Persistence of Metarhizium brunneum (Ascomycota: Hypocreales) in the Soil Is Affected by Formulation Type as Shown by Strain-Specific DNA Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Whole Genome Shotgun Sequencing and Assembly
2.3. Marker Design and Validation
2.4. Preparation of Prototypes
2.5. Field Trial Design and Implementation
2.6. Soil Sampling
2.7. DNA Extraction from Soil Samples
2.8. Strain Quantification from Field Samples
2.9. Weather Data
3. Results
3.1. Whole Genome Shotgun Sequencing
3.2. Marker Design and Validation
3.3. Persistence of EAMb 09/01-Su in the Soil of Field Trials
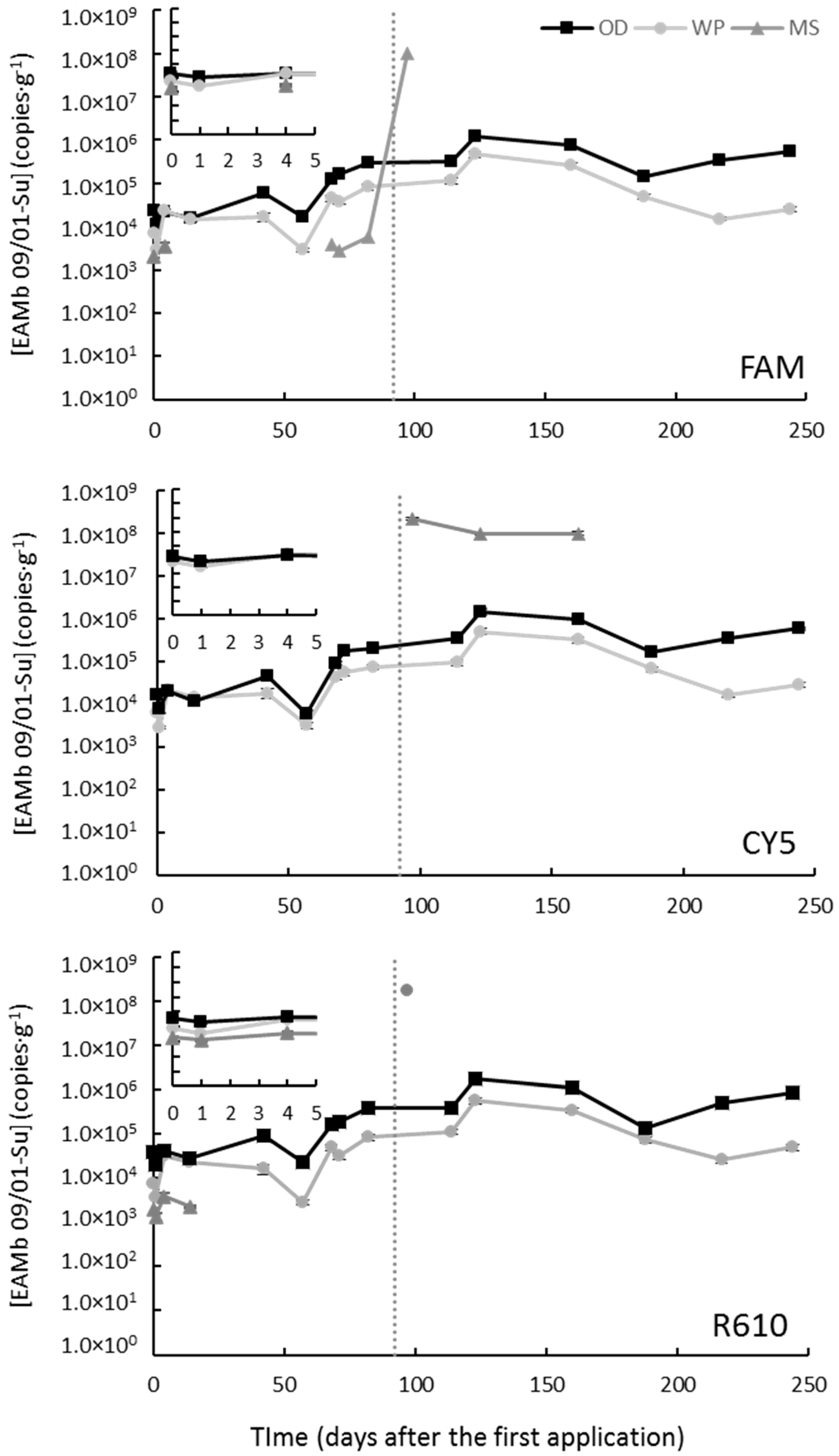
3.3.1. Mont-Roig del Camp
3.3.2. Avinyó Nou
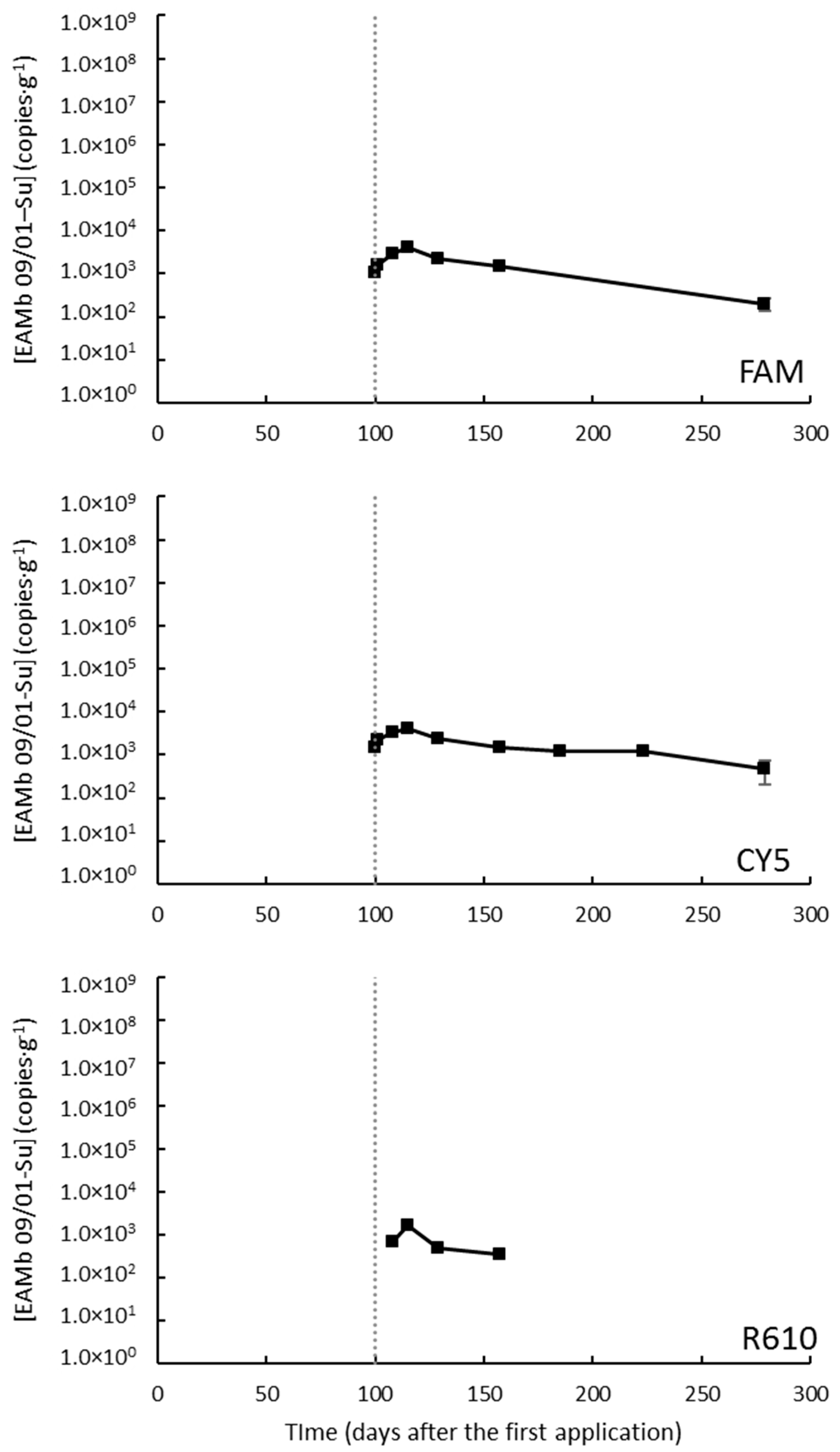
3.3.3. Caldes de Montbui I: Super-Intensive Regime
3.3.4. Caldes de Montbui II: Traditional Regime
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, G. Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Sci. Technol. 2007, 17, 879–920. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Martin-Carballo, I.; Garrido-Jurado, I.; Cándido, S.-Á. Horizontal transmission of Metarhizium anisopliae among laboratory populations of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Biol. Control 2008, 47, 115–124. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Ruiz-García, A.; Santiago-Alvarez, C. Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adults of Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2006, 99, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Alba-Ramírez, C.; Garrido Jurado, I.; Mateu, J.; Raya Díaz, S.; Valverde-García, P.; Quesada-Moraga, E. Metarhizium brunneum (Ascomycota; Hypocreales) treatments targeting olive fly in the soil for sustainable crop production. Front. Plant Sci. 2018, 9, 1. [Google Scholar] [CrossRef]
- Yousef, M.; Garrido-Jurado, I.; Quesada-Moraga, E. One Metarhizium brunneum strain, two uses to control Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2014, 107, 1736–1744. [Google Scholar] [CrossRef]
- Yousef, M.; Garrido-Jurado, I.; Ruíz-Torres, M.; Quesada-Moraga, E. Reduction of adult olive fruit fly populations by targeting preimaginals in the soil with the entomopathogenic fungus Metarhizium brunneum. J. Pest Sci. 2017, 90, 345–354. [Google Scholar] [CrossRef]
- Yousef, M.; Lozano-Tovar, M.D.; Garrido-Jurado, I.; Quesada-Moraga, E. Biocontrol of Bactrocera oleae (Diptera: Tephritidae) with Metarhizium brunneum and its extracts. J. Econ. Entomol. 2013, 106, 1118–1125. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Valverde-García, P.; Quesada-Moraga, E. Use of a multiple logistic regression model to determine the effects of soil moisture and temperature on the virulence of entomopathogenic fungi against pre-imaginal Mediterranean fruit fly Ceratitis capitata. Biol. Control 2011, 59, 366–372. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Torrent, J.; Barrón, V.; Corpas, A.; Quesada-Moraga, E. Soil properties affect the availability, movement, and virulence of entomopathogenic fungi conidia against puparia of Ceratitis capitata (Diptera: Tephritidae). Biol. Control 2011, 58, 277–285. [Google Scholar] [CrossRef]
- Yousef, M.; Quesada-Moraga, E.; Garrido-Jurado, I. Compatibility of herbicides used in olive orchards with a Metarhizium brunneum strain used for the control of preimaginal stages of tephritids in the soil. J. Pest Sci. 2015, 88, 605–612. [Google Scholar] [CrossRef]
- Lozano-Tovar, M.D.; Garrido-Jurado, I.; Lafont, F.; Quesada-Moraga, E. Insecticidal activity of a destruxin-containing extract of Metarhizium brunneum against Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2015, 108, 462–472. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Ruano, F.; Campos, M.; Quesada-Moraga, E. Effects of soil treatments with entomopathogenic fungi on soil dwelling non-target arthropods at a commercial olive orchard. Biol. Control 2011, 59, 239–244. [Google Scholar] [CrossRef]
- Burges, H.D. Formulation of Mycoinsecticides. In Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments; Burges, H.D., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 131–185. [Google Scholar]
- Birnbaum, N.; Reingold, V.; Matveev, S.; Kottakota, C.; Davidovitz, M.; Mani, K.A.; Feldbaum, R.; Yaakov, N.; Mechrez, G.; Ment, D. Not Only a Formulation: The Effects of Pickering Emulsion on the Entomopathogenic Action of Metarhizium brunneum. J. Fungi 2021, 7, 499. [Google Scholar] [CrossRef]
- Lei, C.J.; Halim, N.A.; Asib, N.; Zakaria, A.; Azmi, W.A. Conidial Emulsion Formulation and Thermal Storability of Metarhizium anisopliae against Red Palm Weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Microorganisms 2022, 10, 1460. [Google Scholar] [CrossRef]
- Fernandes, É.K.; Rangel, D.E.; Braga, G.U.; Roberts, D.W. Tolerance of entomopathogenic fungi to ultraviolet radiation: A review on screening of strains and their formulation. Curr. Genet. 2015, 61, 427–440. [Google Scholar] [CrossRef]
- Gillespie, A.T. The Potential of Entomogenous Fungi to Control Glasshouse Pests and Brown Planthopper of Rice. Ph.D. Thesis, University of Southampton, Southampton, UK, 1984. [Google Scholar]
- Pilz, C.; Enkerli, J.; Wegensteiner, R.; Keller, S. Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J. Appl. Entomol. 2011, 135, 393–403. [Google Scholar] [CrossRef]
- Zottele, M.; Mayerhofer, J.; Embleton, H.; Wechselberger, K.; Enkerli, J.; Strasser, H. Biological Diabrotica Management and Monitoring of Metarhizium Diversity in Austrian Maize Fields Following Mass Application of the Entomopathogen Metarhizium brunneum. Appl. Sci. 2021, 11, 9445. [Google Scholar] [CrossRef]
- Bischoff, J.F.; Rehner, S.A.; Humber, R.A. A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 2009, 101, 512–530. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Bolchacova, E.; Voigt, K.; Crous, P.W.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Schneider, S.; Rehner, S.A.; Widmer, F.; Enkerli, J. A PCR-based tool for cultivation-independent detection and quantification of Metarhizium clade 1. J. Invertebr. Pathol. 2011, 108, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Widmer, F.; Jacot, K.; Kölliker, R.; Enkerli, J. Spatial distribution of Metarhizium clade 1 in agricultural landscapes with arable land and different semi-natural habitats. Appl. Soil Ecol. 2012, 52, 20–28. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Lutz, A.; Dennert, F.; Rehner, S.A.; Kepler, R.M.; Widmer, F.; Enkerli, J. A species-specific multiplexed PCR amplicon assay for distinguishing between Metarhizium anisopliae, M. brunneum, M. pingshaense and M. robertsii. J. Invertebr. Pathol. 2019, 161, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tranchida-Lombardo, V.; Aiese Cigliano, R.; Anzar, I.; Landi, S.; Palombieri, S.; Colantuono, C.; Bostan, H.; Termolino, P.; Aversano, R.; Batelli, G.; et al. Whole-genome re-sequencing of two Italian tomato landraces reveals sequence variations in genes associated with stress tolerance, fruit quality and long shelf-life traits. DNA Res. 2018, 25, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Hernández, I.; Sant, C.; Martínez, R.; Fernández, C. Design of bacterial strain-specific qPCR assays using NGS data and publicly available resources and its application to track biocontrol strains. Front. Microbiol. 2020, 11, 208. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Mathulwe, L.; Malan, A.; Stokwe, N. Mass production of entomopathogenic fungi, Metarhizium robertsii and Metarhizium pinghaense, for commercial application against insect pests. J. Vis. Exp. 2022, 181, e63246. [Google Scholar] [CrossRef]
- Mascarin, G.M.; Kobori, N.N.; de Jesus Vital, R.C.; Jackson, M.A.; Quintela, E.D. Production of microsclerotia by Brazilian strains of Metarhizium spp. using submerged liquid culture fermentation. World J. Microbiol. Biotechnol. 2014, 30, 1583–1590. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jaronski, S.T. Production of microsclerotia of the fungal entomopathogen Metarhizium anisopliae and their potential for use as a biocontrol agent for soil-inhabiting insects. Mycol. Res. 2009, 113, 842–850. [Google Scholar] [CrossRef]
- Servei Meteorològic de Catalunya. Xarxa d’Estacions Meteorològiques Automàtiques. Available online: https://www.meteo.cat/wpweb/serveis/cataleg-de-serveis/dades-meteorologiques/ (accessed on 16 January 2022).
- Hermosa, M.R.; Grondona, I.; Diaz-Minguez, J.M.; Iturriaga, E.A.; Monte, E. Development of a strain-specific SCAR marker for the detection of Trichoderma atroviride 11, a biological control agent against soilborne fungal plant pathogens. Curr. Genet. 2001, 38, 343–350. [Google Scholar] [CrossRef]
- Dauch, A.L.; Watson, A.K.; Jabaji-Hare, S.H. Detection of the biocontrol agent Colletotrichum coccodes (183088) from the target weed velvetleaf and from soil by strain-specific PCR markers. J. Microbiol. Methods 2003, 55, 51–64. [Google Scholar] [CrossRef]
- Felici, C.; Vettori, L.; Toffanin, A.; Nuti, M. Development of a strain-specific genomic marker for monitoring a Bacillus subtilis biocontrol strain in the rhizosphere of tomato. FEMS Microbiol. Ecol. 2008, 65, 289–298. [Google Scholar] [CrossRef]
- Rotolo, C.; De Miccolis Angelini, R.M.; Pollastro, S.; Faretra, F. A TaqMan-based qPCR assay for quantitative detection of the biocontrol agents Bacillus subtilis strain QST713 and Bacillus amyloliquefaciens subsp. plantarum strain D747. BioControl 2016, 61, 91–101. [Google Scholar] [CrossRef]
- Daranas, N.; Bonaterra, A.; Frances, J.; Cabrefiga, J.; Montesinos, E.; Badosa, E. Monitoring viable cells of the biological control agent Lactobacillus plantarum PM411 in aerial plant surfaces by means of a strain-specific viability quantitative PCR method. Appl. Environ. Microbiol. 2018, 84, e00107-18. [Google Scholar] [CrossRef]
- Soto-Muñoz, L.; Teixidó, N.; Usall, J.; Viñas, I.; Torres, R. Detection and quantification by PCR assay of the biocontrol agent Pantoea agglomerans CPA-2 on apples. Int. J. Food Microbiol. 2014, 175, 45–52. [Google Scholar] [CrossRef]
- Yousef-Yousef, M.; Romero-Conde, A.; Quesada-Moraga, E.; Garrido-Jurado, I. Production of Microsclerotia by Metarhizium sp., and Factors Affecting Their Survival, Germination, and Conidial Yield. J. Fungi 2022, 8, 402. [Google Scholar] [CrossRef]
- Fernández-Bravo, M.; Gschwend, F.; Mayerhofer, J.; Hug, A.; Widmer, F.; Enkerli, J. Land-use type drives soil population structures of the entomopathogenic fungal genus Metarhizium. Microorganisms 2021, 9, 1380. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Hodgson, E.W.; Gassmann, A.J. Abundance of soil-borne entomopathogenic fungi in organic and conventional fields in the midwestern USA with an emphasis on the effect of herbicides and fungicides on fungal persistence. PLoS ONE 2015, 10, e0133613. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.B.; Pauli, G.; Mascarin, G.M.; Faria, M. Protection of entomopathogenic conidia against chemical fungicides afforded by an oil-based formulation. Biocontrol Sci. Technol. 2011, 21, 125–137. [Google Scholar] [CrossRef]
- Alves, R.T.; Bateman, R.P.; Prior, C.; Leather, S.R. Effects of simulated solar radiation on conidial germination of Metarhizium anisopliae in different formulations. Crop Protect. 1998, 17, 675–679. [Google Scholar] [CrossRef]
- Faria, M.; Hajek, A.E.; Wraight, S.P. Imbibitional damage in conidia of the entomopathogenic fungi Beauveria bassiana, Metarhizium acridum, and Metarhizium anisopliae. Biol. Control 2009, 51, 346–354. [Google Scholar] [CrossRef]
- Inglis, G.D.; Ivie, T.J.; Duke, G.M.; Goettel, M.S. Influence of rain and conidial formulation on persistence of Beauveria bassiana on potato leaves and colorado potato beetle larvae. Biol. Control 2000, 18, 55–64. [Google Scholar] [CrossRef]
- Hedimbi, M.; Kaaya, G.P.; Singh, S.; Chimwamurombe, P.M.; Gindin, G.; Glazer, I.; Samish, M. Protection of Metarhizium anisopliae conidia from ultra-violet radiation and their pathogenicity to Rhipicephalus evertsi evertsi ticks. Exp. Appl. Acarol. 2008, 46, 149–156. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E. Application parameters affecting field efficacy of Beauveria bassiana foliar treatments against colorado potato beetle Leptinotarsa decemlineata. Biol. Control 2002, 23, 164–178. [Google Scholar] [CrossRef]
- Moore, D.; Bridge, P.D.; Higgins, P.M.; Bateman, R.P.; Prior, C. Ultra-violet radiation damage to Metarhizium flavoviride conidia and the protection given by vegetable and mineral oils and chemical sunscreens. Ann. Appl. Biol. 1993, 122, 605–616. [Google Scholar] [CrossRef]
- Malsam, O.; Kilian, M.; Oerke, E.-C.; Dehne, H.-W. Oils for increased efficacy of Metarhizium anisopliae to control whiteflies. Biocontrol Sci. Technol. 2002, 12, 337–348. [Google Scholar] [CrossRef]


| Mont-Roig del Camp | Avinyó Nou | Caldes de Montbui I | Caldes de Montbui II | |
|---|---|---|---|---|
| Coordinates | 41.0530, 0.95679 | 41.37614, 1.77810 | 41.63020, 2.15113 | 41.63066, 2.14766 |
| Variety | Biancolilla | Picual | Arbequina | Vera del Vallès |
| Tree spacing (m × m) | 6 × 7 | 6 × 6 | 1.5 × 4 | 5 × 7–7 × 7 1 |
| Prototypes tested | WP 2, OD 3 and MS 4 | WP, OD and MS | OD | OD |
| Treated/untreated area (ha) | 0.25 5/0.25 5 | 0.2 5/0.2 5 | 1.66 5/1.09 5 | 1.72 6/1.72 6 |
| Date A 7 (mm/dd/yyyy) | 25 November 2019 | 25 November 2019 | 17 November 2020 | 17 November 2020 |
| Concentration A (%; v:v) | 1 | 1 | 1 | 1 |
| Volume A (L·ha−1) | 1500 8 | 1500 8 | 500 | 500 |
| Dose A (CFU·ha−1; ×1010) | 11.9, 22.5, 187.5 9 | 13.5, 31.5, 375 9 | 4.0–4.6 1 | 3.4 |
| Date B 10 (mm/dd/yyyy) | 25 February 2020 | 4 February 2020 | 25 February 2021 | 9 February 2021 |
| Concentration B (%; v:v) | 1 | 1 | 1 | 1 |
| Volume B (L·ha−1) | 1500 8 | 1500 8 | 500 | 500 |
| Dose B (CFU·ha−1; ×1010) | 4.8, 4.35, 2434 | 2.94, 3.0, 195 4 | 1075–3645 | 340–360 |
| Machinery 11 | Quad; 200-L auto-propelled deposit with integrated mixing; hose with a standard gardening nozzle | Tractor; 600-L auto-propelled deposit with integrated mixing; herbicide application bar with 2 nozzles (110°) 50 cm apart, 40–50 cm above the soil | ||
| Statistic | EAMb 09/01-Su | ARSEF 3297 |
| Total length (bp) | 38,285,473 | 37,066,166 |
| Contig number | 278 | 92 |
| Longest scaffold (bp) | 7,146,780 | 7,151,295 |
| N50 | 1,825,093 | 1,825,569 |
| N’s per 100 kbp | 223.06 | 264.87 |
| Complete and single copy | 286 | 285 |
| Complete and duplicated | 4 | 5 |
| Fragmented | 0 | 0 |
| Missing | 0 | 0 |
| Marker | Oligo | Sequence (5′→3′) | 5′ Label | 3′ Label |
|---|---|---|---|---|
| H977_1 | FWD primer | CGTAGTAGTCGCGGGCTATC | N/A | N/A |
| RCP primer | GCTCCAATGCCTCCGTAATA | N/A | N/A | |
| Probe | CCTGCCCAACCATCCATCCA | FAM | BHQ-1 | |
| H977_9 | FWD primer | GGCATCAGAGCACATGAAGA | N/A | N/A |
| RCP primer | GCCACGCCTCTAGAACAAAG | N/A | N/A | |
| Probe | TGTGGGTCACCGCGTCCAAA | CY5 | BBQ | |
| H977_12 | FWD primer | AGTGGTGGATGGCAAAGTTC | N/A | N/A |
| RCP primer | CAGCGCGTTATTTGTGCTTA | N/A | N/A | |
| Probe | CGGCACGGTCAACTGCTCCC | R610 | BHQ-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, I.; Sant, C.; Martínez, R.; Almazán, M.; Caminal, M.; Quero, V.; El-Adak, M.; Casanova, A.; Garrido-Jurado, I.; Yousef-Yousef, M.; et al. Persistence of Metarhizium brunneum (Ascomycota: Hypocreales) in the Soil Is Affected by Formulation Type as Shown by Strain-Specific DNA Markers. J. Fungi 2023, 9, 229. https://doi.org/10.3390/jof9020229
Hernández I, Sant C, Martínez R, Almazán M, Caminal M, Quero V, El-Adak M, Casanova A, Garrido-Jurado I, Yousef-Yousef M, et al. Persistence of Metarhizium brunneum (Ascomycota: Hypocreales) in the Soil Is Affected by Formulation Type as Shown by Strain-Specific DNA Markers. Journal of Fungi. 2023; 9(2):229. https://doi.org/10.3390/jof9020229
Chicago/Turabian StyleHernández, Iker, Clara Sant, Raquel Martínez, Marta Almazán, Marta Caminal, Víctor Quero, Mohammed El-Adak, Albert Casanova, Inmaculada Garrido-Jurado, Meelad Yousef-Yousef, and et al. 2023. "Persistence of Metarhizium brunneum (Ascomycota: Hypocreales) in the Soil Is Affected by Formulation Type as Shown by Strain-Specific DNA Markers" Journal of Fungi 9, no. 2: 229. https://doi.org/10.3390/jof9020229
APA StyleHernández, I., Sant, C., Martínez, R., Almazán, M., Caminal, M., Quero, V., El-Adak, M., Casanova, A., Garrido-Jurado, I., Yousef-Yousef, M., Quesada-Moraga, E., Lara, J. M., & Fernández, C. (2023). Persistence of Metarhizium brunneum (Ascomycota: Hypocreales) in the Soil Is Affected by Formulation Type as Shown by Strain-Specific DNA Markers. Journal of Fungi, 9(2), 229. https://doi.org/10.3390/jof9020229








