Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles stephensi (Diptera: Culicidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Ethical Clearance (IEC)
2.2. Maintenance of An. stephensi Colonies
2.3. Fungal Isolates
2.4. Experiment 1
2.4.1. Preparation of Conidial Suspension
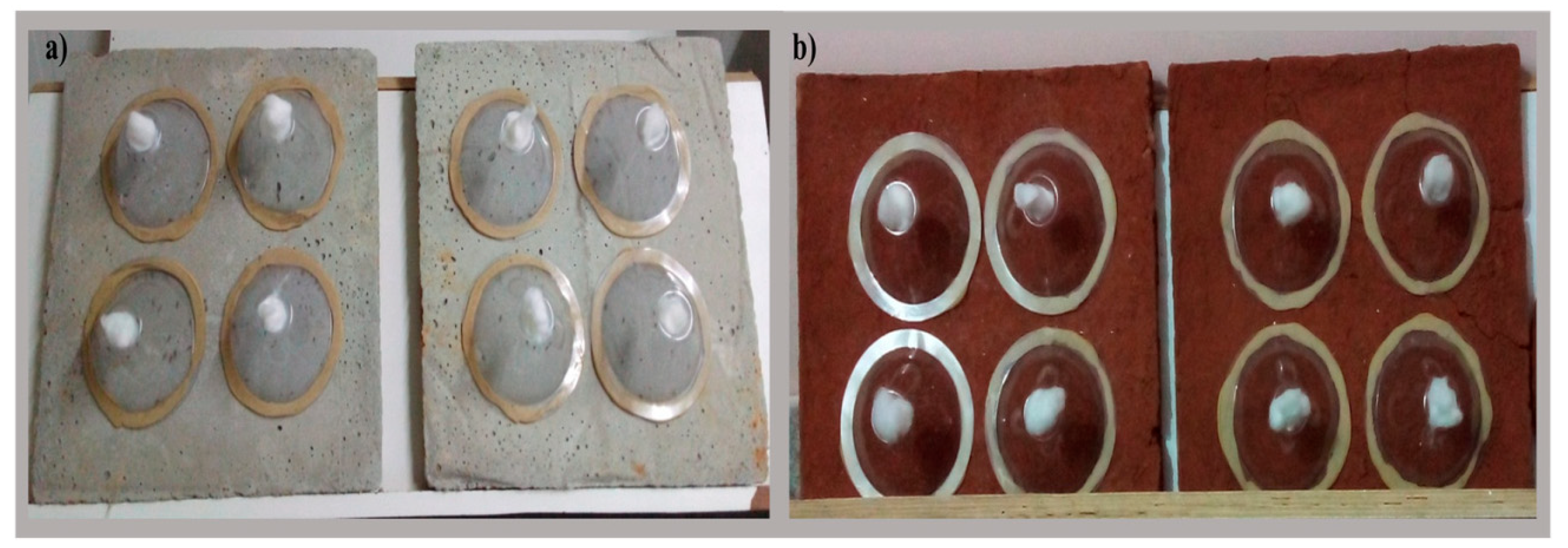
2.4.2. Test Panel Preparation
2.4.3. Cone Bioassay Test
2.5. Experiment 2
2.5.1. Preparation of Conidia and Blastospores Suspension
2.5.2. Larval Susceptibility Test
2.6. Statistical Analysis
3. Results
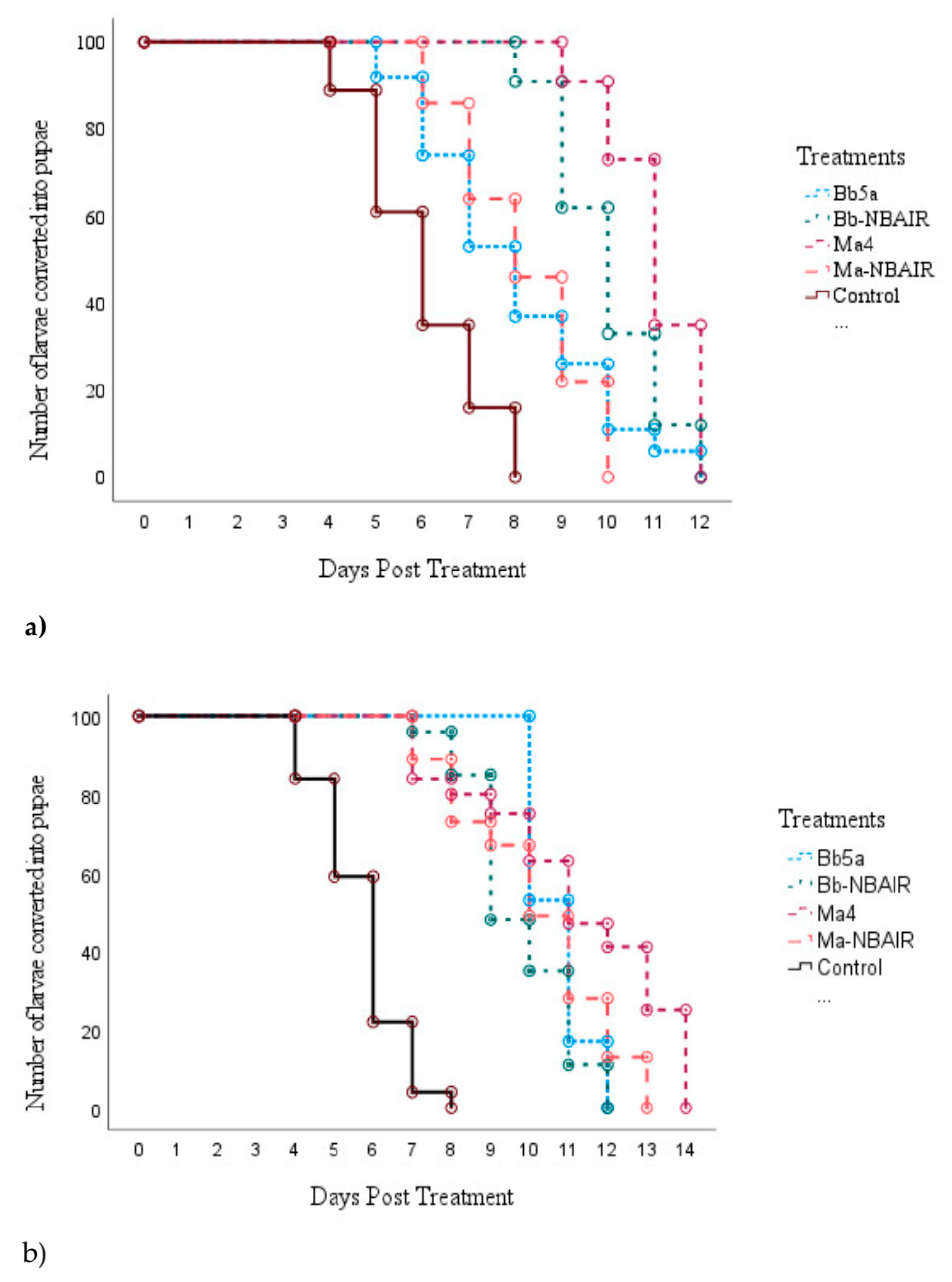
3.1. Experiment 1
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Rosenfeld, L.C.; Lim, S.S.; Andrews, K.G.; Foreman, K.J.; Haring, D.; Fullman, N.; Naghavi, M.; Lozano, R.; Lopez, A.D. Global Malaria Mortality between 1980 and 2010: A Systematic Analysis. Lancet 2012, 379, 413–431. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240040496. [Google Scholar]
- Menard, D.; Dondorp, A. Antimalarial Drug Resistance: A Threat to Malaria Elimination. Cold Spring Harb. Perspect. Med. 2017, 7, a025619. [Google Scholar] [CrossRef] [PubMed]
- Imwong, M.; Hien, T.T.; Thuy-Nhien, N.T.; Dondorp, A.M.; White, N.J. Spread of a Single Multidrug Resistant Malaria Parasite Lineage (PfPailin) to Vietnam. Lancet Infect. Dis. 2017, 17, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Amato, R.; Pearson, R.D.; Almagro-Garcia, J.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Drury, E.; Stalker, J.; Miotto, O.; et al. Origins of the Current Outbreak of Multidrug-Resistant Malaria in Southeast Asia: A Retrospective Genetic Study. Lancet Infect. Dis. 2018, 18, 337–345. [Google Scholar] [CrossRef]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 7, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Raymond, M.; Qiao, C.-L. Insecticide Resistance in Vector Mosquitoes in China. Pest Manag. Sci. 2006, 62, 1013–1022. [Google Scholar] [CrossRef]
- Castrillo, L.A.; Griggs, M.H.; Liu, H.; Bauer, L.S.; Vandenberg, J.D. Assessing Deposition and Persistence of Beauveria bassiana GHA (Ascomycota: Hypocreales) Applied for Control of the Emerald Ash Borer, Agrilus planipennis (Coleoptera: Buprestidae), in a Commercial Tree Nursery. Biol. Control 2010, 54, 61–67. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model Application of Entomopathogenic Fungi as Alternatives to Chemical Pesticides: Prospects, Challenges, and Insights for Next-Generation Sustainable Agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef]
- Kidanu, S.; Hagos, L. Entomopathogenic Fungi as a Biological Pest Management Option: A Review. Int. J. Res. Stud. Agric. Sci. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Skinner, M.; Parker, B.L.; Kim, J.S. Role of entomopathogenic fungi in integrated pest management. In Integrated Pest Management; Elsevier: Amsterdam, The Netherlands, 2014; pp. 169–191. ISBN 9780123985293. [Google Scholar]
- Ramanujam, B.; Krishna, J.; Poornesha, B. Evaluation of Entomopathogenic Fungi Against Bemisia tabaci (Gennadius) in Capsicum Under Protected Cultivation. Indian J. Entomol. 2022, 84, 160–163. [Google Scholar] [CrossRef]
- Ramanujam, B.; Kandan, A.; Poornesha, B.; Shylesha, A.N.; Gandhi, G.; Mohan, M. Pathogenicity of Beauveria assiana and Metarhizium anisopliae on Aak Grasshopper, Poekilocerus pictus Fabr. (Orthoptera: Acrididea). Int. J. Trop. Insect. Sci. 2022, 42, 2023–2026. [Google Scholar] [CrossRef]
- Renuka, S.; Ramanujam, B.; Poornesha, B. Colonization of Beauveria bassiana (Balsamo) Vuillemin Strains in Maize (Zea mays L.) and Their Efficacy against Stem Borer Chilo partellus (Swinhoe). J. Biol. Control 2017, 31, 28–37. [Google Scholar] [CrossRef]
- Clarkson, J.M.; Charnley, A.K. New Insights into the Mechanisms of Fungal Pathogenesis in Insects. Trends Microbiol. 1996, 4, 197–203. [Google Scholar] [CrossRef]
- Pedrini, N.; Crespo, R.; Juárez, M.P. Biochemistry of Insect Epicuticle Degradation by Entomopathogenic Fungi. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2007, 146, 124–137. [Google Scholar] [CrossRef]
- Valero-Jiménez, C.A.; Debets, A.J.; van Kan, J.A.; Schoustra, S.E.; Takken, W.; Zwaan, B.J.; Koenraadt, C.J. Natural Variation in Virulence of the Entomopathogenic Fungus Beauveria bassiana against Malaria Mosquitoes. Malar. J. 2014, 13, 479. [Google Scholar] [CrossRef]
- Howard, A.F.; Koenraadt, C.J.; Farenhorst, M.; Knols, B.G.; Takken, W. Pyrethroid Resistance in Anopheles gambiae Leads to Increased Susceptibility to the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana. Malar. J. 2010, 9, 168. [Google Scholar] [CrossRef]
- Mnyone, L.L.; Kirby, M.J.; Lwetoijera, D.W.; Mpingwa, M.W.; Simfukwe, E.T.; Knols, B.G.; Takken, W.; Russell, T.L. Tools for Delivering Entomopathogenic Fungi to Malaria Mosquitoes: Effects of Delivery Surfaces on Fungal Efficacy and Persistence. Malar. J. 2010, 9, 246. [Google Scholar] [CrossRef]
- Blanford, S.; Jenkins, N.E.; Christian, R.; Chan, B.H.; Nardini, L.; Osae, M.; Koekemoer, L.; Coetzee, M.; Read, A.F.; Thomas, M.B. Storage and Persistence of a Candidate Fungal Biopesticide for Use against Adult Malaria Vectors. Malar. J. 2012, 11, 354. [Google Scholar] [CrossRef]
- Okumu, F.O.; Madumla, E.P.; John, A.N.; Lwetoijera, D.W.; Sumaye, R.D. Attracting, Trapping and Killing Disease-Transmitting Mosquitoes Using Odor-Baited Stations—The Ifakara Odor-Baited Stations. Parasites Vectors 2010, 3, 12. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Takken, W.; Knols, B.G.J. Infection of Adult Aedes aegypti and Ae. albopictus Mosquitoes with the Entomopathogenic Fungus Metarhizium anisopliae. Acta Trop. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- Blanford, S.; Chan, B.H.K.; Jenkins, N.; Sim, D.; Turner, R.J.; Read, A.F.; Thomas, M.B. Fungal Pathogen Reduces Potential for Malaria Transmission. Science 2005, 308, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Darbro, J.M.; Johnson, P.H.; Thomas, M.B.; Ritchie, S.A.; Kay, B.H.; Ryan, P.A. Effects of Beauveria bassiana on Survival, Blood-Feeding Success, and Fecundity of Aedes aegypti in Laboratory and Semi-Field Conditions. Am. J. Trop. Med. Hyg. 2012, 86, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.-J.; Knols, B.G.J.; Takken, W. Infection of the Malaria Mosquito Anopheles gambiae with the Entomopathogenic Fungus Metarhizium anisopliae Reduces Blood Feeding and Fecundity. J. Invertebr. Pathol. 2006, 91, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Pinto, J. Training Manual on Malaria Entomology; United States Agency for International Development: Washington, DC, USA, 2012. [Google Scholar]
- Nagpal, B.N.; Sharma, V.P. Indian Anophelines. In Indian Anophelines; Science Publishers, Inc.: Lebanon, NH, USA, 1995; 416p, ISBN 9781886106093. [Google Scholar]
- Hicks, B.J.; Watt, A.D.; Cosens, D. The Potential of Beauveria bassiana (Hyphomycetes: Moniliales) as a Biological Control Agent against the Pine Beauty Moth, Panolis flammea (Lepidoptera: Noctuidae). For. Ecol. Manag. 2001, 149, 275–281. [Google Scholar] [CrossRef]
- Johnson, D.M.; White, R.L.; Pereira, R.M.; Geden, C.J. Beauveria bassiana Culturing and Harvesting for Bioassays with House Flies. J. Insect Sci. 2020, 20, 14. [Google Scholar] [CrossRef]
- Oxborough, R.M.; Kitau, J.; Jones, R.; Mosha, F.W.; Rowland, M.W. Experimental Hut and Bioassay Evaluation of the Residual Activity of a Polymer-Enhanced Suspension Concentrate (SC-PE) Formulation of Deltamethrin for IRS Use in the Control of Anopheles arabiensis. Parasites Vectors 2014, 7, 454. [Google Scholar] [CrossRef]
- Spence, E.L.; Chandler, D.; Edgington, S.; Berry, S.D.; Martin, G.; O’Sullivan, C.; Svendsen, C.; Hesketh, H. A Standardised Bioassay Method Using a Bench-top Spray Tower to Evaluate Entomopathogenic Fungi for Control of the Greenhouse Whitefly, Trialeurodes Vaporariorum. Pest Manag. Sci. 2020, 76, 2513–2524. [Google Scholar] [CrossRef]
- Allossogbe, M.; Gnanguenon, V.; Yovogan, B.; Akinro, B.; Anagonou, R.; Agossa, F.; Houtoukpe, A.; Padonou, G.G.; Akogbeto, M. WHO Cone Bio-Assays of Classical and New-Generation Long-Lasting Insecticidal Nets Call for Innovative Insecticides Targeting the Knock-down Resistance Mechanism in Benin. Malar. J. 2017, 16, 77. [Google Scholar] [CrossRef]
- Blanford, S.; Shi, W.; Christian, R.; Marden, J.H.; Koekemoer, L.L.; Brooke, B.D.; Coetzee, M.; Read, A.F.; Thomas, M.B. Lethal and Pre-Lethal Effects of a Fungal Biopesticide Contribute to Substantial and Rapid Control of Malaria Vectors. PLoS ONE 2011, 6, e23591. [Google Scholar] [CrossRef]
- Alkhaibari, A.M.; Carolino, A.T.; Bull, J.C.; Samuels, R.I.; Butt, T.M. Differential Pathogenicity of Metarhizium Blastospores and Conidia Against Larvae of Three Mosquito Species. J. Med. Entomol. 2017, 54, 696–704. [Google Scholar] [CrossRef]
- Alkhaibari, A.M.; Carolino, A.T.; Yavasoglu, S.I.; Maffeis, T.; Mattoso, T.C.; Bull, J.C.; Samuels, R.I.; Butt, T.M. Metarhizium brunneum Blastospore Pathogenesis in Aedes Aegypti Larvae: Attack on Several Fronts Accelerates Mortality. PLoS Pathog. 2016, 12, e1005715. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Snetselaar, J.; Andriessen, R.; Suer, R.A.; Osinga, A.J.; Knols, B.G.; Farenhorst, M. Development and Evaluation of a Novel Contamination Device That Targets Multiple Life-Stages of Aedes aegypti. Parasites Vectors 2014, 7, 200. [Google Scholar] [CrossRef]
- Vatandoost, H.; Shamspour, S.; Abai, M.R. Relative Efficacy of Different Synthetic Pyrethroids Impregnated Fabrics (ITNs) Against Anopheles stephensi in Iran. Pak. J. Biol. Sci. 2006, 9, 503–506. [Google Scholar] [CrossRef]
- Howard, A.F.; N’Guessan, R.; Koenraadt, C.J.; Asidi, A.; Farenhorst, M.; Akogbéto, M.; Thomas, M.B.; Knols, B.G.; Takken, W. The Entomopathogenic Fungus Beauveria bassiana Reduces Instantaneous Blood Feeding in Wild Multi-Insecticide-Resistant Culex qQuinquefasciatus Mosquitoes in Benin, West Africa. Parasites Vectors 2010, 3, 87. [Google Scholar] [CrossRef]
- Farenhorst, M.; Farina, D.; Scholte, E.-J.; Takken, W.; Hunt, R.H.; Coetzee, M.; Knols, B.G.J. African Water Storage Pots for the Delivery of the Entomopathogenic Fungus Metarhizium anisopliae to the Malaria Vectors Anopheles gambiae s.s. and Anopheles funestus. Am. J. Trop. Med. Hyg. 2008, 78, 910–916. [Google Scholar] [CrossRef]
- Scholte, E.-J.; Knols, B.G.J.; Samson, R.A.; Takken, W. Entomopathogenic Fungi for Mosquito Control: A Review. J. Insect Sci. 2004, 4, 19. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Khachatourians, G.G. Infection Sites of the Entomopathogenic Fungus Beauveria bassiana in the Larvae of the Mosquito Aedes aegypti. Entomol. Exp. Appl. 1991, 59, 19–27. [Google Scholar] [CrossRef]
- Clark, T.B.; Kellen, W.R.; Fukuda, T.; Lindegren, J.E. Field and Laboratory Studies on the Pathogenicity of the Fungus Beauveria bassiana to Three Genera of Mosquitoes. J. Invertebr. Pathol. 1968, 11, 1–7. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Khachatourians, G.G. N-Acetyl-D-Glucosamine-Mediated Regulation of Extracellular Protease in the Entomopathogenic Fungus Beauveria bassiana. Appl. Environ. Microbiol. 1988, 54, 2699–2704. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Khachatourians, G.G. Regulation of Extracellular Protease in the Entomopathogenic Fungus Beauveria bassiana. Exp. Mycol. 1988, 12, 161–168. [Google Scholar] [CrossRef]
- Miranpuri, G.S.; Khachatourians, G.G. Larvicidal Activity of Blastospores and Conidiospores of Beauveria bassiana (Strain GK 2016) against Age Groups of Aedes aegypti. Vet. Parasitol. 1990, 37, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, T.; Takken, W.; Koenraadt, C.J. Development of Metarhizium anisopliae and Beauveria bassiana Formulations for Control of Malaria Mosquito Larvae. Parasites Vectors 2011, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Hamama, H.M.; Zyaan, O.H.; Abu Ali, O.A.; Saleh, D.I.; Elakkad, H.A.; El-Saadony, M.T.; Farag, S.M. Virulence of Entomopathogenic Fungi against Culex pipiens: Impact on Biomolecules Availability and Life Table Parameters. Saudi J. Biol. Sci. 2022, 29, 385–393. [Google Scholar] [CrossRef]
- Quintero-Zapata, I.; Flores-González, M.S.; Luna-Santillana, E.J.; Arroyo-González, N.; Gandarilla-Pacheco, F.L. Late Effects of Beauveria bassiana on Larval Stages of Aedes Aegypti Linneo, 1762 (Diptera: Culicidae). Braz. J. Biol. 2022, 82, e237789. [Google Scholar] [CrossRef]
- Lee, J.Y.; Woo, R.M.; Choi, C.J.; Shin, T.Y.; Gwak, W.S.; Woo, S.D. Beauveria bassiana for the Simultaneous Control of Aedes albopictus and Culex pipiens Mosquito Adults Shows High Conidia Persistence and Productivity. AMB Expr. 2019, 9, 206. [Google Scholar] [CrossRef]

| EPF Isolate | Institute Isolate Code | Source of Isolation | ICAR-NBAIM Accession Number | NCBI Genbank Accession Number |
|---|---|---|---|---|
| Bb-5a | PDBC Bb-5a | Coffee berry borer (Hypothenemus hampei) cadaver | NAIMCC-F-00396 | JF837134 |
| Bb- NBAIR | PDBC Bb-3 | Mottled water hyacinth weevil (Neochetina bruchi) cadaver | NAIMCC-F-00393 | JF837139 |
| Ma-4 | PDBC Ma-4 | Cashew stem and root borer (Plocaederus ferrugineus) cadaver | NAIMCC-F-01296 | JF837157 |
| Ma –NBAIR | PDBC Ma-15 | Soil sample | NAIMCC-F-01306 | JF837154 |
| Mud Panel | |||||||
|---|---|---|---|---|---|---|---|
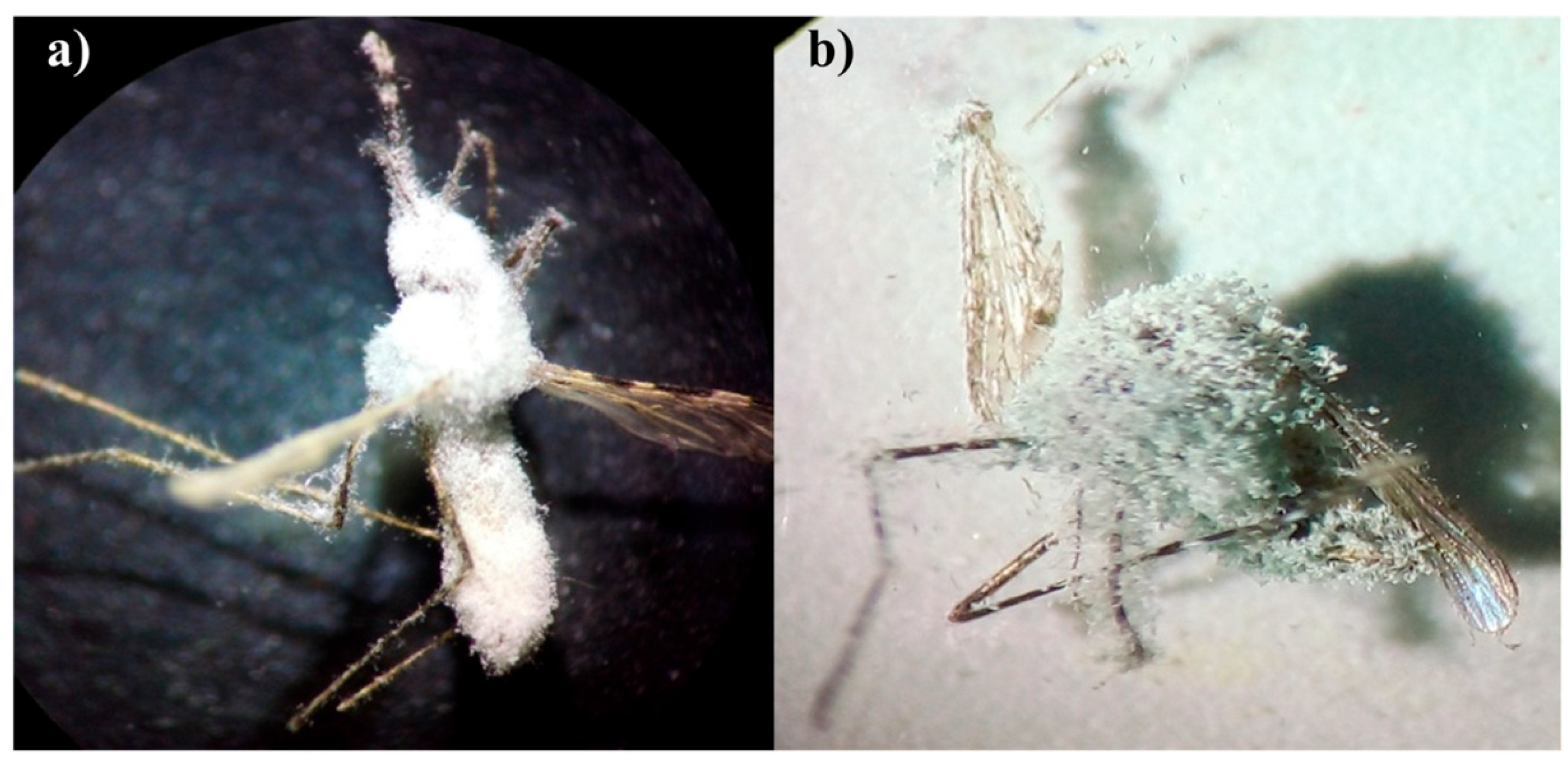
| Mosquito Species | EPF Isolate | Percent Mortality | Mean | Standard Deviation | Percent Mycosis | Mean | Standard Deviation |
| An. stephensi | Bb5a | 88.75 a | 44.37 | 4.74 | 86.25 a | 43.12 | 4.62 |
| Bb-NBAIR | 72.50 b | 36.25 | 3.89 | 62.50 b | 31.25 | 3.34 | |
| Ma4 | 57.50 b | 28.75 | 3.12 | 12.50 c | 5.62 | 7.76 | |
| Ma-NBAIR | 48.75 c | 24.37 | 2.63 | 11.25 c | 6.25 | 7.90 | |
| Control | 0.00 d | - | - | 0.00 d | - | - | |
| CD (p ≤ 0.01) | 13.11 | 10.66 | |||||
| Cement Panel | |||||||
|---|---|---|---|---|---|---|---|
| Mosquito Species | EPF Isolate | Percent Mortality | Mean | Standard Deviation | Percent Mycosis | Mean | Standard Deviation |
| An. stephensi | Bb5a | 86.25 a | 43.1 | 4.61 | 83.75 a | 41.87 | 4.47 |
| Bb-NBAIR | 72.50 b | 36.25 | 3.88 | 70.00 b | 35.00 | 3.76 | |
| Ma4 | 36.25 c | 18.12 | 1.99 | 11.25 c | 5.62 | 6.78 | |
| Ma-NBAIR | 33.75 c | 16.87 | 1.86 | 6.25 c | 3.12 | 3.72 | |
| Control | 0.00 d | - | - | 0.00 d | - | - | |
| CD (p ≤ 0.01) | 12.58 | 8.42 | |||||
| MST (Days) | ||
|---|---|---|
| Fungal Isolates | Cement Panel | Mud Panel |
| Bb5a | 6 | 6 |
| Bb-NBAIR | 7 | 7 |
| Ma4 | 10 | 9 |
| Ma-NBAIR | 10 | 10 |
| Control | 10 | 10 |
| Fungal Isolates | Mean ± SE (Days) | |
|---|---|---|
| Conidia | Blastospores | |
| Bb5a | 7.99 ± 0.19 | 10.70 ± 0.07 |
| Bb-NBAIR | 9.98 ± 0.11 | 9.75 ± 0.13 |
| Ma4 | 10.99 ± 0.09 | 11.15 ± 0.25 |
| Ma-NBAIR | 8.18 ± 0.13 | 10.19 ± 0.19 |
| Control | 6.01 ± 0.12 | 5.69 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renuka, S.; Vani H, C.; Alex, E. Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles stephensi (Diptera: Culicidae). J. Fungi 2023, 9, 223. https://doi.org/10.3390/jof9020223
Renuka S, Vani H C, Alex E. Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles stephensi (Diptera: Culicidae). Journal of Fungi. 2023; 9(2):223. https://doi.org/10.3390/jof9020223
Chicago/Turabian StyleRenuka, Siddaramegowda, Chalageri Vani H, and Eapen Alex. 2023. "Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles stephensi (Diptera: Culicidae)" Journal of Fungi 9, no. 2: 223. https://doi.org/10.3390/jof9020223
APA StyleRenuka, S., Vani H, C., & Alex, E. (2023). Entomopathogenic Fungi as a Potential Management Tool for the Control of Urban Malaria Vector, Anopheles stephensi (Diptera: Culicidae). Journal of Fungi, 9(2), 223. https://doi.org/10.3390/jof9020223






