Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries
Abstract
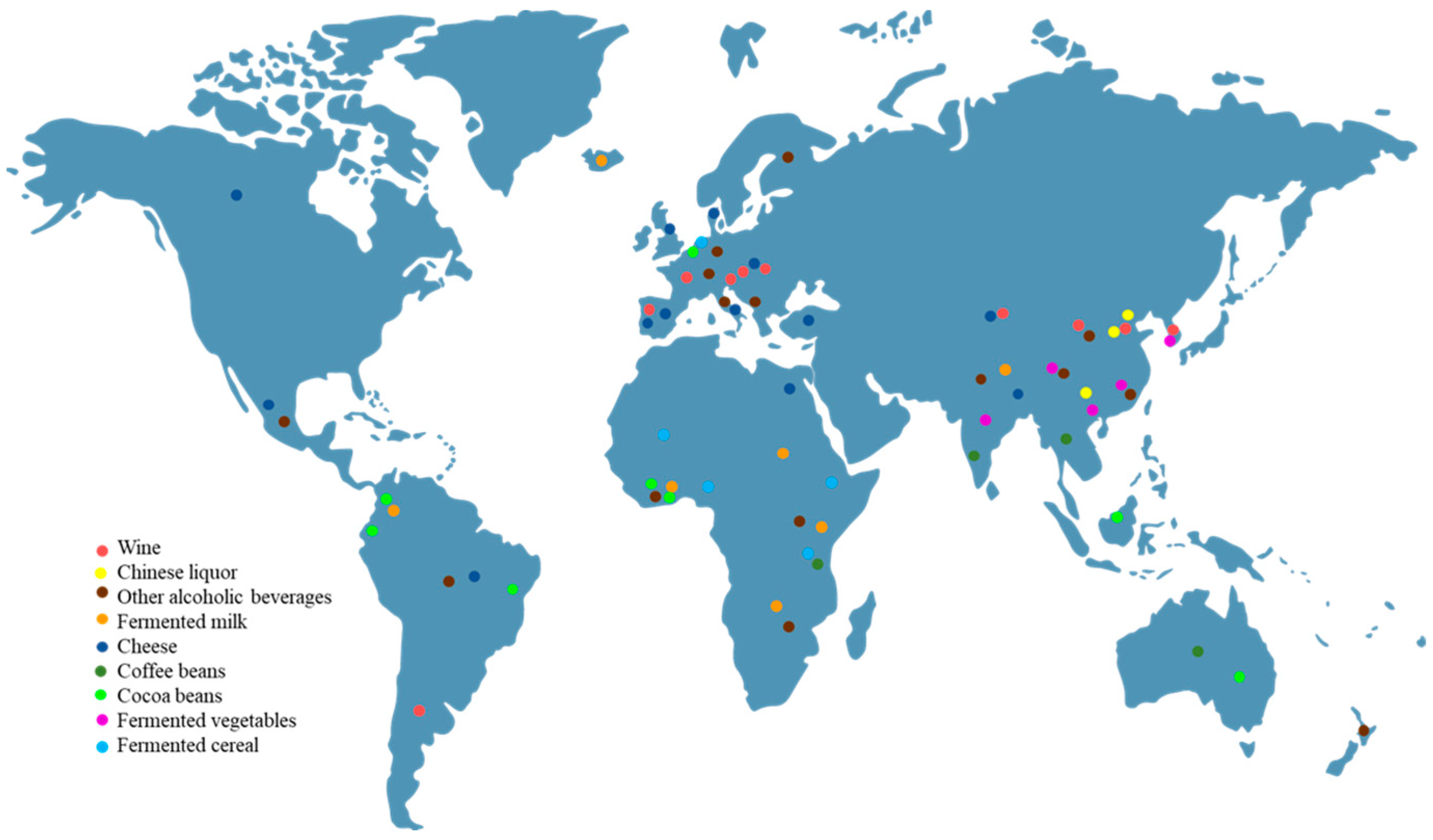
1. Introduction
2. Taxonomy and Physiology of P. kudriavzevii
3. Application in Fermented Foods and Feed Industry
3.1. Fermented Alcoholic Beverages
3.1.1. Wine
3.1.2. Chinese Liquor
3.1.3. Other Alcoholic Beverages
3.2. Dairy Products
3.2.1. Fermented Milk
3.2.2. Cheese
3.3. Coffee Beans and Cocoa Beans
3.4. Fermented Vegetables
3.5. Fermented Cereals
3.6. Applications as a Feeding Additive
4. Applications in Biotechnology
4.1. Biochemicals Production
4.1.1. Bioethanol
| Strains | Substrates | Ethanol Production | Fermentation Conditions | References |
|---|---|---|---|---|
| MF-121 | Glucose | 5.50% (v/v) | 10 g/L Na2SO4, pH 2.0 | [96] |
| RZ8-1 | Glucose | 69.9 g/L, 1.46 g/L·h | 40 °C | [101] |
| RZ8-1 | Sugarcane bagasse hydrolysate | 33.8 g/L, 1.41 g/L·h | 40 °C | [101] |
| IPE 100 | Cornstalk hydrolysate | 45.9 g/L, 0.96 g/L·h | 42 °C | [100] |
| IPE 100 | Sweet sorghum stalk | 0.25 g/g-dry stalk | Solid state fermentation | [102] |
| Unnamed | Sugarcane juice | 71.9 g/L, 4.0 g/L·h | 40 °C | [98] |
| ITV-S42 | Glucose | 66.2 g/L, 2.67 g/L·h | 200 g/L initial glucose concentration | [103] |
| HOP-1 | Alkali-treated rice straw | 24.3 g/L, 1.10 g/L·h | SSF, 40 °C, pH 5.0 | [92] |
| HOP-1 | Sweet sorghum bagasse hydrolysate | 26.8 g/L, 0.56 g/L·h | SSF, 35 °C, pH 5.0 | [104] |
| KJ27-7 | Wheat straw hydrolysate | 10.3 g/L, 0.43 g/L·h, 0.5 g/g glucose | 26 °C, pH 5.0 | [99] |
| 4A | Malted barley | 115.1 g/L, 4.80 g/L·h | 40 °C, pH 5.0 | [105] |
| LC375240 | Cassava peel | 5.4%(w/v), 1.45 g/L·h, 0.38 g/g glucose | SSF, 40 °C | [106] |
| SI | Rice straw | 33.4 g/L, 1.07 g/L·h | SSF, 42 °C, uncontrolled pH | [84] |
| KVMP10 | Citrus peel waste hydrolysate | 30.7 g/L | 42 °C | [107] |
| NBRC1664 | Japanese cedar without pretreatment | 23.8 g/L, 0.74 g/L·h | SSF, 35 °C | [108] |
| DMKU3-ET15 | Cassava starch hydrolysate | 7.35% (w/v), 2.23 g/L·h | 40 °C, pH 5.0 | [109] |
| Pa27 | Xylose | 14.6 g/L, 0.27 g/g xylose | 42 °C | [110] |
| Brk | Undetoxified acid- hydrolyzed corncob | 3.64 g/L, 28% of the theoretical yield | 42 °C | [111] |
| Brk | Detoxified acid- hydrolyzed corncob | 3.93 g/L, 32% of the theoretical yield | 42 °C | [111] |
4.1.2. Organic Acids
| Organic Acids | Strains | Metabolic Engineering Strategies | Production | Fermentation Conditions | References |
|---|---|---|---|---|---|
| Succinic acid | SD108 |
| 11.6 g/L, 0.12 g/g, 0.11 g/L·h | 30 °C, uncontrolled pH | [119] |
| CY902 |
| 3.60 g/L | 30 °C, uncontrolled pH | [112] | |
| 13171 |
| 23.0 g/L, 0.26 g/L·h | 30 °C, pH 3.0 | [120] | |
| 13723 |
| 48.20 g/L, 0.45 g/g glucose, 0.97 g/L·h | 30 °C, pH 3.0 | [113] | |
| Lactic acid | CD1690 |
| 88 g/L, 2.6 g/L·h | 38–40 °C, pH 5.5 | [114] |
| NG7 |
| ① 135 g/L, 3.66 g/L·h ② 154 g/L, 4.16 g/L·h | ① 30 °C, pH 3.6 ② 30 °C, pH 4.7 | [85] | |
| Itaconic acid | YB4010 |
| 1232 mg/L, 29.0 mg/g-glucose, 51 g/L·h | 30 °C, uncontrolled pH | [118] |
| D-xylonate | VTT C-79090T |
| ① 146 g/L, 1.2 g/L·h ② 171 g/L, 1.4 g/L·h | ① 30 °C, pH 3.0 ② 30 °C, pH 5.5 | [116] |
4.1.3. Glycerol
4.1.4. 2-Phenylethanol
4.1.5. Single-Cell Oils
4.2. Applications in Environmental Management
4.3. Biological Control
5. Pathogenicity of P. kudriavzevii
6. Concluding Remarks and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nielsen, J. Yeast Systems Biology: Model Organism and Cell Factory. Biotechnol. J. 2019, 14, e1800421. [Google Scholar] [CrossRef]
- Thorwall, S.; Schwartz, C.; Chartron, J.W.; Wheeldon, I. Stress-tolerant non-conventional microbes enable next-generation chemical biosynthesis. Nat. Chem. Biol. 2020, 16, 113–121. [Google Scholar] [CrossRef]
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Mukherjee, V.; Radecka, D.; Aerts, G.; Verstrepen, K.J.; Lievens, B.; Thevelein, J.M. Phenotypic landscape of non-conventional yeast species for different stress tolerance traits desirable in bioethanol fermentation. Biotechnol. Biofuels 2017, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Semkiv, M.V.; Ruchala, J.; Dmytruk, K.V.; Sibirny, A.A. 100 years later, what is new in glycerol bioproduction? Trends Biotechnol. 2020, 38, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ong, K.L.; Cui, Z.; Sang, Z.; Li, X.; Patria, R.D.; Qi, Q.; Fickers, P.; Yan, J.; Lin, C.S.K. Promising advancement in fermentative succinic acid production by yeast hosts. J. Hazard. Mater. 2021, 401, 123414. [Google Scholar] [CrossRef]
- Yadav, J.S.; Bezawada, J.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Candida krusei: Biotechnological potentials and concerns about its safety. Can. J. Microbiol. 2012, 58, 937–952. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Robnett, C.J.; Basehoar-Powers, E. Phylogenetic relationships among species of Pichia, Issatchenkia and Williopsis determined from multigene sequence analysis, and the proposal of Barnettozyma gen. nov., Lindnera gen. nov. and Wickerhamomyces gen. nov. FEMS Yeast Res. 2008, 8, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Douglass, A.P.; Offei, B.; Braun-Galleani, S.; Coughlan, A.Y.; Martos, A.A.R.; Ortiz-Merino, R.A.; Byrne, K.P.; Wolfe, K.H. Population genomics shows no distinction between pathogenic Candida krusei and environmental Pichia kudriavzevii: One species, four names. PLoS Pathog. 2018, 14, e1007138. [Google Scholar] [CrossRef]
- van Rijswijck, I.M.; Dijksterhuis, J.; Wolkers-Rooijackers, J.C.; Abee, T.; Smid, E.J. Nutrient limitation leads to penetrative growth into agar and affects aroma formation in Pichia fabianii, P. kudriavzevii and Saccharomyces cerevisiae. Yeast 2015, 32, 89–101. [Google Scholar]
- Gomez-Gaviria, M.; Mora-Montes, H.M. Current aspects in the biology, pathogeny, and treatment of Candida krusei, a Neglected Fungal Pathogen. Infect. Drug Resist. 2020, 13, 1673–1689. [Google Scholar] [CrossRef]
- Ji, H.; Xu, K.; Dong, X.; Sun, D.; Peng, R.; Lin, S.; Zhang, K.; Jin, L. Transcriptional profiling reveals molecular basis and the role of arginine in response to low-pH stress in Pichia kudriavzevii. J. Biosci. Bioeng. 2020, 130, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Villa, S.; Hamideh, M.; Weinstock, A.; Qasim, M.N.; Hazbun, T.R.; Sellam, A.; Hernday, A.D.; Thangamani, S. Transcriptional control of hyphal morphogenesis in Candida albicans. FEMS Yeast Res. 2020, 20, foaa005. [Google Scholar] [CrossRef]
- Schnierda, T.; Bauer, F.F.; Divol, B.; van Rensburg, E.; Gorgens, J.F. Optimization of carbon and nitrogen medium components for biomass production using non-Saccharomyces wine yeasts. Lett. Appl. Microbiol. 2014, 58, 478–485. [Google Scholar] [CrossRef]
- Koutinas, M.; Patsalou, M.; Stavrinou, S.; Vyrides, I. High temperature alcoholic fermentation of orange peel by the newly isolated thermotolerant Pichia kudriavzevii KVMP10. Lett. Appl. Microbiol. 2016, 62, 75–83. [Google Scholar] [CrossRef]
- Radecka, D.; Mukherjee, V.; Mateo, R.Q.; Stojiljkovic, M.; Foulquie-Moreno, M.R.; Thevelein, J.M. Looking beyond Saccharomyces: The potential of non-conventional yeast species for desirable traits in bioethanol fermentation. FEMS Yeast Res. 2015, 15, fov053. [Google Scholar] [CrossRef] [PubMed]
- Mateus, D.; Sousa, S.; Coimbra, C.; Rogerson, F.S.; Simoes, J. Identification and characterization of non-saccharomyces species isolated from port wine spontaneous fermentations. Foods 2020, 9, 120. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuo, X.; Hu, L.; Zhang, X. Effects of crude beta-glucosidases from Issatchenkia terricola, Pichia kudriavzevii, Metschnikowia pulcherrima on the flavor complexity and characteristics of wines. Microorganisms 2020, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- del Mónaco, S.M.; Barda, N.B.; Rubio, N.C.; Caballero, A.C. Selection and characterization of a Patagonian Pichia kudriavzevii for wine deacidification. J. Appl. Microbiol. 2014, 117, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Hong, Y.A.; Park, H.D. Co-fermentation of grape must by Issatchenkia orientalis and Saccharomyces cerevisiae reduces the malic acid content in wine. Biotechnol. Lett. 2008, 30, 1633–1638. [Google Scholar] [CrossRef]
- Zhu, L.X.; Wang, G.Q.; Aihaiti, A. Combined indigenous yeast strains produced local wine from over ripen Cabernet Sauvignon grape in Xinjiang. World J. Microbiol. Biotechnol. 2020, 36, 122. [Google Scholar] [CrossRef]
- Li, X.R.; Ma, E.B.; Yan, L.Z.; Meng, H.; Du, X.W.; Quan, Z.X. Bacterial and fungal diversity in the starter production process of Fen liquor, a traditional Chinese liquor. J. Microbiol. 2013, 51, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, L.; Tan, Y.; Wang, H.; Yang, F.; Chen, L.; Hao, F.; Lv, X.; Du, H.; Xu, Y. Effect of Pichia on shaping the fermentation microbial community of sauce-flavor Baijiu. Int. J. Food Microbiol. 2021, 336, 108898. [Google Scholar] [CrossRef]
- Liu, P.; Xiong, X.; Wang, S.; Miao, L. Population dynamics and metabolite analysis of yeasts involved in a Chinese miscellaneous-flavor liquor fermentation. Ann. Microbiol. 2017, 67, 553–565. [Google Scholar] [CrossRef]
- Li, R.R.; Xu, M.; Zheng, J.; Liu, Y.J.; Sun, C.H.; Wang, H.; Guo, X.W.; Xiao, D.G.; Wu, X.L.; Chen, Y.F. Application potential of Baijiu non-Saccharomyces yeast in winemaking through sequential fermentation with Saccharomyces cerevisiae. Front. Microbiol. 2022, 13, 902597. [Google Scholar] [CrossRef]
- Wang, X.; Du, H.; Zhang, Y.; Xu, Y. Environmental microbiota drives microbial succession and metabolic profiles during Chinese liquor fermentation. Appl. Environ. Microbiol. 2018, 84, e02369-17. [Google Scholar] [CrossRef]
- Du, H.; Wang, X.; Zhang, Y.; Xu, Y. Exploring the impacts of raw materials and environments on the microbiota in Chinese Daqu starter. Int. J. Food Microbiol. 2019, 297, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fan, Y.; Lu, T.; Kang, J.; Pang, X.; Han, B.; Chen, J. Composition and metabolic functions of the microbiome in fermented grain during light-flavor Baijiu fermentation. Microorganisms 2020, 8, 1281. [Google Scholar] [CrossRef]
- Deng, N.; Du, H.; Xu, Y. Cooperative response of Pichia kudriavzevii and Saccharomyces cerevisiae to lactic acid stress in Baijiu fermentation. J. Agric. Food Chem. 2020, 68, 4903–4911. [Google Scholar] [CrossRef]
- Coulibaly, W.H.; Boli, Z.; Bouatenin, K.M.J.; M’Bra, A.A.; Kouhounde, S.H.S.; Dje, K.M. Identification of non-Saccharomyces yeast strains isolated from local traditional sorghum beer produced in Abidjan district (Cote d’Ivoire) and their ability to carry out alcoholic fermentation. BMC Microbiol. 2022, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- van Rijswijck, I.M.H.; Wolkers-Rooijackers, J.C.M.; Abee, T.; Smid, E.J. Performance of non-conventional yeasts in co-culture with brewers’ yeast for steering ethanol and aroma production. Microb. Biotechnol. 2017, 10, 1591–1602. [Google Scholar] [CrossRef]
- Nyanga, L.K.; Nout, M.J.; Gadaga, T.H.; Theelen, B.; Boekhout, T.; Zwietering, M.H. Yeasts and lactic acid bacteria microbiota from masau (Ziziphus mauritiana) fruits and their fermented fruit pulp in Zimbabwe. Int. J. Food Microbiol. 2007, 120, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Laaksonen, O.; Marsol-Vall, A.; Zhu, B.; Yang, B. Comparison of volatile composition between alcoholic bilberry beverages fermented with non-Saccharomyces yeasts and dynamic changes in volatile compounds during fermentation. J. Agric. Food Chem. 2020, 68, 3626–3637. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Polo, A.; Celano, G.; Martinovic, A.; Cavoski, I.; Gobbetti, M. Design of potential probiotic yeast starters tailored for making a cornelian cherry (Cornus mas L.) functional beverage. Int. J. Food Microbiol. 2020, 323, 108591. [Google Scholar] [CrossRef] [PubMed]
- Mukisa, I.M.; Byaruhanga, Y.B.; Muyanja, C.; Langsrud, T.; Narvhus, J.A. Production of organic flavor compounds by dominant lactic acid bacteria and yeasts from Obushera, a traditional sorghum malt fermented beverage. Food Sci. Nutr. 2017, 5, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.X.; Mutukumira, A.N. Isolation and characterisation of dominant acetic acid bacteria and yeast isolated from Kombucha samples at point of sale in New Zealand. Curr. Res. Food Sci. 2022, 5, 835–844. [Google Scholar] [CrossRef]
- Bai, M.; Qing, M.; Guo, Z.; Zhang, Y.; Chen, X.; Bao, Q.; Zhang, H.; Sun, T.S. Occurrence and dominance of yeast species in naturally fermented milk from the Tibetan Plateau of China. Can. J. Microbiol. 2010, 56, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Lore, T.A.; Mbugua, S.K.; Wangoh, J. Enumeration and identification of microflora in suusac, a Kenyan traditional fermented camel milk product. LWT 2005, 38, 125–130. [Google Scholar] [CrossRef]
- Abdelgadir, W.; Nielsen, D.S.; Hamad, S.; Jakobsen, M. A traditional Sudanese fermented camel’s milk product, Gariss, as a habitat of Streptococcus infantarius subsp. infantarius. Int. J. Food Microbiol. 2008, 127, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Akabanda, F.; Owusu-Kwarteng, J.; Tano-Debrah, K.; Glover, R.L.; Nielsen, D.S.; Jespersen, L. Taxonomic and molecular characterization of lactic acid bacteria and yeasts in nunu, a Ghanaian fermented milk product. Food Microbiol. 2013, 34, 277–283. [Google Scholar] [CrossRef]
- Srimahaeak, T.; Petersen, M.A.; Lillevang, S.K.; Jespersen, L.; Larsen, N. Spoilage potential of contaminating yeast species Kluyveromyces marxianus, Pichia kudriavzevii and Torulaspora delbrueckii during cold storage of skyr. Foods 2022, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Chapter 5-Yeast Spoilage of Foods and Beverages the Yeasts, 5th ed.; Elseviver: Amsterdam, The Netherlands, 2011; pp. 53–63. [Google Scholar]
- Chaves-Lopez, C.; Tofalo, R.; Serio, A.; Paparella, A.; Sacchetti, G.; Suzzi, G. Yeasts from Colombian Kumis as source of peptides with angiotensin I converting enzyme (ACE) inhibitory activity in milk. Int. J. Food Microbiol. 2012, 159, 39–46. [Google Scholar] [CrossRef]
- Chaves-Lopez, C.; Serio, A.; Paparella, A.; Martuscelli, M.; Corsetti, A.; Tofalo, R.; Suzzi, G. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiol. 2014, 42, 117–121. [Google Scholar] [CrossRef]
- Lavoie, K.; Touchette, M.; St-Gelais, D.; Labrie, S. Characterization of the fungal microflora in raw milk and specialty cheeses of the pro vince of Quebec. Dairy Sci. Technol. 2012, 92, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, K.; Shi, X.; Ni, Y.; Li, B.; Zhuge, B. Potential characterization of yeasts isolated from Kazak artisanal cheese to produce flavoring compounds. Microbiologyopen 2018, 7, e00533. [Google Scholar] [CrossRef] [PubMed]
- Merchan, A.V.; Ruiz-Moyano, S.; Vazquez Hernandez, M.; Benito, M.J.; Aranda, E.; Rodriguez, A.; Martin, A. Characterization of autochthonal yeasts isolated from Spanish soft raw ewe milk protected designation of origin cheeses for technological application. J. Dairy Sci. 2022, 105, 2931–2947. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, Y.; Li, J.; Shi, X.; Deng, L.; Wang, B. Evaluation of the effect of auxiliary starter yeasts with enzyme activities on Kazak cheese quality and flavor. Front. Microbiol. 2020, 11, 614208. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Yin, P.; Zhang, K.; Liu, Y.; Gao, Y.; Li, Y.; Wang, T.; Lu, S.; Li, B. Probiotic potential of gamma-aminobutyric acid (GABA)-producing yeast and its influence on the quality of cheese. J. Dairy Sci. 2021, 104, 6559–6576. [Google Scholar] [CrossRef]
- Helmy, E.A.; Soliman, S.A.; Abdel-Ghany, T.M.; Ganash, M. Evaluation of potentially probiotic attributes of certain dairy yeast isolated from buffalo sweetened Karish cheese. Heliyon 2019, 5, e01649. [Google Scholar] [CrossRef]
- Geronikou, A.; Larsen, N.; Lillevang, S.K.; Jespersen, L. Occurrence and identification of yeasts in production of white-brined cheese. Microorganisms 2022, 10, 1079. [Google Scholar] [CrossRef]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and control of spoilage fungi in dairy products: An update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef]
- Gilberto, V.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2015, 57, 2775–2788. [Google Scholar]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. The crucial role of yeasts in the wet fermentation of coffee beans and quality. Int. J. Food Microbiol. 2020, 333, 108796. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Magalhaes Junior, A.I.; Vasquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and biochemical performances of six yeast species as potential starter cultures for wet fermentation of coffee beans. LWT 2021, 137, 110430. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Frank, D.; Zhao, J. Microbiological and chemical characteristics of wet coffee fermentation inoculated with Hansinaspora uvarum and Pichia kudriavzevii and their impact on coffee sensory quality. Front. Microbiol. 2021, 12, 713969. [Google Scholar] [CrossRef]
- Krajangsang, S.; Seephin, P.; Tantayotai, P.; Mahingsapun, R.; Meeampun, Y.; Panyachanakul, T.; Samosorn, S.; Dolsophon, K.; Jiamjariyatam, R.; Lorliam, W.; et al. New approach for screening of microorganisms from Arabica coffee processing for their ability to improve Arabica coffee flavor. 3 Biotech 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.R.; Sneha, H.P.; Prakash, I.; Khan, M.; HN, P.K.; Om, H.; Basavaraj, K.; Murthy, P.S. Microbial ecology and functional coffee fermentation dynamics with Pichia kudriavzevii. Food Microbiol. 2022, 105, 104012. [Google Scholar]
- Figueroa-Hernandez, C.; Mota-Gutierrez, J.; Ferrocino, I.; Hernandez-Estrada, Z.J.; Gonzalez-Rios, O.; Cocolin, L.; Suarez-Quiroz, M.L. The challenges and perspectives of the selection of starter cultures for fermented cocoa beans. Int. J. Food Microbiol. 2019, 301, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Triboletti, S.; Alessandria, V.; Serio, A.; Sergi, M.; Paparella, A.; Rantsiou, K.; Chaves-Lopez, C. Functional biodiversity of yeasts isolated from Colombian fermented and dry cocoa beans. Microorganisms 2020, 8, 1086. [Google Scholar] [CrossRef]
- Viesser, J.A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Favero, G.R.; de Carvalho, J.C.; Goés-Neto, A.; Rogez, H.; Soccol, C.R. Global cocoa fermentation microbiome: Revealing new taxa and microbial functions by next generation sequencing technologies. World J. Microbiol. Biotechnol. 2021, 37, 118. [Google Scholar] [CrossRef]
- Samagaci, L.; Ouattara, H.; Niamké, S.; Lemaire, M. Pichia kudrazevii and Candida nitrativorans are the most well-adapted and relevant yeast species fermenting cocoa in Agneby-Tiassa, a local Ivorian cocoa producing region. Food Res. Int. 2016, 89, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Alvarez, J.P.; Neto, D.P.d.C.; Soccol, V.T.; Tanobe, V.O.A.; Rogez, H.; Góes-Neto, A.; Soccol, C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT 2017, 84, 290–297. [Google Scholar] [CrossRef]
- Chagas Junior, G.C.A.; Ferreira, N.R.; Gloria, M.B.A.; Martins, L.; Lopes, A.S. Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chem. 2021, 338, 127834. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Ospina, J.; Acquaticci, L.; Molina-Hernandez, J.B.; Rantsiou, K.; Martuscelli, M.; Kamgang-Nzekoue, A.F.; Vittori, S.; Paparella, A.; Chaves-Lopez, C. Exploring the capability of yeasts isolated from Colombian fermented cocoa beans to form and degrade biogenic amines in a lab-scale model system for cocoa fermentation. Microorganisms 2020, 9, 28. [Google Scholar] [CrossRef]
- Moon, S.H.; Chang, M.; Kim, H.Y.; Chang, H.C. Pichia kudriavzevii is the major yeast involved in film-formation, off-odor production, and texture-softening in over-ripened Kimchi. Food Sci. Biotechnol. 2014, 23, 489–497. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, J.Y.; Oh, Y.J.; Ko, H.I.; Roh, S.W.; Hong, S.W.; Kwon, H.C.; Han, S.G.; Kim, T.W. Safety assessment of white colony-forming yeasts in kimchi. Food Microbiol. 2022, 106, 104057. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Sanni, A.I.; Agrawal, R. Probiotic potentials of yeasts isolated from some cereal-based Nigerian traditional fermented food products. J. Appl. Microbiol. 2015, 119, 797–808. [Google Scholar] [CrossRef]
- Hjortmo, S.B.; Hellstrom, A.M.; Andlid, T.A. Production of folates by yeasts in Tanzanian fermented togwa. FEMS Yeast Res. 2008, 8, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Greppi, A.; Saubade, F.; Botta, C.; Humblot, C.; Guyot, J.P.; Cocolin, L. Potential probiotic Pichia kudriavzevii strains and their ability to enhance folate content of traditional cereal-based African fermented food. Food Microbiol. 2017, 62, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, O.R.; Agrawal, R.; Sanni, A.I. Development of cereal-based functional food using cereal-mix substrate fermented with probiotic strain—Pichia kudriavzevii OG. Food Sci. Nutr. 2015, 3, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Ogunremi, O.R.; Agrawal, R.; Sanni, A. Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. J. Genet. Eng. Biotechnol. 2020, 18, 16. [Google Scholar] [CrossRef]
- Fernandes, T.; Carvalho, B.F.; Mantovani, H.C.; Schwan, R.F.; Avila, C.L.S. Identification and characterization of yeasts from bovine rumen for potential use as probiotics. J. Appl. Microbiol. 2019, 127, 845–855. [Google Scholar] [CrossRef]
- Suntara, C.; Cherdthong, A.; Wanapat, M.; Uriyapongson, S.; Leelavatcharamas, V.; Sawaengkaew, J.; Chanjula, P.; Foiklang, S. Isolation and characterization of yeasts from rumen fluids for potential use as additives in ruminant feeding. Vet. Sci. 2021, 8, 52. [Google Scholar] [CrossRef]
- Santos, M.C.; Lock, A.L.; Mechor, G.D.; Kung, L., Jr. Effects of a spoilage yeast from silage on in vitro ruminal fermentation. J. Dairy Airy Sci. 2015, 98, 2603–2610. [Google Scholar] [CrossRef]
- Suntara, C.; Cherdthong, A.; Uriyapongson, S.; Wanapat, M.; Chanjula, P. Comparison effects of ruminal crabtree-negative yeasts and crabtree-positive yeasts for improving ensiled rice straw quality and ruminal digestion using in vitro gas production. J. Fungi 2020, 6, 109. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, X.; Liu, Z.; Wang, W.; Yan, H.; Liu, X. Effects of bovine Pichia kudriavzevii T7, Candida glabrata B14, and Lactobacillus plantarum Y9 on milk production, quality and digestive tract microbiome in dairy cows. Microorganisms 2022, 10, 842. [Google Scholar] [CrossRef]
- Intanoo, M.; Kongkeitkajorn, M.B.; Pattarajinda, V.; Bernard, J.K.; Callaway, T.R.; Suriyasathaporn, W.; Phasuk, Y. Isolation and screening of aflatoxin-detoxifying yeast and bacteria from ruminal fluids to reduce aflatoxin B1 contamination in dairy cattle feed. J. Appl. Microbiol. 2018, 125, 1603–1613. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Rodriguez, M.C.; Poloni, V.L.; Rojo, M.C.; Combina, M.; Chiacchiera, S.M.; Dalcero, A.M.; Cavaglieri, L.R. Novel yeast isolated from broilers’ feedstuff, gut and faeces as aflatoxin B1 adsorbents. J. Appl. Microbiol. 2016, 121, 1766–1776. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Rodriguez, M.C.; Gonzalez Pereyra, M.L.; Poloni, V.L.; Peralta, M.F.; Nilson, A.J.; Miazzo, R.D.; Bagnis, G.; Chiacchiera, S.M.; Cavaglieri, L.R. Use of yeast (Pichia kudriavzevii) as a novel feed additive to ameliorate the effects of aflatoxin B1 on broiler chicken performance. Mycotoxin Res. 2017, 33, 273–283. [Google Scholar] [CrossRef]
- Yuan, S.F.; Guo, G.L.; Hwang, W.S. Ethanol production from dilute-acid steam exploded lignocellulosic feedstocks using an isolated multistress-tolerant Pichia kudriavzevii strain. Microb. Biotechnol. 2017, 10, 1581–1590. [Google Scholar] [CrossRef]
- Park, H.J.; Bae, J.H.; Ko, H.J.; Lee, S.H.; Sung, B.H.; Han, J.I.; Sohn, J.H. Low-pH production of d-lactic acid using newly isolated acid tolerant yeast Pichia kudriavzevii NG. Biotechnol. Bioeng. 2018, 115, 2232–2242. [Google Scholar] [CrossRef]
- Baptista, M.; Domingues, L. Kluyveromyces marxianus as a microbial cell factory for lignocellulosic biomass valorisation. Biotechnol. Adv. 2022, 60, 108027. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Kar Fai, C.; Mohamed, M.S.; Abdul Rahman, N.; Halim, M. Production of single cell oil by Yarrowia lipolytica JCM 2320 using detoxified desiccated coconut residue hydrolysate. Peer J. 2022, 10, e12833. [Google Scholar] [CrossRef] [PubMed]
- Konzock, O.; Zaghen, S.; Norbeck, J. Tolerance of Yarrowia lipolytica to inhibitors commonly found in lignocellulosic hydrolysates. BMC Microbiol. 2021, 21, 77. [Google Scholar] [CrossRef]
- Lin, X.; Qi, Y.; Yan, D.; Liu, H.; Chen, X.; Liu, L. CgMED3 changes membrane sterol composition to help Candida glabrata tolerate low-pH stress. Appl. Environ. Microbiol. 2017, 83, e00972-17. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Stratford, M.; Steels, H.; Fernandez-Espinar, M.T.; Querol, A. Physiological characterization of spoilage strains of Zygosaccharomyces bailii and Zygosaccharomyces rouxii isolated from high sugar environments. Int. J. Food Microbiol. 2007, 114, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Babbar, N.; Sandhu, S.K.; Dhaliwal, S.S.; Kaur, U.; Chadha, B.S.; Bhargav, V.K. Ethanol production from alkali-treated rice straw via simultaneous saccharification and fermentation using newly isolated thermotolerant Pichia kudriavzevii HOP-1. J. Ind. Microbiol. Biotechnol. 2012, 39, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Park, E.H.; Kim, M.D. Isolation of thermotolerant yeast Pichia kudriavzevii from nuruk. Food Sci. Biotechnol. 2017, 26, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Lee, H.J.; Lee, J.E.; Kim, S.; Lee, D.Y.; Kim, K.H.; Park, Y.C. Physiological and metabolomic analysis of Issatchenkia orientalis MTY1 with multiple tolerance for cellulosic bioethanol production. Biotechnol. J. 2017, 12, 1700110. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, Q.; Wang, Y.; Yang, X.; Chen, S.; Zhao, Y.; Wu, Y.; Li, L. Salt stress improves thermotolerance and high-temperature bioethanol production of multi-stress-tolerant Pichia kudriavzevii by stimulating intracellular metabolism and inhibiting oxidative damage. Biotechnol. Biofuels 2021, 14, 222. [Google Scholar] [CrossRef]
- Isono, N.; Hayakawa, H.; Usami, A.; Mishima, T.; Hisamatsu, M. A comparative study of ethanol production by Issatchenkia orientalis strains under stress conditions. J. Biosci. Bioeng 2012, 113, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Techaparin, A.; Thanonkeo, P.; Klanrit, P. High-temperature ethanol production using thermotolerant yeast newly isolated from Greater Mekong Subregion. Braz. J. Microbiol. 2017, 48, 461–475. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Oberoi, H.S.; Sandhu, S.K.; Nanda, D.; Kumar, D.; Uppal, S.K. Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresour. Technol. 2011, 102, 5968–5975. [Google Scholar] [CrossRef]
- Zwirzitz, A.; Alteio, L.; Sulzenbacher, D.; Atanasoff, M.; Selg, M. Ethanol production from wheat straw hydrolysate by Issatchenkia orientalis isolated from waste cooking oil. J. Fungi 2021, 7, 121. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Ma, A.Z.; Li, Q.; Wang, F.; Zhuang, G.Q.; Liu, C.Z. Effect of lignocellulosic inhibitory compounds on growth and ethanol fermentation of newly-isolated thermotolerant Issatchenkia orientalis. Bioresour. Technol. 2011, 102, 8099–8104. [Google Scholar] [CrossRef]
- Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production. Braz J. Microbiol. 2018, 49, 378–391. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Wang, F.; Liu, C.Z. Deep-bed solid state fermentation of sweet sorghum stalk to ethanol by thermotolerant Issatchenkia orientalis IPE 100. Bioresour. Technol. 2011, 102, 11262–11265. [Google Scholar] [CrossRef]
- Diaz-Nava, L.E.; Montes-Garcia, N.; Dominguez, J.M.; Aguilar-Uscanga, M.G. Effect of carbon sources on the growth and ethanol production of native yeast Pichia kudriavzevii ITV-S42 isolated from sweet sorghum juice. Bioprocess. Biosyst. Eng. 2017, 40, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Lavudi, S.; Oberoi, H.S.; Mangamoori, L.N. Ethanol production from sweet sorghum bagasse through process optimization using response surface methodology. 3 Biotech 2017, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Sarabia, V.L.; Ballinas-Cesatti, C.B.; Melgar-Lalanne, G.; Cristiani-Urbina, E.; Morales-Barrera, L. Isolation, identification, and kinetic and thermodynamic characterization of a Pichia kudriavzevii yeast strain capable of fermentation. Food Bioprod. Process. 2022, 131, 109–124. [Google Scholar] [CrossRef]
- Murata, Y.; Nwuche, C.O.; Nweze, J.E.; Ndubuisi, I.A.; Ogbonna, J.C. Potentials of multi-stress tolerant yeasts, Saccharomyces cerevisiae and Pichia kudriavzevii for fuel ethanol production from industrial cassava wastes. Process. Biochem. 2021, 111, 305–314. [Google Scholar] [CrossRef]
- Patsalou, M.; Samanides, C.G.; Protopapa, E.; Stavrinou, S.; Vyrides, I.; Koutinas, M. A citrus peel waste biorefinery for ethanol and methane production. Molecules 2019, 24, 2451. [Google Scholar] [CrossRef]
- Akita, H.; Goshima, T.; Suzuki, T.; Itoiri, Y.; Kimura, Z.-i.; Matsushika, A. Application of Pichia kudriavzevii NBRC1279 and NBRC1664 to Simultaneous Saccharification and Fermentation for Bioethanol Production. Fermentation 2021, 7, 83. [Google Scholar] [CrossRef]
- Yuangsaard, N.; Yongmanitchai, W.; Yamada, M.; Limtong, S. Selection and characterization of a newly isolated thermotolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Antonie Van Leeuwenhoek 2013, 103, 577–588. [Google Scholar] [CrossRef]
- Nweze, J.E.; Ndubuisi, I.; Murata, Y.; Omae, H.; Ogbonna, J.C. Isolation and evaluation of xylose-fermenting thermotolerant yeasts for bioethanol production. Biofuels 2019, 12, 961–970. [Google Scholar] [CrossRef]
- Sunkar, B.; Bhukya, B. Bi-phasic hydrolysis of corncobs for the extraction of total sugars and ethanol production using inhibitor resistant and thermotolerant yeast, Pichia kudriavzevii. Biomass Bioenerg. 2021, 153, 106230. [Google Scholar] [CrossRef]
- Xi, Y.; Zhan, T.; Xu, H.; Chen, J.; Bi, C.; Fan, F.; Zhang, X. Characterization of JEN family carboxylate transporters from the acid-tolerant yeast Pichia kudriavzevii and their applications in succinic acid production. Microb Biotechnol. 2021, 14, 1130–1147. [Google Scholar] [CrossRef]
- Rush, B.J.; Fosmer, A.M. Method for Succinate Production. WO2013/112939A3, 2 October 2014. [Google Scholar]
- Suominen, P.; Aristidou, A.; Penttila, M.; Ilmen, M.; Ruohonen, L.; Koivuranta, K.; Roberg-Perez, K. Genetically Modified Yeast of the Species Issatchenkia orientalis and Closely Related Species, and Fermentation Processes Using Same. U.S. Patent 2009/0226989 A1, 10 September 2009. [Google Scholar]
- Baek, S.H.; Kwon, E.Y.; Kim, Y.H.; Hahn, J.S. Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2737–2748. [Google Scholar] [CrossRef] [PubMed]
- Toivari, M.; Vehkomaki, M.L.; Nygard, Y.; Penttila, M.; Ruohonen, L.; Wiebe, M.G. Low pH D-xylonate production with Pichia kudriavzevii. Bioresour. Technol. 2013, 133, 555–562. [Google Scholar] [CrossRef]
- Ji, H.; Xu, K.; Dong, X.; Sun, D.; Jin, L. Sequential Production of ᴅ-xylonate and Ethanol from Non-Detoxified Corncob at Low-pH by Pichia kudriavzevii via a Two-Stage Fermentation Strategy. J. Fungi 2021, 7, 1038. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Vila-Santa, A.; Liu, N.; Prozorov, T.; Xie, D.; Faria, N.T.; Ferreira, F.C.; Mira, N.P.; Shao, Z. Metabolic engineering of an acid-tolerant yeast strain Pichia kudriavzevii for itaconic acid production. Metab. Eng. Commun. 2020, 10, e00124. [Google Scholar] [CrossRef]
- Xiao, H.; Shao, Z.; Jiang, Y.; Dole, S.; Zhao, H. Exploiting Issatchenkia orientalis SD108 for succinic acid production. Microb. Cell. Fact. 2014, 13, 121. [Google Scholar] [CrossRef]
- Finley, K.R.; Huryta, J.M.; Mastel, B.M.; Mcmullin, T.W.; Poynter, G.M.; Rush, B.J.; Watts, K.T.; Fosmer, A.M.; McIntosh, V.L., Jr.; Brady, K.M. Compositions and methods for succinate production. US20130302866A1, 14 November 2013. [Google Scholar]
- Zhuge, J.; Fang, H.Y.; Wang, Z.X.; Chen, D.Z.; Jin, H.R.; Gu, H.L. Glycerol production by a novel osmotolerant yeast Candida glycerinogenes. Appl. Microbiol. Biot. 2001, 55, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Xie, D. Improvement of glycerol production by Candida krusei in batch and continuous cultures using corn steep liquor. Biotechnol. Lett. 2002, 24, 1539–1542. [Google Scholar] [CrossRef]
- Chen, G.; Yao, S.; Guan, Y. Influence of osmoregulators on osmotolerant yeast Candida krusei for the production of glycerol. Chin. J. Chem. Eng. 2006, 14, 371–376. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Su, Q.; Xie, D. Glycerol production by Candida krusei employing NaCl as an osmoregulator in batch and continuous fermentations. Biotechnol. Lett. 2002, 24, 1137–1140. [Google Scholar] [CrossRef]
- Fan, G.; Cheng, L.; Fu, Z.; Sun, B.; Teng, C.; Jiang, X.; Li, X. Screening of yeasts isolated from Baijiu environments for 2-phenylethanol production and optimization of production conditions. 3 Biotech 2020, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Avila, O.; Munoz-Torrero, P.; Sanchez, A.; Font, X.; Barrena, R. Valorization of agro-industrial wastes by producing 2-phenylethanol via solid-state fermentation: Influence of substrate selection on the process. Waste Manag. 2021, 121, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-phenylethanol (rose aroma) production potential of an isolated Pichia kudriavzevii through solid-state fermentation. Process. Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Sankh, S.; Thiru, M.; Saran, S.; Rangaswamy, V. Biodiesel production from a newly isolated Pichia kudriavzevii strain. Fuel 2013, 106, 690–696. [Google Scholar] [CrossRef]
- Xue, S.J.; Chi, Z.; Zhang, Y.; Li, Y.F.; Liu, G.L.; Jiang, H.; Hu, Z.; Chi, Z.M. Fatty acids from oleaginous yeasts and yeast-like fungi and their potential applications. Crit. Rev. Biotechnol. 2018, 38, 1049–1060. [Google Scholar] [CrossRef]
- Diwan, B.; Gupta, P. Comprehending the influence of critical cultivation parameters on the oleaginous behaviour of potent rotten fruit yeast isolates. J. Appl. Microbiol. 2018, 125, 490–505. [Google Scholar] [CrossRef]
- Bettencourt, S.; Miranda, C.; Pozdniakova, T.A.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Single cell oil production by oleaginous yeasts grown in synthetic and waste-derived volatile fatty acids. Microorganisms 2020, 8, 1809. [Google Scholar] [CrossRef]
- Ubeda, J.F.; Maldonado, M.; Briones, A.I.; Francisco, J.F. Bio-prospecting of distillery yeasts as bio-control and bio-remediation agents. Curr. Microbiol. 2014, 68, 594–602. [Google Scholar] [CrossRef]
- Li, C.; Yu, J.; Wang, D.; Li, L.; Yang, X.; Ma, H.; Xu, Y. Efficient removal of zinc by multi-stress-tolerant yeast Pichia kudriavzevii A16. Bioresour. Technol. 2016, 206, 43–49. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Li, L.; Yang, X.; Wang, Y. Acid stress induces cross-protection for cadmium tolerance of multi-stress-tolerant Pichia kudriavzevii by regulating cadmium transport and antioxidant defense system. J. Hazard. Mater. 2019, 366, 151–159. [Google Scholar] [CrossRef]
- Mesquita, V.A.; Silva, C.F.; Soares, E.V. Toxicity induced by a metal mixture (Cd, Pb and Zn) in the yeast Pichia kudriavzevii: The role of oxidative stress. Curr. Microbiol. 2016, 72, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Tondee, T.; Sirianuntapiboon, S.; Ohmomo, S. Decolorization of molasses wastewater by yeast strain, Issatchenkia orientalis No. SF9-246. Bioresour. Technol. 2008, 99, 5511–5519. [Google Scholar] [CrossRef] [PubMed]
- Pajot, H.F.; Delgado, O.D.; de Figueroa, L.I.; Farina, J.I. Unraveling the decolourizing ability of yeast isolates from dye-polluted and virgin environments: An ecological and taxonomical overview. Antonie Van Leeuwenhoek 2011, 99, 443–456. [Google Scholar] [CrossRef]
- Rosu, C.M.; Vochita, G.; Mihasan, M.; Avadanei, M.; Mihai, C.T.; Gherghel, D. Performances of Pichia kudriavzevii in decolorization, biodegradation, and detoxification of C.I. Basic Blue 41 under optimized cultural conditions. Environ. Sci. Pollut. Res. Int. 2019, 26, 431–445. [Google Scholar] [CrossRef]
- Lin, H.; Ye, J.; Sun, W.; Yu, Q.; Wang, Q.; Zou, P.; Chen, Z.; Ma, J.; Wang, F.; Ma, J. Solar composting greenhouse for organic waste treatment in fed-batch mode: Physicochemical and microbiological dynamics. Waste Mana.g 2020, 113, 1–11. [Google Scholar] [CrossRef]
- Nakasaki, K.; Araya, S.; Mimoto, H. Inoculation of Pichia kudriavzevii RB1 degrades the organic acids present in raw compost material and accelerates composting. Bioresour. Technol. 2013, 144, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Nakasaki, K.; Hirai, H. Temperature control strategy to enhance the activity of yeast inoculated into compost raw material for accelerated composting. Waste Manag. 2017, 65, 29–36. [Google Scholar] [CrossRef]
- Bleve, G.; Grieco, F.; Cozzi, G.; Logrieco, A.; Visconti, A. Isolation of epiphytic yeasts with potential for biocontrol of Aspergillus carbonarius and A. niger on grape. Int. J. Food Microbiol. 2006, 108, 204–209. [Google Scholar] [CrossRef]
- Madbouly, A.K.; Abo Elyousr, K.A.M.; Ismail, I.M. Biocontrol of Monilinia fructigena, causal agent of brown rot of apple fruit, by using endophytic yeasts. Biol. Control 2020, 144, 104239. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Raina, S.; Singh, S. Killer toxin from a novel killer yeast Pichia kudriavzevii RY55 with idiosyncratic antibacterial activity. J. Basic Microbiol. 2013, 53, 645–656. [Google Scholar] [CrossRef]
- Delali, K.I.; Chen, O.; Wang, W.J.; Yi, L.H.; Deng, L.L.; Zeng, K.F. Evaluation of yeast isolates from kimchi with antagonistic activity against green mold in citrus and elucidating the action mechanisms of three yeast: P. kudriavzevii, K. marxianus, and Y. lipolytica. Postharvest Biol. Technol. 2021, 176. [Google Scholar] [CrossRef]
- Chi, M.; Li, G.; Liu, Y.; Liu, G.; Li, M.; Zhang, X.; Sun, Z.; Sui, Y.; Liu, J. Increase in antioxidant enzyme activity, stress tolerance and biocontrol efficacy of Pichia kudriavzevii with the transition from a yeast-like to biofilm morphology. Biol. Control. 2015, 90, 113–119. [Google Scholar] [CrossRef]
- Choinska, R.; Piasecka-Jozwiak, K.; Chablowska, B.; Dumka, J.; Lukaszewicz, A. Biocontrol ability and volatile organic compounds production as a putative mode of action of yeast strains isolated from organic grapes and rye grains. Antonie Van Leeuwenhoek 2020, 113, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Yang, H.; Li, L.; Jiang, Y.; Li, Y.; Zheng, J. Pichia kudriavzevii retards fungal decay by influencing the fungal community succession during cherry tomato fruit storage. Food Microbiol. 2020, 88, 103404. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.Y.; Wang, Z.W.Z.; Zhang, X.Q.; Wei, Z.; Zhang, X.; Li, L.; Fu, Y.N.; Gao, J.H.; Zhao, X.; Guo, J.; et al. Enhancement of biocontrol efficacy of Pichia kudriavzevii induced by Ca Ascorbate against Botrytis cinerea in cherry tomato fruit and the possible mechanisms of action. Microbiol. Spectr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY antifungal surveillance program: Results for Candida species from 1997–2016. Open Forum. Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef]
- Nagarathnamma, T.; Chunchanur, S.K.; Rudramurthy, S.M.; Vineetha, K.R.; Ramamurthy, K.; Joseph, J.; Ambica, R. Outbreak of Pichia kudriavzevii fungaemia in a neonatal intensive care unit. J. Med. Microbiol. 2017, 66, 1759–1764. [Google Scholar] [CrossRef]
- Aslani, N.; Janbabaei, G.; Abastabar, M.; Meis, J.F.; Babaeian, M.; Khodavaisy, S.; Boekhout, T.; Badali, H. Identification of uncommon oral yeasts from cancer patients by MALDI-TOF mass spectrometry. BMC Infect. Dis. 2018, 18, 24. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Desnos-Ollivier, M.; Bretagne, S.; Dromer, F.; French Mycoses Study, G. The risk and clinical outcome of candidemia depending on underlying malignancy. Intensive Care Med. 2017, 43, 652–662. [Google Scholar] [CrossRef]
- Kaur, H.; Shankarnarayana, S.A.; Hallur, V.; Muralidharan, J.; Biswal, M.; Ghosh, A.K.; Ray, P.; Chakrabarti, A.; Rudramurthy, S.M. Prolonged outbreak of Candida krusei candidemia in paediatric ward of tertiary care hospital. Mycopathologia 2020, 185, 257–268. [Google Scholar] [CrossRef]
- Salehi, M.; Ahmadikia, K.; Mahmoudi, S.; Kalantari, S.; Jamalimoghadamsiahkali, S.; Izadi, A.; Kord, M.; Dehghan Manshadi, S.A.; Seifi, A.; Ghiasvand, F.; et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020, 63, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Angebault, C.; Djossou, F.; Abelanet, S.; Permal, E.; Ben Soltana, M.; Diancourt, L.; Bouchier, C.; Woerther, P.L.; Catzeflis, F.; Andremont, A.; et al. Candida albicans is not always the preferential yeast colonizing humans: A study in Wayampi Amerindians. J. Infect. Dis. 2013, 208, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Domingues Bianchin, M.; Borowicz, S.M.; da Rosa Monte Machado, G.; Pippi, B.; Staniscuaski Guterres, S.; Raffin Pohlmann, A.; Meneghello Fuentefria, A.; Clemes Kulkamp-Guerreiro, I. Lipid core nanoparticles as a broad strategy to reverse fluconazole resistance in multiple Candida species. Colloids Surf. B Biointerfaces 2019, 175, 523–529. [Google Scholar] [CrossRef]
- Patriota, L.L.S.; Procópio, T.F.; de Souza, M.F.D.; de Oliveira, A.P.S.; Carvalho, L.V.N.; Pitta, M.G.R.; Rego, M.J.B.M.; Paiva, P.M.G.; Pontual, E.V.; Napoleão, T.H. A Trypsin inhibitor from Tecoma stans Leaves inhibits growth and promotes ATP depletion and lipid peroxidation in Candida albicans and Candida krusei. Front. Microbiol. 2016, 7, 611. [Google Scholar] [CrossRef] [PubMed]
- International Dairy Federation. Inventory of microbial food cultures with safety demonstration in fermented food products (Bulletin of the IDF n514/2022). Int. J. Food Microbiol. 2022, 154, 87. [Google Scholar] [CrossRef]
- Cao, M.; Fatma, Z.; Song, X.; Hsieh, P.H.; Tran, V.G.; Lyon, W.L.; Sayadi, M.; Shao, Z.; Yoshikuni, Y.; Zhao, H. A genetic toolbox for metabolic engineering of Issatchenkia orientalis. Metab. Eng. 2020, 59, 87–97. [Google Scholar] [CrossRef]
- Tran, V.G.; Cao, M.; Fatma, Z.; Song, X.; Zhao, H. Development of a CRISPR/Cas9-based tool for gene deletion in Issatchenkia orientalis. mSphere 2019, 4, e00345-19. [Google Scholar] [CrossRef]
| Non-Conventional Yeasts | Glucose (w/v) | Ethanol (v/v) | Temperature (°C) | Acetic Acid (g/L) | Furfural (g/L) | 5-HMF (g/L) | Low-pH | Reference |
|---|---|---|---|---|---|---|---|---|
| Pichia kudriavzevii | 48% | 13% | 50 | 18 | 5 | 7 g/L | 1.5 | [4,17,84,85] |
| Kluyveromyces lactis | 48% | 10% | 39 | 4 | - | 4 g/L | - | [4] |
| Kluyveromyces marxianus | 40% | 7% | 52 | 15 | 3 | 7 g/L | 2.5 | [4,17,86] |
| Yarrowia lipolytica | 50% | 5% | 30 | 5 | 2.9 | 5 g/L | - | [4,87,88] |
| Candida glabrata | 50% | 11% | 41 | - | - | 7 g/L | 2.0 | [4,89] |
| Zygosaccharomyces rouxii | 70% | 13 % | 30 | - | - | 5 g/L | 2.2 | [4,90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Li, M.; Jin, J.; Dong, X.; Xu, K.; Jin, L.; Qiao, Y.; Ji, H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. J. Fungi 2023, 9, 170. https://doi.org/10.3390/jof9020170
Chu Y, Li M, Jin J, Dong X, Xu K, Jin L, Qiao Y, Ji H. Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. Journal of Fungi. 2023; 9(2):170. https://doi.org/10.3390/jof9020170
Chicago/Turabian StyleChu, Yunfei, Mengmeng Li, Jiahui Jin, Xiameng Dong, Ke Xu, Libo Jin, Yanming Qiao, and Hao Ji. 2023. "Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries" Journal of Fungi 9, no. 2: 170. https://doi.org/10.3390/jof9020170
APA StyleChu, Y., Li, M., Jin, J., Dong, X., Xu, K., Jin, L., Qiao, Y., & Ji, H. (2023). Advances in the Application of the Non-Conventional Yeast Pichia kudriavzevii in Food and Biotechnology Industries. Journal of Fungi, 9(2), 170. https://doi.org/10.3390/jof9020170






