. Expr. Purif. 2007, 53, 255–263. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce Acceleration-Supported Software for Integrated Quality Control and Preprocessing of High-Throughput Sequencing Data. GigaScience 2018, 7, gix120. [Google Scholar] [CrossRef]
- Magnan, C.; Yu, J.; Chang, I.; Jahn, E.; Kanomata, Y.; Wu, J.; Zeller, M.; Oakes, M.; Baldi, P.; Sandmeyer, S. Sequence Assembly of Yarrowia Lipolytica Strain W29/CLIB89 Shows Transposable Element Diversity. PLoS ONE 2016, 11, e0162363. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Gao, C.-H.; Cao, H.; Ju, F.; Xiao, K.-Q.; Cai, P.; Wu, Y.; Huang, Q. Emergent Transcriptional Adaption Facilitates Convergent Succession within a Synthetic Community. ISME Commun. 2021, 1, 46. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]


| Cu(mM) Day | 0 | 0.001 | 0.002 | 0.003 | 0.004 | 0.005 | 0.05 | 0.5 | 1 | 2 | 4 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| 1 | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| 2 | × | × | × | × | × | × | × | × | × | × | × | × | × | × |
| 3 | × | × | × | × | × | × | × | × | × | × | × | √ | × | × |
| 4 | × | × | × | × | × | × | × | × | × | × | √ | √ | × | × |
| 5 | × | × | × | × | × | × | × | × | × | √ | √ | √ | × | × |
| 6 | × | × | × | × | × | × | × | × | √ | √ | √ | √ | × | × |
| 7 | × | × | × | × | × | × | × | √ | √ | √ | √ | √ | × | × |
| 8 | × | × | × | × | × | × | √ | √ | √ | √ | √ | √ | × | × |
| 9 | × | × | × | × | √ | √ | √ | √ | √ | √ | √ | √ | × | × |
| 10 | × | × | × | √ | √ | √ | √ | √ | √ | √ | √ | √ | × | × |
| 11 | × | × | × | √ | √ | √ | √ | √ | √ | √ | √ | √ | × | × |
| 12 | × | × | × | √ | √ | √ | √ | √ | √ | √ | √ | √ | × | × |
| KOG Group | KOG Class | Yeast to Transition (up/dn) | Transition to Hyphae (up/dn) | Yeast to Hyphae (up/dn) | Total (Unique) |
|---|---|---|---|---|---|
| CELLULAR PROCESSES AND SIGNALING | Cell wall/membrane/envelope biogenesis | 2/2 | 11/5 # | 13/3 # | 24 |
| Cytoskeleton | 2/5 | 32/3 # | 29/4 # | 44 | |
| Defense mechanisms | 1/3 | 6/9 | 3/4 | 16 | |
| Extracellular structures | 0/3 | 5/1 | 3/3 | 8 | |
| Intracellular trafficking, secretion, and vesicular transport | 13/9 | 48/22 # | 61/17 # | 114 | |
| Nuclear structure | 2/1 | 4/4 | 4/0 | 10 | |
| Posttranslational modification, protein turnover, chaperones | 71/24 # | 52/94 | 63/53 | 224 | |
| Signal transduction mechanisms | 11/15 | 54/32 | 49/39 | 128 | |
| INFORMATION STORAGE AND PROCESSING | Chromatin structure and dynamics | 6/1 # | 2/10 # | 6/3 | 21 |
| Replication, recombination and repair | 15/9 | 12/43 # | 10/27 # | 72 | |
| RNA processing and modification | 7/3 | 4/10 # | 10/10 | 37 | |
| Transcription | 6/5 | 13/26 | 18/22 | 62 | |
| Translation, ribosomal structure and biogenesis | 4/7 | 95/7 # | 78/7 # | 122 | |
| METABOLISM | Amino acid transport and metabolism | 25/21 | 40/38 | 35/34 | 120 |
| Carbohydrate transport and metabolism | 23/18 | 34/26 | 35/28 | 102 | |
| Cell cycle control, cell division, chromosome partitioning | 16/8 | 29/16 | 28/11 # | 68 | |
| Coenzyme transport and metabolism | 5/10 | 21/4 # | 12/8 | 36 | |
| Energy production and conversion | 17/35 # | 62/30 # | 27/27 | 124 | |
| Inorganic ion transport and metabolism | 16/23 | 14/11 | 18/30 | 64 | |
| Lipid transport and metabolism | 44/10 # | 28/60 # | 51/36 | 140 | |
| Nucleotide transport and metabolism | 4/6 | 12/7 | 8/7 | 28 | |
| Secondary metabolites biosynthesis, transport and catabolism | 21/19 | 13/18 | 23/28 | 69 | |
| CELLULAR PROCESSES AND SIGNALING | Cell motility | 0/1 | 0/1 | 1 | |
| POORLY CHARACTERIZED | Function unknown | 19/17 | 33/35 | 25/30 | 104 |
| General function prediction only | 43/45 | 95/102 | 82/95 | 286 |
| GeneID | Hyphae /Yeast 1 | Transition /Yeast 2 | Hyphae /Transition 3 | Description of the Encoded Protein 4 | Ref. 5 |
|---|---|---|---|---|---|
| YALI1_B07500g | −1.237 | C2H2-type domain-containing protein | this study | ||
| YALI1_C12900g | 1.216 | 1.757 | HABP4_PAI-RBP1 domain-containing protein | this study | |
| YALI1_E04033g | 3.289 | 3.228 | EF-hand protein | this study | |
| YALI1_F29317g | 1.079 | 1.312 | Transcription initiation factor IIA subunit 2 | this study | |
| YALI1_B11983g | −1.579 | 1.589 | Superoxide dismutase | this study | |
| YALI1_B19932g | 2.218 | 2.915 | Thiol-specific antioxidant | this study | |
| YALI1_B23771g | 1.928 | Similar to copper transport protein, ATX1 | this study | ||
| YALI1_C05880g | −1.998 | 2.018 | Similar to copper transport protein, SMF1 | this study | |
| YALI1_C13106g | −1.537 | 1.48 | NADPH-dependent diflavin oxidoreductase 1 | this study | |
| YALI1_C28396g | −5.962 | −9.551 | 3.589 | Similar to copper transport protein, CTR1 | this study |
| YALI1_D01404g | 2.539 | 2.311 | Oxidoreductase activity | this study | |
| YALI1_E14988g | 2.407 | 1.352 | 1.055 | Superoxide dismutase [Cu-Zn] | this study |
| YALI1_B13328g | 2.738 | 4.643 | Downstream target genes of Mhy1 | [40] | |
| YALI1_B17773g | 3.689 | 3.624 | BHLH domain-containing protein | [41] | |
| YALI1_B28150g | 1.673 | 3.112 | Mhy1, C2H2-type zinc finger protein | [40,42] | |
| YALI1_C15610g | 2.436 | −1.932 | 4.368 | YlRim101- and Mhy1-coregulated gene | [39] |
| YALI1_C21578g | 6.252 | 6.359 | Downstream target genes of Mhy1 | [40] | |
| YALI1_C32352g | 6.352 | 5.541 | Downstream target genes of Mhy1 | [40] | |
| YALI1_D06131g | 4.353 | 4.323 | 1,3-beta-glucanosyltransferase, YlPhr1 | [39] | |
| YALI1_D07729g | 2.306 | 2.106 | 1,3-beta-glucanosyltransferase, YlPhr2 | [39] | |
| YALI1_D11653g | 6.574 | 1.078 | 5.496 | Similar to the S. cerevisiae a-agglutinin Aga1 | [39] |
| YALI1_D18089g | 1.424 | Zn(2)-C6 domain-containing protein | [41] | ||
| YALI1_D34684g | 1.137 | 1.243 | YlGpi7 | [43] | |
| YALI1_E15718g | 1.449 | YlCdc25 | [43] | ||
| YALI1_E26257g | 1.681 | −1.575 | 3.929 | Cell wall protein precursor YlCWP1 | [39] |
| YALI1_E27731g | −0.861 | Fts2, C2H2-type zinc finger protein | [41] | ||
| YALI1_E30639g | 1.257 | 1.48 | YlRac1 | [44] | |
| YALI1_F20378g | −1.551 | YITec1 | [45] | ||
| YALI1_F25382g | 4.347 | 3.870 | Similar to S. cerevisiae flocculin Flo11 | [39] | |
| YALI1_F30395g | 1.888 | 2.849 | −1.888 | YlRsr1 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, M.; Zhao, G.; Jiao, L.; Gu, Z.; Yang, K.; Wang, L.; Cao, X.; Xu, L.; Yan, J.; Yan, Y.; et al. Copper Ion Mediates Yeast-to-Hypha Transition in Yarrowia lipolytica. J. Fungi 2023, 9, 249. https://doi.org/10.3390/jof9020249
Ran M, Zhao G, Jiao L, Gu Z, Yang K, Wang L, Cao X, Xu L, Yan J, Yan Y, et al. Copper Ion Mediates Yeast-to-Hypha Transition in Yarrowia lipolytica. Journal of Fungi. 2023; 9(2):249. https://doi.org/10.3390/jof9020249
Chicago/Turabian StyleRan, Mengqu, Guowei Zhao, Liangcheng Jiao, Zhaorui Gu, Kaixin Yang, Lishuang Wang, Xinghong Cao, Li Xu, Jinyong Yan, Yunjun Yan, and et al. 2023. "Copper Ion Mediates Yeast-to-Hypha Transition in Yarrowia lipolytica" Journal of Fungi 9, no. 2: 249. https://doi.org/10.3390/jof9020249
APA StyleRan, M., Zhao, G., Jiao, L., Gu, Z., Yang, K., Wang, L., Cao, X., Xu, L., Yan, J., Yan, Y., Xie, S., & Yang, M. (2023). Copper Ion Mediates Yeast-to-Hypha Transition in Yarrowia lipolytica. Journal of Fungi, 9(2), 249. https://doi.org/10.3390/jof9020249










