Abstract
The deviation of conventional fungal niches is an important factor in the implications of hidden fungal diversity and global fungal numbers. The Xylariomycetidae (Sordariomycetes, Ascomycota), which is also referred to as xylarialean taxa, has a wide range of species that demonstrate a high degree of variation in their stromatic characteristics, showing either conspicuous or inconspicuous forms. In this study, samples were collected while focusing on temporal and spatial parameters and substrate characteristics. Based on internal transcribed spacer (ITS), 28S large subunit rDNA (LSU), RNA polymerase II second largest subunit (RPB2), and β-tubulin (TUB2) multigene phylogeny and morphology, five new species are introduced as Muscodor brunneascosporus, M. lamphunensis (Xylariaceae), Nigropunctata hydei, N. saccata (Incertae sedis), and Xenoanthostomella parvispora (Gyrotrichaceae). Plant substrates in the early stages of decay and attached to the host were feasible sample niches, with an emphasis on the collection of inconspicuous, hidden xylarialean species. The appearance of inconspicuous saprobic xylarialean forms during the rainy season may be linked to the change in nutritional mode, from endophytic mode during the dry season to saprobic in the wet. Therefore, it would be fascinating to concentrate future research on how seasonal fluctuations affect nutritional mode shifts, especially in northern Thailand, which would provide the optimal spatial characteristics. In order to establish a comprehensive linkage between endophytic and saprobic modes, it is imperative to have a substantial representation of endophytic isolate sequences resembling inconspicuous xylariaceous fungi within publicly accessible databases.
Keywords:
diversity; Muscodor; Nigropunctata; nutritional modes; phylogeny; species; taxonomy; Xenoanthostomella 1. Introduction
A fungal stroma is a dense cluster of hyphal cells enveloped by a melanized outer layer of substances. Stromatic structures play a crucial role in the life cycle of various fungi, serving as the site for conidial and ascospore development [1]. The characteristics of the substrate or the processes influencing growth are the primary drivers of stromal diversity [2]. According to in vitro studies on Dussiella tuberiformis (Clavicipitaceae, Hypocreales), the development of the stromata is primarily influenced by non-reducing sugars, and the formation of the perithecia may be prompted by nutrition and moisture restriction [3]. Stromata appear to have evolved convergently among ascomycetes and basidiomycetes, which act as a resilient propagule that can withstand harsh environmental conditions [1]. Furthermore, stromata development is important for optimum temperature regulation for viable spores and maintaining the water content of the stromata to be able to spread spores, as well as for insects that need an appropriate temperature for their survival [4]. Importantly, early fungal classifications have been focused on the presence or absence, nature, and interior development of like characters for the delimitation of systematic groups of major ranks [5].
The stromatic characters play a major role in the generic and family level classifications of species [6] in the Xylariomycetidae (referred to as “xylarialean taxa” herein). In certain xylarialean species, there is a specialized character known as the clypeus. It consists of stromatic tissue or melanized hyphae that form above the partially submerged or immersed ascomata, which are shield-shaped structures with variable development [2]. Daranagama et al. [7] focused on those stromatic differences of xylarialean taxa and discussed the taxonomic significance of micro-xylariaceous (with inconspicuous ascomata) and macro-xylariaceous (with conspicuous stromata) genera. Following seven stromatic characters among the xylarialean taxa, Samarakoon et al. [8] performed an ancestral character analysis. The results indicated that xylarialean taxa most likely diverged from inconspicuous forms when they first appeared. Additionally, the majority of the xylarialean taxa’s undiscovered sexual morphs are presumably inconspicuous forms. However, there are limited studies on inconspicuous forms compared to conspicuous forms [7].
Taxonomic studies focusing on inconspicuous xylarialean taxa have been limited, probably due to a lack of fresh collections, and many species were described long ago and have never been recollected, especially from tropical locations [7]. It is interesting to note that the genera transferred to new families were previously accepted but had uncertain morphologies and phylogenies. In contrast to conspicuous stromatic xylarialean taxa, those transferred taxa are morphologically unique in having inconspicuous, immersed ascomata that do not have key stromatic characters for delimiting higher ranks. Still, more than 50 incertae sedis genera, mostly inconspicuous forms, demanding fresh collections and taxonomic and phylogenetic studies [8].
Hyde et al. [9] provided an outline for the Sordariomycetes, including the Xylariomycetidae, with three orders, as Amphisphaeriales (17 families), Delonicicolales (2 families), and Xylariales (15 families), based on morpho-molecular studies. In addition, due to a lack of molecular data, the Myelospermataceae is treated as a Xylariomycetidae family incertae sedis. Samarakoon et al. [8] revisited the Xylariomycetidae following morphology, internal transcribed spacer (ITS), 28S large subunit rDNA (LSU), RNA polymerase II second largest subunit (RPB2), β-tubulin (TUB2), and translation elongation factor-1α (TEF-1α) phylogeny, divergence time estimation, and ancestral character state reconstruction, including fresh collections from China, Italy, Russia, Thailand, and UK. The divergence time estimations showed that Amphisphaeriales and Xylariales diverged 154 (117–190) MYA, whereas Delonicicolales diverged at 161 (123–197) MYA, with crown ages of 127 (92–165) MYA and 147 (111–184) MYA, respectively [8]. The Xylariomycetidae currently comprises 40 families: Amphisphaeriales (21 families), Delonicicolales (2 families), Xylariales (16 families), and Xylariomycetidae family incertae sedis (1 family).
Thailand is part of the Indo-Malayan hub of biodiversity, which is distinguished by a special combination of vascular plants and, as a result, many related species of fungi [10]. Using polyphasic approaches and significant advancements in the hierarchical structure of classification, significant progress has been achieved in our understanding of the fungi in northern Thailand. Since 2017, our research has focused on the taxonomy, phylogeny, evolution, and secondary metabolites of xylarialean taxa in northern Thailand. In the provinces of Chiang Mai, Chiang Rai, Lampang, Nan, Phayao, and Phrae, we have introduced 20 new species and five new genera (Magnostiolata, Melanostictus, Neoamphisphaeria, Nigropunctata, and Paravamsapriya) until 2022 [8,11,12,13]. Based on our previous studies, there is a high possibility of the addition of new inconspicuous xylarialean species from northern Thailand. In this study, we introduce five new inconspicuous xylarialean species collected from northern Thailand and describe them based on morphology and ITS, LSU, RPB2, and TUB2 multigene phylogeny.
2. Materials and Methods
2.1. Collection, Isolation, and Morphological Studies
Fresh specimens were collected mainly during the rainy season in 2022 from northern Thailand. External examinations were carried out using a stereomicroscope (Leica M205 FCA-FA, Leica Microsystems Ltd., Hessian, Germany) and photographed with a Leica DMC6200 digital microscope camera. Microscopic photography was performed using a Nikon DS-Ri2 camera connected with a Nikon ECLIPSE Ni (Tokyo, Japan) compound microscope, as described by Samarakoon et al. [8]. Where necessary, Melzer’s reagent, Congo red, and Indian ink were used. The photographs included in the figures were edited using Adobe Photoshop CS6 (Adobe Systems, San Jose, CA, USA) and measured using the Tarosoft (R) Image Framework (v. 0.9.7).
The herbarium specimens were deposited in the Chiang Mai University Herbarium (CMUB), Sustainable Development of Biological Resources Research Herbarium (SDBR), Center of Microbial Diversity, Sustainable Utilization, Department of Biology, Faculty of Science, Chiang Mai University, the Mae Fah Luang University Herbarium (MFLU), Chiang Rai, Thailand, and the Herbarium of Cryptogams Kunming Institute of Botany Academia Sinica (KUN-HKAS), Chinese Academy of Sciences, Kunming, China. New taxa were linked with the MycoBank (https://www.mycobank.org, accessed on 18 October 2023).
2.2. DNA Extraction, PCR Amplification, and Sequencing
DNA extractions were performed directly from the fruiting bodies. Total DNA extraction kits were used according to the manufacturer’s instructions (PureDireX, Genomic DNA Isolation Kit, The BIO-HELIX Co., Ltd., New Taipei City, Taiwan).
The ITS (ITS5/ITS4; [14]), LSU (LR0R/LR5; [15]), 18S small subunit rDNA (SSU) (NS1/NS4; [14]), RPB2 (fRPB2-5f/fRPB2-7cR; [16]), TUB2 (T1/T22; [17]), and TEF-1α (EF1-983F/EF1-2218R; [18]) gene regions were amplified using the PCR protocols described by Samarakoon et al. [8]. The total volume of 25 μL contained 12.5 μL of 2X Taq Plus Master Mix (Taq DNA Polymerase, dNTP, an optimized buffer system, and Dye Plus) (Nanjing Vazyme Biotech Co., Ltd., Nanjing, China), 1 μL of each primer, 9.5 μL of double-distilled water, and 1 μL (25–50 ng) of DNA template. All of the PCR products were immediately subjected to 4 °C and visualized on 1% agarose electrophoresis gels using a 100 bp + 1.5 kb DNA ladder labeled with stain (0.01% bromophenol blue, 0.25 M EDTA, 50% glycerol) (SibEnzyme, Nowosibirsk, Russia) and SafeView I nuclear staining dye (1 µL/10 mL of agarose). PCR products were sent to Apical Scientific SDN. BHD. (Seri Kembangan, Malaysia) for purification and DNA sequencing.
2.3. Phylogenetic Analyses
All of contig sequences were searched in BLASTn (https://www.ncbi.nlm.nih.gov, accessed on 18 July 2023) [19]. Related sequences for newly acquired sequences were downloaded from GenBank based on the BLASTn results and recent publications (Table 1). FFT-NS-2 Tree-based Progressive Method, 20 PAM/k = 2, was used to align individual loci with the scoring matrix for nucleotide sequences and the 1.0 Gap opening penalty settings of MAFFT V.7.036 (http://mafft.cbrc.jp/alignment/server/, accessed on 20 July 2023). Alignments were manually improved as needed in BioEdit v. 7.0 [20]. TrimAl (v.1.0) (gappyout option) was used to trim the ITS and LSU sequences [21]. Exon regions of RPB2 and TUB2 were extracted with reference to Barrmaelia macrospora (CBS 142768).
Characters were assessed as unordered and equally weighted. The best evolutionary model for each gene was found using MrModeltest 2.3 and the Akaike Information Criterion (AIC). Phylogenetic trees were constructed using single and merged alignments of the genetic markers ITS, LSU, RPB2, and TUB2. Both maximum likelihood (ML) and Bayesian Inference (BI) methods were employed. The newly obtained sequences have been archived in GenBank for future research reference (Table 1). ML analyses were conducted using RAxMLGUI v.1.3 [22], employing the ML+rapid bootstrap configuration with 1000 replicates. The Bayesian tree was constructed using MCMC sampling within MrBayes v3.1.2 [23,24], comprising 1,000,000 MCMC generations utilizing four chains and partition analysis with 100 sampling frequencies. The initial 2500 trees (25% of the total) were designated as the burn-in phase and were subsequently excluded from the analysis. Posterior probabilities (PP) were calculated using the remaining 7500 trees. The generated trees were visualized using the FigTree v.1.4.0 program [25], and the final figures were crafted using Adobe Illustrator® CS5 (Version 15.0.0, Adobe Systems, San Jose, CA, USA).

Table 1.
Names, codes, and corresponding GenBank accession numbers of the taxa used in the phylogenetic analyses of this study.
Table 1.
Names, codes, and corresponding GenBank accession numbers of the taxa used in the phylogenetic analyses of this study.
| Taxa | Original Code | GenBank Accession Numbers | References | |||
|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | TUB2 | |||
| Achaetomium macrosporum | CBS 532.94 | KX976574 | KX976699 | KX976797 | KX976915 | [26] |
| Alloanthostomella rubicola | MFLUCC 16-0479 T | KX533455 | KX533456 | N/A | N/A | [27] |
| Amphirosellinia nigrospora | HAS T 91092308 T | GU322457 | N/A | GQ848340 | GQ495951 | [28] |
| Anthostomella formosa | MFLUCC 14-0170 | MW240652 | MW240582 | N/A | MW820917 | [8] |
| A. helicofissa | MFLUCC 14-0173 T | MW240653 | MW240583 | KP340534 | KP406617 | [8,29] |
| A. lamiacearum | MFLU 18-0101 T | MW240669 | MW240599 | MW658648 | N/A | [8] |
| A. obesa | MFLUCC 14-0171 T | KP297405 | KP340546 | KP340533 | N/A | [29] |
| Anthostomelloides krabiensis | MFLUCC 15-0678 T | KX305927 | KX305928 | KX305929 | N/A | [30] |
| Astrocystis sublimbata | HAS T 89032207 | GU322447 | N/A | GQ844834 | GQ495940 | [28] |
| Barrmaelia macrospora | CBS 142768 T | KC774566 | KC774566 | MF488995 | MF489014 | [31,32] |
| B. moravica | CBS 142769 T | MF488987 | MF488987 | MF488996 | MF489015 | [32] |
| B. rappazii | CBS 142771 T | MF488989 | MF488989 | MF488998 | MF489017 | [32] |
| Biscogniauxia nummularia | MUCL 51395 T | KY610382 | KY610427 | KY624236 | KX271241 | [33] |
| Brunneiperidium involucratum | MFLUCC 14-0009 T | KP297399 | KP340541 | KP340527 | KP406610 | [29] |
| Camillea obularia | A TCC 28093 | KY610384 | KY610429 | KY624238 | KX271243 | [33] |
| Chaetomium elatum | CBS 374.66 | KC109758 | KC109758 | KF001820 | KC109776 | [34] |
| Circinotrichum circinatum | CBS 148326 | ON400743 | ON400796 | ON399328 | N/A | [35] |
| C. maculiforme | CBS 140016 T | KR611874 | KR611895 | ON399338 | N/A | [35] |
| Clypeosphaeria mamillana | CBS 140735 T | KT949897 | KT949897 | MF489001 | MH704637 | [32,36,37] |
| Collodiscula leigongshanensis | GZUH 0107 T | KP054281 | KP054282 | KR002588 | KR002587 | [38] |
| Coniocessia maxima | CBS 593.74 T | GU553332 | MH878275 | N/A | N/A | [39,40] |
| C. nodulisporioides | CBS 281.77 T | MH861061 | MH872831 | N/A | N/A | [40] |
| Creosphaeria sassafras | S TMA 14087 | KY610411 | KY610468 | KY624265 | KX271258 | [33] |
| Digitodochium amoenum | CBS 147285 T | ON869303 | ON869303 | ON808481 | ON808525 | [41] |
| Emarcea castanopsidicola | CBS 117105 T | AY603496 | MK762717 | MK791285 | MK776962 | [12,42] |
| E. eucalyptigena | CBS 139908 T | KR476733 | MK762718 | MK791286 | MK776963 | [12,43] |
| Entalbostroma erumpens | ICMP 21152 T | KX258206 | N/A | KX258204 | KX258205 | [44] |
| Entoleuca mammata | J.D.R. 100 | GU300072 | N/A | GQ844782 | GQ470230 | [28] |
| Entosordaria perfidiosa | CBS 142773 T | MF488993 | MF488993 | MF489003 | MF489021 | [32] |
| E. quercina | CBS 142774 T | MF488994 | MF488994 | MF489004 | MF489022 | [32] |
| Graphostroma platystomum | CBS 270.87 T | JX658535 | DQ836906 | KY624296 | HG934108 | [33,45,46,47] |
| Gyrothrix encephalarti | CBS 146684 T | MT373376 | MT373358 | ON399342 | N/A | [35,48] |
| G. eucalypti | CBS 146023 T | MN562109 | MN567617 | ON399346 | N/A | [35,49] |
| G. podosperma | MFLUCC 16-0243 T | KX505957 | KX505958 | KX789496 | KX789495 | [27] |
| G. podosperma | CBS 148804 | ON400756 | ON400810 | ON399343 | N/A | [35] |
| G. verticillata | CBS 148806 | ON400759 | ON400813 | ON399318 | N/A | [35] |
| Hansfordia pruni | CBS 194.56 T | MK442585 | MH869122 | KU684307 | N/A | [40,50,51] |
| H. pulvinata | CBS 144422 | MK442587 | MK442527 | N/A | N/A | [50] |
| Hypocreodendron sanguineum | J.D.R. 169 T | GU322433 | N/A | GQ844819 | GQ487710 | [28] |
| Induratia apiospora | A TCC 60639 T | OP862879 | OP862881 | OP879469 | OP879468 | [52] |
| Kretzschmaria deusta | CBS 163.93 | KC477237 | KY610458 | KY624227 | KX271251 | [33,53] |
| K. guyanensis | HAS T 89062903 | GU300079 | N/A | GQ844792 | GQ478214 | [28] |
| Kretzschmariella culmorum | J.D.R. 88 | KX430043 | N/A | KX430045 | KX430046 | [44] |
| Linosporopsis ischnotheca | CBS 145761 T | MN818952 | MN818952 | MN820708 | MN820715 | [54] |
| L. ochracea | CBS 145999 T | MN818958 | MN818958 | MN820714 | MN820721 | [54] |
| Lopadostoma dryophilum | CBS 133213 T | KC774570 | KC774570 | KC774526 | MF489023 | [31,32] |
| L. quercicola | CBS 133212 T | KC774610 | KC774610 | KC774558 | N/A | [31] |
| L. turgidum | CBS 133207 T | KC774618 | KC774618 | KC774563 | MF489024 | [31] |
| Magnostiolata mucida | MFLU 19-2133 T | MW240673 | MW240603 | MW658652 | MW775618 | [8] |
| Melanographium phoenicis | MFLUCC 18-1481 T | MN482677 | MN482678 | N/A | N/A | [55] |
| M. smilacis | MFLU 21-0075 T | MZ538514 | MZ538548 | N/A | N/A | [56] |
| Microdochium lycopodinum | CBS 125585 T | JF440979 | JF440979 | KP859125 | KP859080 | [57,58] |
| M. phragmitis | CBS 285.71 T | KP859013 | KP858949 | KP859122 | KP859077 | [58] |
| Muscodor albus | 9_6 | HM034857 | HM034865 | N/A | HM034844 | [59] |
| M. albus | MON T 620 T | AF324336 | N/A | N/A | N/A | [60] |
| M. brasiliensis | LGMF1255 | KY924493 | N/A | N/A | N/A | [61] |
| M. brasiliensis | LGMF1256 T | KY924494 | N/A | N/A | N/A | [61] |
| M. brunneascosporus | CMUB 40020 T | OR507145 | OR507158 | OR504420 | OR519978 | This study |
| M. brunneascosporus | MFLU 23-0406 | OR507146 | OR507159 | N/A | N/A | This study |
| M. camphorae | NFCCI 3236 T | KC481681 | N/A | N/A | N/A | [62] |
| M. cinnanomi | BCC 38842 T | GQ848369 | N/A | N/A | N/A | [63] |
| M. coffeanum | COAD 1842 T | KM514680 | N/A | KP862881 | N/A | [64] |
| M. coffeanum | MFLUCC 13-0159 | MK634693 | MK634694 | MK644942 | MK644943 | [65] |
| M. coffeanum | COAD 1900 | KP862879 | N/A | KP862880 | N/A | [64] |
| M. coffeanum | CMUB 40022 | OR507147 | OR507160 | N/A | N/A | This study |
| M. crispans | MON T 2347 T | EU195297 | N/A | N/A | N/A | [66] |
| M. equiseti | JCM 18233 T | JX089322 | N/A | N/A | N/A | [67] |
| M. fengyangensis | CGMCC 2863 | HM034855 | HM034861 | HM034851 | HM034842 | [59] |
| M. fengyangensis | CGMCC 2862 T | HM034856 | HM034859 | HM034849 | HM034843 | [59] |
| M. ghoomensis | NFCCI 3234 T | KF537625 | N/A | N/A | N/A | [68] |
| M. kashayum | NFCCI 2947 T | KC481680 | N/A | N/A | N/A | [69] |
| M. lamphunensis | CMUB 40021 T | OR507148 | OR507161 | OR504421 | OR519979 | This study |
| M. lamphunensis | MFLU 23-0408 | OR507149 | OR507162 | N/A | N/A | This study |
| M. musae | JCM 18230 T | JX089323 | N/A | N/A | N/A | [67] |
| M. oryzae | JCM 18231 T | JX089321 | N/A | N/A | N/A | [67] |
| M. roseus | MON T 2098 T | AH010859 | N/A | N/A | N/A | [70] |
| Muscodor sp. | SMH 1255 | MN250031 | AY780069 | N/A | AY780119 | [12,71] |
| M. strobelii | NFCCI 2907 T | JQ409999 | N/A | N/A | N/A | [72] |
| M. suthepensis | JCM 18232 T | JN558830 | N/A | N/A | N/A | [67] |
| M. sutura | MSUB 2380 T | JF938595 | N/A | N/A | N/A | [73] |
| M. thailandica | HKAS 102323 | MK762708 | MK762715 | MK791284 | MK776961 | [12] |
| M. thailandica | MFLUCC 17-2669 T | MK762707 | MK762714 | MK791283 | MK776960 | [12] |
| M. tigerensis | NFCCI 3172 T | JQ409998 | N/A | N/A | N/A | [74] |
| M. vitigenus | MON T P-15 T | AY100022 | N/A | N/A | N/A | [75] |
| M. vitigenus | CE-QCA-O1100 | KC771512 | N/A | N/A | N/A | Unpublished |
| M. yucatanensis | MEXU 25511 T | FJ917287 | N/A | N/A | N/A | [76] |
| M. yucatanensis | CDA744 | KU094056 | N/A | N/A | N/A | Unpublished |
| M. yunnanensis | CGMCC 3.18908 T | MG866046 | MG866038 | MG866059 | MG866066 | [77] |
| M. ziziphi | MFLUCC 17-2662 T | MK762705 | MK762712 | MK791281 | MK776958 | [12] |
| M. ziziphi | HKAS 102300 | MK762706 | MK762713 | MK791282 | MK776959 | [12] |
| Nemania abortiva | BISH 467 T | GU292816 | N/A | GQ844768 | GQ470219 | [28] |
| N. macrocarpa | WSP 265 T | GU292823 | MH874423 | GQ844776 | GQ470226 | [28,40] |
| N. primolutea | HAS T 91102001 T | EF026121 | N/A | GQ844767 | EF025607 | [28] |
| Neoanthostomella bambusicola | MFLU 18-0796 T | MW240657 | MW240587 | MW658641 | MW775610 | [8] |
| N. fici | MFLU 19-2765 T | MW114390 | MW114445 | MW177711 | N/A | [78] |
| N. pseudostromatica | MFLU 15-1190 T | KU940158 | KU863146 | N/A | N/A | [79] |
| Neogyrothrix oleae | CBS 146068 | MN562137 | MN567644 | N/A | N/A | [49] |
| N. oleae | CBS 146069 T | MN562136 | MN567643 | N/A | N/A | [49] |
| Nigropunctata bambusicola | MFLU 19-2134 | MW240662 | MW240592 | MW658644 | N/A | [8] |
| N. bambusicola | MFLU 19-2145 T | MW240664 | MW240594 | MW658646 | N/A | [8] |
| N. hydei | CMUB 40018 T | OR507150 | OR507163 | OR504422 | N/A | This study |
| N. hydei | MFLU 23-0410 | OR507151 | OR507164 | N/A | N/A | This study |
| N. nigrocircularis | MFLU 19-2130 T | MW240661 | MW240591 | N/A | MW775612 | [8] |
| N. saccata | MFLU 19-2144 T | MW240663 | MW240593 | MW658645 | MW775613 | This study |
| N. saccata | MFLU 18-0804 | MW240658 | MW240588 | MW658642 | MW775611 | This study |
| Nigropunctata sp. | HKAS 122747 | OQ158966 | OQ170888 | N/A | N/A | Unpublished |
| N. thailandica | MFLU 19-2118 T | MW240659 | MW240589 | MW658643 | N/A | [8] |
| N. thailandica | HKAS 106975 | MW240660 | MW240590 | N/A | N/A | [8] |
| Occultitheca rosae | HKAS 102393 T | MW240672 | MW240602 | MW658651 | MW775617 | [8] |
| Paraxylaria rosacearum | TASM 6132 T | MG828941 | MG829050 | N/A | N/A | [80] |
| Peglionia verticiclada | CBS 127654 T | ON400763 | ON400815 | ON399352 | N/A | [35] |
| Pirozynkiomyces brasiliensis | CBS 112314 T | ON400767 | ON400819 | ON399341 | N/A | [35] |
| Podosordaria mexicana | WSP 176 | GU324762 | N/A | GQ853039 | GQ844840 | [28] |
| Poronia punctata | CBS 656.78 T | KT281904 | KY610496 | KY624278 | KX271281 | [33,81] |
| Pseudoanthostomella delitescens | MFLUCC 16-0477 | KX533451 | KX533452 | KX789491 | KX789490 | [27] |
| P. pini-nigrae | MFLUCC 16-0478 T | KX533453 | KX533454 | KX789492 | N/A | [27] |
| Pseudoceratocladium polysetosum | FMR 10750 T | KY853430 | KY853490 | ON399348 | N/A | [35,82] |
| P. polysetosum | CBS 126092 | MH864077 | MH875534 | ON399347 | N/A | [35,40] |
| Pseudocircinotrichum papakurae | CBS 101373 | KR611876 | KR611897 | N/A | N/A | [35] |
| P. papakurae | CBS 140221 | ON400768 | ON400820 | ON399349 | N/A | [35] |
| Rosellinia buxi | J.D.R. 99 | GU300070 | N/A | GQ844780 | GQ470228 | [28] |
| R. necatrix | HAS T 89062904 | EF026117 | KF719204 | GQ844779 | EF025603 | [28] |
| Sarcoxylon compunctum | CBS 359.61 | KT281903 | KT281898 | KY624230 | KX271255 | [33,81] |
| Selenodriella brasiliana | CBS 140227 T | ON400769 | ON400821 | ON399356 | N/A | [35] |
| S. cubensis | CBS 683.96 T | KP859053 | KP858990 | N/A | N/A | [58] |
| Spiririma gaudefroyi | CBS 147284 T | ON869320 | ON869320 | ON808497 | ON808541 | [41] |
| Sordaria fimicola | CBS 723.96 | MH862606 | MH874231 | DQ368647 | N/A | [40,83] |
| Stilbohypoxylon elaeicola | HAS T 94082615 | GU322440 | N/A | GQ844827 | GQ495933 | [28] |
| Xenoanthostomella calami | MFLUCC 14-0617A T | ON650684 | ON650706 | N/A | ON745964 | [84] |
| X. calami | MFLUCC 14-0617B | ON650685 | ON650707 | N/A | ON745965 | [84] |
| X. chromolaenae | MFLUCC 17-1484 T | MN638863 | MN638848 | MN648729 | N/A | [55] |
| X. chromolaenae | CBS 148702 | ON400784 | ON400841 | N/A | N/A | [35] |
| X. chromolaenae | MFLU 18-0840 | MW240668 | MW240598 | N/A | N/A | [8] |
| X. cycadis | CBS 137969 T | KJ869121 | KJ869178 | ON399350 | N/A | [35] |
| X. cycadis | CPC 25749 | ON400786 | ON400843 | N/A | N/A | [35] |
| X. olivacea | CBS 101185 | ON400787 | ON400844 | ON399351 | N/A | [35] |
| X. parvispora | CMUB 40019 T | OR507143 | OR507156 | OR504419 | OR519977 | This study |
| X. parvispora | MFLU 23-0409 | OR507144 | OR507157 | N/A | N/A | This study |
| Xenoanthostomella sp. | MFLUCC 23-0098 | OR438208 | OR438209 | N/A | N/A | Unpublished |
| Xenoanthostomella sp. | MFLUCC 23-182 | OR438210 | N/A | N/A | N/A | Unpublished |
| Xylaria adscendens | J.D.R. 865 | GU322432 | N/A | GQ844818 | GQ487709 | [28] |
| X. arbuscula | CBS 126415 | KY610394 | KY610463 | KY624287 | KX271257 | [33] |
| X. bambusicola | WSP 205 T | EF026123 | N/A | GQ844802 | AY951762 | [28] |
| X. cubensis | J.D.R. 860 | GU991523 | N/A | GQ848365 | GQ502700 | [28] |
| X. discolor | HAS T 131023 T | JQ087405 | N/A | JQ087411 | JQ087414 | [28] |
| X. hypoxylon | CBS 122620 T | AM993141 | KM186301 | KM186302 | KM186300 | [29,85] |
Abbreviations: ATCC: American Type Culture Collection, Manassas, VA, USA; BCC: BIOTEC Culture Collection, National Center for Genetic Engineering and Biotechnology, Khlong Luang, Thailand; BISH: Bishop Museum, Honolulu, HI, USA; CBS: Westerdijk Fungal Biodiversity Institute, Utrecht, the Netherlands; CDA: Fitopatologia, Universidade de Brasilia, Campus Universitario Darcy Ribeiro, Asa Norte, Brasilia, Distrito Federal 36570000, Brazil; CE-QCA: The collection of endophytes of the Pontificia Universidad Catolica del Ecuador, Ecuador; CGMCC: China General Microbiological Culture Collection Center, Beijing, China; COAD: Otávio de Almeida Drumond Culture Collection, Universidade Federal de Viçosa, Brazil; CPC: Culture collection of Pedro Crous, housed at CBS; FMR: Culture collection of the Faculty of Medicine at the Rovira i Virgili University, Reus, Spain; GZUH: Guizhou University, Guiyang, China; HAST: Academia Sinica, Taipei, Taiwan; HKAS: Herbarium of Cryptogams Kunming Institute of Botany Academia Sinica, China; ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; J.D.R.: Jack D. Rogers, Washington State University, Pullman, WA, USA; JCM: Japan Collection of Microorganisms, Japan; LGMF: LabGeM Culture Collection, Federal University of Parana (UFPR), Curitiba, Brazil; MEXU: Instituto de Biología, Universidad Nacional Autónoma de México, Mexico; MFLU, MFLUCC: Mae Fah Luang University, Chiang Rai, Thailand; MONT: Montana State University Herbarium, Plant Sciences and Plant Pathology, Montana State University, Bozeman, MT, USA; MSUB: Mycological collection of Montana State University, Bozeman, MT, USA; MUCL: Université Catholique de Louvain, Louvain-la-Neuve, Belgium; NFCCI: National Fungal Culture Collection of India (NFCCI), India; SMH: S.M. Huhndorf; STMA: Marc Stadler, Helmholtz-Zentrum für Infektionsforschung, Braunschweig, Germany; TASM: Tashkent Mycological Herbarium of the Institute of Botany, Uzbekistan; WSP: Washington State University, Pullman, WA, USA. Type, authentic and reference collections are denoted in “T”; N/A not available.
3. Results
3.1. Phylogenetic Analyses
Based on the preliminary BLASTn search and morphological studies, new collections were identified into two distinct groups. Therefore, two phylogenetic analyses were conducted as core Xylariaceae and Xylariales genera incertae sedis for better resolution of the phylogenetic affinities. The overall tree topology for each analysis was consistent. All the gene regions resulted in the GTR + I + G model. The RAxML analyses of the combined ITS, LSU, RPB2, and TUB2 datasets yielded the best-scoring trees (Figure 1 and Figure 2). Bayesian posterior probabilities from MCMC were evaluated when the final average standard deviation of split frequencies was less than 0.01.
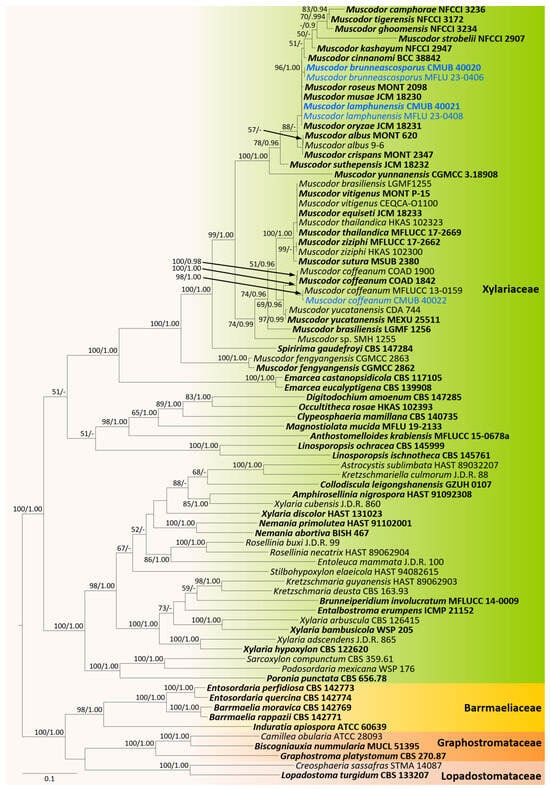
Figure 1.
Phylogram generated from maximum likelihood analysis based on combined ITS, LSU, RPB2, and TUB2 sequence data. The tree is rooted to taxa from the Barrmaeliaceae, Graphostromataceae, and Lopadostomataceae. Bootstrap support values for ML equal to or greater than 50%, PP equal to or greater than 0.9 (ML/PP) are given above or below the nodes. The newly generated sequences are in blue. Type collections are in bold.
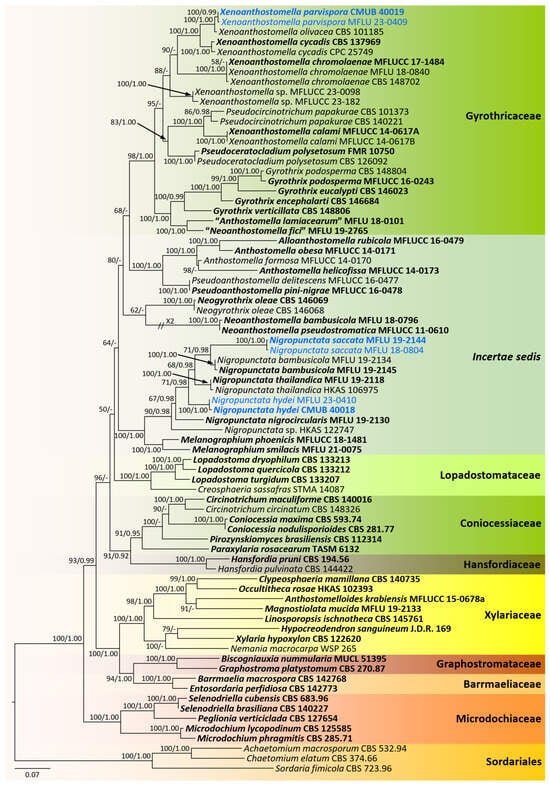
Figure 2.
Phylogram generated from maximum likelihood analysis based on combined ITS, LSU, RPB2, and TUB2 sequence data. The tree is rooted to the taxa belonging to the Sordariales. Bootstrap support values for ML equal to or greater than 50%, PP equal to or greater than 0.9 (ML/PP) are given above or below the nodes. The newly generated sequences are in blue. Type collections are in bold.
Analysis 1: The combined sequence alignment comprised 80 strains with 3704 characters, including gaps (ITS: 1–737, LSU: 738–1578, RPB2: 1579–2665, TUB2: 2666–3704). Single gene analyses were also performed, and topology and clade stability were compared from combined gene analyses. Tree topology from ML analysis was similar to BI analysis. The best scoring RAxML tree with a final likelihood value of −44754.1890 is presented in Figure 1. The matrix had 2133 (=57.5864% of all sites) constants or ambiguous constants, 1280 parsimony informative sites, and 1862 distinct site patterns. Estimated base frequencies were as follows: A = 0.2057, C = 0.3148, G = 0.2654, T = 0.2141; substitution rates: AC = 1.3568, AG = 4.5921, AT = 1.6135, CG = 0.9632, CT = 7.3071, GT = 1.000; gamma distribution shape parameter α = 0.492847. The completed alignment and tree were archived in TreeBASE under the submission ID: 30765.
Analysis 2: The combined sequence alignment comprised 77 strains with 3612 characters, including gaps (ITS: 1–745, LSU: 746–1629, RPB2: 1630–2673, TUB2: 2674–3612). Single gene analyses were also performed, and topology and clade stability were compared from combined gene analyses. Tree topology from ML analysis was similar to that from BI analysis. The best scoring RAxML tree with a final likelihood value of −44146.7002 is presented in Figure 2. The matrix had 1889 (52.2979% of all sites) constants or ambiguous constants, 1334 parsimony informative sites, and 2113 distinct site patterns. Estimated base frequencies were as follows: A = 0.21, C = 0.3068, G = 0.2629, T = 0.2203; substitution rates: AC = 1.1403, AG = 3.2723, AT = 1.5904, CG = 0.8353, CT = 5.4212, GT = 1.000; gamma distribution shape parameter α = 0.7252. The completed alignment and tree were archived in TreeBASE under the submission ID: 30764.
CMUB 40020, CMUB 40021, CMUB 40022, MFLU 23-0406, and MFLU 23-0408 cluster with Muscodor in the Xylariaceae, according to the first phylogenetic tree, which focuses on the core Xylariaceae (Figure 1). The second phylogenetic tree focuses on the Xylariales genera incertae sedis, and CMUB 40018, CMUB 40019, MFLU 18-0804, MFLU 19-2144, MFLU 23-0409, and MFLU 23-0410 form distinct clades in Nigropunctata and Xenoanthostomella (Figure 2).
3.2. Taxonomy
3.2.1. Gyrotrichaceae Hern.-Restr. & Crous, in Hernández-Restrepo et al., Persoonia 49: 111 (2022) [35]
MycoBank: MB845985.
Notes: Hernández-Restrepo et al. [35] introduced the Gyrothricaceae into Xylariales to accommodate genera Gyrothrix, Neogyrothrix, Pseudoceratocladium, Pseudocircinotrichum, and Xenoanthostomella. The family is characterized by having gyrothrix- and circinotrichum-like asexual morphs and anthostomella-like sexual morphs.
Xenoanthostomella Mapook & K.D. Hyde, in Hyde et al., Fungal Divers. 100: 235 (2020) [55].
MycoBank: MB558737.
Notes: Xenoanthostomella was introduced by Hyde et al. [55] with the type X. chromolaenae on Chromolaena odorata from Thailand and accepted in Xylariales, genera incertae sedis, due to phylogenetic uncertainty. The genus has immersed ascomata beneath the clypeus, cylindrical to broadly filiform asci, and broadly fusiform, aseptate ascospores. Even though Xenoanthostomella is morphologically similar to the species of “Anthostomella helicofissa,” Samarakoon et al. [8] treated it as a separate genus until further studies were undertaken. Based on phylogeny and sexual morphology, Hernández-Restrepo et al. [35] discovered two Malaysian strains associated with Albizia falcataria petioles and Falacia moluccana seed pods that were connected to X. chromolaenae. In addition, Xenoanthostomella was accommodated into a family, the Gyrotrichaceae [35]. Currently, four Xenoanthostomella species are accepted: X. calami [84], X. chromolaenae [55], X. cycadis (≡ Circinotrichum cycadis), and X. olivacea (≡ Helicotrichum olivaceum) [35].
Xenoanthostomella parvispora Samarak., sp. nov. (Figure 3).

Figure 3.
Xenoanthostomella parvispora (CMUB 40019, holotype): (a,b) Ascomata on the host surface; (c) Vertical section of ascomata; (d) Peridium; (e) Paraphyses; (f) Apical ring bluing in Melzer’s reagent; (g–k) Asci; (l–s) Ascospores. Scale bars: (a,b) = 500 μm; (c) = 100 μm; (d,g–k) = 20 μm; (e,f,l–s) = 5 μm.
MycoBank: MB850550.
Etymology: The specific epithet refers to the small ascospores.
Holotype: CMUB 40019.
Saprobic on the bark of a dead branch. Sexual morph: Ascomata 110–180 μm high, 70–110 μm diam., immersed, beneath clypeus, erumpent, visible as blackened, uni- or multilocular, in cross-section globose to obpyriform, coriaceous, solitary to scattered. Clypeus extending outwards around the ascomata, comprising dark fungal hyphae and host epidermal cells, thicker around the papilla. Ostioles centric or eccentric, ostiolar canal periphysate. Peridium 9–16 μm ( = 12.5 μm, n = 10) wide, thinner between two ascomata, with two cell layers: outer layer thick, comprising yellowish brown, thick-walled cells of textura angularis, and inner layer thin, composed of hyaline, thin-walled cells of textura angularis. Paraphyses 2–4 μm ( = 2.8 μm, n = 20) diam., intermingled among asci, prominent, hyphae-like, hyaline, smooth, septate, branched, thin-walled, guttulate, apically blunt. Asci 50–70 × 5–7 μm ( = 61.5 × 6.1 μm, n = 25), 8-spored, unitunicate, cylindrical, short-pedicellate, apically rounded with a 1.7–2.3 μm high, 0.6–1.5 μm diam., discoid apical ring, J+ in Melzer’s reagent. Ascospores 7.5–11 × 2.8–4.5 μm ( = 9.2 × 3.6 μm, n = 30), L/W 2.5, uniseriate, brown, inequilaterally ellipsoidal, aseptate, 1–2-guttulate, lacking a germ slit and appendage. Asexual morph: Undetermined.
Material examined: Thailand, Lamphun Province, on the bark of a dead branch of an unidentified dicotyledonous tree, 22 October 2022, M.C. Samarakoon, MC22-021 (CMUB 40019, holotype), (MFLU 23-0409, isotype). Additional sequences: CMUB 40019: OR507134 (SSU), OR504425 (TEF-1α).
Notes: Xenoanthostomella parvispora is described on the bark of a dead branch of an unidentified dicotyledonous tree from northern Thailand, which possesses generic characters such as immersed ascomata beneath a rudimentary clypeus, cylindrical to broadly filiform asci with a J+ apical ring, and broadly fusiform, aseptate ascospores. Xenoanthostomella parvispora differs from X. chromolaenae in having smaller ascomata (110–180 μm high, 70–110 μm diam. vs. 190–220 µm high, 175–210 µm diam.) and smaller ascospores (9.2 × 3.6 µm vs. 12 × 5 µm). Compared to X. calami and X. chromolaenae, X. parvispora lacks a germ slit. However, X. cycadis and X. olivacea are only known from their asexual morphs, thus morphological comparisons are not possible. The ITS, LSU, and RPB2 sequences of X. parvispora are similar to those of X. olivacea CBS 101,185 (99%, 1/533 gaps; 99%, 0/871 gaps; 98%, 0/647 gaps), X. cycadis CBS 137,969 (97%, 7/550 gaps; 99%, 0/887 gaps; 91%, 0/720 gaps), and X. chromolaenae MFLU 18-0840 (88%, 27/570 gaps; 98%, 2/887 gaps; N/A). In our ITS, LSU, RPB2, and TUB2 phylogeny (Figure 2), two of the isolates (CMUB 40,019 and MFLU 23-0409) form a monophyletic group sister to X. olivacea CBS 101,185 with strong statistical support (100% ML/1.00 PP). Here, we introduce X. parvispora as a new Xenoanthostomella species from Thailand.
3.2.2. Xylariaceae Tul. & C. Tul. [as ‘Xylariei’], Select. fung. carpol. (Paris) 2: 3 (1863)
MycoBank: MB81528.
Notes: Xylariaceae is a well-established family in Xylariales including both conspicuous and inconspicuous forms of xylarialean taxa. Following several recent studies, Hyde et al. [9] accepted 32 genera in the Xylariaceae. However, with recent consecutive studies, several genera previously accepted in genera incertae sedis or uncertain taxonomy have been placed in the Xylariaceae based on morpho-molecular studies.
Muscodor Worapong, Strobel & W.M. Hess, Mycotaxon 79: 71 (2001).
MycoBank: MB28513.
Notes: Muscodor was introduced by Worapong et al. [60] based on characteristic volatile organic compounds (VOCs) profiles, SSU and ITS phylogeny, and hyphal morphologies such as coiling, ropiness, and branching patterns. The genus is typified by M. albus from the small limbs of Cinnamomum zeylanicum from Honduras. While numerous investigations have been carried out over the past two decades to identify novel Muscodor species as sterile mycelia and their VOCs, neither the sexual nor asexual morphs of the genus were recorded until 2020. Australia, Bolivia, Ecuador, India, and Thailand are among the tropical countries where a majority of Muscodor species have been described (e.g., [67,69,70,74,75]). In 2020, Samarakoon et al. [12] described two sexual morphic species that were closely related to Muscodor species phylogenetically. These two new taxa shared characteristics with the previously reported genus Induratia, such as apiosporous ascospores and inconspicuous ascomata forms. However, the herbarium specimen and molecular data of the generic type, I. apiospora (PDD 44399), were unavailable. Meanwhile, an Induratia sp. (SMH 1255) specimen originating from Puerto Rico [71] showed a close affinity to the above-mentioned two new sexual morphs. While considering the morphology of I. apiospora (PDD 44399; iconotype) and the morpho-molecular characteristics of Induratia sp. (SMH 1255), Samarakoon et al. [12] synonymized Muscodor into Induratia and introduced those two sexual morphic taxa as new species, I. thailandica (MFLU 18-0784; MFLUCC 17-2669) and I. ziziphi (MFLU 18-0107; MFLUCC 17-2662), and a new family, the Induratiaceae. Recently, Cedeño-Sanchez et al. [52] encountered an unpublished ex-holotype strain of I. apiospora among the holdings of the ATCC collection and conducted a detailed morpho-molecular study. The results showed that I. apiospora (ATCC 60639) clustered in the Barrmaeliaceae, and Muscodor was resurrected within the Xylariaceae. After recent crucial revisions of the genus, Muscodor accommodates 25 species (http://www.indexfungorum.org, accessed on 20 October 2023) and is accepted in the Xylariaceae. In this study, we introduce two new Muscodor species and an additional collection of M. coffeanus.
Muscodor brunneascosporus Samarak., sp. nov. (Figure 4).
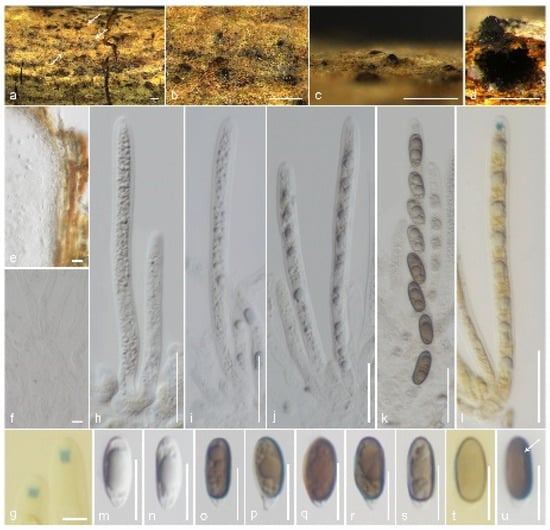
Figure 4.
Muscodor brunneascosporus (CMUB 40020, holotype): (a–c) Ascomata on the host surface (ascomata shown in white arrows); (d) Vertical section of ascoma; (e) Section of peridium; (f) Paraphyses; (g) Apical ring bluing in Melzer’s reagent; (h–l) Asci ((l) in Melzer’s reagent); (m–u) Ascospores; (t) in Melzer’s reagent; (u) germ slit shows in a white arrow. Scale bars: (a–c) = 500 µm; (d) = 200 µm; (h–l) = 20 µm; (e,m–u) = 10 µm; (f,g) = 5 µm.
MycoBank number: MB850551.
Etymology: The specific epithet refers to the Latin word that means ‘‘brown ascospores’.
Holotype: CMUB 40020.
Saprobic on a dead branch. Sexual morph: Ascomata 220–250 μm high, 225–325 μm diam., immersed, raised, visible as blackened, carbonaceous, solitary, in cross-section globose to sub-globose, tightly attached to substrate with a broad base. Clypeus black, thick-walled, short, comprising dark fungal hyphae and host epidermal cells. Ostioles raised from the center of ascomata. Peridium 11.5–16.5 μm ( = 13.8 μm, n = 12) thick, composed of two layers, occasionally not distinguished; inner layer hyaline, thin walled, 3–4 cell layers of textura angularis; outer layer yellowish brown, thick-walled, 4–6 cell layers of textura angularis. Paraphyses 3–5.5 μm ( = 4.2 μm, n = 30) diam., intermingled among asci, prominent, hyphae-like, hyaline, smooth, septate, thin-walled, guttulate, apically blunt. Asci 85–105 × 5.5–7.5 μm ( = 95 × 6.7 μm, n = 25), 8-spored, unitunicate, cylindrical, short-pedicellate, apically rounded with a 2–2.5 μm high, 1.1–1.6 μm diam., cylindrical, apical ring, J+ in Melzer’s reagent. Ascospores 13–16.5 × 4–6 μm ( = 14.5 × 5.3 μm, n = 30), L/W 2.7, uniseriate, ellipsoidal, constricted apiosporous; basal cell 1.5–3 μm ( = 2.2 μm, n = 30) length, hyaline, conical shape, guttulate; apical cell 10.5–12.5 μm ( = 11.4 μm, n = 30) length, brown, guttulate with a short germ slit, lacking an appendage. Asexual morph: Undetermined.
Material examined: Thailand, Lamphun Province, Ban Thi district, on a dead branch of an unidentified dicotyledonous tree, 24 September 2022, M.C. Samarakoon, MC22-011 (CMUB 40020, holotype), (MFLU 23-0406, isotype). Additional sequences: CMUB 40020: OR507135 (SSU), OR504426 (TEF-1α).
Notes: Muscodor brunneascosporus has inconspicuous stromatic forms with a rudimentary clypeus, 8-spored, unitunicate asci with a J+ apical ring, and uniseriate, ellipsoidal, constricted apiosporous ascospores. However, M. brunneascosporus differs from all the known sexual morphic Muscodor species in having apiosporous ascospores with hyaline basal cells and brown apical cells with a short germ slit. Similar ascospores can be observed among the inconspicuous xylarialeans in Xylariales genera incertae sedis. Anthostomella sabiniana is similar to M. brunneascosporus in having immersed ascomata (250 μm high, 240 μm diam. vs. 220–250 μm high, 225–325 μm diam.), filamentous paraphyses (4 μm vs. 4.2 μm wide), and apiosporous brown ascospores (13.5–16 × 5–7 μm vs. 13–16.5 × 4–6 μm). However, A. sabiniana differs from M. brunneascosporus in having larger asci (105–150 × 9–10 μm vs. 85–105 × 5.5–7.5 μm), a wedge-shaped apical ring, and a germ slit extending the full length of the basal brown cell [86]. Brunneiapiospora species are similar to M. brunneascosporus in having immersed ascomata under a clypeus, numerous paraphyses, and apiosporous ascospores with large brown apical cells [87]. However, none of the species is similar to M. brunneascosporus based on the combined morphological characters. The LSU sequence of M. brunneascosporus (CMUB 40020) is similar to that of M. yunnanensis W-S-38 (97%, 6/1193 gaps), M. thailandica HKAS 102,323 (96%, 7/1217 gaps), and M. coffeanum MFLUCC 13-0159 (96%, 5/1125 gaps). Isolates CMUB 40020 and MFLU 23-0406 form a clade with close affinity to M. musae JCM 18230 and M. roseus MONT 2098 (Figure 1). However, the species segregation among the taxa in a multigene phylogeny is not distinct. Since we failed to obtain cultures of these collections, the comparison of hyphal and culture morphologies is not possible. Based on the available data, we introduce M. brunneascosporus as a new species from Thailand.
Muscodor coffeanus A.A.M. Gomes, Pinho & O.L. Pereira [as ‘coffeanum’], in Hongsanan et al., Cryptog. Mycol. 36: 368 (2015) (Figure 5).

Figure 5.
Muscodor coffeanus (CMUB 40022): (a–c) Ascomata on the host surface (ascomata shown in white arrows); (d,e) Vertical sections of ascomata; (f) Section of peridium; (g) Paraphyses; (h) Apical ring bluing in Melzer’s reagent; (i–l) Asci (l in Congo Red); (m–t) Ascospores. Scale bars: (a–c) = 500 µm; (e) = 200 µm; (i–l) = 20 µm; (f,g,m–t) = 10 µm; (h) = 5 µm.
MycoBank: MB836101.
Saprobic on the bark of a dead branch. Sexual morph: Ascomata 275–350 μm high, 190–245 μm diam., semi-immersed, raised, visible as blackened, carbonaceous, solitary, in cross-section globose to sub-globose, tightly attached to substrate. Clypeus black, thick-walled, short, comprising dark fungal hyphae and host epidermal cells. Ostioles raised from the center of ascomata. Peridium 17.5–22 μm ( = 19.6 μm, n = 8) wide, composed of two layers; inner layer hyaline, thin-walled, 3–4 cell layers of textura angularis; outer layer yellowish brown, thick-walled, 3–4 cell layers of textura angularis. Paraphyses 2.5–5 μm ( = 3.6 μm, n = 30) diam., intermingled among asci, prominent, hyphae-like, hyaline, smooth, septate, thin-walled, guttulate, apically blunt, at times breaking into segments. Asci 90–125 × 8–9.5 μm ( = 111.5 × 8.7 μm, n = 25), 8-spored, unitunicate, cylindrical, short-pedicellate, apically rounded with a 3.4–4 μm high, 1.5–1.7 μm diam., cylindrical, apical ring, J+ in Melzer’s reagent. Ascospores 18–25 × 5–6.5 μm ( = 22.5 × 5.8 μm, n = 40), L/W 3.9, overlapping uniseriate, hyaline, bicellular, fusiform, ellipsoid-equilateral, with one median slightly constricted septum, lack constriction when immature, with rounded ends, guttulate, with polar cap-like sheaths, lacking a germ slit and appendage. Asexual morph: Undetermined.
Material examined: Thailand, Lamphun Province, on the bark of a dead branch of an unidentified dicotyledonous tree, 22 October 2022, M.C. Samarakoon, MC22-018 (CMUB 40022, MFLU 23-0407). Additional sequence: CMUB 40022: OR507136 (SSU).
Notes: Muscodor coffeanus was introduced by Hongsanan et al. [64] as an endophyte in stems of Coffea arabica from Brazil. The preliminary studies showed that the VOCs produced by M. coffeanus completely inhibited the growth of Aspergillus ochraceous, A. niger, A. flavus, and Fusarium coffeanus on PDA. Hyphal morphologies and ITS gene phylogeny have been used to introduce this species. Li and Kang [65] described the first report of the sexual morph of M. coffeanus, which was collected on the dead wood of an unknown plant from Chiang Mai. Our collection is morphologically similar to the M. coffeanus (MFLU 12-2129) sexual morph with slight differences in the ascomata and length of asci. MFLU 12-2129 has larger ascomata (380–450 μm high, 580–650 μm diam. vs. 275–350 μm high, 190–245 μm diam.) and longer asci (140–320 × 7–11.5 µm vs. 90–125 × 8–9.5 μm) compared to CMUB 40022. The ascomata (see Figure 2a,b in [65]) size and shape differences might be influenced by the texture of the substrate, as ascomata in our specimen were found immersed in the thick bark while those of Li and Kang [65] were in the soft xylem tissues. However, the size of 2-celled ascospores with equal divisions is in the overlapping range (18.5–22.5 × 6–8 µm vs. 18–25 × 5–6.5 μm). The ITS sequence of CMUB 40022 is similar to that of M. yucatanensis isolate 43 (99%, 0/512 gaps), M. coffeanum CDA739 (99%, 0/511 gaps), M. coffeanum MFLUCC 13-0159 (100%, 0/501 gaps), and M. coffeanum CDA743 (99%, 0/511 gaps), while the LSU sequence is similar to that of M. coffeanum MFLUCC 13-0159 (100%, 0/1114 gaps). Combined gene phylogenies also show that our new collection clusters in the M. coffeanum clade with strong statistical support sister to M. coffeanum MFLUCC 13-0159. Based on the similar morphologies and geography coupled with molecular analysis (Figure 1), our new material is added to M. coffeanum as an additional collection.
Muscodor lamphunensis Samarak., sp. nov. (Figure 6).
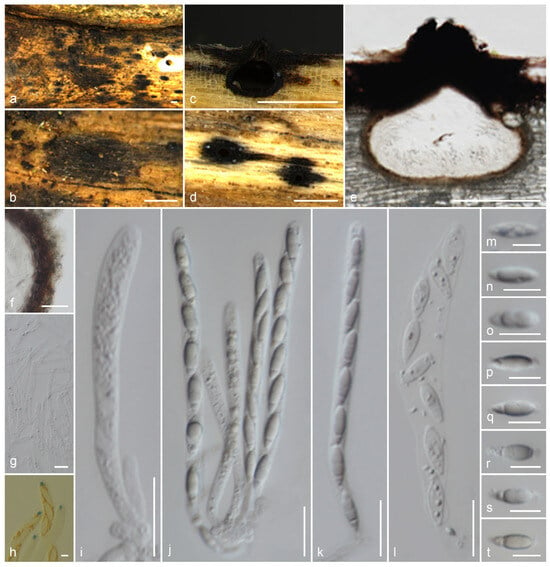
Figure 6.
Muscodor lamphunensis (CMUB 40021, holotype): (a,b) Ascomata on the host surface; (c,e) Vertical sections of ascomata; (d) Longitudinal section of ascomata; (f) Section of peridium; (g) Paraphyses; (h) Apical ring bluing in Melzer’s reagent; (i–l) Asci; (m–t) Ascospores. Scale bars: (a–d) = 500 µm; (e) = 200 µm; (f,i–l) = 20 µm; (g,m–t) = 10 µm; (h) = 5 µm.
MycoBank number: MB850552.
Etymology: The specific epithet refers to Lamphun province, where the species was first collected.
Holotype: CMUB 40021.
Saprobic on a decorticated dead branch. Sexual morph: Ascomata 345–420 μm high, 365–410 μm diam., immersed, raised, visible as blackened, carbonaceous, solitary, in cross-section globose to sub-globose, tightly attached to substrate with a broad base. Clypeus black, thick-walled, short, comprising dark fungal hyphae and host epidermal cells. Ostioles raised from the center of ascomata. Peridium 16.5–35 μm ( = 24.6 μm, n = 10) wide, composed of two layers, occasionally not distinguished; inner layer hyaline, thin-walled, 3–4 cell layers of textura angularis; outer layer reddish brown, thick-walled, 6–8 cell layers of textura angularis. Paraphyses 2.5–4.5 μm ( = 3.6 μm, n = 30) diam., intermingled among asci, prominent, hyphae-like, hyaline, smooth, septate, thin-walled, guttulate, apically blunt. Asci 75–130 × 5.5–8.5 μm ( = 100.5 × 7 μm, n = 25), 8-spored, unitunicate, cylindrical, short-pedicellate, apically rounded with a 2.5–3.4 μm high, 1.7–2.7 μm diam., cylindrical, apical ring, J+ in Melzer’s reagent. Ascospores 13–17 × 3.8–5.8 μm ( = 15.1 × 5 μm, n = 35), L/W 3.02, uniseriate, ellipsoidal, mostly hyaline, constricted apiosporous; basal cell 2.5–6 μm ( = 4.3 μm, n = 30) length, conical shape, guttulate with remnant at the top; apical cell 9.5–12.7 μm ( = 11 μm, n = 30) length, rarely brown, guttulate with remnant at the base, lacking a germ slit and appendage. Asexual morph: Undetermined.
Material examined: Thailand, Lamphun Province, Ban Thi district, on a decorticated dead branch of an unidentified dicotyledonous tree, 24 September 2022, M.C. Samarakoon, MC22-008 (CMUB 40021, holotype), (MFLU 23-0408, isotype). Additional sequences: CMUB 40021: OR507137 (SSU), OR504427 (TEF-1α).
Notes: Muscodor lamphunensis is similar to M. thailandica (MFLU 18-0784) and M. ziziphi (MFLU 18-0107) in having inconspicuous stromatic forms with rudimentary clypeus, 8-spored, unitunicate, cylindrical asci with a J+ apical ring, and uniseriate, naviculate to ellipsoidal, mostly hyaline, smooth-walled constricted apiosporous ascospores with remnant at both polars [12]. Muscodor lamphunensis has larger ascomata and thick peridium compared to M. thailandica and M. ziziphi. On the substrate, we can observe a remarkably distributed black clypeus around the neck area in our collection. Muscodor lamphunensis and M. ziziphi share Type 1 paraphyses, as described by Samuels et al. [88]. The ITS sequence of M. lamphunensis CMUB 40021 is similar to that of M. musae CMU MU3 (99%, 1/601 gaps) and M. albus MONT 620 (99%, 1/601 gaps), while the LSU sequence is similar to that of M. yunnanensis W-S-38 (97%, 6/1193 gaps), M. thailandica HKAS 102,323 (96%, 6/1200 gaps), and M. coffeanum MFLUCC 13-0159 (96%, 5/1125 gaps). Few RPB2 and TUB2 sequences are publicly available, and the species have not been identified among some of them. Therefore, the sequence comparisons are incomplete for the related taxa. The sequence comparisons of the RPB2 sequences from our new collection and the previously described three collections show significant differences as: M. thailandica MFLUCC 17-2669 (84%, 22/1086 gaps), M. ziziphi MFLUCC 17-2662 (83%, 22/1028 gaps), and M. coffeanum MFLUCC 13-0159 (85%, 16/1070 gaps). In our multigene phylogeny, two collections of M. lamphunensis (CMUB 40021 and MFLU 23-0408) cluster with M. musae JCM 18230, M. oryzae JCM 18231, and M. roseus MONT 2098 (Figure 1). However, the species segregation is not clear, probably due to the lack of other gene regions. There are no sexual or asexual morphologies of any species in this cluster. Since we failed to obtain a culture of this collection, the hyphal and culture morphologies are not possible. Even though M. musae JCM 18230 and M. oryzae JCM 18231 have been described from the same region, there is insufficient data to make any more comparisons. Based on the available morpho-molecular analyses, we introduce our new collection as a new species, M. lamphunensis from Thailand. However, it would not be surprising to find other species with asexual and sexual relationships, multigene phylogenies, and variable VOC profiles in the future, and to lump them together.
3.2.3. Xylariales, Genera Incertae Sedis
Nigropunctata Samarak. & K.D. Hyde, in Samarakoon et al. Fungal Divers. 112: 68 (2022) [8].
MycoBank: MB558737.
Notes: Nigropunctata was introduced by Samarakoon et al. [8] to accommodate three new species, including the type species, N. bambusicola. Nigropunctata species are characterized by immersed ascomata; white or yellow ectostroma; cylindrical, short pedicel, apically rounded asci with J+, discoid or inverted hat-shaped apical ring, and cylindrical to broadly ellipsoidal, aseptate ascospores. Sugita et al. [89] introduced the Spirodecosporaceae to accommodate Spirodecospora, which has similar ascomata and asci morphologies to Nigropunctata. However, Spirodecospora species are characterized by broadly ellipsoidal to fusoid, aseptate, brown, verruculose ascospores with spirally or almost straight linear ornamentation. Interestingly, species from both Nigropunctata and Spirodecospora are described from bamboo substrates only. In this study, we introduced two new Nigropuntata species from Thailand, which are also collected from dead bamboo branches.
Nigropunctata saccata Samarak., sp. nov. (Figure 7).

Figure 7.
Nigropunctata saccata (MFLU 19-2144, holotype): (a) Substrate; (b–d) Ascomata on the host surface; (e–g) Vertical sections of ascomata; (h) Peridium; (i) Paraphyses; (j–m) Asci; (n) Sterile ascus like structure; (o) Apical ring bluing in Melzer’s reagent; (p–w) Ascospores (germ slits shown in white arrows). Scale bars: (a) = 1 cm; (b,c) = 1000 μm; (e) = 500 μm; (d,f) = 200 μm; (g) = 100 μm; (j–m) = 20 μm; (h,i,n–w) = 10 μm.
MycoBank number: MB850553.
Etymology: The specific epithet refers to empty sac-like structures in ascomata.
Holotype: MFLU 19-2144.
Saprobic on a dead branch of bamboo. Sexual morph: Ascomata 290–370 μm high, 365–440 μm diam., immersed, dome-shaped areas, visible as black dots surrounded by round yellowish-brown patch, solitary or aggregated, in cross-section globose to sub-globose with an inwardly depressed base. Clypeus black, thick-walled, short, comprising dark fungal hyphae and host epidermal cells, flattened at the top. Ostioles centric or eccentric, periphysate ostiolar canal. Peridium 10–15 μm ( = 13 μm, n = 10) wide, yellowish white ectostroma, with two cell layers: outer layer thick, comprising brown, thick-walled cells of textura angularis, and inner layer thin, composed of hyaline, thin-walled cells of textura angularis. Paraphyses 2–3.3 μm ( = 2.6 μm, n = 15) diam., longer than the asci, numerous, hyaline, filamentous, sinuous, septate, constricted at septum, rarely branched, guttulate, blunt end paraphyses embedded in a gelatinous matrix. Asci 90–130 × 7–10 μm ( = 113 × 7.9 μm, n = 25), 8-spored, unitunicate, cylindrical, short pedicel, apically rounded with a discoid apical ring, J+ in Melzer’s reagent, often with broad sac like structures among asci. Ascospores 10.5–18 × 4.5–8 μm ( = 14.8 × 6.5 μm, n = 50), L/W 2.3, uniseriate or overlapping uniseriate, brown to dark brown, broadly ellipsoidal, aseptate, 1–2 guttules, germ slit on ventral side of the ascospore, straight, across the entire spore length. Asexual morph: Undetermined.
Material examined: Thailand, Chiang Rai, Amphoe Mueang district, on dead bamboo branches (Poaceae), 15 June 2019, M.C. Samarakoon, SAMC227 (MFLU 19-2144 holotype), (HKAS 107001, isotype). Chiang Mai Province, Amphoe Fang, on dead bamboo branches (Poaceae), 27 September 2016, M.C. Samarakoon, SAMC104 (MFLU 18-0804, HKAS 102304, paratype).
Notes: Nigropunctata saccata differs from other Nigropunctata species in having dome-shaped ostioles visible as black dots surrounded by a round yellowish-brown patch, a large clypeus, a white ectostroma, an inwardly depressed base, hyaline, globular sac-like structures among asci, a thin discoid apical ring, and a lack of mucilaginous sheath around ascospores. Our two collections share similar morphology, except MFLU 19-2144 possesses a thin peridium (10–15 μm), while MFLU 18-0804 possesses a thick peridium (18–23 μm). The ITS sequence of N. saccata is similar to that of Anthostomella lamiacearum MFLU 18-0101 (85%, 23/483 gaps), Anthostomelloides leucospermi CBS 110,126 (89%, 11/369 gaps), while the LSU sequence is similar to that of Melanographium phoenicis MFLUCC 18-1481 (94%, 4/857 gaps), Xenoanthostomella chromolaenae MFLUCC 17-1484 (93%, 10/854 gaps), Haploanthostomella elaeidis MFLU 20-0522 (93%, 18/857 gaps), Gyrothrix encephalarti CPC 35,966 (93%, 10/855 gaps), and X. cycadis CBS 137,969 (93%, 8/854 gaps). The RPB2 sequence of N. saccata is similar to that of N. bambusicola MFLU 19-2145 (99%, 0/875 gaps), N. thailandica MFLU 19-2118 (94%, 0/873 gaps), and Circinotrichum australiense CBS 148,706 (80%, 8/724 gaps). In the multigene phylogeny, N. saccata forms a sister clade to N. bambusicola with medium statistical support (71% ML/0.98PP). Based on similar morphology and phylogeny, here we treat our new collection as a species of Nigropunctata and introduce a new species, N. saccata.
Nigropunctata hydei Samarak., sp. nov. (Figure 8).

Figure 8.
Nigropunctata hydei (CMUB 40018, holotype): (a) Substrate; (b,c) Ascomata on the host surface; (d) Vertical sections of ascoma; (e) Peridium; (f) Paraphyses; (g) Apical ring bluing in Melzer’s reagent; (h–l) Asci; (m–t) Ascospores (s,t in Indian ink). Scale bars: (b,c) = 500 μm; (d) = 200 μm; (h–l) = 50 μm; (g,m–t) = 10 μm; (e,f) = 5 μm.
MycoBank number: MB850554.
Etymology: Named in honor of British mycologist Kevin D. Hyde, for his immense contributions to ascomycete taxonomy.
Holotype: CMUB 40018.
Saprobic on a dead branch of bamboo. Sexual morph: Ascomata 400–520 μm high, 485–575 μm diam., immersed, dome-shaped areas, visible as black dots surrounded by round yellowish-brown patches, solitary or aggregated, in cross-section globose to sub-globose with flattened bases. Clypeus black, thick-walled, short, comprising dark fungal hyphae and host epidermal cells, flattened at the top. Ostioles centric or eccentric, periphysate ostiolar canal. Peridium composed of two layers, occasionally not distinguished; 16.5–31 μm ( = 23.2 μm, n = 10) wide, yellowish-brown ectostroma, with two cell layers: outer layer thick, comprising brown, thick-walled cells of textura angularis, and inner layer thin, composed of hyaline, thin-walled cells of textura angularis. Paraphyses 3.5–5.6 μm ( = 4.6 μm, n = 30) diam., longer than the asci, numerous, hyaline, filamentous, sinuous, septate, constricted at septum, rarely branched, guttulate, blunt end paraphyses embedded in a gelatinous matrix. Asci 150–185 × 11.5–16.5 μm ( = 168.5 × 13.7 μm, n = 25), 8-spored, unitunicate, cylindrical, short-pedicellate, apically rounded with a 1.8–3.4 μm high, 3.8–5.2 μm diam., discoid apical ring, J+ in Melzer’s reagent. Ascospores 13.5–18 × 7–10 μm ( = 15.8 × 8.2 μm, n = 40), L/W 1.93, uniseriate or overlapping uniseriate, brown to dark brown, broadly ellipsoidal, aseptate, 1–2 guttules, covered with a thick, mucilaginous sheath, occasionally slightly constricted at the center, lacking a germ slit. Asexual morph: Undetermined.
Material examined: Thailand, Lamphun Province, on a dead branch of bamboo, 22 October 2022, M.C. Samarakoon, MC22-020 (CMUB 40018, holotype), (MFLU 23-0410, isotype). Additional sequence: CMUB 40018: OR507138 (SSU).
Notes: Nigropunctata hydei is similar to the other Nigropunctata species in having immersed ascomata with thick clypeus, ectostroma, cylindrical, short pedicel, apically rounded asci with J+, discoid apical ring, and broadly ellipsoidal, aseptate ascospores. As described in N. saccata, we noticed that the apical ring of N. hydei stained blue with Melzer’s reagent for only a short period. Nigropunctata hydei has larger ascomata (400–520 μm high, 485–575 μm diam.), wider peridium (16.5–31 μm wide), and larger asci (150–185 × 11.5–16.5 μm) compared to N. bambusicola (285–315 μm high, 260–340 μm diam., ascomata; 10–18 μm wide, peridium; 95–140 × 9.5–12.5 μm, asci) and N. saccata (290–370 μm high, 365–440 μm diam., ascomata; 10–15 μm wide, peridium; 90–130 × 7–10 μm, asci). Nigropunctata hydei differs from all Nigropunctata by lacking a germ slit. Anthostomella zongluensis, A. lugubris, and A. tumulosa share a similar size range of J+ apical ring and ellipsoidal, aseptate ascospores with a thick, mucilaginous sheath but lacking a germ slit. However, the ascomata and asci characters differ from those of N. hydei [86]. The ITS, LSU, and RPB2 sequences of N. hydei are similar to those of N. bambusicola (92%, 12/558 gaps; 97%, 3/892 gaps; 91%, 0/963 gaps; 93%, 0/961 gaps) and N. thailandica (91%, 11/566 gaps; 97%, 0/955 gaps; 93%, 0/961 gaps). In the multigene phylogeny, N. hydei forms a sister clade to N. thailandica with medium statistical support. Here, we introduce N. hydei, a new species from Thailand based on morpho-molecular distinctions.
4. Discussion
New additions have occasionally altered the morpho-molecular taxonomy of the Xylariales/Amphisphaeriales/incertae sedis. Recent research on those incertae sedis taxa, which were previously known from a single collection, lacking recent collections, having inconsistent morphologies, no sexual–asexual links, and no molecular evidence, has urged the classification of genus to the family level. Families like the Appendicosporaceae [8], Barrmaeliaceae [32], Fasciatisporaceae [55], Gyrothricaceae [35], Oxydothidaceae [90], and Spirodecosporaceae [89] are some of the examples that have primarily been raised through studies on inconspicuous xylarialean taxa. Prior research has most likely focused on stromatic forms like diatrypoid, hypoxyloid, rosellinioid, and xylarioid rather than inconspicuous stromatic forms like anthostomelloid. The introduction of novel taxa does not adhere to these conservative approaches and emphasizes microscopic characteristics such as the type of ring, the color of the ascospores, and the presence or absence and type of germ slit [8].
Muscodor, Nigropunctata, and Xenoanthostomella are the three genera taxonomically identified during the study to focus on inconspicuous xylarialean taxa. If the stromatic nature was thought of as the key characteristic, those three genera would probably be treated as anthostomella-like taxa. Although incertae sedis taxa, particularly inconspicuous forms, are increasingly being split apart rather than being grouped because of molecular studies, we encountered several problems in each genus in their taxonomy and phylogeny.
With the addition of Muscodor brunneascosporus and M. lamphunensis, the number of species known from their sexual morphs has increased to five, which all are collected from northern Thailand [12,65]. There is a clear differentiation of the ascospore characters in those species. However, obtaining the asexual morphs from cultures has failed; therefore, the sexual–asexual connection is unknown. Due to the fact that many of the available sequences are only ITS, the species delineation based on phylogeny is uncertain. Stadler et al. [91] discussed the intragenomic polymorphism of the ITS region of the Hypoxylaceae and revealed less than 97%, or around 90%, of the overall homology of the ITS sequences. The intragenomic ITS variation among Muscodor species or anthostomella-like taxa needed to be checked to understand the ITS based species demarcation. Nigropunctata and Spirodecospora species are associated with dead bamboo and are similar to inconspicuous stromatic forms [89]. The microscopic morphologies and phylogenetic analyses support accepting them as distinct clades apart from the core Xylariaceae. This is important for the collection of fresh, inconspicuous xylarialean taxa and molecular phylogeny.
In summary, this study introduced five new xylarialean taxa (Muscodor brunneascosporus, M. lamphunensis, Nigropunctata hydei, N. saccata, and Xenoanthostomella parvispora), alongside the inclusion of M. coffeanus from northern Thailand. These species were all found on branches in varying stages of senescence and early decomposition during the rainy season. Nevertheless, the taxonomic classification of inconspicuous xylarialean taxa remains incomplete, and further investigations are needed, particularly with the inclusion of freshly collected specimens. The ongoing exploration of the taxonomy, phylogeny, and secondary metabolites of xylarialean taxa underscores northern Thailand’s significance as a focal point for continued research. Consequently, our future studies will be directed towards comprehensive investigations encompassing morphology, phylogeny, ecology, and the exploration of antimicrobial properties for potential applications in plant disease control.
Author Contributions
Conceptualization, M.C.S.; methodology, M.C.S., I.S.M. and N.S.; resources, M.C.S., I.S.M. and N.S.; writing—original draft preparation, M.C.S.; writing—review and editing, M.C.S., S.L., I.S.M., N.S. and R.C., funding acquisition, M.C.S., S.L. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work (grant no. RGNS 65-078) was supported by Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI). This research was partially supported by Chiang Mai University, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The DNA sequence data obtained from this study have been deposited in GenBank under accession numbers; ITS (OR507143–OR507151, MW240658, MW240663), LSU (OR507156–OR507164, MW240588, MW240593), RPB2 (OR504419–OR504422, MW658642, MW658645), SSU (OR507134–OR507138), TEF-1α (OR504425–OR504427), and TUB2 (OR519977–OR519979, MW775611, MW775613). The alignments are available in TreeBASE under the submission IDs 30764 and 30765.
Acknowledgments
Shaun Pennycook and Konstanze Bensch are thanked for the nomenclatural advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novak, L.A.; Kohn, L.M. Electrophoretic and immunological comparisons of developmentally regulated proteins in members of the Sclerotiniaceae and other sclerotial fungi. Appl. Environ. Microbiol. 1991, 57, 525–534. [Google Scholar] [CrossRef]
- Læssøe, T.; Spooner, B.M. Rosellinia & Astrocystis (Xylariaceae): New species and generic concepts. Kew Bull. 1993, 49, 1–70. [Google Scholar] [CrossRef]
- Koroch, A.; Juliani, H.; Bischoff, J.; Lewis, E.; Bills, G.; Simon, J.; White JR, J. Examination of plant biotrophy in the scale insect parasitizing fungus Dussiella tubericornis. Symbiosis 2004, 37, 267–280. [Google Scholar]
- Lembicz, M.; Miszalski, Z.; Kornaś, A.; Turnau, K. Cooling effect of fungal stromata in the Dactylis-Epichloë-Botanophila symbiosis. Commun. Integr. Biol. 2021, 14, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Orton, C.R. Studies in the morphology of the Ascomycetes I. The stroma and the compound fructification of the Dothideaceae and other groups. Mycologia 1924, 16, 49–95. [Google Scholar] [CrossRef]
- Eriksson, O.E.; Winka, K. Supraordinal taxa of Ascomycota. Myconet 1997, 1, 1–16. [Google Scholar]
- Daranagama, D.A.; Jones, E.B.G.; Liu, X.Z.; To-anun, C.; Stadler, M.; Hyde, K.D. Mycosphere Essays 13—Do xylariaceous macromycetes make up most of the Xylariomycetidae? Mycosphere 2016, 7, 582–601. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Hyde, K.D.; Maharachchikumbura, S.S.N.; Stadler, M.; Gareth Jones, E.B.; Promputtha, I.; Suwannarach, N.; Camporesi, E.; Bulgakov, T.S.; Liu, J.-K. Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 2022, 112, 1–88. [Google Scholar] [CrossRef]
- Hyde, K.D.; Norphanphoun, C.; Maharachchikumbura, S.S.N.; Bhat, D.J.; Jones, E.B.G.; Bundhun, D.; Chen, Y.J.; Bao, D.F.; Boonmee, S.; Calabon, M.S.; et al. Refined families of Sordariomycetes. Mycosphere 2020, 11, 305–1059. [Google Scholar] [CrossRef]
- Vasilyeva, L.N.; Stephenson, S.L.; Hyde, K.D.; Bahkali, A.H. Some stromatic pyrenomycetous fungi from northern Thailand—1. Biscogniuxia, Camillea and Hypoxylon (Xylariaceae). Fungal Divers. 2012, 55, 65–76. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Jack Liu, J.-K.; Hyde, K.D.; Promputtha, I. Two new species of Amphisphaeria (Amphisphaeriaceae) from northern Thailand. Phytotaxa 2019, 391, 207–217. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Thongbai, B.; Hyde, K.D.; Brönstrup, M.; Beutling, U.; Lambert, C.; Miller, A.N.; Liu, J.-K.; Promputtha, I.; Stadler, M. Elucidation of the life cycle of the endophytic genus Muscodor and its transfer to Induratia in Induratiaceae fam. nov., based on a polyphasic taxonomic approach. Fungal Divers. 2020, 101, 177–210. [Google Scholar] [CrossRef]
- Samarakoon, M.C.; Maharachchikumbura, S.S.N.; Liu, J.-K.; Hyde, K.D.; Promputtha, I.; Stadler, M. Molecular phylogeny and morphology of Amphisphaeria (= Lepteutypa) (Amphisphaeriaceae). J. Fungi 2020, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 1997, 7, 103–116. [Google Scholar] [CrossRef]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Zhaxybayeva, O.; Gogarten, J.P. Bootstrap, bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genom. 2002, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v. 1.4.0. Available online: http://tree.bio.ed.ac.uk/%0Asoftware/figtree/ (accessed on 20 August 2023).
- Wang, X.W.; Houbraken, J.; Groenewald, J.Z.; Meijer, M.; Andersen, B.; Nielsen, K.F.; Crous, P.W.; Samson, R.A. Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Stud. Mycol. 2016, 84, 145–224. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Jeewon, R.; Liu, X.; Stadler, M.; Lumyong, S.; Hyde, K.D. Taxonomic rearrangement of Anthostomella (Xylariaceae) based on a multigene phylogeny and morphology. Cryptogam. Mycol. 2016, 37, 509–538. [Google Scholar] [CrossRef]
- Hsieh, H.-M.; Lin, C.-R.; Fang, M.-J.; Rogers, J.D.; Fournier, J.; Lechat, C.; Ju, Y.-M. Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol. Phylogenetics Evol. 2010, 54, 957–969. [Google Scholar] [CrossRef]
- Daranagama, D.A.; Camporesi, E.; Tian, Q.; Liu, X.; Chamyuang, S.; Stadler, M.; Hyde, K.D. Anthostomella is polyphyletic comprising several genera in Xylariaceae. Fungal Divers. 2015, 73, 203–238. [Google Scholar] [CrossRef]
- Tibpromma, S.; Daranagama, D.A.; Boonmee, S.; Promputtha, I.; Nontachaiyapoom, S.; Hyde, K.D. Anthostomelloides krabiensis gen. et sp. nov. (Xylariaceae) from Pandanus odorifer (Pandanaceae). Turk. J. Bot. 2017, 41, 107–116. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Fournier, J.; Rogers, J.D.; Voglmayr, H. Phylogenetic and taxonomic revision of Lopadostoma. Persoonia—Mol. Phylogeny Evol. Fungi 2014, 32, 52–82. [Google Scholar] [CrossRef]
- Voglmayr, H.; Friebes, G.; Gardiennet, A.; Jaklitsch, W.M. Barrmaelia and Entosordaria in Barrmaeliaceae (Fam. Nov., Xylariales) and critical notes on anthostomella-like genera based on multigene phylogenies. Mycol. Prog. 2018, 17, 155–177. [Google Scholar] [CrossRef]
- Wendt, L.; Sir, E.B.; Kuhnert, E.; Heitkämper, S.; Lambert, C.; Hladki, A.I.; Romero, A.I.; Luangsa-ard, J.J.; Srikitikulchai, P.; Peršoh, D.; et al. Resurrection and emendation of the Hypoxylaceae, recognised from a multigene phylogeny of the Xylariales. Mycol. Prog. 2018, 17, 115–154. [Google Scholar] [CrossRef]
- Wang, X.W.; Lombard, L.; Groenewald, J.Z.; Li, J.; Videira, S.I.R.; Samson, R.A.; Liu, X.Z.; Crous, P.W. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia 2016, 36, 83–133. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Restrepo, M.; Decock, C.A.; Costa, M.M.; Crous, P.W. Phylogeny and taxonomy of Circinotrichum, Gyrothrix, Vermiculariopsiella and other setose hyphomycetes. Persoonia 2022, 49, 99–135. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Gardiennet, A.; Voglmayr, H. Resolution of morphology-based taxonomic delusions: Acrocordiella, Basiseptospora, Blogiascospora, Clypeosphaeria, Hymenopleella, Lepteutypa, Pseudapiospora, Requienella, Seiridium and Strickeria. Persoonia—Mol. Phylogeny Evol. Fungi 2016, 37, 82–105. [Google Scholar] [CrossRef]
- Liu, F.; Bonthond, G.; Groenewald, J.Z.; Cai, L.; Crous, P.W. Sporocadaceae, a family of coelomycetous fungi with appendage-bearing conidia. Stud. Mycol. 2019, 92, 287–415. [Google Scholar] [CrossRef]
- Li, Q.R.; Kang, J.C.; Hyde, K.D. Two new species of the genus Collodiscula (Xylariaceae) from China. Mycol Prog. 2015, 14, 52. [Google Scholar] [CrossRef]
- Asgari, B.; Zare, R. Contribution to the taxonomy of the genus Coniocessia (Xylariales). Mycol. Prog. 2011, 10, 189–206. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom Fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Voglmayr, H.; Tello, S.; Jaklitsch, W.M.; Friebes, G.; Baral, H.-O.; Fournier, J. About spirals and pores: Xylariaceae with remarkable germ loci. Persoonia—Mol. Phylogeny Evol. Fungi 2022, 49, 58–98. [Google Scholar] [CrossRef]
- Duong, L.M.; Lumyong, S.; Hyde, K.D.; Jeewon, R. Emarcea castanopsidicola gen. et sp. nov. from Thailand, a new xylariaceous taxon based on morphology and DNA sequences. Stud. Mycol. 2004, 50, 253–260. [Google Scholar]
- Crous, P.W.; Wingfield, M.J.; Guarro, J.; Hernández-Restrepo, M.; Sutton, D.A.; Acharya, K.; Barber, P.A.; Boekhout, T.; Dimitrov, R.A.; Dueñas, M.; et al. Fungal Planet Description Sheets: 320–370. Persoonia—Mol. Phylogeny Evol. Fungi 2015, 34, 167–266. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Rogers, J.D.; Park, D.; Martin, N.A. Entalbostroma Erumpens gen. et sp. nov. (Xylariaceae) from Phormium in New Zealand. Mycotaxon 2016, 131, 765–771. [Google Scholar] [CrossRef]
- Zhang, N.; Castlebury, L.A.; Miller, A.N.; Huhndorf, S.M.; Schoch, C.L.; Seifert, K.A.; Rossman, A.Y.; Rogers, J.D.; Kohlmeyer, J.; Volkmann-Kohlmeyer, B.; et al. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 2006, 98, 1076–1087. [Google Scholar] [CrossRef]
- Stadler, M.; Læssøe, T.; Fournier, J.; Decock, C.; Schmieschek, B.; Tichy, H.-V.; Peršoh, D. A polyphasic taxonomy of Daldinia (Xylariaceae)1. Stud. Mycol. 2014, 77, 1–143. [Google Scholar] [CrossRef]
- Koukol, O.; Kelnarová, I.; Černý, K. Recent observations of sooty bark disease of sycamore maple in Prague (Czech Republic) and the phylogenetic placement of Cryptostroma corticale. For. Pathol. 2015, 45, 21–27. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Chooi, Y.-H.; Gilchrist, C.L.M.; Lacey, E.; Pitt, J.I.; Roets, F.; Swart, W.J.; Cano-Lira, J.F.; Valenzuela-Lopez, N.; et al. Fungal Planet Description Sheets: 1042–1111. Persoonia—Mol. Phylogeny Evol. Fungi 2020, 44, 301–459. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsa-Ard, J.; Thangavel, R.; et al. Fungal Planet Description Sheets: 951–1041. Persoonia 2019, 43, 223–425. [Google Scholar] [CrossRef]
- Crous, P.W.; Schumacher, R.K.; Akulov, A.; Thangavel, R.; Hernández-Restrepo, M.; Carnegie, A.J.; Cheewangkoon, R.; Wingfield, M.J.; Summerell, B.A.; Quaedvlieg, W.; et al. New and Interesting Fungi. 2. Fungal Syst. Evol. 2019, 3, 57–134. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Miadlikowska, J.; Zimmerman, N.B.; Lutzoni, F.; Stajich, J.E.; Arnold, A.E. Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Mol. Phylogenet. Evol. 2016, 98, 210–232. [Google Scholar] [CrossRef]
- Cedeño-Sanchez, M.; Schiefelbein, R.; Stadler, M.; Voglmayr, H.; Bensch, K.; Lambert, C. Redisposition of apiosporous genera Induratia and Muscodor in the Xylariales, following the discovery of an authentic strain of Induratia apiospora. Bot. Stud. 2023, 64, 8. [Google Scholar] [CrossRef]
- Stadler, M.; Kuhnert, E.; Peršoh, D.; Fournier, J. The Xylariaceae as model example for a unified nomenclature following the “One Fungus-One Name” (1F1N) concept. Mycol. Int. J. Fungal Biol. 2013, 4, 5–21. [Google Scholar]
- Voglmayr, H.; Beenken, L. Linosporopsis, a new leaf-inhabiting scolecosporous genus in Xylariaceae. Mycol. Prog. 2020, 19, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Dong, Y.; Phookamsak, R.; Jeewon, R.; Bhat, D.J.; Jones, E.B.G.; Liu, N.-G.; Abeywickrama, P.D.; Mapook, A.; Wei, D.; et al. Fungal Diversity Notes 1151–1276: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2020, 100, 5–277. [Google Scholar] [CrossRef]
- Boonmee, S.; Wanasinghe, D.N.; Calabon, M.S.; Huanraluek, N.; Chandrasiri, S.K.U.; Jones, G.E.B.; Rossi, W.; Leonardi, M.; Singh, S.K.; Rana, S.; et al. Fungal Diversity Notes 1387–1511: Taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 2021, 111, 1–335. [Google Scholar] [CrossRef] [PubMed]
- Jaklitsch, W.M.; Voglmayr, H. Phylogenetic relationships of five genera of Xylariales and Rosasphaeria gen. nov. (Hypocreales). Fungal Divers. 2012, 52, 75–98. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Groenewald, J.Z.; Crous, P.W. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia 2016, 36, 57–82. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Wang, G.-P.; Mao, L.-J.; Komon-Zelazowska, M.; Yuan, Z.-L.; Lin, F.-C.; Druzhinina, I.S.; Kubicek, C.P. Muscodor fengyangensis sp. nov. from southeast China: Morphology, physiology and production of volatile compounds. Fungal Biol. 2010, 114, 797–808. [Google Scholar] [CrossRef]
- Worapong, J.; Strobel, G.; Ford, E.; Li, J.; Baird, G.; Hess, W. Muscodor albus anam. gen. et sp. nov., an endophyte from Cinnamomum zeylanicum. Mycotaxon 2001, 79, 67–79. [Google Scholar]
- Pena, L.C.; Jungklaus, G.H.; Savi, D.C.; Ferreira-Maba, L.; Servienski, A.; Maia, B.H.L.N.S.; Annies, V.; Galli-Terasawa, L.V.; Glienke, C.; Kava, V. Muscodor brasiliensis sp. nov. produces volatile organic compounds with activity against Penicillium digitatum. Microbiol. Res. 2019, 221, 28–35. [Google Scholar] [CrossRef]
- Meshram, V. Muscodor camphora, a new endophytic species from Cinnamomum camphora. Mycosphere 2017, 8, 568–582. [Google Scholar] [CrossRef]
- Suwannarach, N.; Bussaban, B.; Hyde, K.D.; Lumyong, S. Muscodor cinnamomi, a new endophytic species from Cinnamomum bejolghota. Mycotaxon 2011, 114, 15–23. [Google Scholar] [CrossRef]
- Hongsanan, S.; Hyde, K.D.; Bahkali, A.H.; Camporesi, E.; Chomnunti, P.; Ekanayaka, H.; Gomes, A.A.M.; Hofstetter, V.; Jones, E.B.G.; Pinho, D.B.; et al. Fungal Biodiversity Profiles 11–20. Cryptogam. Mycol. 2015, 36, 355–380. [Google Scholar] [CrossRef]
- Li, Q.-R.; Kang, J.-C. The sexual morph of Induratia coffeana, a new record from Thailand. MycoAsia 2022, 1, 1–14. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Strobel, G.A.; Moore, E.; Robison, R.; Sears, J. Volatile antimicrobials from Muscodor crispans, a novel endophytic fungus. Microbiology 2010, 156, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Suwannarach, N.; Kumla, J.; Bussaban, B.; Hyde, K.D.; Matsui, K.; Lumyong, S. Molecular and morphological evidence support four new species in the genus Muscodor from northern Thailand. Ann. Microbiol. 2013, 63, 1341–1351. [Google Scholar] [CrossRef]
- Meshram, V.; Gupta, M.; Saxena, S. Muscodor ghoomensis and Muscodor indica: New endophytic species based on morphological features and molecular and volatile organic analysis from northeast India. Sydowia 2015, 67, 133–146. [Google Scholar] [CrossRef]
- Meshram, V.; Kapoor, N.; Saxena, S. Muscodor kashayum sp. nov.—A new volatile anti-microbial producing endophytic fungus. Mycol. Int. J. Fungal Biol. 2013, 4, 196–204. [Google Scholar] [CrossRef]
- Worapong, J.; Strobel, G.; Daisy, B.; Castillo, U.; Baird, G.; Hess, W. Muscodor roseus anam. sp. nov., an endophyte from Grevillea pteridifolia. Mycotaxon 2002, 81, 463–475. [Google Scholar]
- Miller, A.N.; Huhndorf, S.M. Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi). Mol. Phylogenet. Evol. 2005, 35, 60–75. [Google Scholar] [CrossRef]
- Meshram, V.; Saxena, S.; Kapoor, N. Muscodor strobelii, a new endophytic species from south India. Mycotaxon 2014, 128, 93–104. [Google Scholar] [CrossRef]
- Kudalkar, P.; Strobel, G.; Riyaz-Ul-Hassan, S.; Geary, B.; Sears, J. Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 2012, 53, 319–325. [Google Scholar] [CrossRef]
- Saxena, S.; Meshram, V.; Kapoor, N. Muscodor tigerii sp. nov.-Volatile antibiotic producing endophytic fungus from the northeastern Himalayas. Ann. Microbiol. 2015, 65, 47–57. [Google Scholar] [CrossRef]
- Daisy, B.; Strobel, G.; Ezra, D.; Castillo, U.; Baird, G.; Hess, W. Muscodor vitigentis anam. nov., an endophyte from Paullinia paullinioides. Mycotaxon 2002, 84, 39–50. [Google Scholar]
- González, M.C.; Anaya, A.L.; Glenn, A.E.; Macías-Rubalcava, M.L.; Hernández-Bautista, B.E.; Hanlin, R.T. Muscodor yucatanensis, a new endophytic ascomycete from Mexican Chakah, Bursera simaruba. Mycotaxon 2009, 110, 363–372. [Google Scholar] [CrossRef]
- Chen, J.; Feng, X.; Xia, C.; Kong, D.; Qi, Z.; Liu, F.; Chen, D.; Lin, F.; Zhang, C. Confirming the phylogenetic position of the genus Muscodor and the description of a new Muscodor species. Mycosphere 2019, 10, 187–201. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Kuo, C.-H.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Gentekaki, E.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; De Silva, N.I.; Promputtha, I.; et al. Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers. 2021, 108, 1–215. [Google Scholar] [CrossRef]
- Dai, D.Q.; Phookamsak, R.; Wijayawardene, N.N.; Li, W.J.; Bhat, D.J.; Xu, J.C.; Taylor, J.E.; Hyde, K.D.; Chukeatirote, E. Bambusicolous Fungi. Fungal Divers. 2017, 82, 1–105. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Gareth Jones, E.B.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal Diversity Notes 709–839: Taxonomic and phylogenetic contributions to fungal Taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Bhat, J.D.; Jones, E.B.G.; McKenzie, E.H.C.; Dai, D.Q.; Daranagama, D.A.; Dayarathne, M.C.; Goonasekara, I.D.; et al. Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers. 2015, 73, 73–144. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Gené, J.; Castañeda-Ruiz, R.F.; Mena-Portales, J.; Crous, P.W.; Guarro, J. Phylogeny of saprobic microfungi from southern Europe. Stud. Mycol. 2017, 86, 53–97. [Google Scholar] [CrossRef]
- Tang, A.M.C.; Jeewon, R.; Hyde, K.D. Phylogenetic utility of protein (RPB2, β-Tubulin) and ribosomal (LSU, SSU) gene sequences in the systematics of Sordariomycetes (Ascomycota, Fungi). Antonie Van Leeuwenhoek 2007, 91, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Konta, S.; Tibpromma, S.; Karunarathna, S.; Samarakoon, M.; Steven, L.; Mapook, A.; Boonmee, S.; Senwanna, C.; Balasuriya, A.; Eungwanichayapant, P.; et al. Morphology and multigene phylogeny reveal ten novel taxa in Ascomycota from terrestrial palm substrates (Arecaceae) in Thailand. Mycosphere 2023, 14, 107–152. [Google Scholar] [CrossRef]
- Peršoh, D.; Melcher, M.; Graf, K.; Fournier, J.; Stadler, M.; Rambold, G. Molecular and morphological evidence for the delimitation of Xylaria hypoxylon. Mycologia 2009, 101, 256–268. [Google Scholar] [CrossRef]
- Lu, B.S.; Hyde, K.D. A World Monograph of Anthostomella; Fungal Diversity Press: Hong Kong, China, 2000. [Google Scholar]
- Hyde, K.D.; Fröhlich, J.; Taylor, J.E. Fungi from palms XXXVI. Reflections on unitunicate ascomycetes with aiospores. Sydowia 1998, 50, 21–80. [Google Scholar]
- Samuels, G.J.; Müller, E.; Petrini, O. Studies in the Amphisphaeriaceae (Sensu Lato) 3. New species of Monographella and Pestalosphaeria, and two new genera. Mycotaxon 1987, 28, 473–499. [Google Scholar]
- Sugita, R.; Hirayama, K.; Shirouzu, T.; Tanaka, K. Spirodecosporaceae fam. nov. (Xylariales, Sordariomycetes) and two new species of Spirodecospora. Fungal Syst. Evol. 2022, 10, 217–229. [Google Scholar] [CrossRef]
- Konta, S.; Hongsanan, S.; Tibpromma, S.; Thongbai, B.; Maharachchikumbura, S.S.N.; Bahkali, A.H.; Hyde, K.D.; Boonmee, S. An advance in the endophyte story: Oxydothidaceae fam. nov. with six new species of Oxydothis. Mycosphere 2016, 7, 1425–1446. [Google Scholar] [CrossRef]
- Stadler, M.; Lambert, C.; Wibberg, D.; Kalinowski, J.; Cox, R.J.; Kolařík, M.; Kuhnert, E. Intragenomic polymorphisms in the ITS region of high-quality genomes of the Hypoxylaceae (Xylariales, Ascomycota). Mycol. Prog. 2020, 19, 235–245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).