Effects of Interaction between Claroideogolmus etuicatum and Bacillus aryabhattai on the Utilization of Organic Phosphorus in Camellia oleifera Abel.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials and Growth Substrates
2.2. Three-Compartment Device and Experimental Design
2.3. Mycorrhizal Colonization and Hyphal Density
2.4. Plant Growth and P Content Measurement
2.5. Measurement of Substrate Phosphatase Activities and P Content
2.6. Data Analysis
3. Results
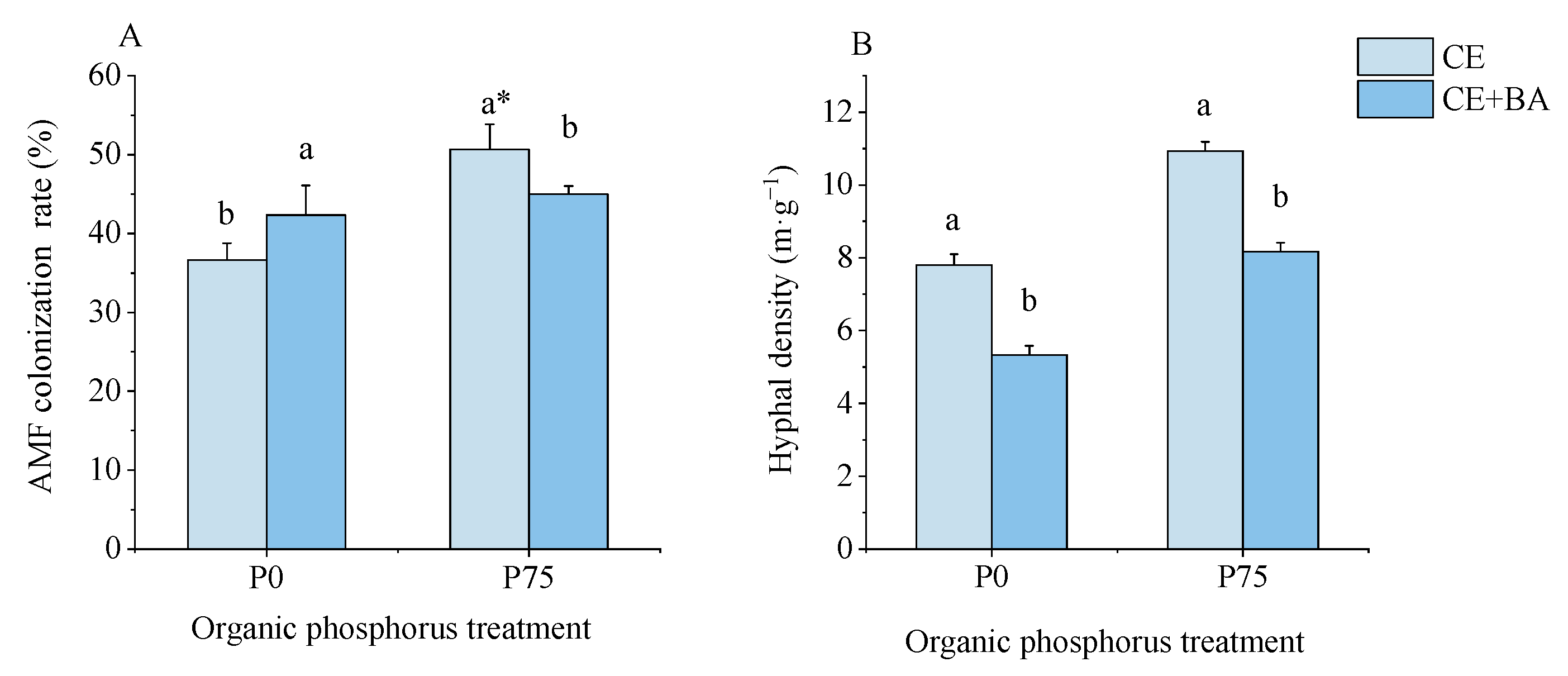
3.1. Mycorrhizal Colonization and Hyphal Density
3.2. Plant Height and Biomass
3.3. P Content of C. oleifera
3.4. Soil Phosphatase and Phytase Activities
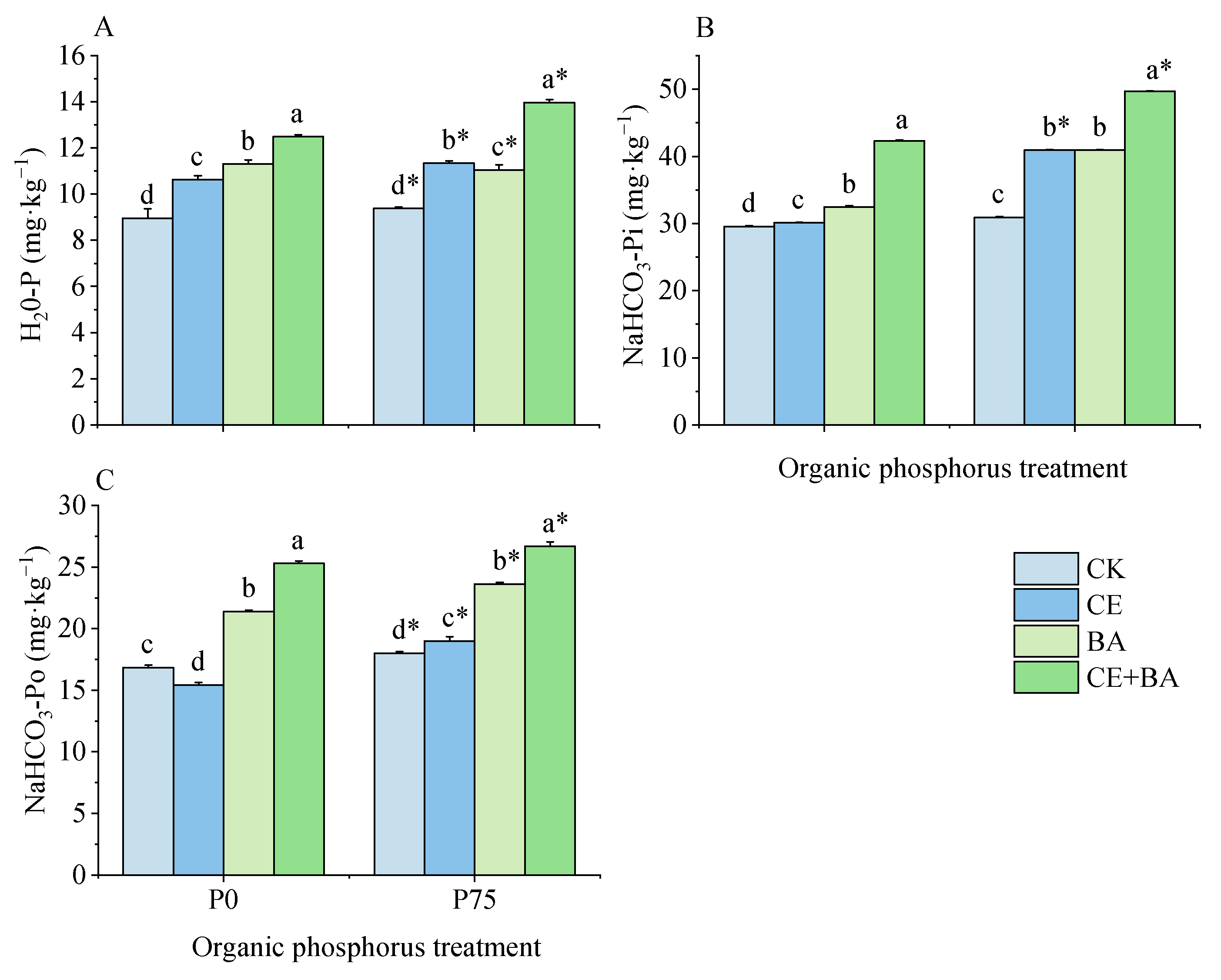
3.5. Soil Liable P Content
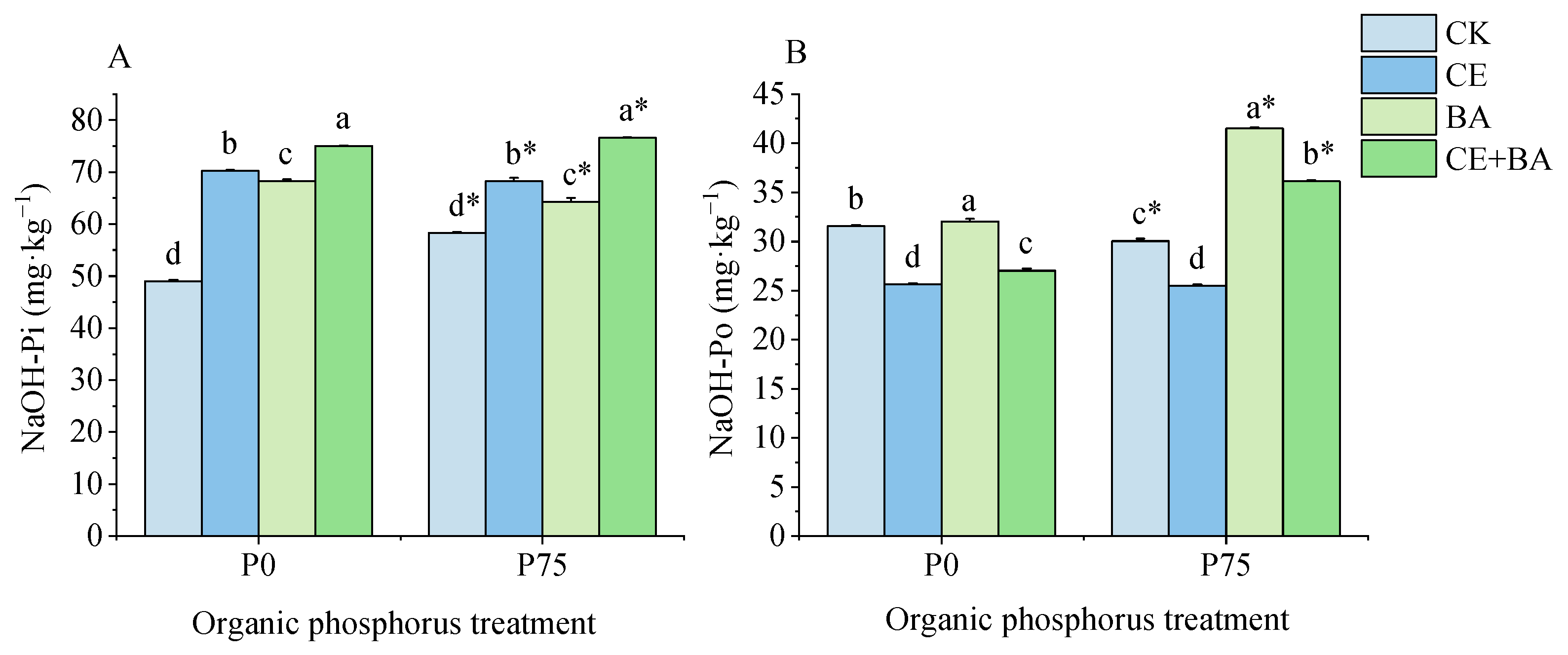
3.6. Soil Moderately Liable P Content
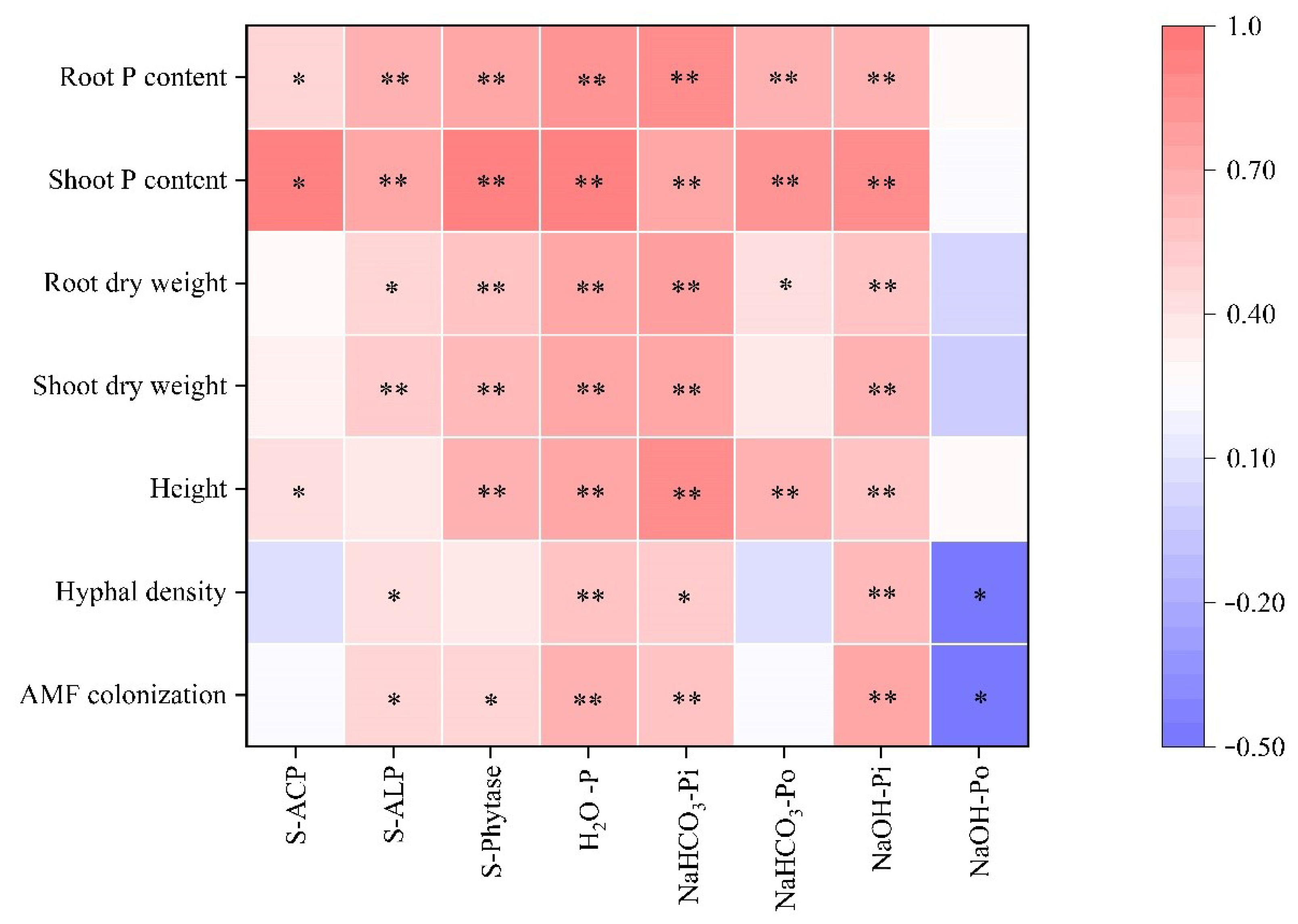
3.7. Correlation Analysis
4. Discussion
4.1. PSB Addition Limits AMF Mycelial Growth and C. oleifera Colonization
4.2. AMF and PSB Interactions Promote C. oleifera Growth and Phosphorus Uptake
4.3. AMF Interacts with PSB to Enhance Soil Phosphatase Activity for Organic P Mineralization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kopittke, P.M.; Dalal, R.C.; Wang, P.; Menzies, N.W. Effects of long-term cultivation on phosphorus (P) in five low-input, subtropical Australian soils. Agric. Ecosyst. Environ. 2018, 252, 191–199. [Google Scholar] [CrossRef]
- Elser, J.J. Phosphorus: A limiting nutrient for humanity? Curr. Opin. Biotechnol. 2012, 23, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Batjes, N. A world dataset of derived soil properties by FAO–UNESCO soil unit for global modelling. Soil Use Manag. 1997, 13, 9–16. [Google Scholar] [CrossRef]
- Cakmak, I. Plant nutrition research: Priorities to meet human needs for food in sustainable ways. Plant Soil 2002, 247, 3–24. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. A review of recent substance flow analyses of phosphorus to identify priority management areas at different geographical scales. Resour. Conserv. Recycl. 2014, 83, 213–228. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera Abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Skinner, M.F.; Attiwill, P.M. The productivity of pine plantations in relation to previous land use. Plant Soil 1981, 61, 329–339. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal symbiosis. Q. Rev. Biol. 2008, 3, 273–281. [Google Scholar]
- Pellegrino, E.; Turrini, A.; Gamper, H.A.; Cafà, G.; Bonari, E.; Young, J.P.W.; Giovannetti, M. Establishment, persistence and effectiveness of arbuscular mycorrhizal fungal inoculants in the field revealed using molecular genetic tracing and measurement of yield components. New Phytol. 2012, 194, 810–822. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.P.; Felix, W.P.; Yano-Melo, A.M. Solubilization of phosphates in vitro by Aspergillus spp. and Penicillium spp. Ecol. Eng. 2012, 42, 85–89. [Google Scholar] [CrossRef]
- Zak, D.H.; Goldhammer, T.; Cabezas, A.; Gelbrecht, J.; Gurke, R.; Wagner, C.; Reuter, H.; Augustin, J.; Klimkowska, A.; McInnes, R. Top soil removal reduces water pollution from phosphorus and dissolved organic matter and lowers methane emissions from rewetted peatlands. J. Appl. Ecol. 2018, 55, 311–320. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhang, Q.; Li, S.; Sun, Y.; Lu, W.; Ma, C. Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express 2020, 10, 200. [Google Scholar] [CrossRef]
- Andrade, G.; Linderman, R.G.; Bethlenfalvay, G.J. Bacterial associations with the mycorrhizosphere and hyphosphere of the arbuscular mycorrhizal fungus Glomus mosseae. Plant Soil 1998, 202, 79–87. [Google Scholar] [CrossRef]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldan, A. Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl. Soil Ecol. 2007, 35, 480–487. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, M.; Liu, Y.; Zhang, F.; Hodge, A.; Feng, G. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Deng, B.; Fang, H.; Jiang, N.; Feng, W.; Luo, L.; Wang, J.; Wang, H.; Hu, D.; Guo, X.; Zhang, L. Biochar Is Comparable to dicyandiamide in the mitigation of nitrous oxide emissions from Camellia oleifera Abel. Fields. Forests 2019, 10, 1076. [Google Scholar] [CrossRef]
- Lee, W.T.; Tung, Y.T.; Wu, C.C.; Tu, P.S.; Yen, G.C. Camellia Oil (Camellia oleifera Abel.) Modifies the composition of gut microbiota and alleviates acetic acid-induced colitis in rats. J. Agric. Food Chem. 2018, 66, 7384–7392. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, Z.; Lin, Y.; Zhang, L. Effects of Funneliformis mosseae on the utilization of organic phosphorus in Camellia oleifera Abel. Can. J. Microbiol. 2021, 67, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.L.; Zhang, Y.; Zhang, L.P.; Wang, S.; Feng, Y.; Rong, N.H. Complete genome sequence of Bacillus megaterium JX285 isolated from Camellia oleifera rhizosphere. Comput. Biol. Chem. 2019, 79, 1–5. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Abbott, L.K.; Robson, A.D.; Boer, G.D. The effect of phosphorus on the formation of hyphae in soil by thevesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytol. 1984, 97, 437–446. [Google Scholar] [CrossRef]
- Hedley, M.; Kirk, G.; Santos, M. Phosphorus efficiency and the forms of soil phosphorus utilized by upland rice cultivars. Plant Soil 1994, 158, 53–62. [Google Scholar] [CrossRef]
- Höflich, G.; Wiehe, W.; Kühn, G. Plant growth stimulation by inoculation with symbiotic and associative rhizosphere microorganisms. Experientia 1994, 50, 897–905. [Google Scholar] [CrossRef]
- Fester, T.; Maier, W.; Strack, D. Accumulation of secondary compounds in barley and wheat roots in response to inoculation with an arbuscular mycorrhizal fungus and co-inoculation with rhizosphere bacteria. Mycorrhiza 1999, 8, 241–246. [Google Scholar] [CrossRef]
- Jyoti, S.; Anamika, J. Impact of a Phosphate solubilizing bacterium and an arbuscular mycorrhizal fungus (Glomus etunicatum) on growth, yield and P concentration in wheat plants. Clean Soil Air Water 2014, 42, 1248–1252. [Google Scholar]
- Deveau, A.; Palin, B.; Delaruelle, C.; Peter, M.; Kohler, A.; Pierrat, J.C.; Sarniguet, A.; Garbaye, J.; Martin, F.; Frey-Klett, P. The mycorrhiza helper pseudomonas fluorescens BBc6R8 has a specific priming effect on the growth, morphology and gene expression of the ectomycorrhizal fungus Laccaria bicolor S238N. New Phytol. 2007, 175, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Mosse, B. The establishment of vesicular-arbuscular mycorrhiza under aseptic conditions. Microbiology 1962, 27, 509–520. [Google Scholar] [CrossRef]
- Toljander, J.F.; Artursson, V.; Paul, L.R.; Jansson, J.K.; Finlay, R.D. Attachment of different soil bacteria to arbuscular mycorrhizal fungal extraradical hyphae is determined by hyphal vitality and fungal species. FEMS Microbiol. Lett. 2006, 254, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H. Enhanced Phosphorus Fertilizer Use Efficiency with Microorganisms. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 215–245. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of phosphate solubilizing microorganisms from managing soil phosphorus deficiency to mediating biogeochemical P cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Shah, C.; Mali, H.; Mesara, S.N.; Dhameliya, H.A.; Subramanian, R.B. Combined inoculation of phosphate solubilizing bacteria with mycorrhizae to alleviate the phosphate deficiency in Banana. Biologia 2022, 77, 2657–2666. [Google Scholar] [CrossRef]
- Tate, K. The biological transformation of P in soil. Biol. Process. Soil Fertil. 1984, 76, 245–256. [Google Scholar]
- Feng, G.; Su, Y.; Li, X.; Wang, H.; Zhang, F.; Tang, C.; Rengel, Z. Histochemical visualization of phosphatase released by arbuscular mycorrhizal fungi in soil. J. Plant Nutr. 2002, 25, 1–11. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, R.; Kertesz, M.A.; Zhang, F.; Feng, G. Arbuscular mycorrhizal fungal hyphae mediating acidification can promote phytate mineralization in the hyphosphere of maize (Zea mays L.). Soil Biol. Biochem. 2013, 65, 69–74. [Google Scholar] [CrossRef]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Q.; MacKenzie, A.F.; Liang, B.C.; Drury, C.F. Soil test phosphorus and phosphorus fractions with long-term phosphorus addition and depletion. Soil Sci. Soc. Am. J. 2004, 68, 519–528. [Google Scholar]



| P | Inoculation | Plant Height cm | Shoot Dry Weight g | Root Dry Weight g |
|---|---|---|---|---|
| P0 | CK | 12.63 ± 0.23 b | 0.74 ± 0.04 b | 0.63 ± 0.10 b |
| CE | 13.03 ± 0.84 b | 0.95 ± 0.03 a | 1.20 ± 0.22 a | |
| BA | 13.53 ± 0.47 b | 0.94 ± 0.09 a | 0.74 ± 0.08 b | |
| CE + BA | 15.30 ± 0.20 a | 1.06 ± 0.12 a | 1.08 ± 0.18 a | |
| P75 | CK | 14.30 ± 1.15 b | 0.86 ± 0.16 b | 0.46 ± 0.02 c * |
| CE | 16.40 ± 0.40 a * | 1.86 ± 0.17 a * | 1.74 ± 0.17 a * | |
| BA | 15.60 ± 0.70 a * | 1.05 ± 0.25 b | 1.05 ± 0.13 b * | |
| CE + BA | 16.93 ± 0.21 a * | 1.93 ± 0.09 a * | 1.79 ± 0.11 a * | |
| Two-way ANOVA | ||||
| P | 75.185 ** | 81.637 ** | 36.747 ** | |
| Inoculation | 18.747 ** | 35.750 ** | 60.061 ** | |
| P × Inoculation | 2.607 NS | 16.655 ** | 11.227 ** | |
| Index | P | Inoculation | P × Inoculation |
|---|---|---|---|
| Shoot P content | 2.217 NS | 191.010 ** | 3.595 * |
| Root P content | 463.154 ** | 253.411 ** | 91.575 ** |
| Index | P | Inoculation | P × Inoculation |
|---|---|---|---|
| S-ACP activity | 0.211 NS | 337.277 ** | 4.153 * |
| S-ALP activity | 1.237 NS | 32.167 ** | 20.049 ** |
| S-phytase activity | 5.559 * | 51.282 ** | 0.802 NS |
| H20-P | 53.255 ** | 424.131 ** | 19.824 ** |
| NaHCO3-Pi | 16,946.042 ** | 14,928.094 ** | 1406.401 ** |
| NaHCO3-Po | 508.959 ** | 2092.466 ** | 34.980 ** |
| NaOH-Pi | 54.846 ** | 3193.198 ** | 316.006 ** |
| NaOH-Po | 3378.228 ** | 3975.991 ** | 1615.785 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Lin, Y.; Zhang, L.; Wu, F.; Zhang, Y.; Huang, S. Effects of Interaction between Claroideogolmus etuicatum and Bacillus aryabhattai on the Utilization of Organic Phosphorus in Camellia oleifera Abel. J. Fungi 2023, 9, 977. https://doi.org/10.3390/jof9100977
Huang Y, Lin Y, Zhang L, Wu F, Zhang Y, Huang S. Effects of Interaction between Claroideogolmus etuicatum and Bacillus aryabhattai on the Utilization of Organic Phosphorus in Camellia oleifera Abel. Journal of Fungi. 2023; 9(10):977. https://doi.org/10.3390/jof9100977
Chicago/Turabian StyleHuang, Yuxuan, Yulan Lin, Linping Zhang, Fei Wu, Yang Zhang, and Shaohua Huang. 2023. "Effects of Interaction between Claroideogolmus etuicatum and Bacillus aryabhattai on the Utilization of Organic Phosphorus in Camellia oleifera Abel." Journal of Fungi 9, no. 10: 977. https://doi.org/10.3390/jof9100977
APA StyleHuang, Y., Lin, Y., Zhang, L., Wu, F., Zhang, Y., & Huang, S. (2023). Effects of Interaction between Claroideogolmus etuicatum and Bacillus aryabhattai on the Utilization of Organic Phosphorus in Camellia oleifera Abel. Journal of Fungi, 9(10), 977. https://doi.org/10.3390/jof9100977






