FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Media, and Culture Conditions
2.2. Gene Deletion and Complementation
2.3. Construction of Fusion Vectors
2.4. RNA Extraction and Quantitative Reverse Transcription PCR
2.5. Subcellular Localization Assay
2.6. Yeast Two-Hybrid
2.7. Pathogenicity and DON Production Assays
3. Results
3.1. FgAP1σ Gene Deletion and Confirmation
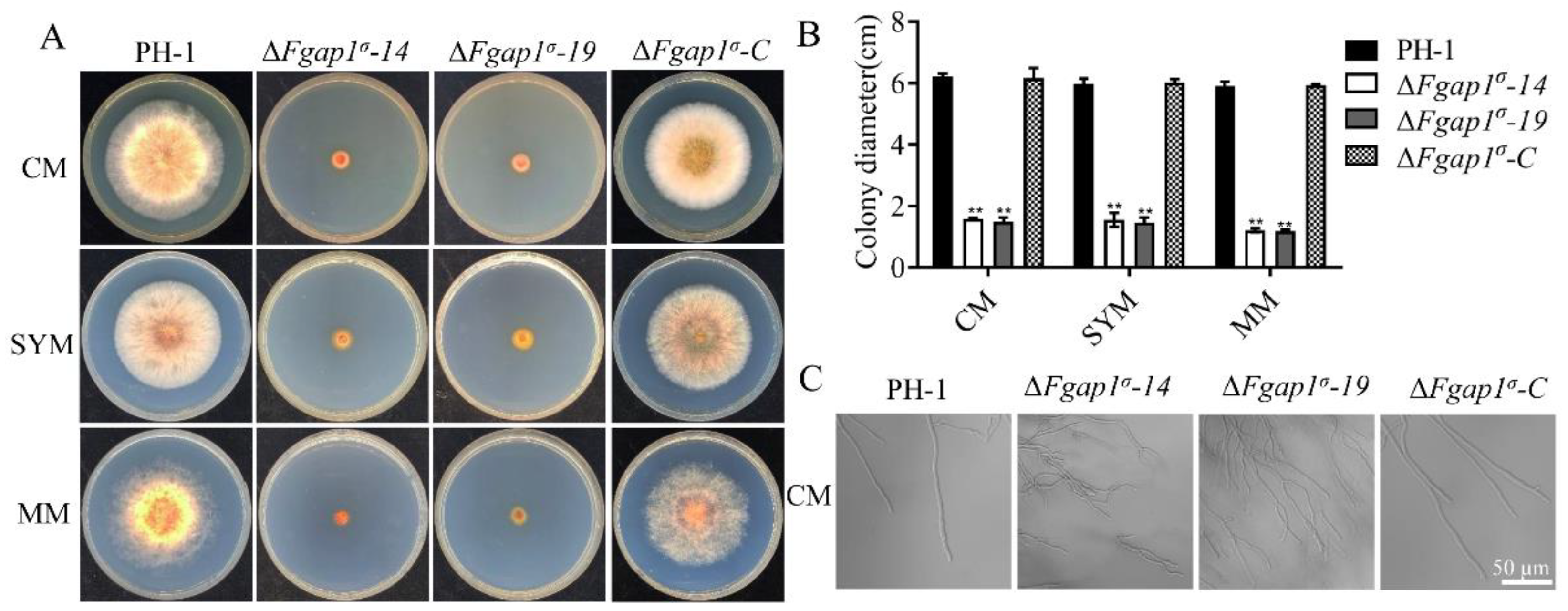
3.2. FgAP1σ Plays an Important Role in Vegetative Growth of F. graminearum
3.3. FgAP1σ Is Required for Conidiation and Sexual Development of F. graminearum
3.4. FgAP1σ Is Critical for F. graminearum Virulence and DON Production
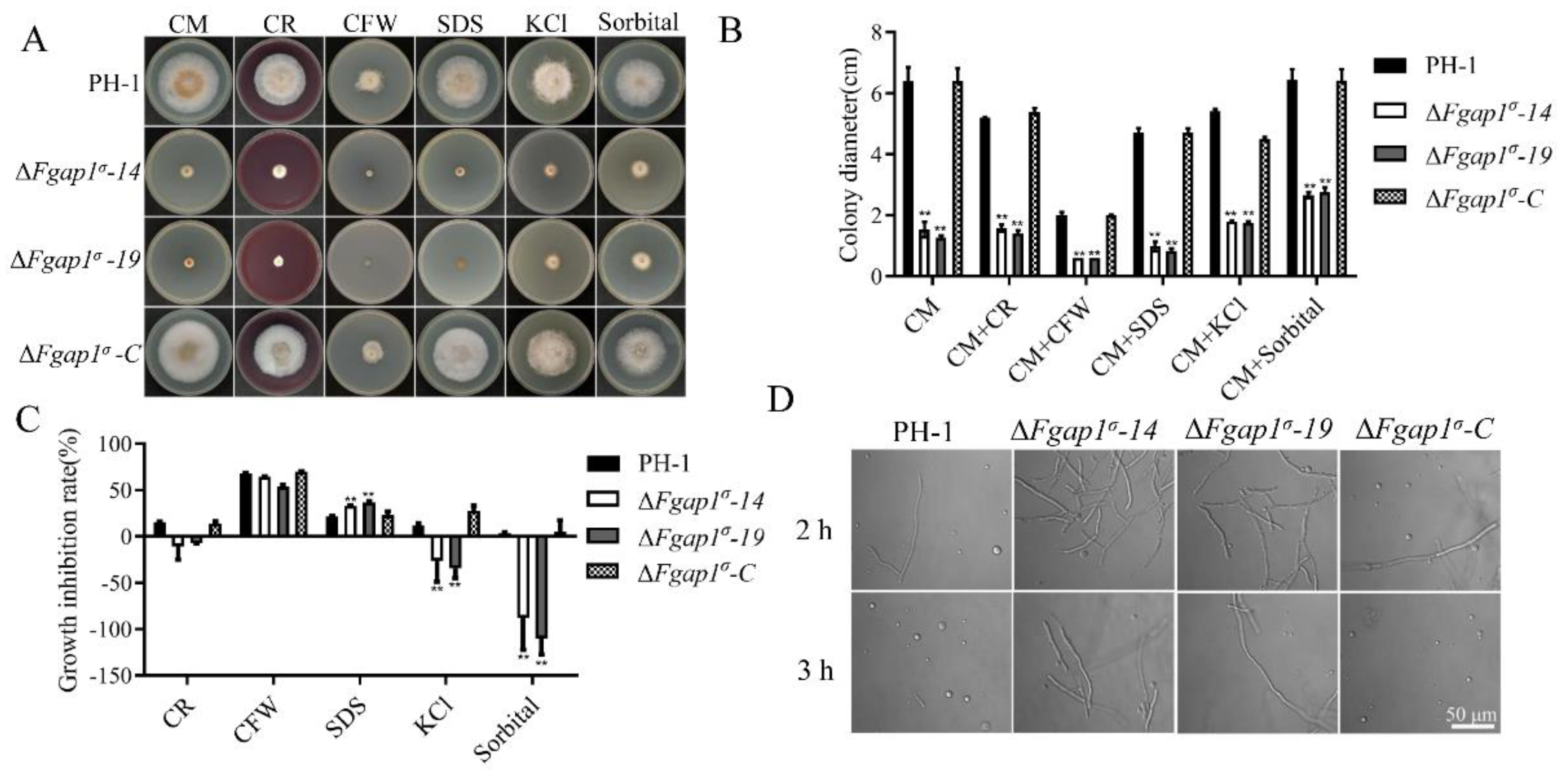
3.5. FgAP1σ Is Important for Cell Wall Integrity and Response to Osmotic Stress in F. graminearum
3.6. FgAP1σ Localizes to Endosomes and Golgi Apparatus
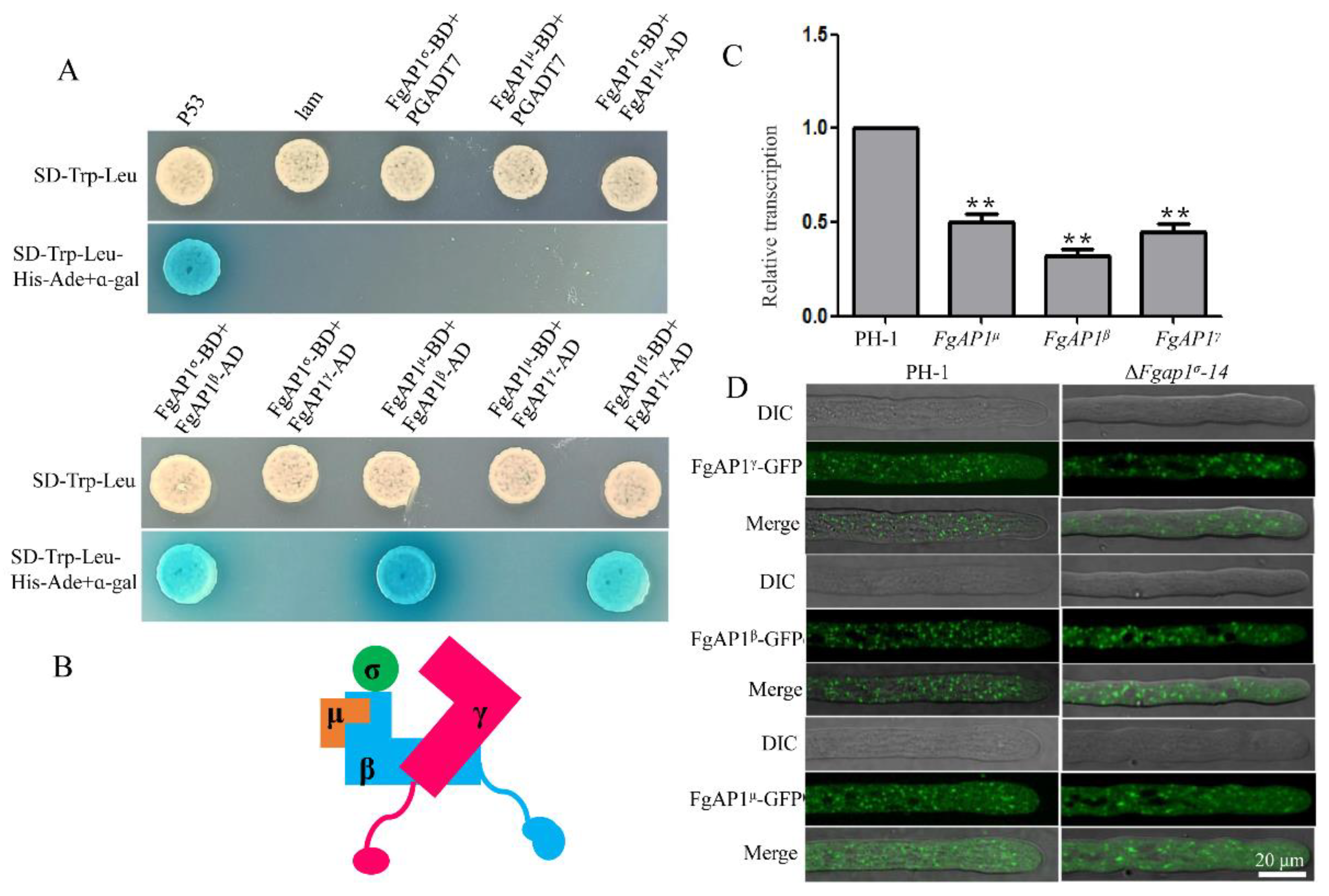
3.7. The Relationship of FgAP1σ with Other Subunits of AP1 Complex
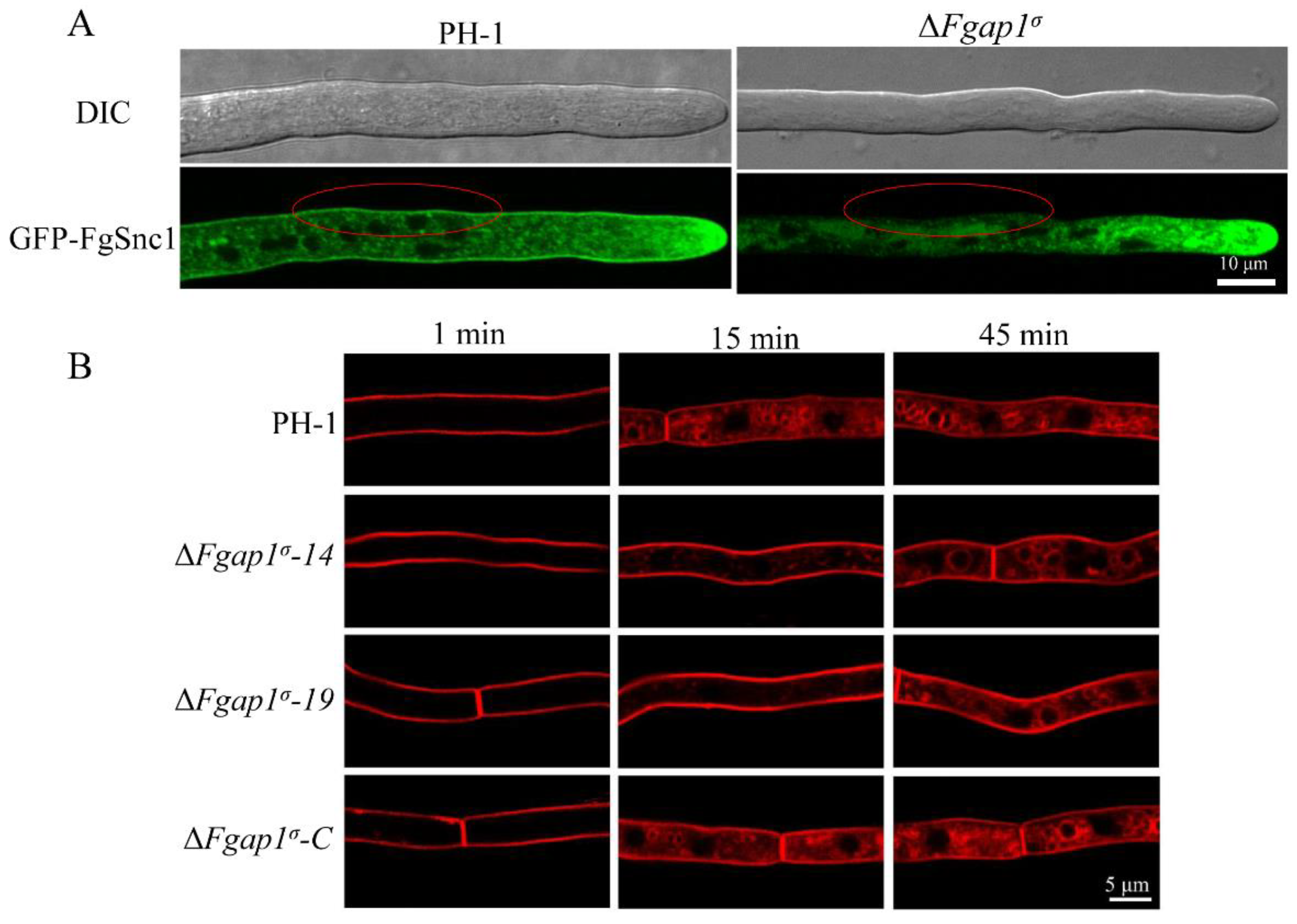
3.8. FgAP1σ Is Required for Plasma Membrane Localization of the v-SNARE FgSnc1
3.9. Loss of FgAP1σ Results in Delayed Internalization of FM4-64 into Vacuole Membrane
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmid, S.L. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annu. Rev. Biochem. 1997, 66, 511–548. [Google Scholar] [CrossRef]
- Hirst, J.; Robinson, M.S. Clathrin and adaptors. Biochim. Biophys. Acta 1998, 14, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.J.; Collins, B.M.; Evans, P.R. Adaptors for clathrin coats: Structure and function. Annu. Rev. Cell Dev. Biol. 2004, 20, 153–191. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H. Clathrin-associated adaptor protein complexes. J. Cell Sci. 2006, 119, 3719–3721. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Deyev, I.E.; Petrenko, A.G. Clathrin-mediated endocytosis and adaptor proteins. Acta Nat. 2013, 5, 62–73. [Google Scholar] [CrossRef]
- Martzoukou, O.; Amillis, S.; Zervakou, A.; Christoforidis, S.; Diallinas, G. The AP-2 complex has a specialized clathrin-independent role in apical endocytosis and polar growth in fungi. Elife 2017, 21, 20083. [Google Scholar] [CrossRef]
- Li, X.; Ortega, B.; Kim, B.; Welling, P.A. A Common Signal Patch Drives AP-1 Protein-dependent Golgi Export of Inwardly Rectifying Potassium Channels. J. Biol. Chem. 2016, 291, 14963–14972. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.S. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004, 14, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Beacham, G.M.; Partlow, E.A.; Hollopeter, G. Conformational regulation of AP1 and AP2 clathrin adaptor complexes. Traffic 2019, 20, 741–751. [Google Scholar] [CrossRef]
- Traub, L.M.; Kornfeld, S.; Ungewickell, E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J. Biol. Chem. 1995, 270, 4933–4942. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Bonifacino, J.S. Adaptins: The final recount. Mol. Biol. Cell 2001, 12, 2907–2920. [Google Scholar] [CrossRef] [PubMed]
- Heldwein, E.E.; Macia, E.; Wang, J.; Yin, H.L.; Kirchhausen, T.; Harrison, S.C. Crystal structure of the clathrin adaptor protein 1 core. Proc. Natl. Acad. Sci. USA 2004, 101, 14108–14113. [Google Scholar] [CrossRef] [PubMed]
- Zysnarski, C.J.; Lahiri, S.; Javed, F.T.; Martínez-Márquez, J.Y.; Trowbridge, J.W.; Duncan, M.C. Adaptor protein complex-1 (AP-1) is recruited by the HEATR5 protein Laa1 and its co-factor Laa2 in yeast. J. Biol. Chem. 2019, 294, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Seaman, M.N.; Sowerby, P.J.; Robinson, M.S. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J. Biol. Chem. 1996, 271, 25446–25451. [Google Scholar] [CrossRef] [PubMed]
- Sauvageau, E.; McCormick, P.J.; Lefrancois, S. In vivo monitoring of the recruitment and activation of AP-1 by Arf1. Sci Rep. 2017, 7, 7148. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Fournier, M.C.; Poy, G.; Bonifacino, J.S. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 1996, 271, 29009–29015. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, I.; Chen, Y.C.; Cupers, P.; Shoelson, S.E.; Kirchhausen, T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998, 17, 2148–2155. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Larocque, G.; Wood, K.M.; Morris, K.L.; Roseman, A.M.; Sessions, R.B.; Royle, S.J.; Smith, C.J. Multi-modal adaptor-clathrin contacts drive coated vesicle assembly. EMBO J. 2021, 40, 6. [Google Scholar] [CrossRef]
- Doray, B.; Lee, I.; Knisely, J.; Bu, G.; Kornfeld, S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol. Biol. Cell 2007, 18, 1887–1896. [Google Scholar] [CrossRef] [PubMed]
- Paraan, M.; Mendez, J.; Sharum, S.; Kurtin, D.; He, H.; Stagg, S.M. The structures of natively assembled clathrin-coated vesicles. Sci. Adv. 2020, 6, eaba8397. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.C. New directions for the clathrin adaptor AP-1 in cell biology and human disease. Curr. Opin. Cell Biol. 2022, 76, 13. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.L.; Finlay, J.A.; Chu, D.S.; Tan, P.K.; Kirchhausen, T.; Payne, G.S. The Saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: Evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 1994, 13, 1706–1717. [Google Scholar] [CrossRef]
- Ma, Y.; Takeuchi, M.; Sugiura, R.; Sio, S.O.; Kuno, T. Deletion mutants of AP-1 adaptin subunits display distinct phenotypes in fission yeast. Genes Cells 2009, 14, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Martzoukou, O.; Diallinas, G.; Amillis, S. Secretory Vesicle Polar Sorting, Endosome Recycling and Cytoskeleton Organization Require the AP-1 Complex in Aspergillus nidulans. Genetics 2018, 209, 1121–1138. [Google Scholar] [CrossRef]
- Venugopal, K.; Werkmeister, E.; Barois, N.; Saliou, J.-M.; Poncet, A.; Huot, L.; Sindikubwabo, F.; Hakimi, M.A.; Langsley, G.; Lafont, F.; et al. Dual role of the Toxoplasma gondii clathrin adaptor AP1 in the sorting of rhoptry and microneme proteins and in parasite division. PLoS Pathog. 2017, 13, e1006331. [Google Scholar] [CrossRef]
- Park, M.; Song, K.; Reichardt, I.; Kim, H.; Mayer, U.; Stierhof, Y.D.; Hwang, I.; Jürgens, G. Arabidopsis μ-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc. Natl. Acad. Sci. USA 2013, 110, 10318–10323. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, Y.; Wang, H.; Zeng, Y.; Zhuang, X.; Li, B.; Jiang, L. Trans-Golgi network-located AP1 gamma adaptins mediate dileucine motif-directed vacuolar targeting in Arabidopsis. Plant Cell 2014, 26, 4102–4118. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yan, X.; Wang, Y.; Liu, C.; Yang, Q.; Tian, D.; Bednarek, S.Y.; Pan, J.; Wang, C. ADAPTOR PROTEIN-1 complex-mediated post-Golgi trafficking is critical for pollen wall development in Arabidopsis. New Phytol. 2022, 235, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Nakatsu, F.; Ohmae, M.; Sugihara, K.; Shioda, N.; Takahashi, D.; Obata, Y.; Furusawa, Y.; Fujimura, Y.; Yamashita, T.; et al. AP-1B—Mediated Protein Sorting Regulates Polarity and Proliferation of Intestinal Epithelial Cells in Mice. Gastroenterology 2013, 145, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Glyvuk, N.; Tsytsyura, Y.; Geumann, C.; D’Hooge, R.; Hüve, J.; Kratzke, M.; Baltes, J.; Boening, D.; Klingauf, J.; Schu, P. AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 2010, 29, 1318–1330. [Google Scholar] [CrossRef]
- Zizioli, D.; Meyer, C.; Guhde, G.; Saftig, P.; von Figura, K.; Schu, P. Early embryonic death of mice deficient in gamma-adaptin. J. Biol. Chem. 1999, 274, 5385–5390. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Zizioli, D.; Lausmann, S.; Eskelinen, E.-L.; Hamann, J.; Saftig, P.; Von Figura, K.; Schu, P. μ1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000, 19, 2193–2203. [Google Scholar] [CrossRef]
- Johnson, K.R.; Gagnon, L.H.; Chang, B. A hypomorphic mutation of the gamma-1 adaptin gene (Ap1g1) causes inner ear, retina, thyroid, and testes abnormalities in mice. Mamm. Genome 2016, 27, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.; Côté, S.; Brustein, E.; Drouin, C.A.; Lapointe, L.; Boudreau, M.; Meloche, C.; Drouin, R.; Hudson, T.J.; Drapeau, P.; et al. Disruption of AP1S1, Causing a Novel Neurocutaneous Syndrome, Perturbs Development of the Skin and Spinal Cord. PLoS Genet. 2008, 4, e1000296. [Google Scholar] [CrossRef]
- Fölsch, H.; Pypaert, M.; Schu, P.; Mellman, I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J. Cell Biol. 2001, 152, 595–606. [Google Scholar] [CrossRef]
- Setta-Kaffetzi, N.; Simpson, M.; Navarini, A.A.; Patel, V.M.; Lu, H.-C.; Allen, M.H.; Duckworth, M.; Bachelez, H.; Burden, A.D.; Choon, S.E.; et al. AP1S3 Mutations Are Associated with Pustular Psoriasis and Impaired Toll-like Receptor 3 Trafficking. Am. J. Hum. Genet. 2014, 94, 790–797. [Google Scholar] [CrossRef]
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Trail, F.; Xu, J.R.; San Miguel, P.; Halgren, R.G.; Kistler, H.C. Analysis of expressed sequence tags from Gibberella zeae (ana-morph Fusarium graminearum). Fungal Genet. Biol. 2003, 38, 187–197. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kistler, H.C.; Ma, Z. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, M.; Li, J.; Yang, C.; Abubakar, Y.S.; Chen, X.; Zheng, W.; Wang, Z.; Zheng, H.; Zhou, J. The Small GTPase FgRab1 Plays Indispensable Roles in the Vegetative Growth, Vesicle Fusion, Autophagy and Pathogenicity of Fusarium graminearum. Int. J. Mol. Sci. 2022, 23, 895. [Google Scholar] [CrossRef]
- Zheng, Q.; Yu, Z.; Yuan, Y.; Sun, D.; Abubakar, Y.S.; Zhou, J.; Wang, Z.; Zheng, H. The GTPase-Activating Protein FgGyp1 Is Important for Vegetative Growth, Conidiation, and Virulence and Negatively Regulates DON Biosynthesis in Fusarium graminearium. Front Microbiol. 2021, 12, 621519. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, J.; Chen, X.; Zhang, X.; Liao, D.; Yun, Y.; Zheng, W.; Abubakar, Y.S.; Li, G.; Wang, Z.; et al. FgVps9, a Rab5 GEF, Is Critical for DON Biosynthesis and Pathogenicity in Fusarium graminearum. Front. Microbiol. 2020, 11, 1714. [Google Scholar] [CrossRef]
- Adnan, M.; Fang, W.; Sun, P.; Zheng, Y.; Abubakar, Y.S.; Zhang, J.; Lou, Y.; Zheng, W.; Lu, G.-D. R-SNARE FgSec22 is essential for growth, pathogenicity and DON production of Fusarium graminearum. Curr. Genet. 2020, 66, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Chen, A.; Zhang, Y.; Yuan, M.; Xie, W.; Zhang, C.; Zheng, W.; Wang, Z.; Li, G.; Zhou, J. Component Interaction of ESCRT Complexes Is Essential for Endocytosis-Dependent Growth, Reproduction, DON Production and Full Virulence in Fusarium graminearum. Front. Microbiol. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Zhao, R.; Kou, R.; Zheng, X.; Zhang, H. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS Pathog. 2019, 15, e1007754. [Google Scholar] [CrossRef]
- Zhang, J.; Yun, Y.; Lou, Y.; Abubakar, Y.S.; Guo, P.; Wang, S.; Li, C.; Feng, Y.; Adnan, M.; Zhou, J.; et al. FgAP-2 complex is es-sential for pathogenicity and polarised growth and regulates the apical localisation of membrane lipid flippases in Fusarium graminearum. Cell Microbiol. 2019, 21, 17. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, S.; Zhou, X.; Wang, C.; Xiang, P.; Zheng, Q.; Xu, J.R. The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS ONE 2012, 7, 14. [Google Scholar] [CrossRef]
- Wu, C.; Guo, Z.; Zhang, M.; Chen, H.; Peng, M.; Abubakar, Y.S.; Zheng, H.; Yun, Y.; Zheng, W.; Wang, Z.; et al. Golgi-localized calcium/manganese transporters FgGdt1 and FgPmr1 regulate fungal development and virulence by maintaining Ca2+ and Mn2+ homeostasis in Fusarium graminearum. Environ. Microbiol. 2022, 15, 1462–2920. [Google Scholar]
- Hou, Z.; Xue, C.; Peng, Y.; Katan, T.; Kistler, H.C.; Xu, J.R. A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation, and plant infection. Mol. Plant-Microbe Interact. 2002, 15, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Catlett, N.L.; Lee, B.N.; Yoder, O.C.; Turgeon, B.G. Split-Marker Recombination for Efficient Targeted Deletion of Fungal Genes. Fungal Genet. Newsl. 2003, 50, 9–11. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Clontech Laboratories. Matchmaker ™ GAL4 Two-Hybrid System 3 & Libraries User Manual. 3 April 2007. Available online: http://www.clontech.com/images/pt/PT3247-1.PDF (accessed on 30 December 2022).
- Yeung, B.G.; Phan, H.L.; Payne, G.S. Adaptor complex-independent clathrin function in yeast. Mol. Biol. Cell 1999, 10, 3643–3659. [Google Scholar] [CrossRef]
- Sanger, A.; Hirst, J.; Davies, A.K.; Robinson, M.S. Adaptor protein complexes and disease at a glance. J. Cell Sci. 2019, 132, 222992. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Tian, D.; Xu, M.; Pan, J.; Wu, H.; Wang, C.; Otegui, M.S. AP1/2β-mediated exocytosis of tapetum-specific transporters is required for pollen development in Arabidopsis thaliana. Plant Cell 2022, 34, 3961–3982. [Google Scholar] [CrossRef] [PubMed]
- Cabib, E.; Roh, D.-H.; Schmidt, M.; Crotti, L.B.; Varma, A. The Yeast Cell Wall and Septum as Paradigms of Cell Growth and Morphogenesis. J. Biol. Chem. 2001, 276, 19679–19682. [Google Scholar] [CrossRef] [PubMed]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Xu, L.; Wang, M.; Tang, G.; Ma, Z.; Shao, W. The endocytic cargo adaptor complex is required for cell-wall integrity via interacting with the sensor FgWsc2B in Fusarium graminearum. Curr. Genet. 2019, 65, 1071–1080. [Google Scholar] [CrossRef]
- Riquelme, M.; Aguirre, J.; Bartnicki-García, S.; Braus, G.H.; Feldbrügge, M.; Fleig, U.; Hansberg, W.; Herrera-Estrella, A.; Kämper, J.; Kück, U.; et al. Fungal Morphogenesis, from the Polarized Growth of Hyphae to Complex Reproduction and Infection Structures. Microbiol. Mol. Biol. Rev. 2018, 82, e00068-17. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, R.H.; Baggott, D.; Chuang, J.S.; Schekman, R.W. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2002, 2, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Heinze, L.; Freimuth, N.; Rößling, A.K.; Hahnke, R.; Riebschläger, S.; Fröhlich, A.; Sampathkumar, A.; McFarlane, H.E.; Sauer, M. EPSIN1 and MTV1 define functionally overlapping but molecularly distinct trans-Golgi network subdomains in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 25880–25889. [Google Scholar] [CrossRef]
- Shimizu, Y.; Takagi, J.; Ito, E.; Ito, Y.; Ebine, K.; Komatsu, Y.; Goto, Y.; Sato, M.; Toyooka, K.; Ueda, T.; et al. Cargo sorting zones in the trans-Golgi network visualized by super-resolution confocal live imaging microscopy in plants. Nat. Commun. 2021, 12, 1901. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.E.; Collins, B.M.; McCoy, A.J.; Robinson, M.S.; Owen, D.J. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature 2007, 450, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lin, Y.; Fang, W.; Zhao, X.; Lou, Y.; Wang, G.; Zheng, H.; Liang, Q.; Abubakar, Y.S.; Olsson, S.; et al. The endosomal recycling of FgSnc1 by FgSnx41-FgSnx4 heterodimer is essential for polarized growth and pathogenicity in Fusarium graminearum. New Phytol. 2018, 219, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Li, L.; Yu, Z.; Yuan, Y.; Zheng, Q.; Xie, Q.; Li, G.; Abubakar, Y.S.; Zhou, J.; Wang, Z.; et al. FgSpa2 recruits FgMsb3, a Rab8 GAP, to the polarisome to regulate polarized trafficking, growth and pathogenicity in Fusarium graminearum. New Phytol. 2021, 229, 1665–1683. [Google Scholar] [CrossRef]
- Lewis, M.J.; Nichols, B.J.; Prescianotto-Baschong, C.; Riezman, H.; Pelham, H.R. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 2000, 11, 23–38. [Google Scholar] [CrossRef]
- Canagarajah, B.J.; Ren, X.; Bonifacino, J.S.; Hurley, J.H. The clathrin adaptor complexes as a paradigm for membrane-associated allostery. Protein Sci. 2013, 22, 517–529. [Google Scholar] [CrossRef]
- Boucrot, E.; Saffarian, S.; Zhang, R.; Kirchhausen, T. Roles of AP-2 in clathrin-mediated endocytosis. PLoS ONE 2010, 5, 0010597. [Google Scholar] [CrossRef]
- A Dahhan, D.; Reynolds, G.D.; Cárdenas, J.J.; Eeckhout, D.; Johnson, A.; Yperman, K.; A Kaufmann, W.; Vang, N.; Yan, X.; Hwang, I.; et al. Proteomic characterization of isolated Arabidopsis clathrin-coated vesicles reveals evolutionarily conserved and plant-specific components. Plant Cell 2022, 34, 2150–2173. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.Y.; Stirling, P.C.; Stimpson, H.E.; Giesselmann, E.; Schmitt, M.J.; Drubin, D.G. A yeast killer toxin screen provides insights into a/b toxin entry, trafficking, and killing mechanisms. Dev. Cell 2009, 17, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Bai, G.H.; Plattner, R.D.; Proctor, R.H. Analysis of aberrant virulence of Gibberella zeae following transformation-mediated complementation of a trichothecene-deficient (Tri5) mutant. Microbiology. 2000, 146, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.Y.; Pasquali, M.; Zhou, X.; Song, J.; Hilburn, K.; McCormick, S.; Dong, Y.; Xu, J.R.; Kistler, H.C. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol. Microbiol. 2009, 72, 354–367. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Chen, H.; Yuan, M.; Zhang, M.; Abubakar, Y.S.; Chen, X.; Zhong, H.; Zheng, W.; Zheng, H.; Zhou, J. FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum. J. Fungi 2023, 9, 145. https://doi.org/10.3390/jof9020145
Wu C, Chen H, Yuan M, Zhang M, Abubakar YS, Chen X, Zhong H, Zheng W, Zheng H, Zhou J. FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum. Journal of Fungi. 2023; 9(2):145. https://doi.org/10.3390/jof9020145
Chicago/Turabian StyleWu, Congxian, Huilin Chen, Mingyue Yuan, Meiru Zhang, Yakubu Saddeeq Abubakar, Xin Chen, Haoming Zhong, Wenhui Zheng, Huawei Zheng, and Jie Zhou. 2023. "FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum" Journal of Fungi 9, no. 2: 145. https://doi.org/10.3390/jof9020145
APA StyleWu, C., Chen, H., Yuan, M., Zhang, M., Abubakar, Y. S., Chen, X., Zhong, H., Zheng, W., Zheng, H., & Zhou, J. (2023). FgAP1σ Is Critical for Vegetative Growth, Conidiation, Virulence, and DON Biosynthesis in Fusarium graminearum. Journal of Fungi, 9(2), 145. https://doi.org/10.3390/jof9020145






