Resistance of Mycorrhizal Cinnamomum camphora Seedlings to Salt Spray Depends on K+ and P Uptake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Determination of Root Morphological Traits and Plant Biomass
2.3. Determination of Root Colonization
2.4. Determination of Elemental Concentrations
2.5. Data Analysis
3. Results
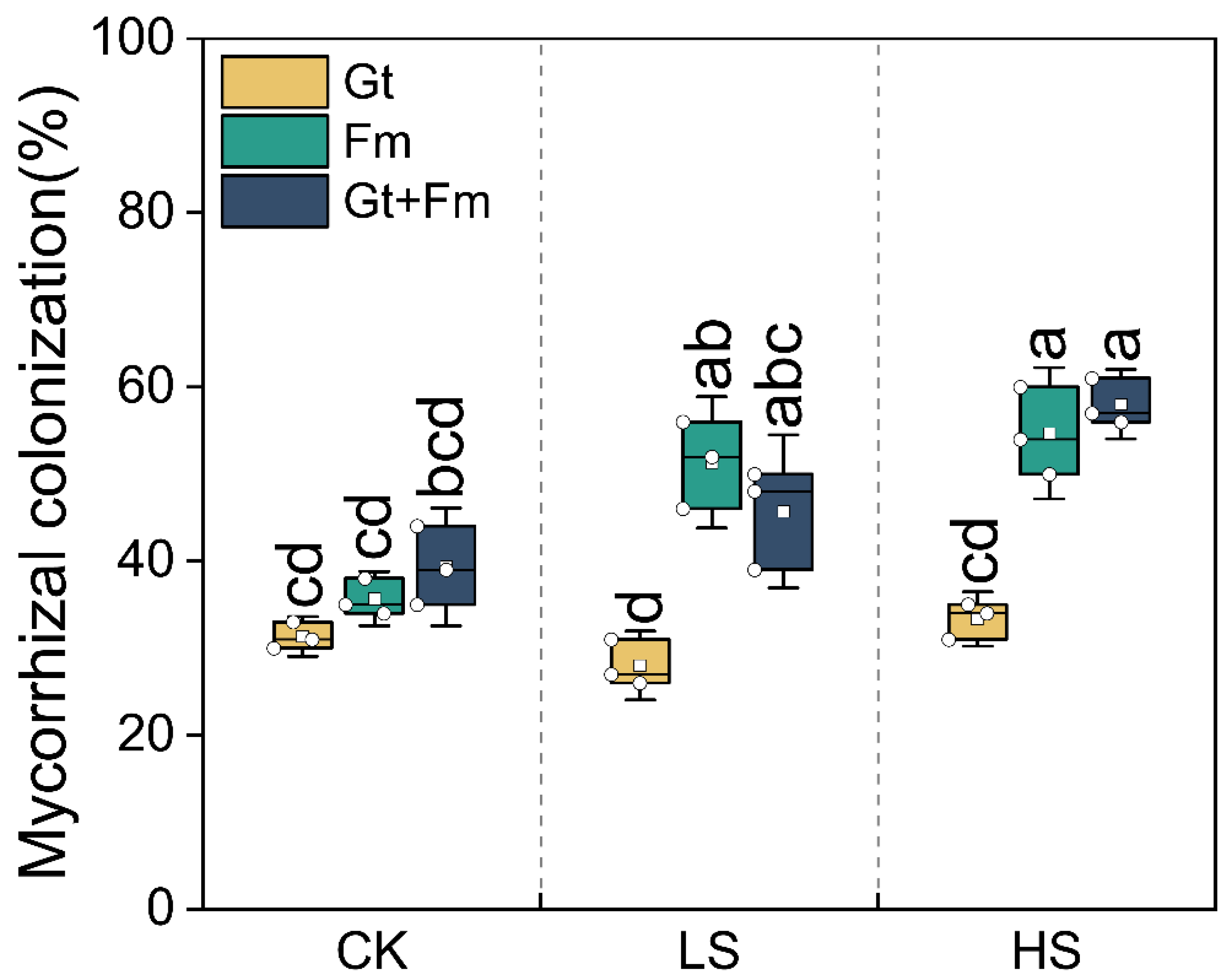
3.1. Mycorrhizal Colonization
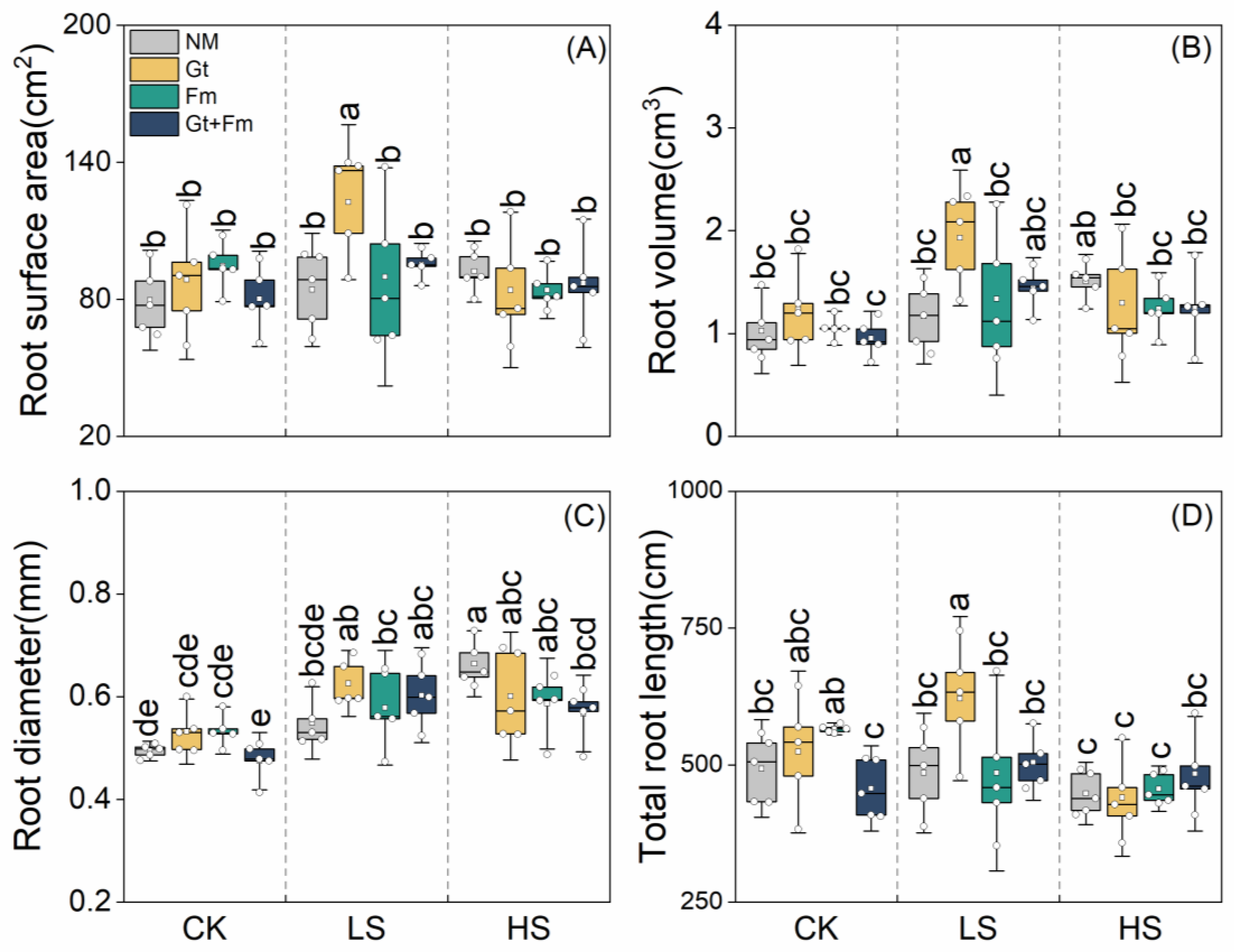
3.2. Root Morphological Traits
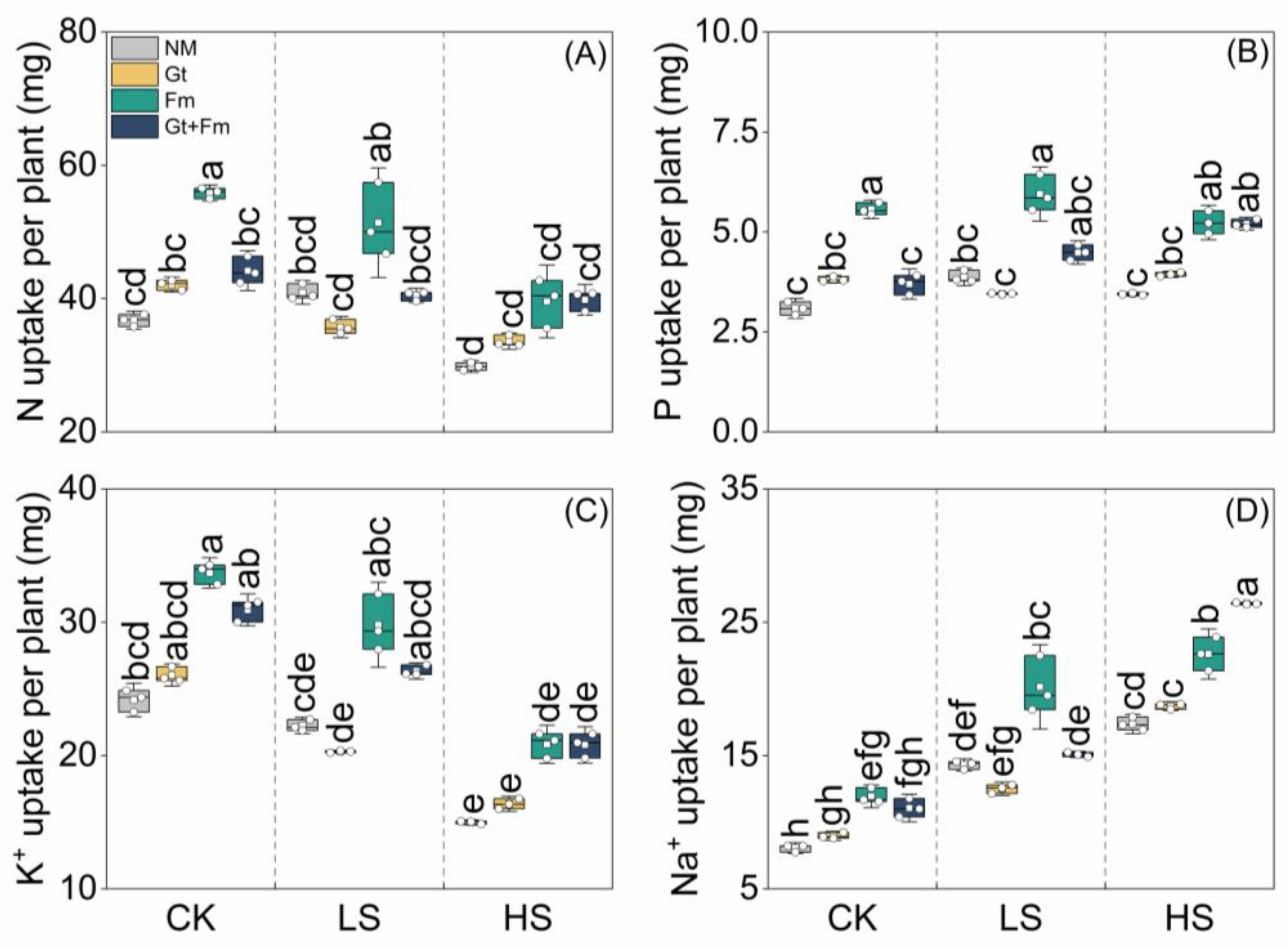
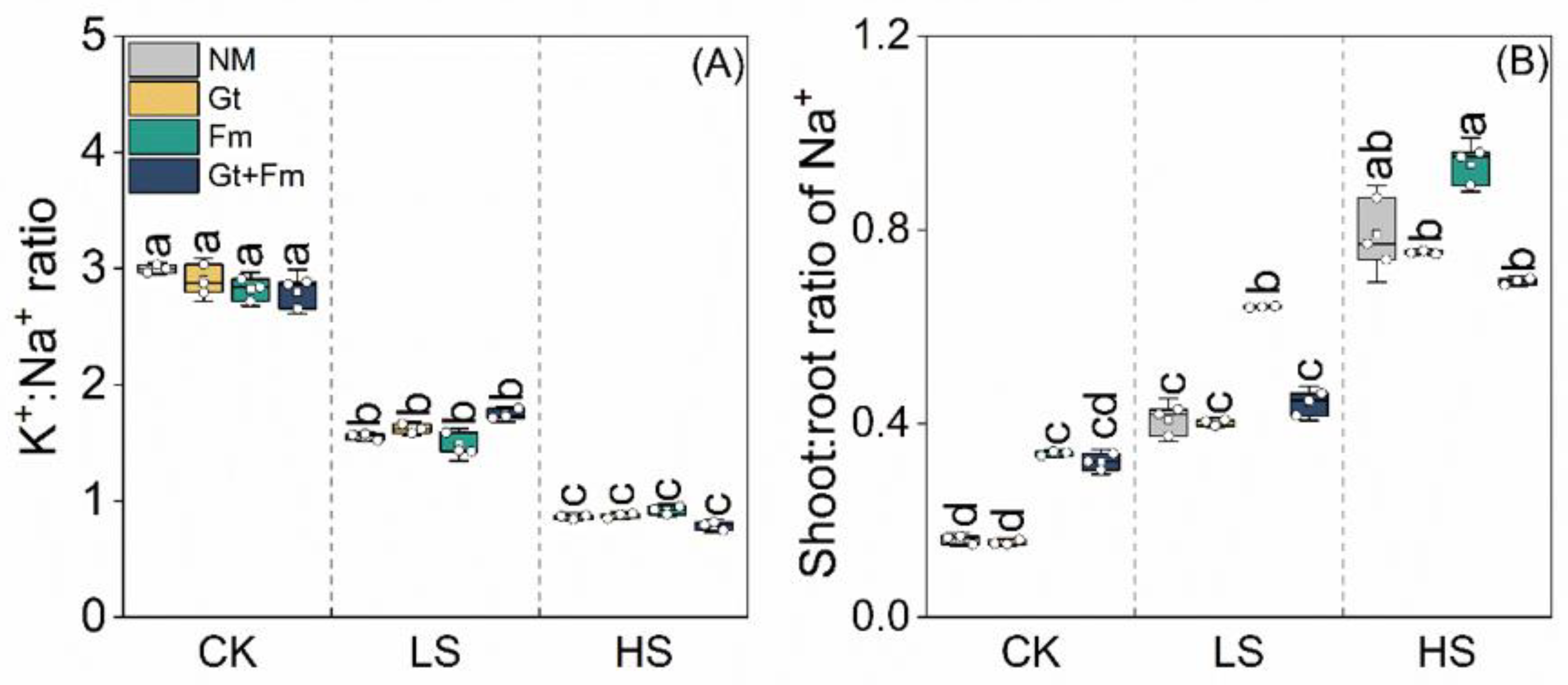
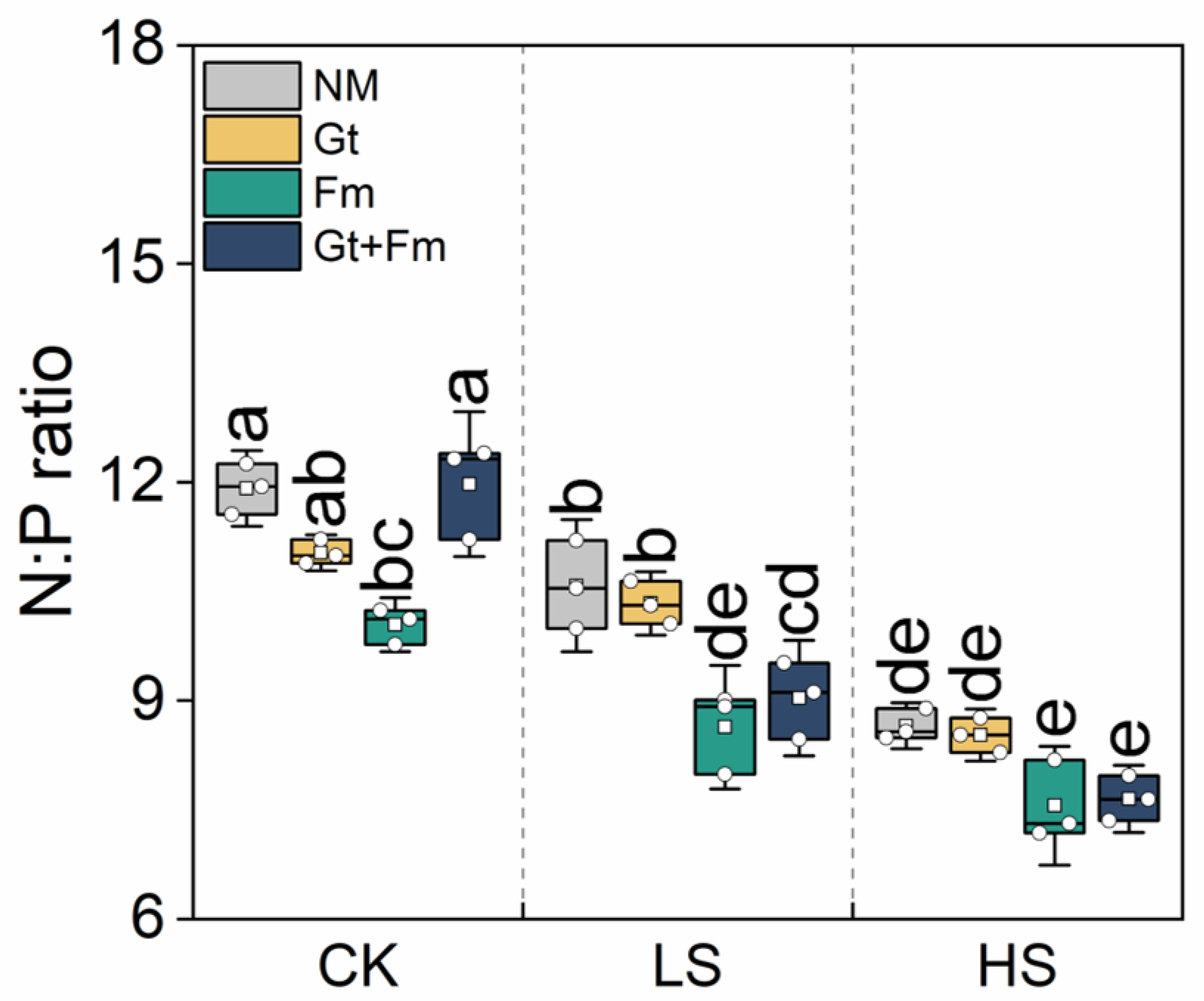
3.3. Elemental Concentrations and Their Ratios
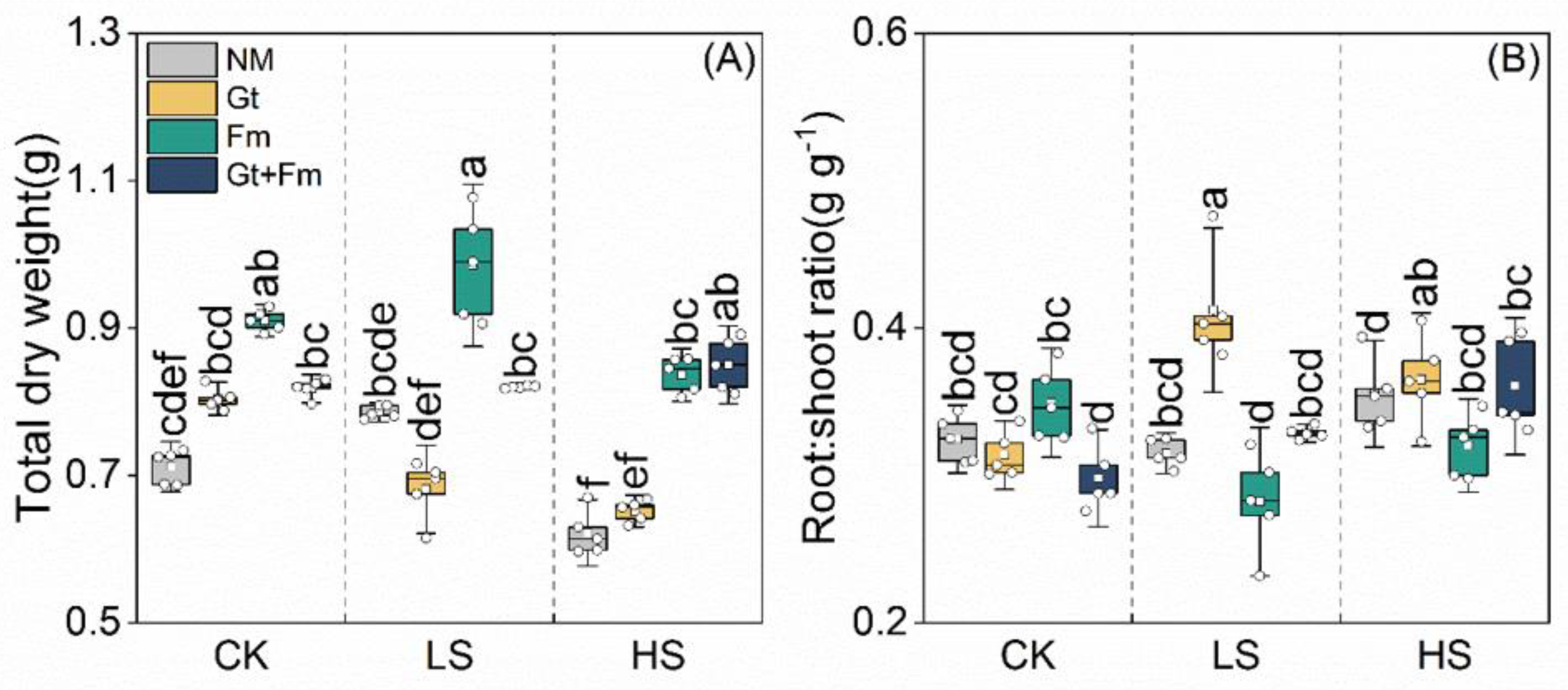
3.4. Biomass Accumulation and Allocation
3.5. Salinity Tolerance, Mycorrhizal Efficiencies, and Their Relationships with Other Plant Traits
4. Discussion
4.1. Mycorrhizal Efficiency on Plant Biomass
4.2. Mycorrhizal Efficiency on Elementary Absorption
4.3. Potential Mechanism of Salt Tolerance Associated with Mycorrhizal Inoculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Wang, X.X.; Lin, J.G.; Liu, J.; Jiang, M.S.; Chu, L.X. Chemical composition and antifungal activity of extracts from the xylem of Cinnamomum camphora. Bioresources 2014, 9, 2560–2571. [Google Scholar] [CrossRef]
- Chen, Z.M.; Chen, Y.X.; Du, G.J.; Wu, X.L.; Li, F. Effects of 60-day NO2 fumigation on growth, oxidative stress and antioxidative response in Cinnamomum camphora seedlings. J. Zhejiang Univ. Sci. B 2010, 11, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Maze, K.M.; Whalley, R.D.B. Effects of salt spray and sand burial on Spinifex sericeus R. Br. Aust. J. Ecol. 1992, 17, 9–19. [Google Scholar] [CrossRef]
- Luo, Y. Sustainability associated coastal eco-environmental problems and coastal science development in China. Bull. Chin. Acad. Sci. 2016, 31, 1133–1142. [Google Scholar] [CrossRef]
- Du, J.H.; Hesp, P.A. Salt spray distribution and its impact on vegetation zonation on coastal dunes: A review. Estuaries Coasts 2020, 43, 1885–1907. [Google Scholar] [CrossRef]
- Textor, C.; Schulz, M.; Guibert, S.; Kinne, S.; Tie, X. Analysis and quantification of the diversities of aerosol life cycles within AeroCom. Atmos. Chem. Phys. 2006, 5, 8331–8420. [Google Scholar] [CrossRef]
- Hesp, P. Foredunes and blowouts: Initiation, geomorphology and dynamics. Geomorphology 2002, 48, 245–268. [Google Scholar] [CrossRef]
- Griffiths, M.E. Salt spray and edaphic factors maintain dwarf stature and community composition in coastal sandplain heathlands. Plant Ecol. 2006, 186, 69–86. [Google Scholar] [CrossRef]
- Boyce, S.G. The salt spray community. Ecol. Monogr. 1954, 24, 29–67. [Google Scholar] [CrossRef]
- de Vos, A.C.; Broekman, R.; Groot, M.P.; Rozema, J. Ecophysiological response of Crambe maritima to airborne and soil-borne salinity. Ann. Bot. 2010, 105, 925–937. [Google Scholar] [CrossRef]
- Mckay, W.; Garland, J.; Livesley, D.; Halliwell, C.; Walker, M. The characteristics of the shore-line sea spray aerosol and the landward transfer of radionuclides discharged to coastal sea water. Atmos. Environ. 1994, 28, 3299–3309. [Google Scholar] [CrossRef]
- Alpha, C.G.; Drake, D.R.; Goldstein, G. Morphological and physiological responses of Scaevola sericea (Goodeniaceae) seedlings to salt spray and substrate salinity. Am. J. Bot. 1996, 83, 86–92. [Google Scholar] [CrossRef]
- Guan, H.; Love, A.J.; Simmons, C.T.; Makhnin, O.; Kayaalp, A.S. Factors influencing chloride deposition in a coastal hilly area and application to chloride deposition mapping. Hydrol. Earth Syst. Sci. 2010, 14, 801–813. [Google Scholar] [CrossRef]
- Silva, G.M.D.; Hesp, P.; Peixoto, J.; Dillenburg, S.R. Foredune vegetation patterns and alongshore environmental gradients: Moçambique Beach, Santa Catarina Island, Brazil. Earth Surf. Process. Landf. 2010, 33, 1557–1573. [Google Scholar] [CrossRef]
- Grythe, H.; Ström, J.; Krejci, R.; Quinn, P.; Stohl, A. A review of sea-spray aerosol source functions using a large global set of sea salt aerosol concentration measurements. Atmos. Chem. Phys. 2014, 14, 1277–1297. [Google Scholar] [CrossRef]
- Griffiths, M.E.; Orians, C.M. Salt spray effects on forest succession in rare coastal sandplain heathlands: Evidence from field surveys and Pinus rigida transplant experiments. J. Torrey Bot. Soc. 2004, 131, 23. [Google Scholar] [CrossRef]
- Munns, R. Physiological processes limiting plant growth in saline soils: Some dogmas and hypotheses. Plant Cell Environ. 1993, 16, 15–24. [Google Scholar] [CrossRef]
- Burkhardt, J.; Kaiser, H.; Kappen, L.; Goldbach, H.E. The possible role of aerosols on stomatal conductivity for water vapour. Basic Appl. Ecol. 2001, 2, 351–364. [Google Scholar] [CrossRef]
- Kouali, A.; Mouradi, M.; Latrach, L.; Khadraji, A.; Elasri, O.; Baicha, Z. Study of effects of seawater salt spray on growth, chlorophyll fluorescence and chlorophyll content in three coastal species of Morocco. J. Mater. Environ. Sci. 2017, 8, 4385–4390. [Google Scholar] [CrossRef]
- Toscano, S.; La Fornara, G.; Romano, D. Salt spray and surfactants induced morphological, physiological, and biochemical responses in Callistemon citrinus (Curtis) plants. Horticulturae 2022, 8, 261. [Google Scholar] [CrossRef]
- Shi, J.C.; Wang, X.l.; Wang, E. Mycorrhizal symbiosis in plant growth and stress adaptation: From genes to ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Courty, P.E.; Smith, P.; Koegel, S.; Redecker, D.; Wipf, D. Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. Crit. Rev. Plant Sci. 2015, 34, 4–16. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Varma, A.; Choudhary, D.K. Mycorrhizosphere and Pedogenesis; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Genre, A.; Lanfranco, L.; Perotto, S.; Bonfante, P. Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 2020, 18, 649–660. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, C.; Qiu, Y.; Yu, S.; Xia, L.; He, X.; Wu, A.; Zhang, N. Arbuscular mycorrhizal fungi protect a subtropical tree species exposed to simulated acid rain by accelerating photosynthetic ability, antioxidant enzymes and osmolyte accumulation. J. Plant Ecol. 2022, 15, 1036–1048. [Google Scholar] [CrossRef]
- Wu, Q.S.; He, X.H.; Zou, Y.N.; Liu, C.Y.; Xiao, J.; Li, Y. Arbuscular mycorrhizas alter root system architecture of Citrus tangerine through regulating metabolism of endogenous polyamines. Plant Growth Regul. 2012, 68, 27–35. [Google Scholar] [CrossRef]
- Martin, F. Molecular Mycorrhizal Symbiosis; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.D.; Akter, M.A.; Okazaki, S. Plant salinity tolerance conferred by arbuscular mycorrhizal fungi and associated mechanisms: A meta-analysis. Front. Plant Sci. 2020, 11, 588550. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Graham, J.-H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Atkinson, D.A.; Black, K.E.; Forbes, P.J.; Hooker, J.E.; Watson, C.A. The influence of arbuscular mycorrhizal colonization and environment on root development in soil. Eur. J. Soil Sci. 2003, 54, 751–757. [Google Scholar] [CrossRef]
- Zhang, D.H.; Hong, C.D.; Shen, X.Z.; Ye, G.F.; Zhang, R.F.; Shi, M.F.; SHi, M.R.; Zhu, Y.X. Experimental study on salinity tolerance in tree species in shoal of Northern Zhejiang. J. Zhejiang For. Sci. Technol. 2002, 12, 166–170. [Google Scholar] [CrossRef]
- Wang, J.P.; Wang, S.T.; Yue, J.M.; Zhang, J.C.; Zhang, L.; You, Y.H.; Zhao, W.R. Physiological response of Cinnamomum camphora seedlings to NaCl stress. Sci. Soil Water Conserv. China 2016, 14, 82–89. [Google Scholar] [CrossRef]
- Yan, M.; Zhong, Z.C. Effects of aluminum stress on the growth of camphor seedlings infected with arbuscular mycorrhizal fungi. For. Sci. 2007, 43, 59–65. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Boughattas, S.; Hu, S.j.; Oh, S.H.; Sa, T.M. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, T.T.; Wu, A.P.; Li, Y.; Zhang, N.l. Mycorrhizal benefits of salt-stressed Cinnamomum camphora (L.) Presl. may be related to P and Mn2+ contents in shoots, biomass allocation, and K+/Na+ in roots and shoots. Forests 2022, 13, 1882. [Google Scholar] [CrossRef]
- Liang, M.X.; Liu, X.B.; Etienne, R.S.; Huang, F.M.; Wang, Y.F.; Yu, S.X. Arbuscular mycorrhizal fungi counteract the Janzen-Connell effect of soil pathogens. Ecology 2015, 96, 562–574. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, M.Q.; Li, Y.; Wu, A.P.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef]
- McNamara, N.P.; Black, H.I.J.; Beresford, N.A.; Parekh, N.R. Effects of acute gamma irradiation on chemical, physical and biological properties of soils. Appl. Soil Ecol. 2003, 24, 117–132. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Li, Y.; Xiao, Y. Nitrogen fertilizer enhances growth and nutrient uptake of Medicago sativa inoculated with Glomus tortuosum grown in Cd-contaminated acidic soil. Chemosphere 2017, 167, 204–211. [Google Scholar] [CrossRef]
- Wu, Q.S.; Li, Y.; Zou, Y.N.; He, X.H. Arbuscular mycorrhiza mediates glomalin-related soil protein production and soil enzyme activities in the rhizosphere of trifoliate orange grown under different P levels. Mycorrhiza 2015, 25, 121–130. [Google Scholar] [CrossRef]
- Xu, F.; Kang, J.C. The climatological distribution of surface salinity in the East China Sea and adjacent northwest Pacific Ocean during 1981–2010. Mar. Geol. Quat. Geol. 2019, 39, 44–60. [Google Scholar] [CrossRef]
- Guo, D.L.; Xia, M.X.; Wei, X.; Chang, W.J.; Liu, Y.; Wang, Z.Q. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.S.L.; Faccio, A.; Genre, A.; Pieterse, C.M.J.; Bonfante, P.; van der Heijden, M.G.A. Arbuscular mycorrhizal fungi reduce growth and infect roots of the non-host plant Arabidopsis thaliana. Plant Cell Environ. 2013, 36, 1926–1937. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Bei, M.R.; Luo, X.H.; Yang, H.Z. Simultaneous determination of nitrogen, phosphorus and potassium in rubber leaf samples by AA3 continuous flow analyzer(CFA). Chin. J. Trop. Crops 2011, 32, 1258–1264. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and water. Soil Sci. 1961, 93, 68. [Google Scholar] [CrossRef]
- Xia, L.N.; Shao, C.l.; Zhang, N.l.; Wu, A.P.; Xie, J.B.; Qiu, Y.J.; He, X.B.; Pei, J.; Wang, X.D.; Wang, Y.H. Improved tolerance of mycorrhizal Torreya grandis seedlings to sulfuric acid rain related to phosphorus and zinc contents in shoots. J. Fungi 2021, 7, 296. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, P.; Bi, Q.; Zhang, Z.H.; Yang, Y.F. The effect of the arbuscular mycorrhizal fungi on the growth of Leymus chinensis under saline stress of different intensities. J. Ecol. 2016, 36, 10. [Google Scholar] [CrossRef]
- He, X.; Shao, C.; Wu, A.; Xia, L.; Li, T.; Pei, J.; Zhang, N.; Wang, Y. Arbuscular mycorrhizal fungi enhance nutrient acquisition and reduce aluminum toxicity in Lespedeza formosa under acid rain. Environ. Sci. Pollut. Res. 2022, 29, 29904–29916. [Google Scholar] [CrossRef]
- Lubke, R.A.; Avis, A.M. The effect of salt spray on the growth of Scirpus nodosus. S. Afr. J. Bot. 1982, 1, 163–164. [Google Scholar] [CrossRef]
- Cheplick, G.P.; Demetri, H. Impact of saltwater spray andsand deposition on the coastal annual Triplasis purpurea (Poaceae). Am. J. Bot. 1999, 86, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.E.; Orians, C.M. Salt spray differentially affects water status, necrosis, and growth in coastal sandplain heathland species. Am. J. Bot. 2003, 90, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Kekere, O. Effect of air-borne salinity on the growth and appearance of the tropical perennial strandline plant, Commelina erecta subsp. maritima (C.V. Morton) C.V. Morton. Sustain. Agric. Res. 2014, 3, 77–88. [Google Scholar] [CrossRef]
- Goldstein, G.; Drake, D.R.; Alpha, C.; Melcher, P.; Heraux, J.; Azocar, A. Growth and photosynthetic responses of Scaevola sericea, a Hawaiian coastal shrub, to substrate salinity and salt spray. Int. J. Plant Sci. 1996, 157, 171. [Google Scholar] [CrossRef]
- Griffiths, M.E.; Orians, C.M. Responses of common and successional heathland species to manipulated salt spray and water availability. Am. J. Bot. 2003, 90, 1720–1728. [Google Scholar] [CrossRef]
- Romero, F.; Argüello, A.; de Bruin, S.; van der Heijden, M.G.A. The plant–mycorrhizal fungi collaboration gradient depends on plant functional group. Funct. Ecol. 2023, 37, 2386–2398. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Wilson, J.A.; Miller, R.M.; Bowker, M.A. Mycorrhizal phenotypes and the law of the minimum. New Phytol. 2015, 205, 1473–1484. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Microbial mediation of plant competition and community structure. Funct. Ecol. 2013, 27, 865–875. [Google Scholar] [CrossRef]
- Benes, S.E.; Aragüés, R.; Grattan, S.R.; Austin, R.B. Foliar and root absorption of Na+ and Cl− in maize and barley: Implications for salt tolerance screening and the use of saline sprinkler irrigation. Plant Soil 1996, 180, 75–86. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef]
- Sudhakar, C.; Veeranagamallaiah, G.; Nareshkumar, A.; Sudhakarbabu, O.; Sivakumar, M.; Pandurangaiah, M.; Kiranmai, K.; Lokesh, U. Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Rep. 2015, 34, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Van Manen, Y.; Vugts, H.F.; Leusink, A. Airborne and soilborne salinity and the distribution of coastal and inland species of the genus Elytrigia. Acta Bot. Neerl. 1983, 32, 447–456. [Google Scholar] [CrossRef]
- Koide, R.T. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol. 2000, 147, 233–235. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, L.; Liu, P.; Wu, A.; Dong, L.; Wu, Q.; Zhao, M.; Liu, H.; Li, Y.; Zhang, N.; Wang, Y. Resistance of Mycorrhizal Cinnamomum camphora Seedlings to Salt Spray Depends on K+ and P Uptake. J. Fungi 2023, 9, 964. https://doi.org/10.3390/jof9100964
Xue L, Liu P, Wu A, Dong L, Wu Q, Zhao M, Liu H, Li Y, Zhang N, Wang Y. Resistance of Mycorrhizal Cinnamomum camphora Seedlings to Salt Spray Depends on K+ and P Uptake. Journal of Fungi. 2023; 9(10):964. https://doi.org/10.3390/jof9100964
Chicago/Turabian StyleXue, Lin, Peng Liu, Aiping Wu, Lijia Dong, Qiqian Wu, Mingshui Zhao, Hua Liu, Yan Li, Naili Zhang, and Yanhong Wang. 2023. "Resistance of Mycorrhizal Cinnamomum camphora Seedlings to Salt Spray Depends on K+ and P Uptake" Journal of Fungi 9, no. 10: 964. https://doi.org/10.3390/jof9100964
APA StyleXue, L., Liu, P., Wu, A., Dong, L., Wu, Q., Zhao, M., Liu, H., Li, Y., Zhang, N., & Wang, Y. (2023). Resistance of Mycorrhizal Cinnamomum camphora Seedlings to Salt Spray Depends on K+ and P Uptake. Journal of Fungi, 9(10), 964. https://doi.org/10.3390/jof9100964




