Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov.
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Ecology
2.2. Cultivation and Modified Atmosphere Packaging of Strains
2.3. Phylogenetic Analyses
2.4. Fatty Acid Analysis
2.5. Physiology and Chemotaxonomy of Strains WT5 and WR1
3. Results
3.1. Growth Assessment
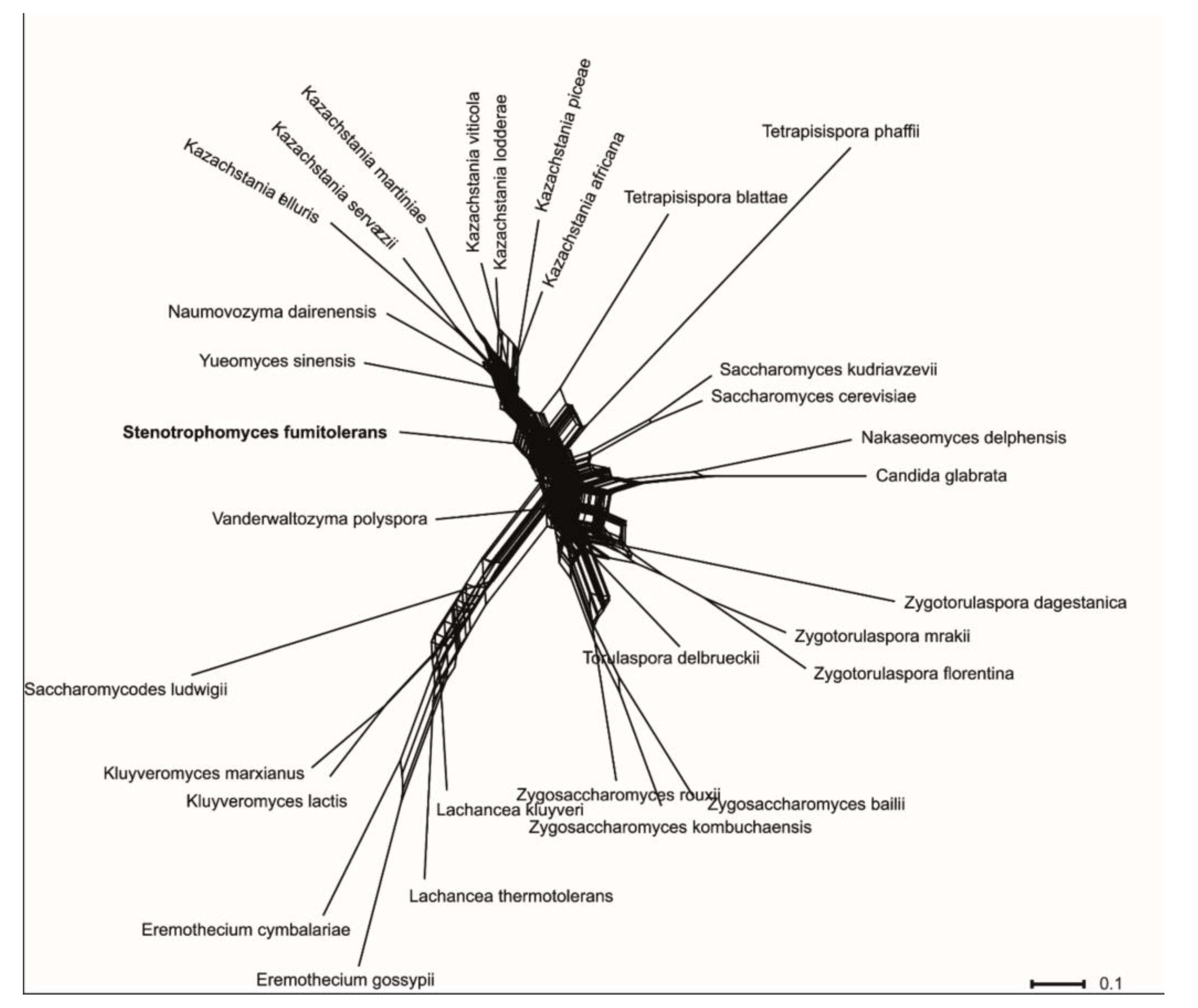
3.2. Phylogenetic Analyses
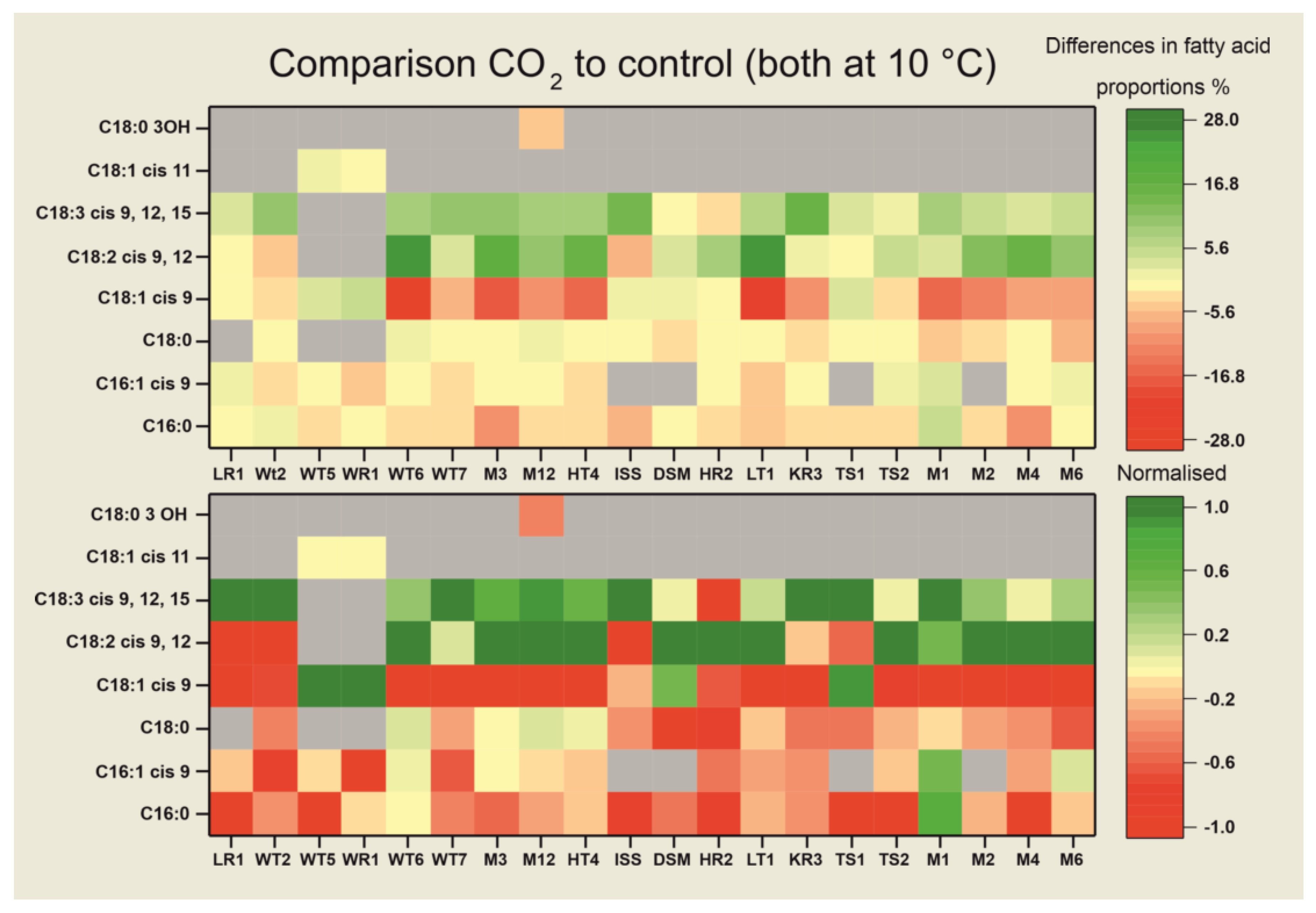
3.3. Fatty Acid Compositions
3.4. Taxonomy of Novel Yeast Species
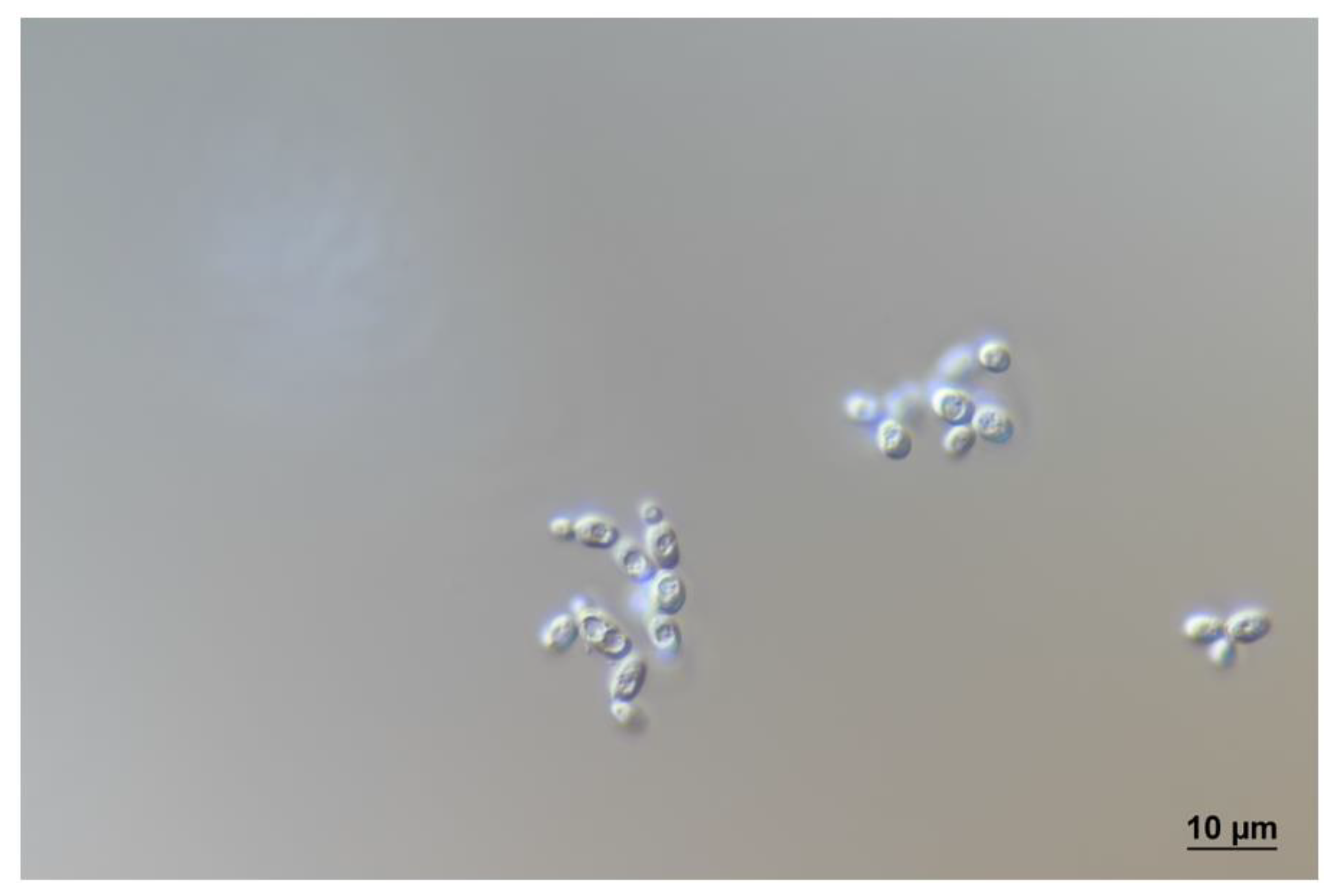
3.5. Description of Stenotrophomyces fumitolerans Gen. Nov., Sp. Nov.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ropars, J.; Giraud, T. Convergence in domesticated fungi used for cheese and dry-cured meat maturation: Beneficial traits, genomic mechanisms, and degeneration. Curr. Opin. Microbiol. 2022, 70, 102236. [Google Scholar] [CrossRef]
- Kure, C.F.; Skaar, I. The fungal problem in cheese industry. Curr. Opin. Food Sci. 2019, 29, 14–19. [Google Scholar] [CrossRef]
- Lambert, A.D.; Smith, J.P.; Dodds, K.L. Shelf life extension and microbiological safety of fresh meat—A review. Food Microbiol. 1991, 8, 267–297. [Google Scholar] [CrossRef]
- Kurtzman, C.P. Identification of food and beverage spoilage yeasts from DNA sequence analyses. Int. J. Food Microbiol. 2015, 213, 71–78. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Fröhlich-Nowoisky, J.; Pickersgill, D.A.; Després, V.R.; Pöschl, U. High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 2009, 106, 12814–12819. [Google Scholar] [CrossRef]
- Mullan, M.; McDowell, D. Modified Atmosphere Packaging. In Food and Beverage Packaging Technology; Coles, R., Kirwan, M., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 263–294. [Google Scholar]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth and mycotoxin production by food spoilage fungi under high carbon dioxide and low oxygen atmospheres. Int. J. Food Microbiol. 2009, 132, 100–108. [Google Scholar] [CrossRef]
- Taniwaki, M.; Hocking, A.; Pitt, J.; Fleet, G. Growth of fungi and mycotoxin production on cheese under modified atmospheres. Int. J. Food Microbiol. 2001, 68, 125–133. [Google Scholar] [CrossRef]
- Gonzalez-Fandos, E.; Maya, N.; Martínez-Laorden, A.; Perez-Arnedo, I. Efficacy of Lactic Acid and Modified Atmosphere Packaging against Campylobacter jejuni on Chicken during Refrigerated Storage. Foods 2020, 9, 109. [Google Scholar] [CrossRef]
- Santillan, E.-F.U.; Shanahan, T.M.; Omelon, C.R.; Major, J.R.; Bennett, P.C. Isolation and characterization of a CO2-tolerant Lactobacillus strain from Crystal Geyser, Utah, U.S.A. Front. Earth Sci. 2015, 3, 41. [Google Scholar] [CrossRef]
- Šibanc, N.; Zalar, P.; Schroers, H.-J.; Zajc, J.; Pontes, A.; Sampaio, J.P.; Maček, I. Occultifur mephitis f.a., sp. nov. and other yeast species from hypoxic and elevated CO2 mofette environments. Int. J. Syst. Evol. Microbiol. 2018, 68, 2285–2298. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Spilimbergo, S.; Elst, K.; Van Ginneken, L.; Debevere, J.; Van Impe, J.; Devlieghere, F. High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future. Int. J. Food Microbiol. 2007, 117, 1–28. [Google Scholar] [CrossRef]
- Isenschmid, A.; Marison, I.W.; von Stockar, U. The influence of pressure and temperature of compressed CO2 on the survival of yeast cells. J. Biotechnol. 1995, 39, 229–237. [Google Scholar] [CrossRef]
- Beccaccioli, M.; Reverberi, M.; Scala, V. Fungal lipids: Biosynthesis and signalling during plant-pathogen interaction. Front. Biosci. (Landmark Ed) 2019, 24, 172–185. [Google Scholar] [CrossRef]
- Pedneault, K.; Angers, P.; Avis, T.J.; Gosselin, A.; Tweddell, R.J. Fatty acid profiles of polar and non-polar lipids of Pleurotus ostreatus and P. cornucopiae var. ‘citrino-pileatus’ grown at different temperatures. Mycol. Res. 2007, 111 Pt 10, 1228–1234. [Google Scholar] [CrossRef]
- Suutari, M. Effect of growth temperature on lipid fatty acids of four fungi (Aspergillus niger, Neurospora crassa, Penicillium chrysogenum, and Trichoderma reesei). Arch. Microbiol. 1995, 164, 212–216. [Google Scholar] [CrossRef]
- Sumner, J.L.; Morgan, E.D.; Evans, H.C. The effect of growth temperature on the fatty acid composition of fungi in the order Mucorales. Can. J. Microbiol. 1969, 15, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Weete, J.D. Lipid Biochemistry of Fungi and Other Organisms; Springer: Boston, MA, USA, 1980. [Google Scholar]
- Phillips, R.L.; Zak, D.R.; Holmes, W.E.; White, D.C. Microbial community composition and function beneath temperate trees exposed to elevated atmospheric carbon dioxide and ozone. Oecologia 2002, 131, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Lipson, D.A.; Wilson, R.F.; Oechel, W.C. Effects of elevated atmospheric CO2 on soil microbial biomass, activity, and diversity in a chaparral ecosystem. Appl. Environ. Microbiol. 2005, 71, 8573–8580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.; Groenewald, M.; Wang, Q.-M.; Boekhout, T. Reclassification of Saccharomycodes sinensis, Proposal of Yueomyces sinensis gen. nov., comb. nov. within Saccharomycetaceae (Saccharomycetales, Saccharomycotina). PLoS ONE 2015, 10, e0136987. [Google Scholar] [CrossRef]
- Simpson, A.C.; Urbaniak, C.; Bateh, J.R.; Singh, N.K.; Wood, J.M.; Debieu, M.; O’hara, N.B.; Houbraken, J.; Mason, C.E.; Venkateswaran, K. Draft Genome Sequences of Fungi Isolated from the International Space Station during the Microbial Tracking-2 Experiment. Microbiol. Resour. Announc. 2021, 10, e0075121. [Google Scholar] [CrossRef]
- James, J.T. The Headache of Carbon Dioxide Exposures; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef]
- Law, J.; Van Baalen, M.; Foy, M.; Mason, S.S.; Mendez, C.; Wear, M.L.; Meyers, V.E.; Alexander, D. Relationship between carbon dioxide levels and reported headaches on the international space station. J. Occup. Environ. Med. 2014, 56, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, N.P.; Babiker, W.M.; Merz, W.G.; Carroll, K.C.; Zhang, S.X. Evaluation of nucleic acid sequencing of the D1/D2 region of the large subunit of the 28S rDNA and the internal transcribed spacer region using SmartGene IDNS corrected software for identification of filamentous fungi in a clinical laboratory. J. Mol. Diagn. 2012, 14, 393–401. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Phylogenetic relationships among yeasts of the ‘Saccharomyces complex’ determined from multigene sequence analyses. FEMS Yeast Res. 2003, 3, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Matheny, P.B.; Liu, Y.J.; Ammirati, J.F.; Hall, B.D. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am. J. Bot. 2002, 89, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kachalkin, A.V.; Abdullabekova, D.A.; Magomedova, E.S.; Yurkov, A.M. Zygotorulaspora dagestanica sp. nov., a novel ascomycetous yeast species associated with the Georgian honeysuckle (Lonicera iberica M. Bieb.). Int. J. Syst. Evol. Microbiol. 2021, 71, 004785. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Yurkov, A.; Guerreiro, M.A.; Sharma, L.; Carvalho, C.; Fonseca, Á. Multigene assessment of the species boundaries and sexual status of the basidiomycetous yeasts Cryptococcus flavescens and C. terrestris (Tremellales). PLoS ONE 2015, 10, e0120400. [Google Scholar] [CrossRef]
- Wu, Q.; James, S.A.; Roberts, I.N.; Moulton, V.; Huber, K.T. Exploring contradictory phylogenetic relationships in yeasts. FEMS Yeast Res. 2008, 8, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.-X.; Opulente, D.A.; Kominek, J.; Zhou, X.; Steenwyk, J.L.; Buh, K.V.; Haase, M.A.; Wisecaver, J.H.; Wang, M.; Doering, D.T.; et al. Tempo and Mode of Genome Evolution in the Budding Yeast Subphylum. Cell 2018, 175, 1533–1545.e20. [Google Scholar] [CrossRef] [PubMed]
- Sasser, M. Identification of Bacteria by Gas Chromatography of Cellular Fatty Acids; Technical Note 101; MIDI-Inc.: Newark, DE, USA, 1990. [Google Scholar]
- Lipski, A.; Altendorf, K. Identification of Heterotrophic Bacteria Isolated from Ammonia-supplied Experimental Biofilters. Syst. Appl. Microbiol. 1997, 20, 448–457. [Google Scholar] [CrossRef]
- van Lent, D.M.; Egert, S.; Wolfsgruber, S.; Kleineidam, L.; Weinhold, L.; Wagner-Thelen, H.; Maier, W.; Jessen, F.; Ramirez, A.; Schmid, M.; et al. Eicosapentaenoic Acid Is Associated with Decreased Incidence of Alzheimer’s Dementia in the Oldest Old. Nutrients 2021, 13, 461. [Google Scholar] [CrossRef]
- Seel, W.; Flegler, A.; Zunabovic-Pichler, M.; Lipski, A. Increased Isoprenoid Quinone Concentration Modulates Membrane Fluidity in Listeria monocytogenes at Low Growth Temperatures. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 87–110. [Google Scholar]
- von Heilborn, D.H.; Reinmüller, J.; Hölzl, G.; Meier-Kolthoff, J.P.; Woehle, C.; Marek, M.; Hüttel, B.; Lipski, A. Sphingomonas aliaeris sp. nov., a new species isolated from pork steak packed under modified atmosphere. Int. J. Syst. Evol. Microbiol. 2021, 71, 004973. [Google Scholar] [CrossRef]
- Lösel, D.M. Lipids in the Structure and Function of Fungal Membranes; Springer: Berlin/Heidelberg, Germany, 1990; pp. 119–133. [Google Scholar] [CrossRef]
- Timke, M.; Wang-Lieu, N.Q.; Altendorf, K.; Lipski, A. Identity, beer spoiling and biofilm forming potential of yeasts from beer bottling plant associated biofilms. Antonie Van Leeuwenhoek 2008, 93, 151–161. [Google Scholar] [CrossRef]
- Loureiro, V. The prevalence and control of spoilage yeasts in foods and beverages. Trends Food Sci. Technol. 1999, 10, 356–365. [Google Scholar] [CrossRef]
- Jones, R.P.; Greenfield, P.F. Effect of carbon dioxide on yeast growth and fermentation. Enzym. Microb. Technol. 1982, 4, 210–223. [Google Scholar] [CrossRef]
- Hutkins, R.W.; Nannen, N.L. pH Homeostasis in Lactic Acid Bacteria. J. Dairy Sci. 1993, 76, 2354–2365. [Google Scholar] [CrossRef]
- Münch, M.; Guillard, V.; Gaucel, S.; Destercke, S.; Thévenot, J.; Buche, P. Composition-based statistical model for predicting CO2 solubility in modified atmosphere packaging application. J. Food Eng. 2023, 340, 111283. [Google Scholar] [CrossRef]
- Tesnière, C. Importance and role of lipids in wine yeast fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 8293–8300. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Rosenfield, C.-L.; Knipple, D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, A.C.; Cliffe, D.E.; Knapper, S. Factors affecting Penicillium roquefortii (Penicillium glaucum) in internally mould ripened cheeses: Implications for pre-packed blue cheeses. Int. J. Food Sci. Technol. 2011, 46, 1586–1590. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Hocking, A.D.; Pitt, J.I.; Fleet, G.H. Growth and mycotoxin production by fungi in atmospheres containing 80% carbon dioxide and 20% oxygen. Int. J. Food Microbiol. 2010, 143, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Castelli, A.; Littarru, G.P.; Barbaresi, G. Effect of pH and CO2 concentration changes on lipids and fatty acids of Saccharomyces cerevisiae. Arch. Mikrobiol. 1969, 66, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, S.; Kienberger, H.; Kleigrewe, K.; Hilgarth, M.; Vogel, R.F. Effect of high levels of CO2 and O2 on membrane fatty acid profile and membrane physiology of meat spoilage bacteria. Eur. Food Res. Technol. 2021, 247, 999–1011. [Google Scholar] [CrossRef]
- Tsuzuki, M.; Ohnuma, E.; Sato, N.; Takaku, T.; Kawaguchi, A. Effects of CO(2) Concentration during Growth on Fatty Acid Composition in Microalgae. Plant Physiol. 1990, 93, 851–856. [Google Scholar] [CrossRef]
- Farber, J.M. Microbiological Aspects of Modified-Atmosphere Packaging Technology—A Review 1. J. Food Prot. 1991, 54, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M.; Hinze, L.L.; Gardner, H.W.; Keller, N.P. Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus spp. Appl. Environ. Microbiol. 1999, 65, 3668–3673. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, G.; Kates, M. Pathways for desaturation of oleoyl chains in Candida lipolytica. Can. J. Biochem. Cell Biol. 1983, 61, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Binkoski, A.E. Polyunsaturated Fatty Acids and Cardiovascular Health. Nut. Rev. 2004, 62, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Klug, M.J. Characterization and differentiation of filamentous fungi based on Fatty Acid composition. Appl. Environ. Microbiol. 1996, 62, 4136–4146. [Google Scholar] [CrossRef] [PubMed]
- Sinensky, M. Homeoviscous adaptation—A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- Krell, T. Cellular Ecophysiology of Microbe: Hydrocarbon and Lipid Interactions; Springer: Berlin/Heidelberg, Germany, 2018; p. 381. [Google Scholar] [CrossRef]
- Al-Fageeh, M.B.; Smales, C.M. Control and regulation of the cellular responses to cold shock: The responses in yeast and mammalian systems. Biochem. J. 2006, 397, 247–259. [Google Scholar] [CrossRef]
- Buzzini, P.; Turchetti, B.; Yurkov, A. Extremophilic yeasts: The toughest yeasts around? Yeast 2018, 35, 487–497. [Google Scholar] [CrossRef]

| Source | Strain | Species According to NCBI BLAST | Sequence Similarity with Closely-Related Type Strain | Sequence Accession No. |
|---|---|---|---|---|
| Vegetarian wrap | WR1 | Torulaspora globosa | 96.9% ‡ (KY109865.1) | OQ255941 |
| WT2 | Pichia fermentans | 99.8% ‡ (MK394169.1) | OQ255937 | |
| WT5 | Vanderwaltozyma verrucispora | 89.0% * (NR_137559.1) | OQ255923 | |
| Torulaspora globosa | 96.9% ‡ (KY109865.1) | OQ255942 | ||
| Further accession numbers for SSU-ITS-LSU fragment, RPB2 and TEF1 | - | OQ708375, OQ715316, OR661267 | ||
| WT6 | Apiotrichum gracile | 99.8% ‡ (KY106124.1) | OQ255938 | |
| WT7 | Candida oleophila | 99.6% ‡ (NG_060820.1) | OQ255939 | |
| Minced Meat | HR2 | Penicillium griseofulvum | 99.8% * (MH854925.1) | OQ255924 |
| Penicillium dipodomyus † | 100% ‡ (MH874450.1) | OQ255931 | ||
| HT4 | Candida sake | 100% ‡ (KY106745.1) | OQ255932 | |
| Salmon | LR1 | Candida zeylanoides | 100% ‡ (NG_060834.1) | OQ255933 |
| LT1 | Didymella corylicola † | 100% ‡ (MN954290.1) | OQ255934 | |
| Coffee | KR3 | Neurospora tetraspora | 98.9% ‡ (NG_068996.1) | OQ255940 |
| Airborne | TS1 | Penicillium tardochrysogenum † | 100% * (MH865983.1) | OQ255929 |
| Penicillium dipodomyus † | 100% ‡ (MH874450.1) | OQ255935 | ||
| TS2 | Penicillium griseofulvum | 99.8% * (MH854925.1) | OQ255930 | |
| Penicillium tardochrysogenum † | 100% ‡ (NG_070021.1) | OQ255936 | ||
| Mofettes | M1 | Rhizopus oryzae | 100% * (DQ641279.1) | OQ255925 |
| M2 | Cladosporium subuliforme † | 100% * (MH864124.1) | OQ255922 | |
| M3 | Rhodotorula alborubescens | 99.6% * (NR_153197.1) | OQ255926 | |
| M4 | Alternaria alstroemeriae † | 100% * (NR_163686.1) | OQ255927 | |
| M6 | Penicillium glandicola | 100% * (MH860946.1) | OQ255928 | |
| M12 | Rhodotorula babjevae | 99.4% * (NR_077096.1) | OQ504373 | |
| International Space Station (ISS) | IF2SW-F4 | Penicillium rubens | 100.0% (MT558923.1) | JACSPE000000000 |
| Cantaloupe melon | DSM 1075 | Penicillium rubens | 100% * (NR_111815.1) | PKG00000000, as published before [22] |
| Organisms | Growth at | C14:0 | C16:0 | C16:1 cis 9 | C18:0 | C18:1 cis 9 | C18:2 cis 9, 12 | C18:3 cis 9, 12, 15 | C18:1 cis 11 | C18:0 3OH | WAMT (°C) | DU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candida zeylanoides LR1 | 10 °C, CO2 | 10.4 ± 0.5 | 8.9 ± 1.8 | <1 | 38.7 ± 3.1 | 24.8 ± 1.9 | 17.3 ± 2.6 | nd | 7.7 ± 0.2 | 1.49 | ||
| 10 °C | <1 | 11.5 ± 1.5 | 8.4 ± 2.1 | 39.6 ± 3.1 | 25.8 ± 0.5 | 14.7 ± 4.0 | nd | 8.7 ± <0.1 | 1.44 | |||
| 25 °C | 15.1 ± 0.6 | 4.5 ± 0.3 | 41.3 ± 1.7 | 27.0 ±0.3 | 12.0 ± 1.3 | 11.5 ± 0.1 | 1.36 | |||||
| Pichia fermentans WT2 | 10 °C, CO2 | 11.4 ± 1.6 | 19.8 ± 1.7 | 0.5 ± 0.2 | 22.9 ± 2.1 | 22.9 ± 1.3 | 22.4 ± 0.4 | nd | 6.4 ± 0.7 | 1.56 | ||
| 10 °C | <1 | 11.1 ± 0.9 | 23.1 ± 0.4 | 1.0 ± 0.1 | 24.9 ± 1.2 | 27.1 ± 1.0 | 12.8 ± 0.5 | nd | 7.6 ± 0.5 | 1.41 | ||
| 25 °C | 14.8 ± 0.4 | 15.7 ± 1.6 | 2.2 ± 0.3 | 21.4 ± 1.3 | 35.6 ± 0.2 | 10.3 ± 0.3 | 9.9 ± 0.6 | 1.39 | ||||
| Stenotrophomyces fumitolerans WT5 | 10 °C, CO2 | 5.6 ± 0.7 | 74.2 ± 2.6 | <1 | 16.4 ± 2.0 | nd | nd | 3.8 ± 0.7 | 7.0 ± 0.7 | 0.94 | ||
| 10 °C | <1 | 8.3 ± 0.3 | 74.5 ± 0.8 | 13.5 ± 0.3 | 3.8 ± 0.7 | nd | 8.4 ± 0.2 | 0.92 | ||||
| 25 °C | 9.3 ± 1.5 | 65.5 ± 3.3 | 18.2 ± 1.9 | 7.0 ± 1.0 | 10.0 ± 1.0 | 0.91 | ||||||
| Stenotrophomyces fumitolerans WR1 | 10 °C, CO2 | 8.6 ± 0.5 | 75.6 ± 0.1 | <1 | 13.1 ± 0.2 | nd | nd | 2.7 ± 0.3 | 8.3 ± 0.3 | 0.91 | ||
| 10 °C | <1 | 9.0 ± 0.5 | 79.7 ± 4.6 | 9.2 ± 3.5 | 2.9 ± 0.8 | nd | 8.2 ± 0.2 | 0.91 | ||||
| 25 °C | 10.5 ± 0.4 | 66.4 ± 1.2 | 17.2 ± 1.0 | 5.9 ± 0.4 | 10.4 ± 0.4 | 0.89 | ||||||
| Apiotrichum gracile WT6 | 10 °C, CO2 | 16.2 ± 1.0 | 1.4 ± 0.3 | 2.4 ± 2.8 | 19.6 ± 1.0 | 47.2 ± 4.2 | 14.0 ± 1.7 | nd | 8.7 ± 2.3 | 1.57 | ||
| 10 °C | <1 | 19.1 ± 1.8 | 2.3 ± 0.4 | 2.3 ± 0.4 | 47.5 ± 3.0 | 22.9 ± 3.0 | 5.9 ± 1.4 | nd | 17.2 ± 2.0 | 1.13 | ||
| 25 °C | 20.8 ± 1.1 | 0.8 ± 0.3 | 3.9 ± 1.2 | 24.0 ± 1.7 | 46.4 ± 2.8 | 4.2 ± 3.9 | 14.9 ± 0.6 | 1.30 | ||||
| Candida oleophila WT7 | 10 °C, CO2 | 12.4 ± 0.5 | 12.4 ± 1.1 | 0.3 ± 0.1 | 35.6 ± 0.4 | 19.1 ± 0.9 | 20.1 ± 0.3 | nd | 8.9 ± 0.3 | 1.37 | ||
| 10 °C | <1 | 14.3 ± 0.5 | 15.7 ± 1.0 | 0.8 ± 0.1 | 41.9 ± 3.8 | 16.8 ± 1.3 | 10.3 ± 2.7 | nd | 12.6 ± 1.1 | 1.22 | ||
| 25 °C | 14.7 ± 0.4 | 1.8 ± 0.1 | 3.7 ± 0.3 | 47.8 ± 0.7 | 23.7 ± 0.4 | 8.2 ± 0.8 | 15.2 ± 0.6 | 1.22 | ||||
| Candida sake HT4 | 10 °C, CO2 | 11.1 ± 0.5 | 11.5 ± 2.7 | 0.7 ± 0.3 | 34.0 ± 8.8 | 28.7 ± 6.8 | 14.0 ± 4.5 | nd | 8.2 ± 0.2 | 1.45 | ||
| 10 °C | <1 | 14.3 ± 1.2 | 14.7 ± 0.1 | 1.1 ± 0.1 | 50.5 ± 3.4 | 13.7 ± 3.1 | 5.8 ± 1.6 | nd | 14.6 ± 1.6 | 1.10 | ||
| 25 °C | 12.3 ± 0.7 | 10.5 ± 0.2 | 1.5 ± 0.3 | 48.0 ± 4.6 | 23.0 ± 3.1 | 4.7 ± 1.3 | 12.8 ± 0.8 | 1.19 | ||||
| Rhodotorula alborubescens M3 | 10 °C, CO2 | 10.4 ± 0.5 | 0.9 ± 0.1 | 1.9 ± 0.1 | 28.5 ± 0.8 | 39.1 ± 1.1 | 19.2 ± 0.4 | nd | 6.4 ± 0.4 | 1.65 | ||
| 10 °C | <1 | 20.4 ± 1.3 | 1.4 ± 0.2 | 2.4 ± 0.5 | 46.8 ± 2.3 | 20.9 ± 2.1 | 8.1 ± 1.4 | nd | 17.9 ± 1.5 | 1.14 | ||
| 25 °C | 18.1 ± 0.5 | 0.4 ± 0.1 | 4.0 ± 0.2 | 54.3 ± 0.8 | 17.6 ± 0.6 | 5.6 ± 0.4 | 19.0 ± 0.3 | 1.06 | ||||
| Rhodotorula babjevae M12 | 10 °C, CO2 | 1.0 ± <0.1 | 13.7 ± 0.3 | 2.1 ± 0.2 | 1.4 ± 0.2 | 25.8 ± 0.9 | 25.6 ± 0.2 | 18.9 ± 0.3 | 11.5 ± 0.5 | 19.2 ± 0.4 | 1.70 | |
| 10 °C | 1.0 ± 0.2 | 16.9 ± 0.9 | 3.2 ± 0.5 | 1.0 ± 0.4 | 36.5 ± 2.8 | 15.5 ± 0.8 | 9.6 ± 2.3 | nd | 16.1 ± 1.3 | 28.7 ± 0.8 | 1.48 | |
| 25 °C | 1.1 ± 0.1 | 17.7 ± 1.2 | 1.2 ± 0.1 | 1.7 ± 0.2 | 39.0 ± 3.3 | 20.2 ± 2.9 | 10.3 ± 1.9 | 8.5 ± 0.5 | 22.1 ± 1.7 | 1.38 |
| Organisms | Growth at | C16:0 | C16:1 cis 9 | C18:0 | C18:1 cis 9 | C18:2 cis 9, 12 | C18:3 cis 9, 12, 15 | WAMT (°C) | DU |
|---|---|---|---|---|---|---|---|---|---|
| Penicillium rubens IF2SW-F4 | 10 °C, CO2 | 8.8 ± 0.5 | 6.5 ± 1.0 | 11.1 ± 1.7 | 43.7 ± 3.8 | 30.0 ± 4.8 | 4.7 ± 0.2 | 1.89 | |
| 10 °C | 14.9 ± 0.6 | <1 | 7.2 ± 1.4 | 10.2 ± 1.2 | 51.0 ± 2.7 | 16.7 ± 0.6 | 10.0 ± 1.7 | 1.64 | |
| 25 °C | 16.1 ± 1.1 | 3.6 ± 0.9 | 8.7 ± 0.5 | 60.0 ± 2.7 | 11.6 ± 1.0 | 8.0 ± 1.3 | 1.63 | ||
| Penicillium rubens DSM 1075 | 10 °C, CO2 | 8.9 ± 0.4 | 6.8 ± 2.9 | 4.2 ± 2.2 | 47.3 ± 4.6 | 32.8 ± 4.5 | 3.6 ± 1.4 | 1.97 | |
| 10 °C | 10.6 ± 1.3 | <1 | 10.1 ± 2.9 | 2.7 ± 0.9 | 44.5 ± 6.2 | 33.0 ± 5.6 | 6.8 ± 1.5 | 1.91 | |
| 25 °C | 16.0 ± 1.6 | 4.0 ± 0.6 | 3.5 ± 0.5 | 61.5 ± 5.2 | 15.1 ± 3.2 | 7.0 ± 1.6 | 1.72 | ||
| Penicillium griseofulvum HR2 | 10 °C, CO2 | 10.5 ± 1.2 | 1.6 ± 0.5 | 2.3 ± 1.1 | 6.9 ± 1.0 | 64.2 ± 3.3 | 14.5 ± 2.3 | 2.7 ± 1.8 | 1.80 |
| 10 °C | 13.0 ± 0.4 | 1.6 ± 0.3 | 4.2 ± 0.8 | 8.0 ± 0.9 | 55.7 ± 3.3 | 17.5 ± 2.2 | 6.0 ± 0.5 | 1.74 | |
| 25 °C | 14.8 ± 0.9 | 0.8 ± 0.1 | 2.9 ± 0.3 | 8.0 ± 0.4 | 70.2 ± 2.2 | 3.4 ± 1.1 | 6.8 ± 0.8 | 1.59 | |
| Didymella corylicola LT1 | 10 °C, CO2 | 14.8 ± 2.3 | 3.9 ± 0.7 | 1.5 ± 0.2 | 12.9 ± 2.2 | 58.6 ± 2.3 | 8.4 ± 2.6 | 6.8 ± 2.2 | 1.59 |
| 10 °C | 18.8 ± 1.5 | 8.1 ± 2.2 | 3.0 ± 1.0 | 33.8 ± 3.9 | 33.8 ± 5.0 | 2.5 ± 0.5 | 15.5 ± 2.0 | 1.17 | |
| 25 °C | 14.4 ± 1.8 | 1.0 ± 0.6 | 2.8 ± 0.1 | 20.7 ± 5.4 | 57.9 ± 6.5 | 3.2 ± 1.5 | 9.1 ± 2.4 | 1.47 | |
| Neurospora tetraspora KR3 | 10 °C, CO2 | 10.2 ± 1.8 | 1.5 ± 1.0 | 1.5 ± 0.4 | 5.8 ± 0.6 | 54.3 ±2.4 | 26.8 ± 3.5 | 1.1 ± 1.7 | 1.96 |
| 10 °C | 12.1 ± 1.3 | 3.2 ± 0.8 | 4.7 ± 1.8 | 15.5 ± 3.2 | 53.2 ± 1.6 | 11.2 ± 1.3 | 7.7 ± 1.6 | 1.59 | |
| 25 °C | 15.0 ± 1.2 | 1.5 ± 0.1 | 8.0 ± 2.8 | 25.7 ± 3.0 | 45.0 ± 3.1 | 4.8 ± 2.2 | 14.4 ± 2.1 | 1.32 | |
| Penicillium tardochrysogenum TS1 | 10 °C, CO2 | 11.0 ± 1.1 | <1 | 4.5 ± 0.4 | 13.4 ± 1.5 | 42.5 ± 4.2 | 28.5 ± 6.5 | 5.4 ± 1.1 | 1.84 |
| 10 °C | 13.8 ± 0.9 | 5.8 ± 0.9 | 10.7 ± 1.3 | 44.0 ± 2.6 | 25.6 ± 3.3 | 7.9 ± 0.4 | 1.76 | ||
| 25 °C | 15.0 ± 0.4 | 2.5 ± 0.4 | 23.3 ± 3.0 | 50.4 ± 6.9 | 8.8 ± 3.3 | 9.4 ± 0.9 | 1.50 | ||
| Penicillium griseofulvum TS2 | 10 °C, CO2 | 10.3 ± 0.8 | 1.7 ± 0.9 | 2.5 ± 1.1 | 6.2 ± 1.4 | 64.6 ± 4.8 | 14.7 ± 2.3 | 2.6 ± 1.5 | 1.81 |
| 10 °C | 12.9 ± 0.6 | 1.6 ± 0.4 | 2.6 ± 0.8 | 8.1 ± 0.8 | 60.8 ± 3.5 | 14.0 ± 4.2 | 4.9 ± 0.6 | 1.73 | |
| 25 °C | 14.6 ± 1.0 | 0.8 ± 0.1 | 3.0 ± 0.6 | 8.6 ± 0.7 | 70.2 ± 3.0 | 2.7 ± 0.9 | 6.9 ± 1.2 | 1.58 | |
| Rhizopus oryzae M1 * | 10 °C, CO2 | 25.5 ± 4.4 | 8.0 ± 2.9 | 2.1 ± 0.3 | 35.1 ± 4.1 | 11.6 ± 2.0 | 17.7 ± 4.8 | 19.2 ± 4.0 | 1.19 |
| 10 °C | 20.3 ± 0.4 | 5.0 ± 1.0 | 6.5 ± 2.5 | 50.4 ± 1.6 | 8.8 ± 2.4 | 9.0 ± 2.0 | 22.0 ± 2.3 | 1.00 | |
| 25 °C | 22.0 ± 3.5 | 0.9 ± 0.4 | 6.1 ± 1.1 | 42.1 ± 1.5 | 19.4 ± 1.5 | 9.5 ± 1.4 | 20.9 ± 2.8 | 1.10 | |
| Cladosporium subuliforme M2 | 10 °C, CO2 | 20.6 ± 2.2 | <1 | 2.4 ± 0.4 | 16.2 ± 0.9 | 49.9 ± 2.1 | 10.9 ± 0.8 | 11.7 ± 1.5 | 1.49 |
| 10 °C | 22.8 ±1.6 | 5.4 ± 2.8 | 28.2 ± 0.2 | 37.3 ± 0.1 | 6.3 ± 0.8 | 18.1 ± 1.0 | 1.22 | ||
| 25 °C | 29.1 ± 2.4 | 8.4 ± 1.8 | 33.7 ± 0.8 | 27.9 ± 4.0 | 0.9 ± 0.2 | 26.1 ± 2.7 | 0.92 | ||
| Alternaria alstroemeriae M4 | 10 °C, CO2 | 14.6 ± 1.5 | 0.9 ± <0.1 | 5.0 ± 1.0 | 8.3 ± 1.6 | 63.7 ± 1.3 | 7.5 ± 2.0 | 8.2 ± 1.7 | 1.59 |
| 10 °C | 24.1 ± 1.3 | 1.3 ± <0.1 | 6.2 ± 1.1 | 16.9 ± 0.6 | 47.6 ± 3.2 | 4.0 ± 0.2 | 17.5 ± 1.9 | 1.25 | |
| 25 °C | 22.3 ± 2.2 | 1.5 ± 0.1 | 6.2 ± 0.6 | 33.9 ± 2.3 | 35.8 ± 0.6 | 0.3 ± <0.1 | 20.0 ± 1.6 | 1.08 | |
| Penicillium glandicola M6 | CO2 | 12.0 ± 0.7 | 2.8 ± 0.3 | 2.4 ± 0.4 | 7.7 ± 1.3 | 61.3 ± 2.0 | 13.9 ± 2.7 | 4.1 ± 0.6 | 1.75 |
| 10 °C | 12.9 ± 0.6 | 1.2 ± 0.1 | 8.3 ± 0.2 | 17.0 ± 1.7 | 50.6 ± 0.9 | 10.0 ± 2.5 | 11.2 ± 0.8 | 1.49 | |
| 25 °C | 16.4 ± 1.4 | 1.4 ± 0.1 | 6.9 ± 1.4 | 36.7 ± 2.1 | 37.3 ± 1.5 | 1.4 ± 0.5 | 16.9 ± 1.8 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidler von Heilborn, D.; Reinmüller, J.; Yurkov, A.; Stehle, P.; Moeller, R.; Lipski, A. Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov. J. Fungi 2023, 9, 1031. https://doi.org/10.3390/jof9101031
Heidler von Heilborn D, Reinmüller J, Yurkov A, Stehle P, Moeller R, Lipski A. Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov. Journal of Fungi. 2023; 9(10):1031. https://doi.org/10.3390/jof9101031
Chicago/Turabian StyleHeidler von Heilborn, David, Jessica Reinmüller, Andrey Yurkov, Peter Stehle, Ralf Moeller, and André Lipski. 2023. "Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov." Journal of Fungi 9, no. 10: 1031. https://doi.org/10.3390/jof9101031
APA StyleHeidler von Heilborn, D., Reinmüller, J., Yurkov, A., Stehle, P., Moeller, R., & Lipski, A. (2023). Fungi under Modified Atmosphere—The Effects of CO2 Stress on Cell Membranes and Description of New Yeast Stenotrophomyces fumitolerans gen. nov., sp. nov. Journal of Fungi, 9(10), 1031. https://doi.org/10.3390/jof9101031








