Comparative Study of the Co-Occurring Alternaria and Colletotrichum Species in the Production of Citrus Leaf Spot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Collection
2.2. Fungal Isolation and Identification
2.3. DNA Extraction and Amplicon Sequencing
2.4. Fungal Inoculation
2.5. RNA Extraction and Transcriptional Sequencing
2.6. Statistical Analysis
3. Results
3.1. The Alternaria and Colletotrichum Isolates from Citrus Leaves with ABS
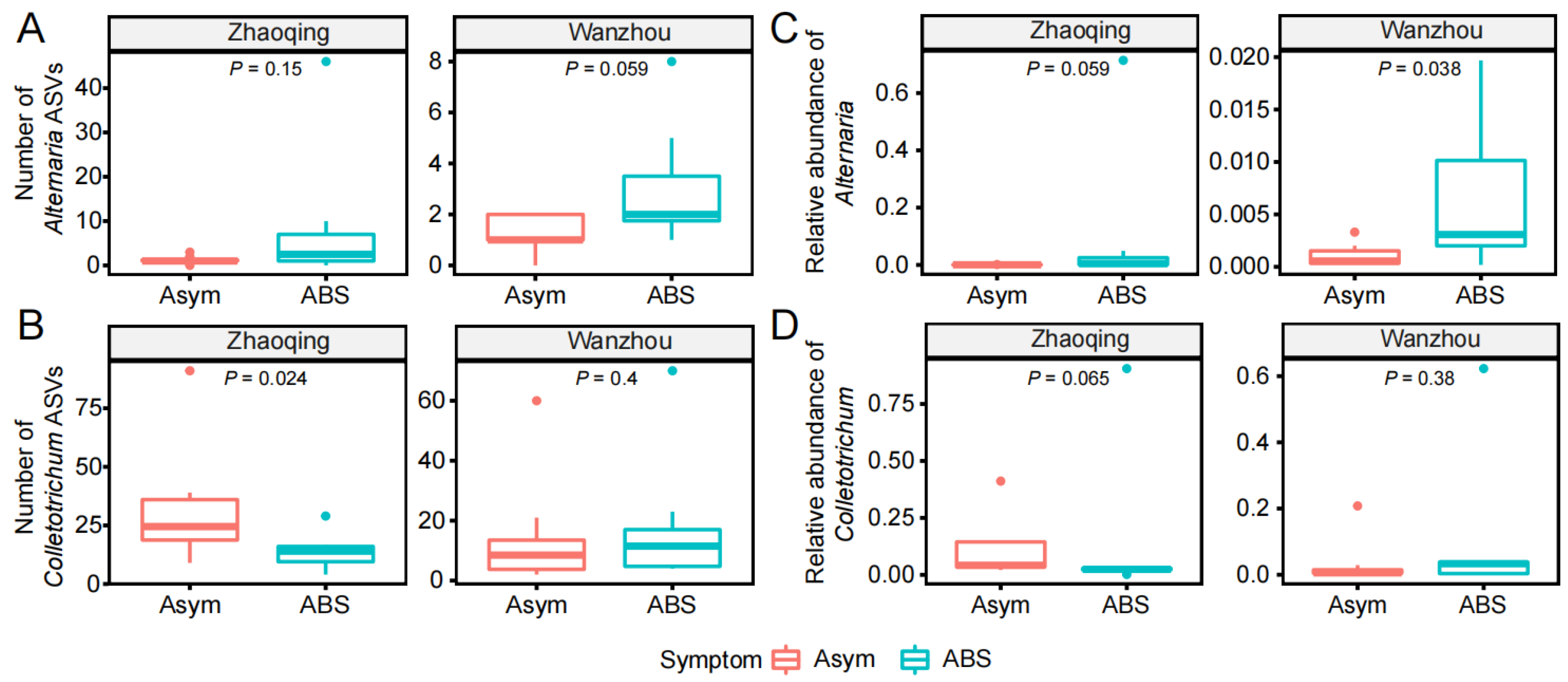
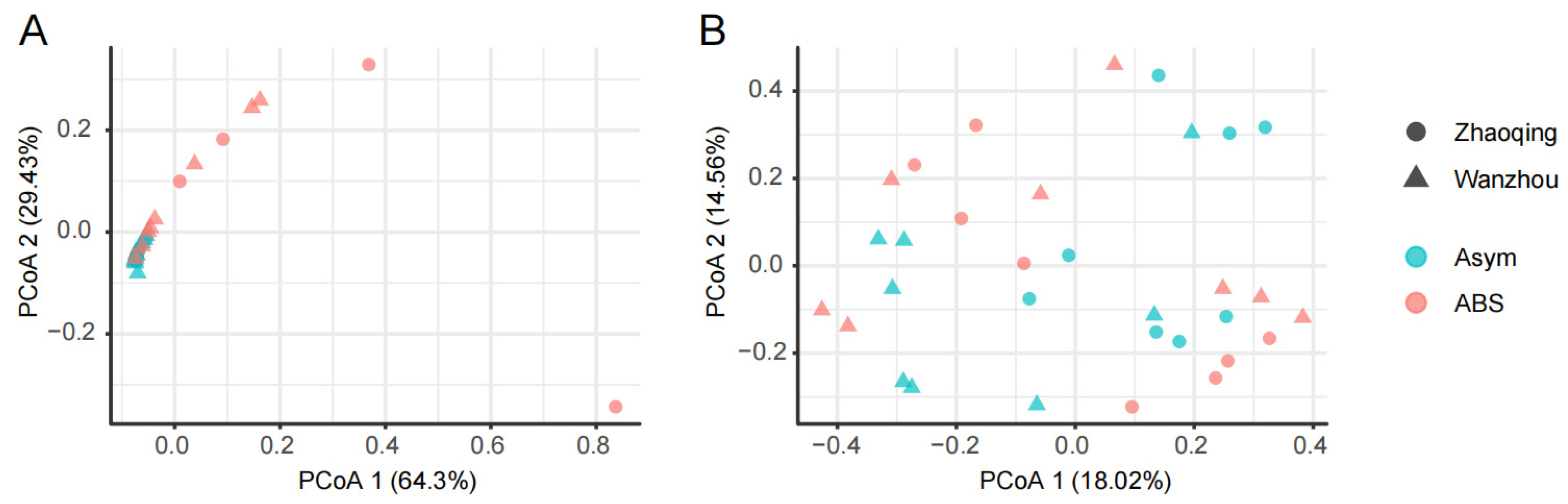
3.2. The Alternaria and Colletotrichum Species in the Fungal Communities of Citrus Leaves without or with ABS
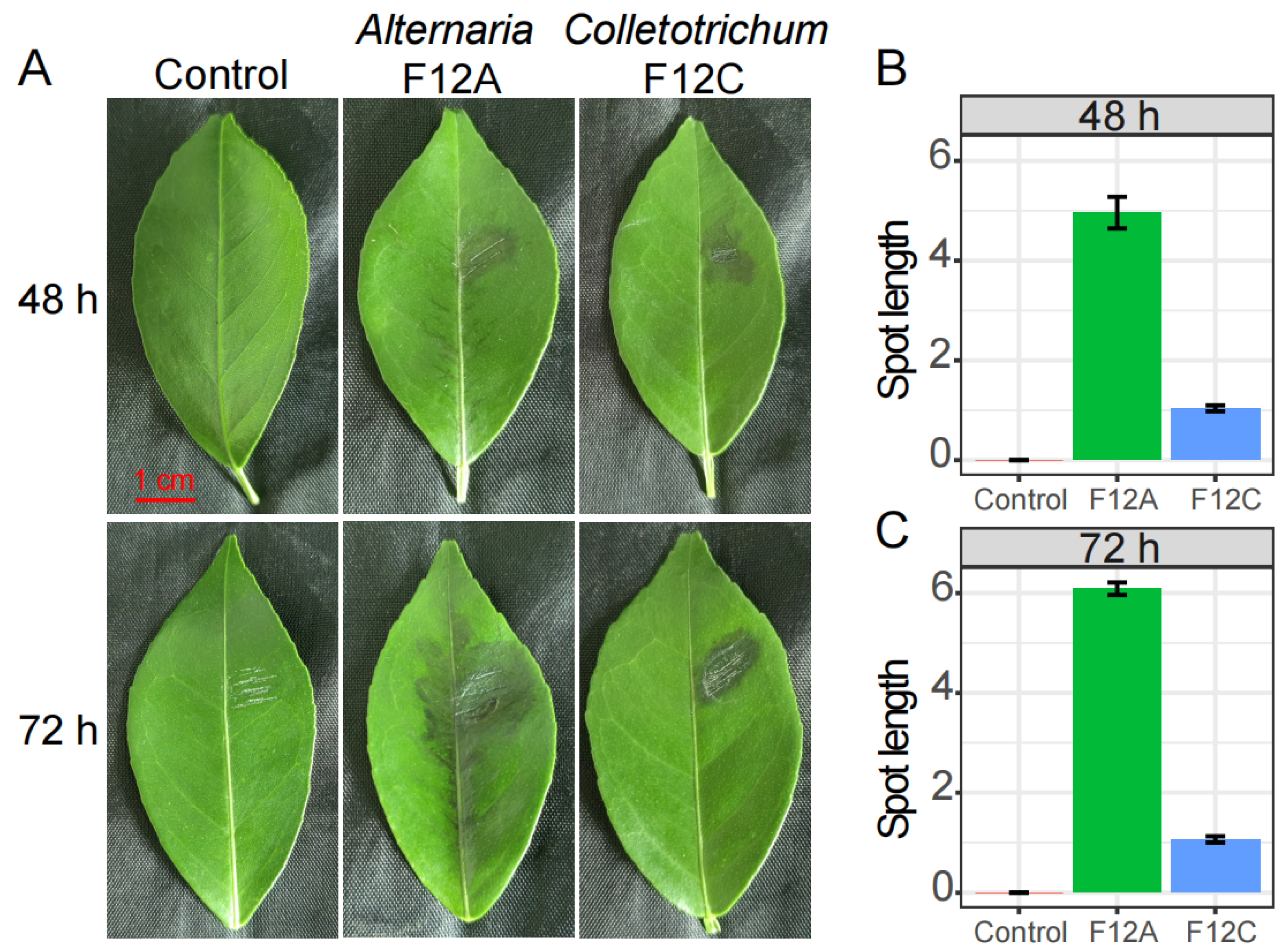
3.3. Host Response to the Infection by Alternaria and Colletotrichum Species
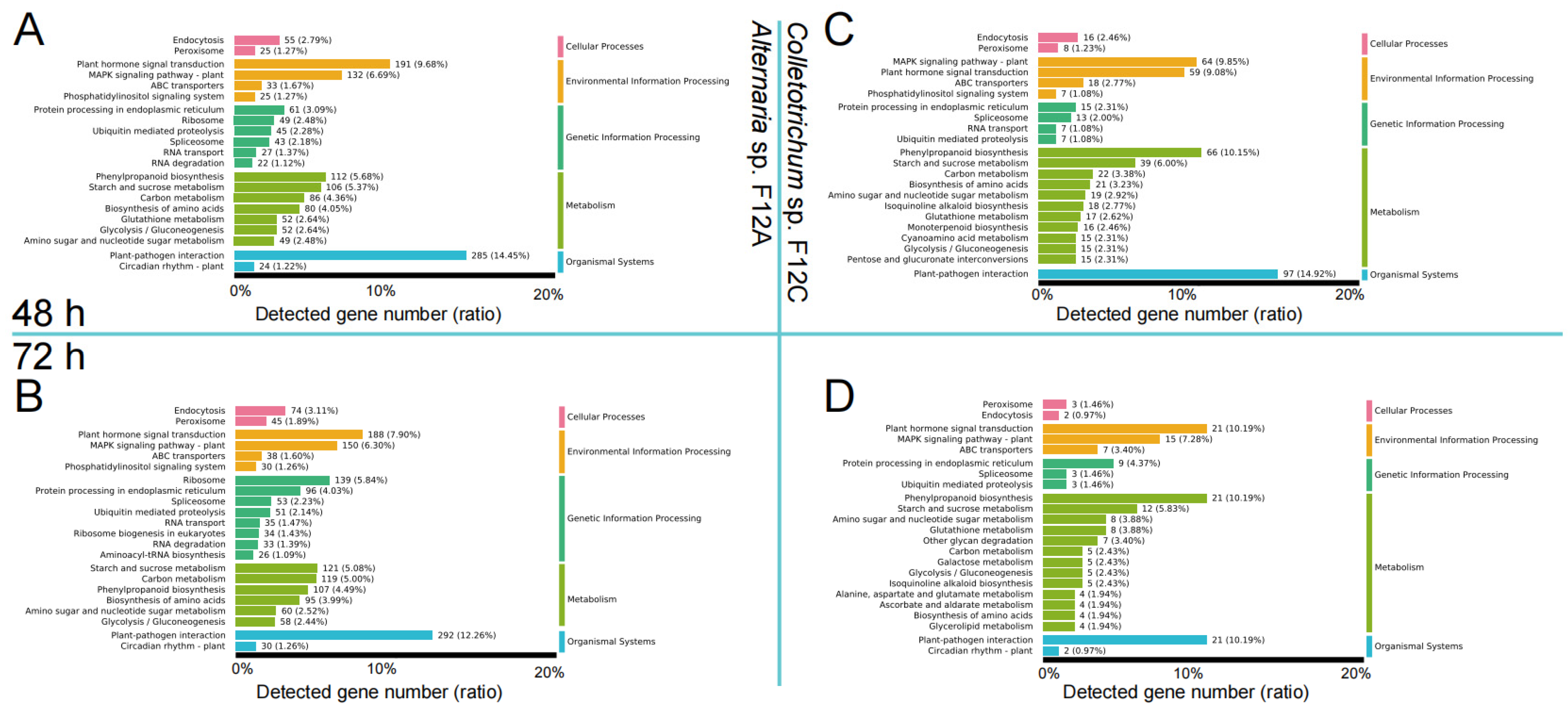
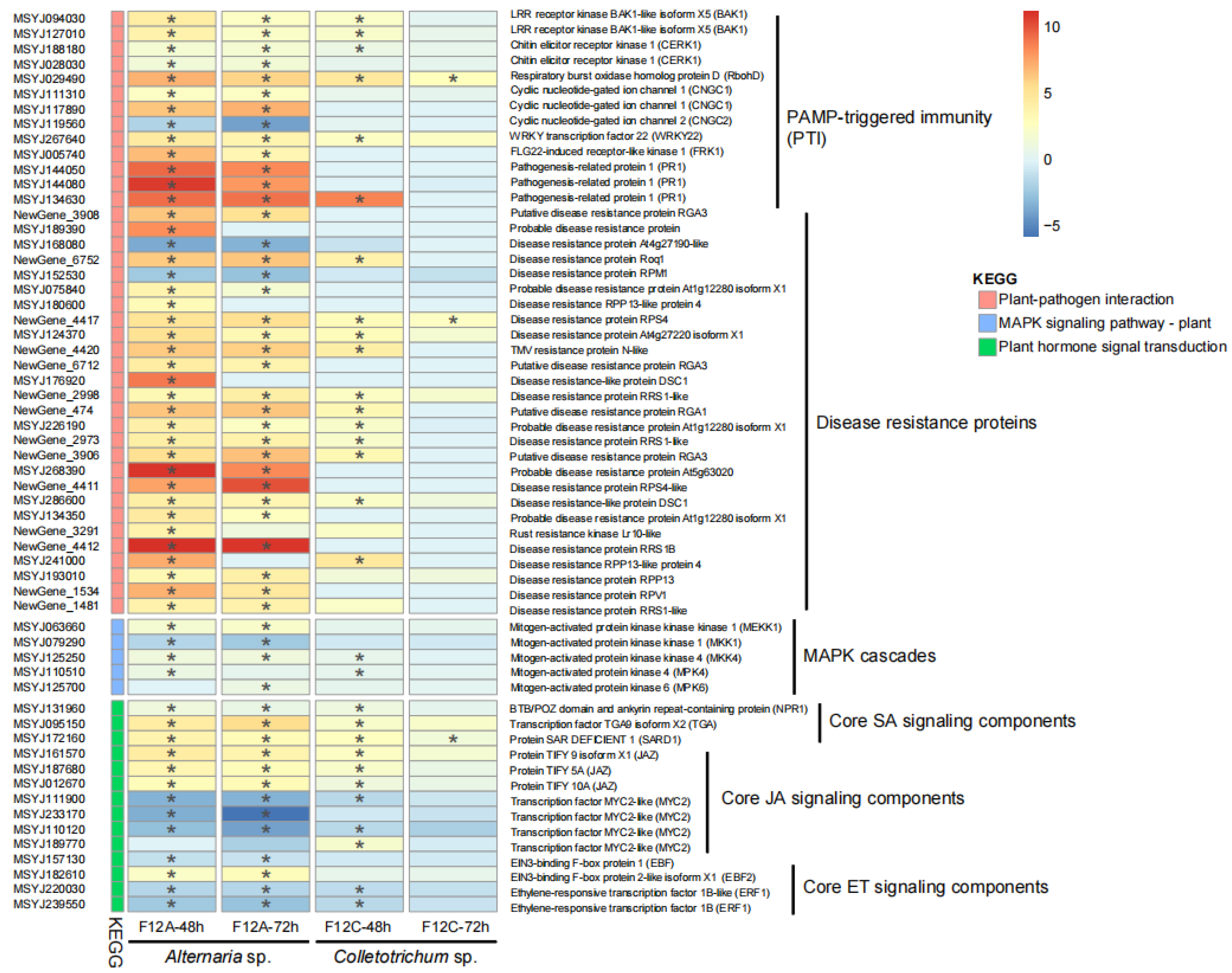
3.4. Host Defense Genes to the Infection by Alternaria and Colletotrichum Species
4. Discussion
4.1. Brown Spot Is Caused by Alternaria Species, but Not Colletotrichum Species
4.2. Citrus Recovers Fast from Anthracnose, but Not ABS
4.3. Host Defense Genes Are More Sensitive to ABS Than Anthracnose Pathogen
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Timmer, L.W.; Garnsey, S.M.; Graham, J.H. Compendium of Citrus Diseases, 2nd ed.; The American Phytopathological Society: Saint Paul, MN, USA, 2000; pp. 5–69. [Google Scholar]
- Knorr, L.C.; Suit, R.F.; Ducharme, E.P. Handbook of Citrus Diseases in Florida; University of Florida: Gainesville, FL, USA, 1957; pp. 11–19. [Google Scholar]
- Gai, Y.; Niu, Q.; Kong, J.; Li, L.; Liang, X.; Cao, Y.; Jiao, C. Genomic and Transcriptomic Characterization of Alternaria alternata during Infection. Agronomy 2023, 13, 809. [Google Scholar] [CrossRef]
- Vega, B.; Liberti, D.; Harmon, P.F.; Dewdney, M.M. A rapid resazurin-based microtiter assay to evaluate QoI sensitivity for Alternaria alternata isolates and their molecular characterization. Plant Dis. 2012, 96, 1262–1270. [Google Scholar] [CrossRef]
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Huang, F.; Cheng, L.; Feng, C.G.; Huang, T.J.; Li, H.Y. Identification of the pathogenic fungus causing brown spot on tangerine (Citrus reticulata CV. Hongjv). Acta Phytopathol. Sin. 2011, 41, 449–455. (In Chinese) [Google Scholar]
- Peng, A.; Chen, Y.; Song, X.; Yao, Y.; Wu, Y. Anthracnose occurrence and control of deqing Citrus gonggan. Guangdong Agric. Sci. 2008, 2, 58–60. (In Chinese) [Google Scholar]
- Yang, T.M.; Deng, M.X.; Wang, M.Z.; Zhang, S.Y.; Men, Y.J.; Tang, M.L.; Zhang, G.B.; Qin, X. Identification of suspicious “acute Anthracnose” on Gonggan. South. Hort. 2011, 22, 30–32. (In Chinese) [Google Scholar]
- Wang, X.F.; Li, Z.A.; Tang, K.Z.; Zhou, C.Y.; Yi, L. First report of Alternaria brown spot of citrus caused by Alternaria alternata in Yunnan Province, China. Plant Dis. 2010, 94, 375. [Google Scholar] [CrossRef]
- Huang, F.; Zhu, L.; Hou, X.; Li, H.Y. Identification of the pathogenic fungus causing brown spot on Ougan. J. Zhejiang Agric. Sci. 2012, 9, 1281–1282. (In Chinese) [Google Scholar]
- Li, Y.; Lv, J.; Zhu, L.; Lu, G.; Wang, Z.; Liu, G. Isolation, identification and phylogenetic analysis of citrus brown spot pathogens in Fujian Province. J. Plant Prot. 2019, 46, 1243–1251. [Google Scholar]
- Wang, Z.; Yang, M.; Yang, Y.; Peng, Z.; Zhou, E. Morphological and molecular identification of citrus anthracnose pathogen from Guangdong Province. Mycosystema 2010, 29, 488–493. (In Chinese) [Google Scholar]
- Ling, J.; Peng, A.; Song, X.; Chen, X. Bioassay research of 17 fungicides against Colletotrichum gloeosporioides Penz. Guangdong Agric. Sci. 2010, 1, 72–76. (In Chinese) [Google Scholar]
- Yang, M.; Feng, S.; He, Y.; Wang, Z.; Zhou, E. Screening of efficient fungicides for the control of citrus anthracnose and the discovery of fungicide-resistant isolates. J. South China Agric. Univ. 2013, 34, 28–31. (In Chinese) [Google Scholar]
- Yu, H.; Song, X.; Zhou, J.; Peng, A.; Ling, J.; Cheng, B.; Chen, X. Efficacy test of carbendazim+flusilazole against gonggan anthracnose. Chin. J. Trop. Agric. 2016, 36, 56–58. (In Chinese) [Google Scholar]
- Huang, F.; Chen, G.Q.; Hou, X.; Fu, Y.S.; Cai, L.; Hyde, K.D.; Li, H.Y. Colletotrichum species associated with cultivated citrus in China. Fungal Divers. 2013, 61, 61–74. [Google Scholar] [CrossRef]
- Peng, L.; Yang, Y.; Bahkali, A.H.; Liu, Z. Colletotrichum species on Citrus leaves in Guizhou and Yunnan provinces, China. Cryptogam. Mycol. 2012, 33, 267–283. [Google Scholar]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Allen, G.C.; Flores-Vergara, M.A.; Krasynanski, S.; Kumar, S.; Thompson, W.F. A modified protocol for rapid DNA isolation from plant tissues using cetyltrimethylammoniumbromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package; R Package Version 2.0; R Foundation: Vienna, Austria, 2013; Available online: https://cran.r-project.org/package=vegan (accessed on 26 June 2022).
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 26 July 2023).
- Kolde, R. Pheatmap: Pretty Heatmaps; R Package Version 1.0.12; R Foundation: Vienna, Austria, 2019; Available online: https://cran.r-project.org/package=pheatmap (accessed on 27 July 2023).
- Akimitsu, K.; Tsuge, T.; Kodama, M.; Yamamoto, M.; Otani, H. Alternaria host-selective toxins: Determinant factors of plant disease. J. Gen. Plant Pathol. 2014, 80, 109–122. [Google Scholar] [CrossRef]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Otani, H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Jones, J.D.; Ding, P. Plant immune networks. Trends Plant Sci. 2022, 27, 255–273. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Berens, M.L.; Berry, H.M.; Mine, A.; Argueso, C.T.; Tsuda, K. Evolution of hormone signaling networks in plant defense. Ann. Rev. Phytopathol. 2017, 55, 401–425. [Google Scholar] [CrossRef] [PubMed]
- Kadota, Y.; Shirasu, K.; Zipfel, C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015, 56, 1472–1480. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Upadhyaya, C.P.; Nookaraju, A.; Pandey, S.K.; Park, S.W. Plant disease resistance genes: Current status and future directions. Physiol. Mol. Plant Pathol. 2012, 78, 51–65. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Kourelis, J.; Van Der Hoorn, R.A. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef]


| Location | Variety | Orchard | No. Segment a | Carbendazol (10 μg/mL) | No. Colony | Alternaria spp. b | Colletotrichum spp. |
|---|---|---|---|---|---|---|---|
| Zhaoqing, Guangdong | Gonggan | ZQ1 | 10 | − | 5 | 0 (0) | 2 (40%) |
| 10 | + | 8 | 4 (50%) | 0 (0) | |||
| ZQ2 | 10 | − | 7 | 1 (14.3%) | 2 (28.6%) | ||
| 10 | + | 5 | 4 (80%) | 1 (20%) | |||
| ZQ3 | 10 | − | 6 | 2 (33.3%) | 1 (16.7%) | ||
| 10 | + | 5 | 3 (60%) | 1 (20%) | |||
| Wanzhou, Chongqing | Hongjv | WZ1 | 10 | − | 7 | 0 (0) | 0 (0) |
| 10 | + | 4 | 0 (0) | 0 (0) | |||
| WZ2 | 10 | − | 5 | 0 (0) | 0 (0) | ||
| 10 | + | 4 | 1 (25%) | 0 (0) | |||
| WZ3 | 10 | − | 6 | 0 (0) | 0 (0) | ||
| 10 | + | 4 | 2 (50%) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, M.; Zhu, C.; Li, L.; Liu, J.; Liu, J.; Huang, F. Comparative Study of the Co-Occurring Alternaria and Colletotrichum Species in the Production of Citrus Leaf Spot. J. Fungi 2023, 9, 1089. https://doi.org/10.3390/jof9111089
Lei M, Zhu C, Li L, Liu J, Liu J, Huang F. Comparative Study of the Co-Occurring Alternaria and Colletotrichum Species in the Production of Citrus Leaf Spot. Journal of Fungi. 2023; 9(11):1089. https://doi.org/10.3390/jof9111089
Chicago/Turabian StyleLei, Mengying, Congyi Zhu, Luoye Li, Jiangshan Liu, Jiashang Liu, and Feng Huang. 2023. "Comparative Study of the Co-Occurring Alternaria and Colletotrichum Species in the Production of Citrus Leaf Spot" Journal of Fungi 9, no. 11: 1089. https://doi.org/10.3390/jof9111089
APA StyleLei, M., Zhu, C., Li, L., Liu, J., Liu, J., & Huang, F. (2023). Comparative Study of the Co-Occurring Alternaria and Colletotrichum Species in the Production of Citrus Leaf Spot. Journal of Fungi, 9(11), 1089. https://doi.org/10.3390/jof9111089





