Ferric Chloride Controls Citrus Anthracnose by Inducing the Autophagy Activity of Colletotrichum gloeosporioides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Pathogens, Citrus, and Metal Salt
2.2. Effect of FeCl3 on C. gloeosporioides In Vitro
2.3. Effect of FeCl3 on C. gloeosporioides In Vivo
2.4. Effect of FeCl3 on C. gloeosporioides Spore Germination
2.5. Observation by OM, SEM, and TEM
2.6. Detection of FeCl3 on the Cell Membrane Integrity of C. gloeosporioides
2.7. Detection of Autophagic Structures, Conidial Viability and ROS Accumulation
2.8. Physiological Qualities Test of FeCl3-Treated Citrus Fruit
2.9. Statistical Analysis
3. Results
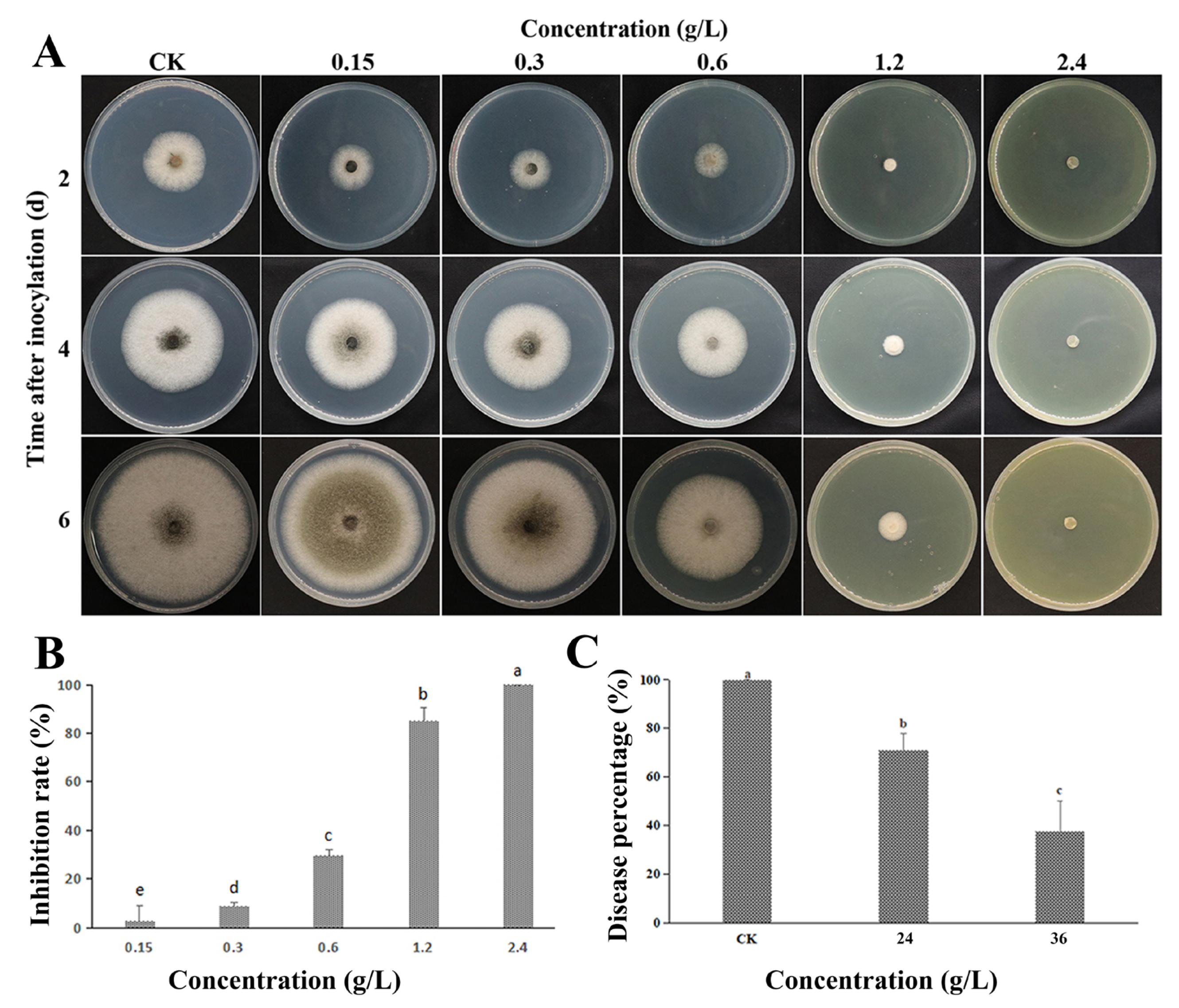
3.1. Inhibiting Effect In Vitro and In Vivo
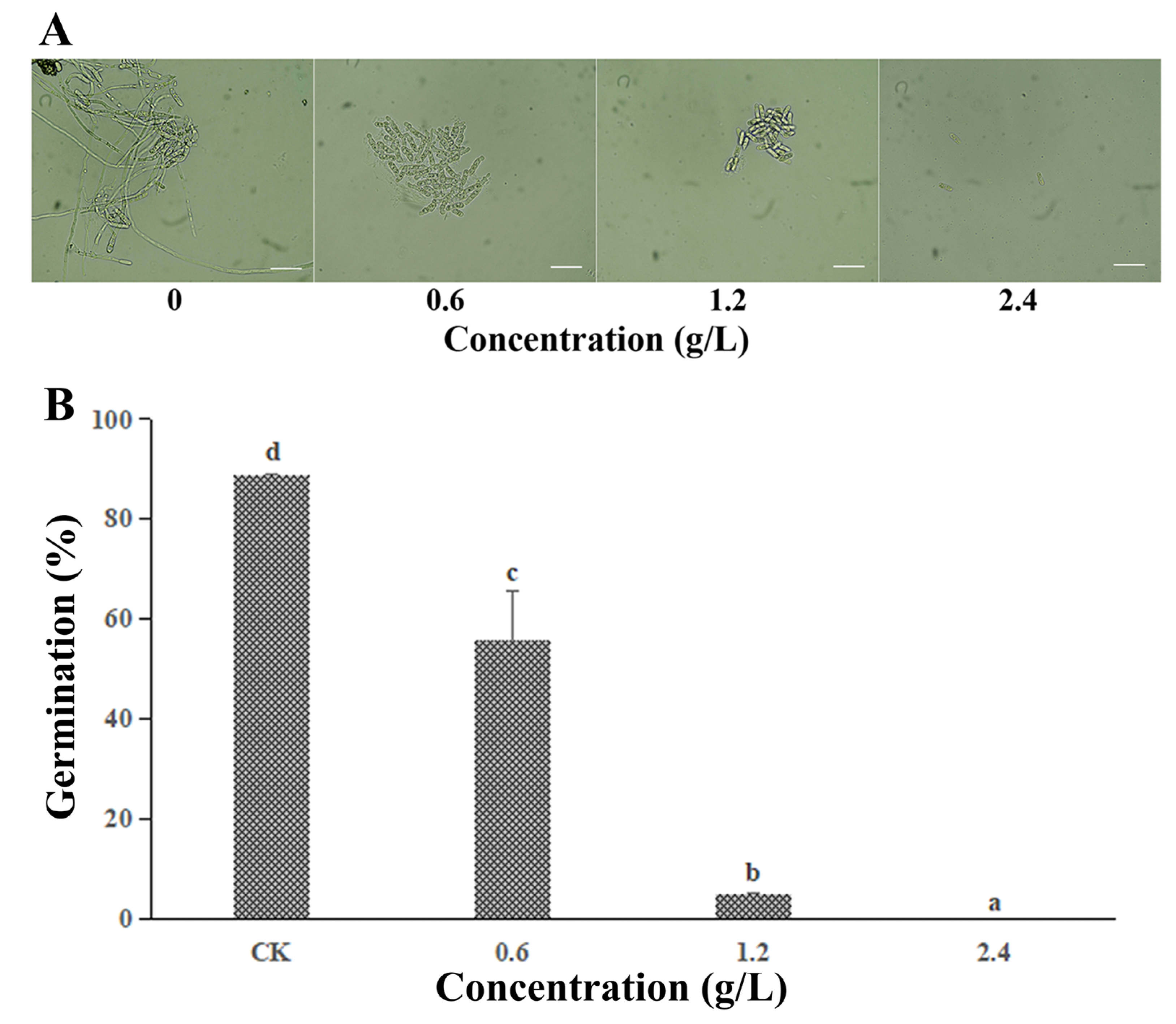
3.2. Effect of FeCl3 on the Spore Germination
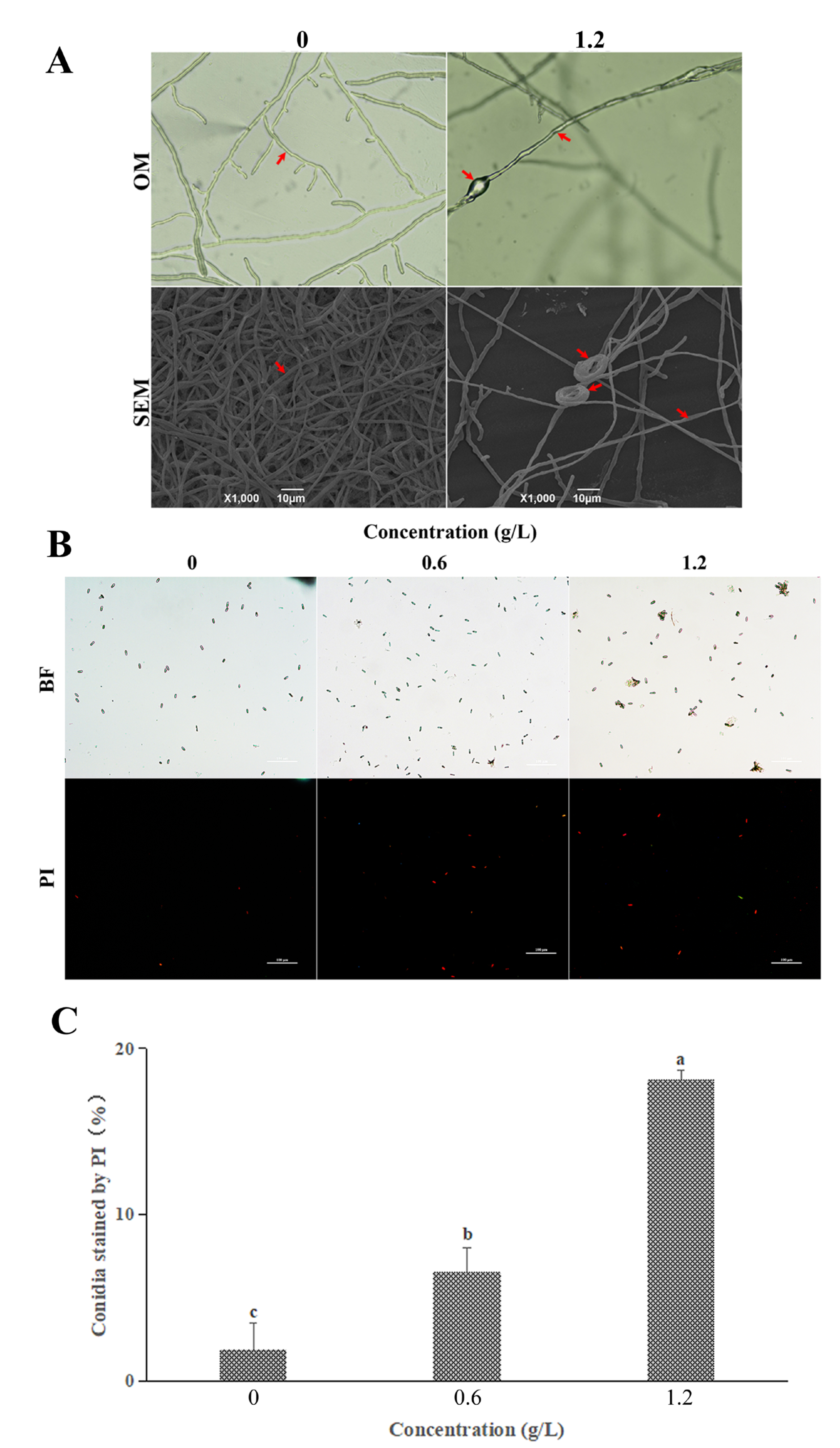
3.3. Effect of FeCl3 on Mycelial Morphology and the Cell Membrane Integrity
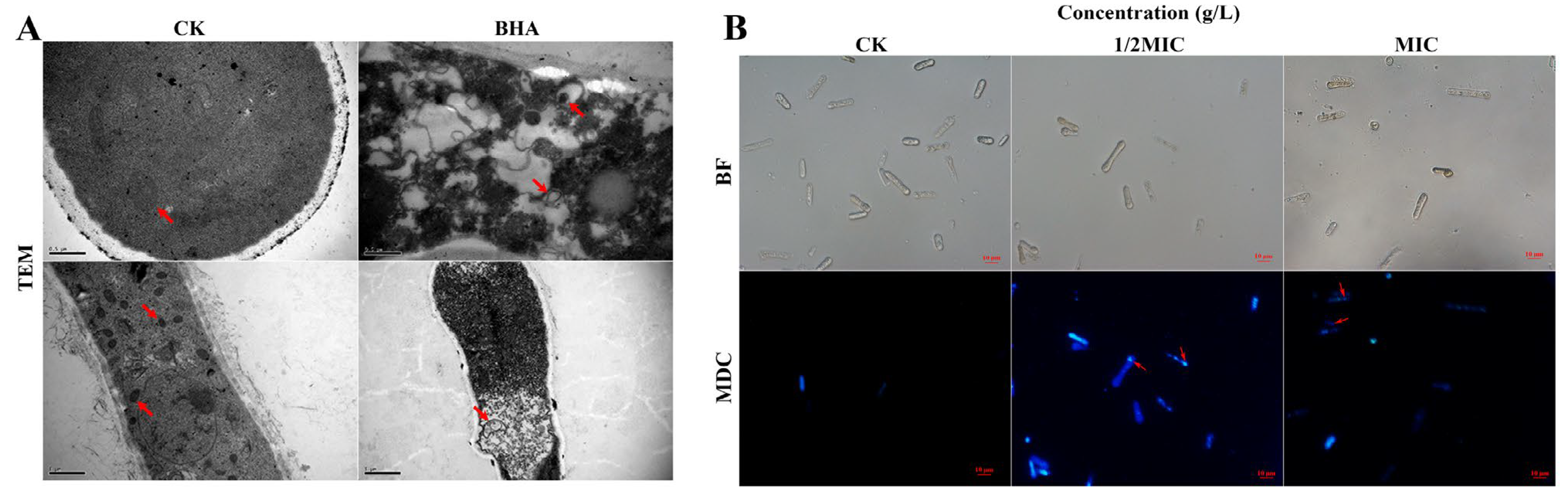
3.4. Effect of FeCl3 on Internal Structure and Autophagic Activity
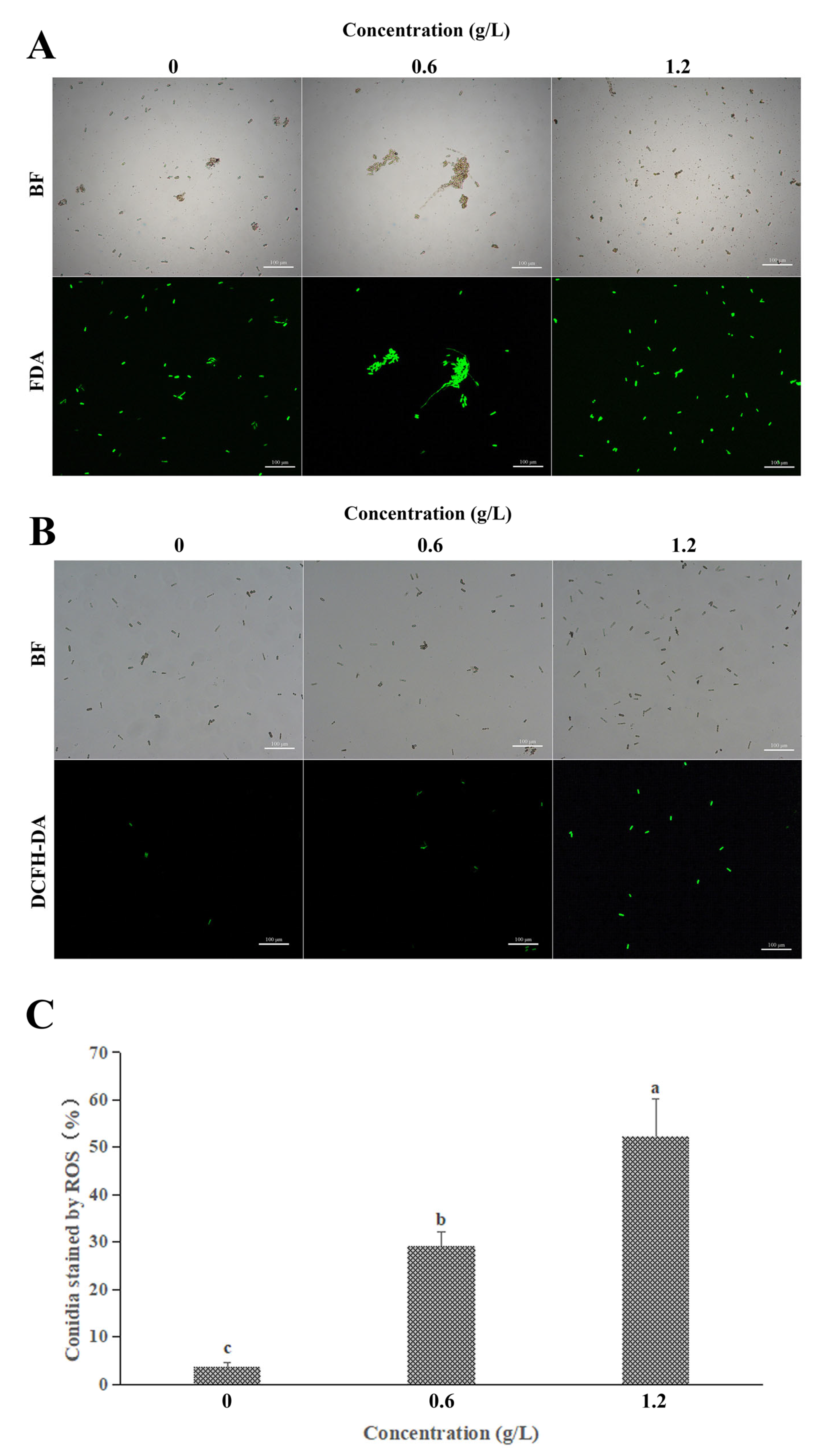
3.5. Effect of FeCl3 on Vital Activity and ROS Accumulation
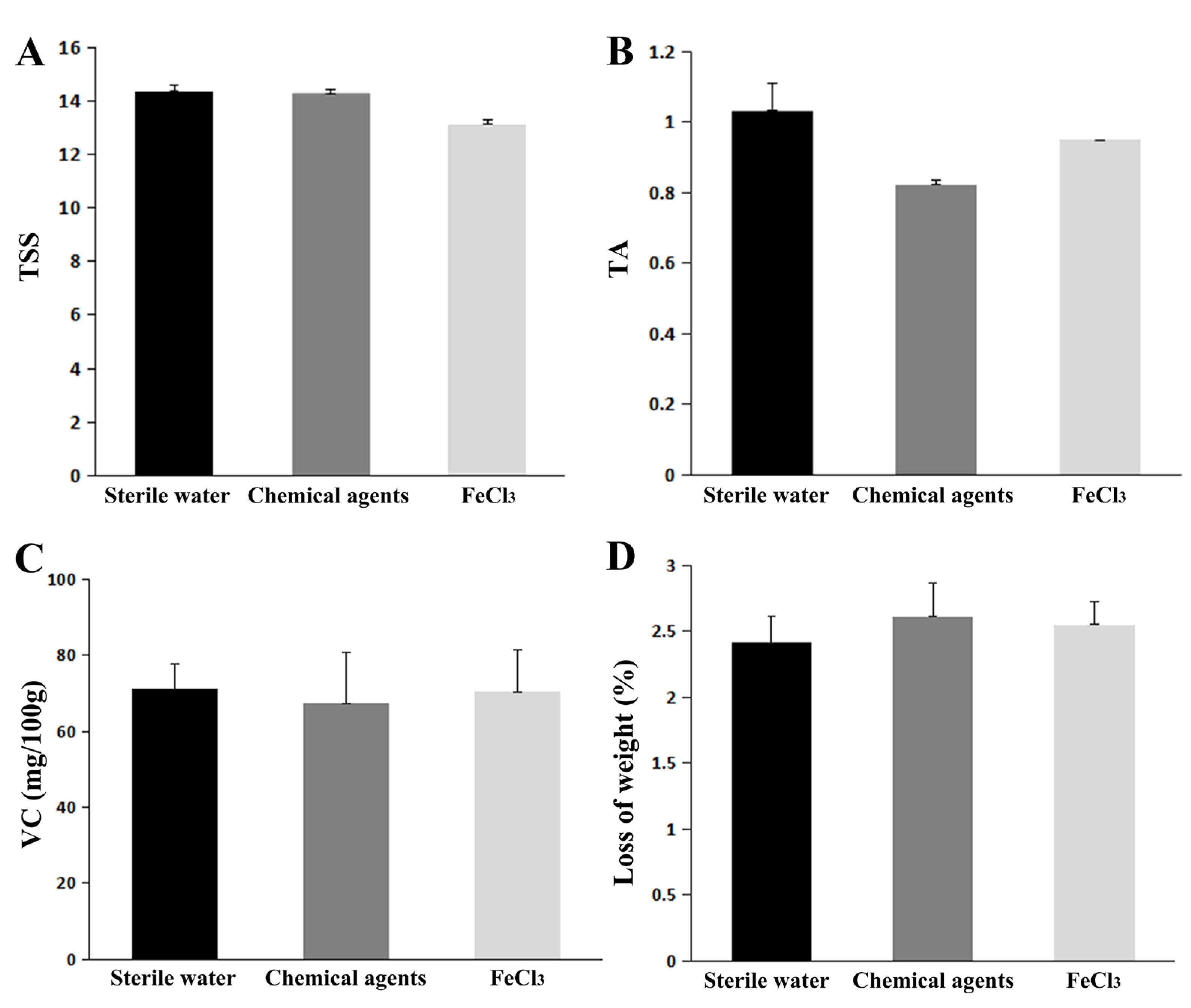
3.6. Physiological Qualities Test of FeCl3-Treated Citrus Fruit
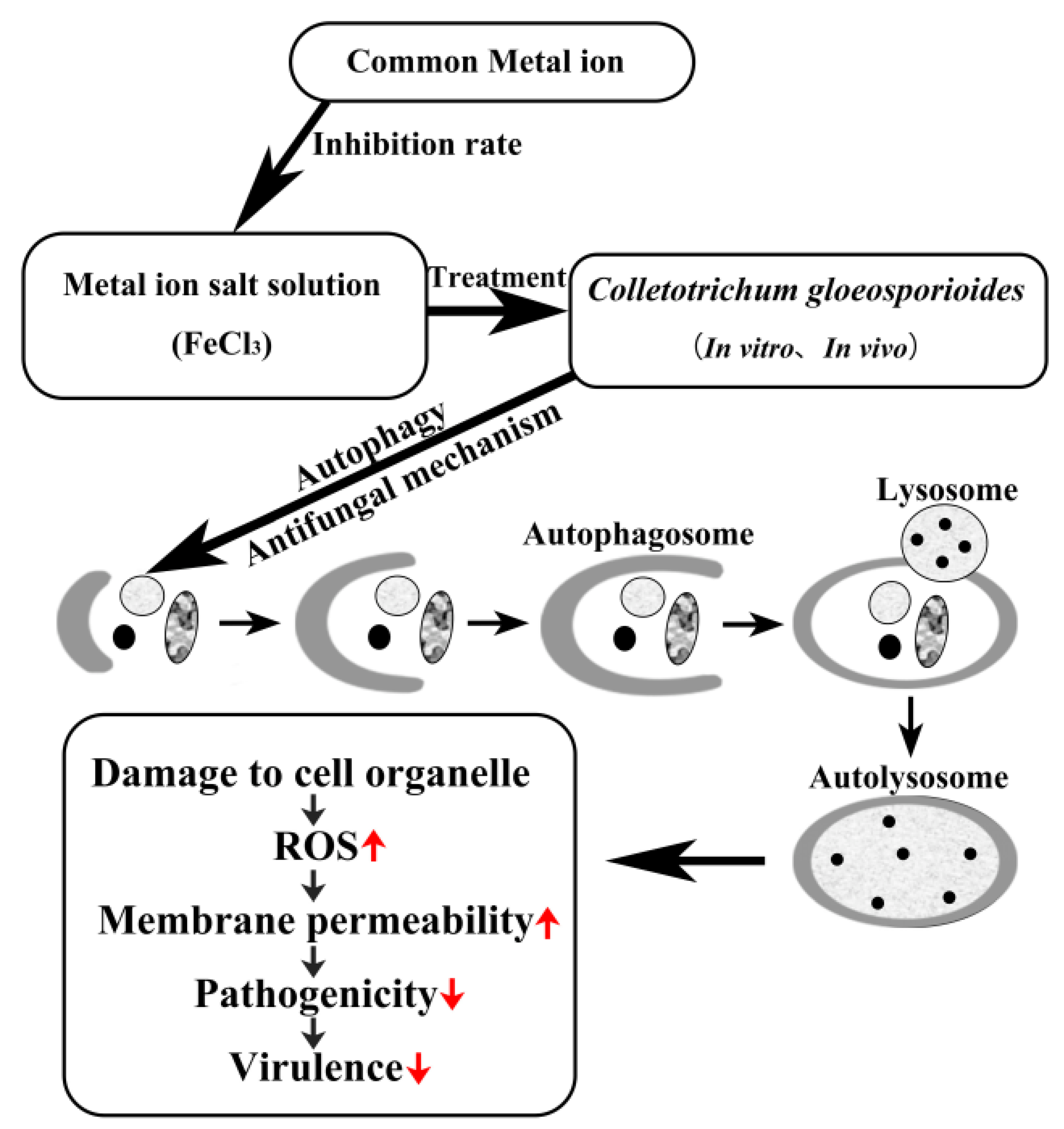
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; de Silva, D.; Moslemi, A.; Edwards, J.; Ades, P.; Crous, P.; Taylor, P.W.J. Colletotrichum Species Causing Anthracnose of Citrus in Australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Timmer, L. Preharvest application of fungicides for postharvest disease control on early season tangerine hybrids in Florida. Crop Prot. 2007, 26, 886–893. [Google Scholar] [CrossRef]
- Mehta, P.; Wiltse, C.; Rooney, W.; Collins, S.; Frederiksen, R.; Hess, D.; Chisi, M.; TeBeest, D.O. Classification and inheritance of genetic resistance to anthracnose in Sorghum Field. Crops Res. 2005, 93, 1–9. [Google Scholar] [CrossRef]
- Giulio, P.; Raffaele, C.; Giancarlo, P.; Antonino, A.; Ernesto, L.; Dolores, F.; Alessandro, V. In vitro and in vivo activity of QoI fungicides against Colletotrichum gloeosporioides causing fruit anthracnose in Citrus sinensis. Sci. Hortic. 2018, 236, 90–95. [Google Scholar] [CrossRef]
- Feng, L.; Wu, F.; Li, J.; Jiang, Y.; Duan, X. Antifungal activities of polyhexamethylene biguanide and polyhexamethylene guanide against the citrus sour rot pathogen Geotrichum citri-aurantii in vitro and in vivo. Postharvest Biol. Technol. 2011, 61, 160–164. [Google Scholar] [CrossRef]
- Cia, P.; Pascholati, S.; Benato, E.; Camili, E.; Santos, C. Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biol. Technol. 2007, 43, 366–373. [Google Scholar] [CrossRef]
- Jeong, S.W.; Kim, H.G.; Park, S.; Lee, J.H.; Kim, Y.-H.; Kim, G.-S.; Jin, J.S.; Kwak, Y.-S.; Huh, M.R.; Lee, J.E.; et al. Variation in flavonoid levels in Citrus benikoji Hort. ex. Tan. infected by Colletotrichum gloeosporioides. Food Chem. 2014, 148, 284–288. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, W.S.; Caudillo-Pérez, C.; Ulises, P.; Salazar-Sánchez; Macías-Sánchez, K.L. Influence of iron and copper on the activity of laccases in Fusarium oxysporum f. sp. Lycopersici. Braz. J. Microbiol. 2018, 49 (Suppl. S1), 269–275. [Google Scholar] [CrossRef]
- Nazik, H.; Penner, J.C.; Ferreira, J.A.; Haagensen, J.A.; Cohen, K.; Spormann, A.M.; Martinez, M.; Chen, V.; Hsu, J.L.; Clemons, K.V.; et al. Effects of Iron Chelators on the Formation and Development of Aspergillus fumigatus Biofilm. Antimicrob. Agents Chemother. 2015, 59, 6514–6520, Erratum in: Antimicrob. Agents Chemother. 2015, 59, 7160. [Google Scholar] [CrossRef]
- Food and Drug Administration. Health and human services. 2009, Pt.184: 73—FR—8607[S]. Available online: https://www.fda.gov/media/77832 (accessed on 9 January 2023).
- Yang, M.; Ismayil, A.; Liu, Y. Autophagy in Plant-Virus Interactions. Annu. Rev. Virol. 2020, 7, 403–419. [Google Scholar] [CrossRef]
- Yin, Z.; Pascual, C.; Klionsky, D. Autophagy: Machinery and regulation Microb. Cell 2016, 3, 588–596. [Google Scholar]
- Klionsky, D.J. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005, 118 Pt 1, 7–18. [Google Scholar] [CrossRef]
- Bursch, W.; Ellinger, A.; Gerner, C.; Schulte-Hermann, R. Autophagocytosis and programmed cell death. In Autophagy; Klionsky Landes, D.J., Ed.; Bioscience: Georgetown, TX, USA, 2004; pp. 287–303. [Google Scholar]
- Bergamini, E.; Cavallini, G.; Donati, A.; Gori, Z. The anti-ageing effects of caloric restriction may involve stimulation of macroautophagy and lysosomal degradation, and can be intensified pharmacologically. Biomed. Pharmacother. 2003, 57, 203–208. [Google Scholar] [CrossRef]
- Longo, V.D.; Finch, C.E. Evolutionary medicine: From dwarf model systems to healthy centenarians? Science 2003, 299, 1342–1346. [Google Scholar] [CrossRef]
- Melendez, A.; Tallóczy, Z.; Seaman, M.; Eskelinen, E.-L.; Hall, D.H.; Levine, B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science 2003, 301, 1387–1391. [Google Scholar] [CrossRef]
- Vellai, T.; Takacs-Vellai, K.; Zhang, Y.; Kovacs, A.L.; Orosz, L.; Muller, F. Influence of TOR kinase on lifespan in C. elegans. Nature 2003, 426, 620. [Google Scholar] [CrossRef]
- Liu, S.Q.; Du, Y.J.; Zhang, D.Y.; Yang, F.; He, X.; Long, C.A. Aluminum sulfate inhibits green mold by inducing chitinase activity of Penicillium digitatum and enzyme activity of citrus fruit. Food Control 2022, 136, 108854. [Google Scholar] [CrossRef]
- Cui, X.; Ma, D.; Liu, X.; Zhang, Z.; Li, B.; Xu, Y.; Chen, T.; Tian, S. Magnolol inhibits gray mold on postharvest fruit by inducing autophagic activity of Botrytis cinerea. Postharvest Biol. Technol. 2021, 180, 111596. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, Y.; Cheon, W.; Park, J.; Kwon, H.T.; Balaraju, K.; Kim, J.; Yoon, Y.J.; Jeon, Y. Characterization of Bacillus velezensis AK-0 as a biocontrol agent against apple bitter rot caused by Colletotrichum gloeosporioides. Sci. Rep. 2021, 11, 626. [Google Scholar] [CrossRef]
- Fang, X.; Chai, W.; Li, S.; Zhang, L.; Yu, H.; Shen, J.; Xiao, W.; Liu, A.; Zhou, B.; Zhang, X. HSP17.4 mediates salicylic acid and jasmonic acid pathways in the regulation of resistance to Colletotrichum gloeosporioides in strawberry. Mol. Plant Pathol. 2021, 22, 817–828. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Sela, N.; Carmeli-Weissberg, M.; Ovadia, R.; Panda, S.; Feygenberg, O.; Maurer, D.; Oren-Shamir, M.; Aharoni, A.; Alkan, N. Induced defense response in red mango fruit against Colletotrichum gloeosporioides. Hortic. Res. 2021, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, D.; Wang, Y.; Yang, F.; Zhao, J.; Du, Y.; Tian, Z.; Long, C. Dimethyl Dicarbonate as a Food Additive Effectively Inhibits Geotrichum citri-aurantii of Citrus. Foods 2022, 11, 2328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Li, L.; Wu, M.; Liang, S.; Shi, H.B.; Liu, X.H.; Lin, F.C. Current opinions on autophagy in pathogenicity of fungi. Virulence 2019, 10, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Cinque, L.; Forrester, A.; Bartolomeo, R.; Svelto, M.; Venditti, R.; Montefusco, S.; Polishchuk, E.; Nusco, E.; Rossi, A.; Medina, D.L.; et al. FGF signalling regulates bone growth through autophagy. Nature 2015, 528, 272–275. [Google Scholar] [CrossRef]
- Hofius, D.; Li, L.; Hafrén, A.; Coll, N.S. Autophagy as an emerging arena for plant-pathogen interactions. Curr. Opin. Plant Biol. 2017, 38, 117–123. [Google Scholar] [CrossRef]
- Talbot, N.; Kershaw, M. The emerging role of autophagy in plant pathogen attack and host defence. Curr. Opin. Plant Biol. 2009, 12, 444–450. [Google Scholar] [CrossRef]
- Ma, D.; Cui, X.; Zhang, Z.; Li, B.; Xu, Y.; Tian, S.; Chen, T. Honokiol suppresses mycelial growth and reduces virulence of Botrytis cinerea by inducing autophagic activities and apoptosis. Food Microbiol. J. 2020, 88, 103411. [Google Scholar] [CrossRef]
- Peng, Q.; Huo, D.; Li, H.; Zhang, B.; Li, Y.; Liang, A.; Wang, H.; Yu, Q.; Li, M. ROS-independent toxicity of Fe3O4 nanoparticles to yeast cells: Involvement of mitochondrial dysfunction. Chem. Biol. Interact. 2018, 287, 20–26. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, C.; Chen, K.; Liu, P.; Fan, Q.; Zhao, J.; Long, C. Biocontrol and the mechanisms of Bacillus sp. w176 against postharvest green mold in citrus. Postharvest Biol. Technol. 2020, 159, 111022. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Yang, Q.; Liu, P.; Du, Y.; Chen, C.; Long, C. Antifungal activities and the mechanisms of biocontrol agent WE-3 against postharvest sour rot in citrus. Eur. J. Plant Pathol. 2021, 161, 723–733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, X.; Lu, Y.; Fu, H.; Liu, S.; Zhao, J.; Long, C. Ferric Chloride Controls Citrus Anthracnose by Inducing the Autophagy Activity of Colletotrichum gloeosporioides. J. Fungi 2023, 9, 230. https://doi.org/10.3390/jof9020230
Wang Y, Wu X, Lu Y, Fu H, Liu S, Zhao J, Long C. Ferric Chloride Controls Citrus Anthracnose by Inducing the Autophagy Activity of Colletotrichum gloeosporioides. Journal of Fungi. 2023; 9(2):230. https://doi.org/10.3390/jof9020230
Chicago/Turabian StyleWang, Yuqing, Xiaoxiao Wu, Yongqing Lu, Huimin Fu, Shuqi Liu, Juan Zhao, and Chaoan Long. 2023. "Ferric Chloride Controls Citrus Anthracnose by Inducing the Autophagy Activity of Colletotrichum gloeosporioides" Journal of Fungi 9, no. 2: 230. https://doi.org/10.3390/jof9020230
APA StyleWang, Y., Wu, X., Lu, Y., Fu, H., Liu, S., Zhao, J., & Long, C. (2023). Ferric Chloride Controls Citrus Anthracnose by Inducing the Autophagy Activity of Colletotrichum gloeosporioides. Journal of Fungi, 9(2), 230. https://doi.org/10.3390/jof9020230






