Seasonal and Spatial Dynamics of Fungal Diversity and Communities in the Intertidal Zones of Qingdao, China
Abstract
:1. Introduction
2. Materials and Methods
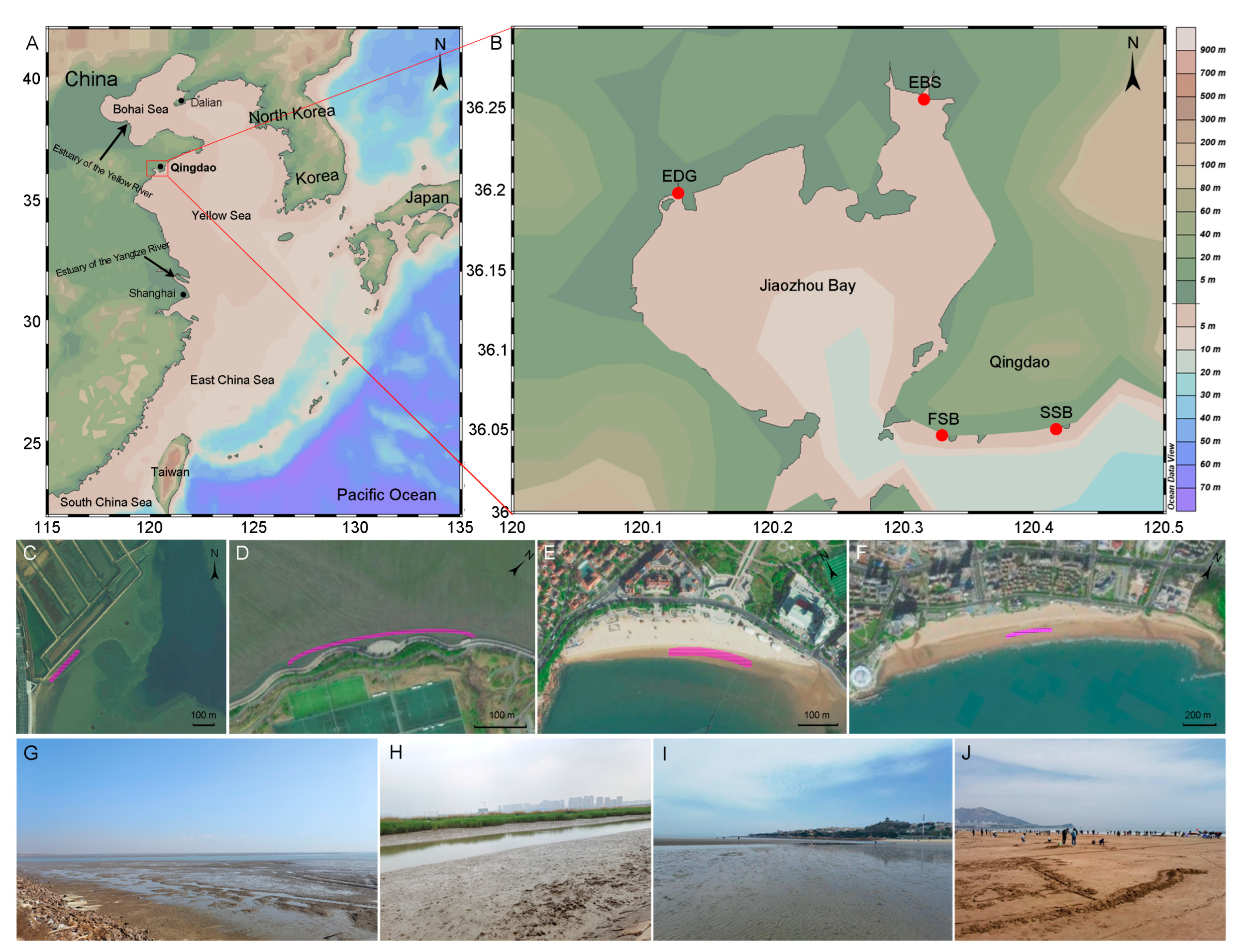
2.1. Sample Collection
2.2. DNA Extraction and High-Throughput Sequencing
2.3. Bioinformatic Analysis
2.4. Statistical Analyses
3. Results
3.1. The Difference of Environmental Parameters across Habitats
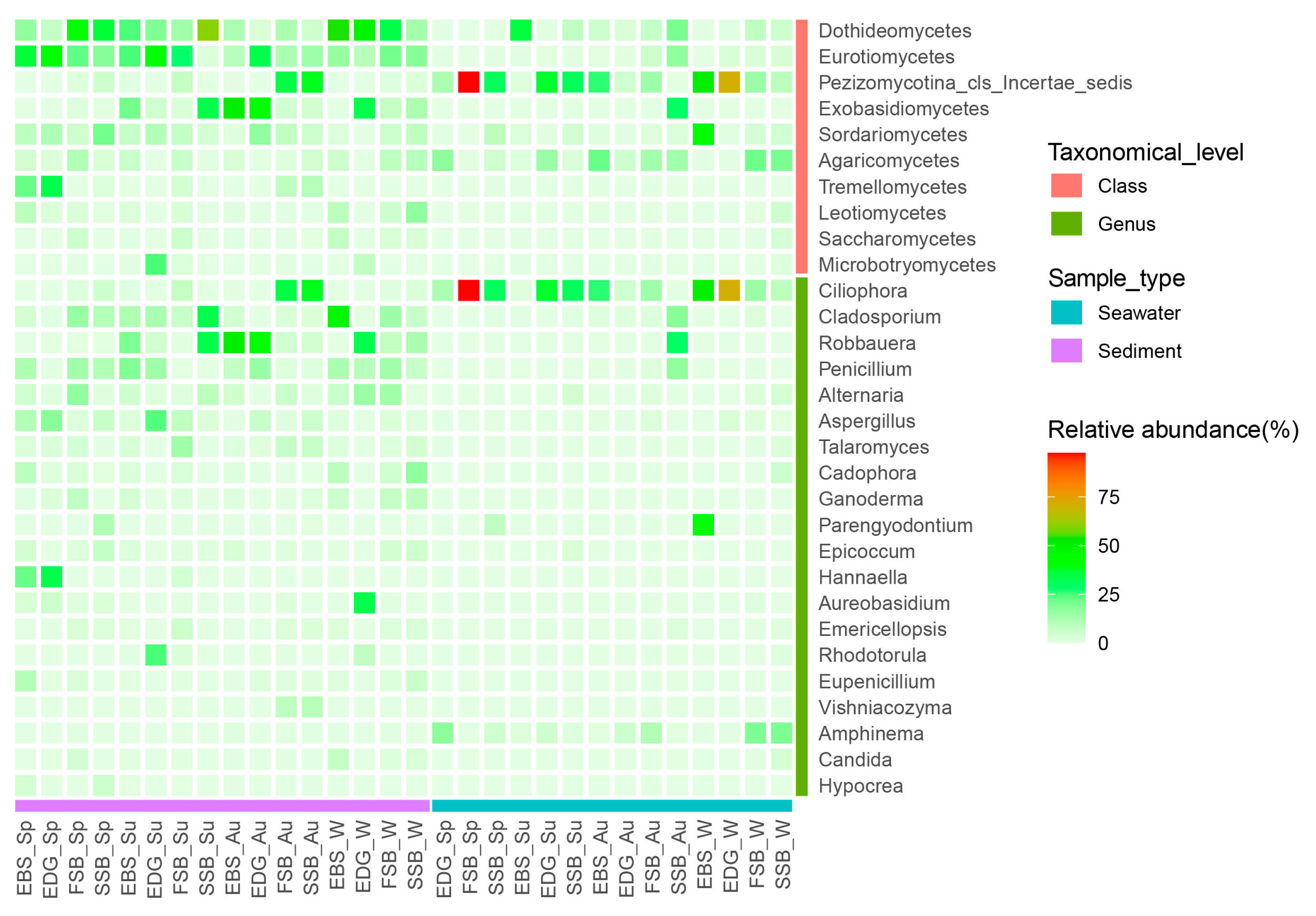
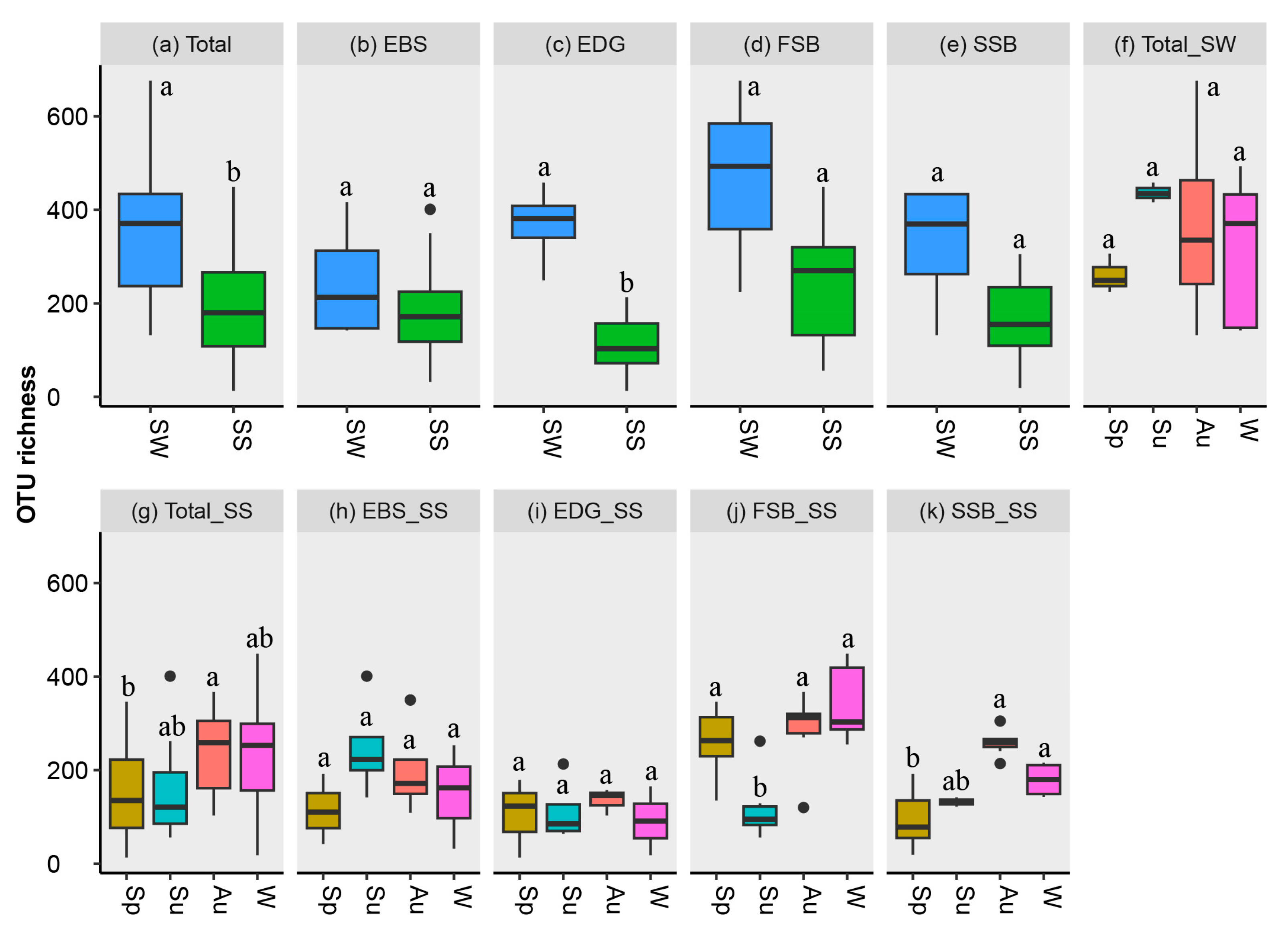
3.2. Taxonomic Composition and α-Diversity of Fungal Community
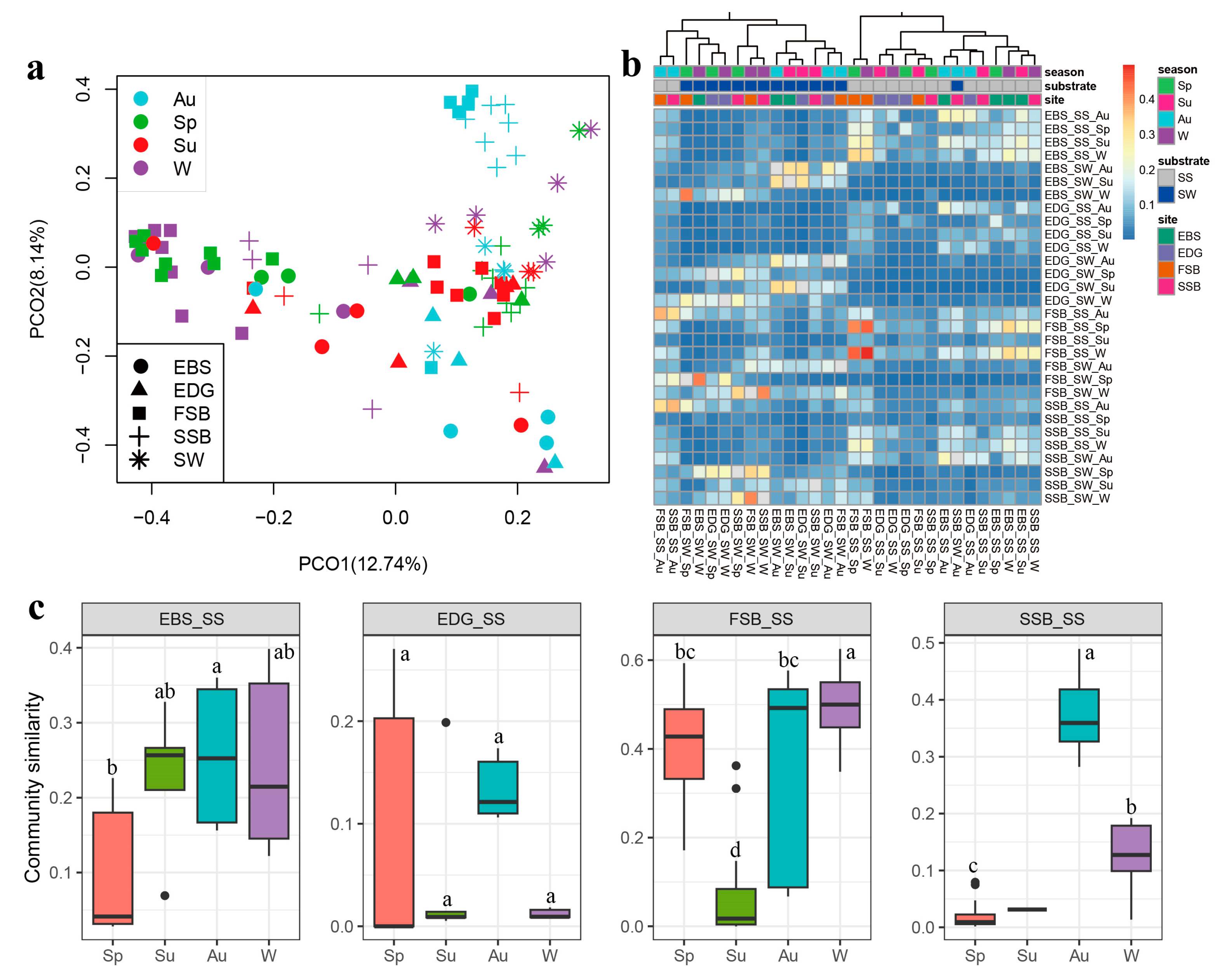
3.3. Space–Time Variation in Community Structure and the Influencing Factors
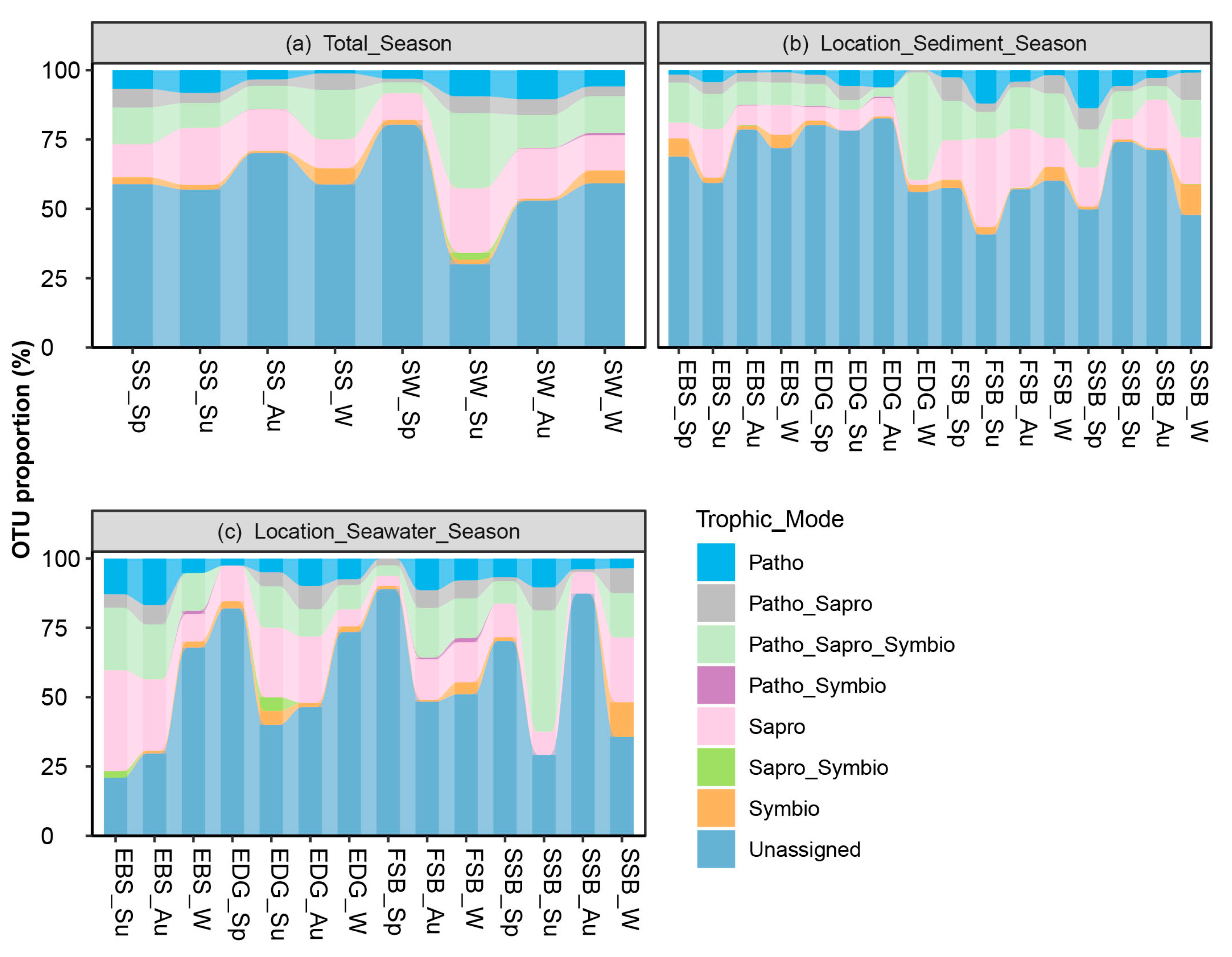
3.4. Trophic Modes of Fungal Community in Intertidal Zone
4. Discussion
4.1. Intertidal Fungi Exhibit Spatiotemporal Variation in Taxonomic Composition
4.2. Potential Ecological Functions of Intertidal Fungi Switch with Habitat and Season
4.3. Environmental and Anthropogenic Factors Strongly Influence the Intertidal Mycobiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burdige, D.J. Estuarine and Coastal Sediments—Coupled Biogeochemical Cycling. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D., Eds.; Academic Press: Waltham, MA, USA, 2012; Volume 9, Chapter 5; pp. 279–316. [Google Scholar]
- Song, J.M. (Ed.) Biogeochemical Processes of Biogenic Elements in China Marginal Seas; Springer: Berlin/Heidelberg: Germany; Zhejiang University Press: Hangzhou, China, 2009. [Google Scholar]
- Costello, M.J.; Tsai, P.; Wong, P.S.; Cheung, A.K.L.; Basher, Z.; Chaudhary, C. Marine biogeographic realms and species endemicity. Nat. Commun. 2017, 8, 1057. [Google Scholar] [CrossRef] [PubMed]
- Burgaud, G.; Edgcomb, V.P.; Hassett, B.T.; Kumar, A.; Li, W.; Mara, P.; Peng, X.F.; Philippe, A.; Phule, P.; Prado, S.; et al. Marine Fungi. In The Marine Microbiome; Stal, L.J., Cretoiu, M.S., Eds.; Springer: Cham, Swizerland, 2022; Volume 2, pp. 243–295. [Google Scholar]
- Calabon, M.; Jones, E.B.G.; Pang, K.L.; Abdel-Wahab, M.; Jin, J.; Devadatha, B.; Sadaba, R.; Apurillo, C.C.S.; Hyde, K. Updates on the classification and numbers of marine fungi. Bot. Mar. 2023, 66, 213–238. [Google Scholar] [CrossRef]
- Calabon, M.S.; Jones, E.B.G.; Promputtha, I.; Hyde, K.D. Fungal Biodiversity in Salt Marsh Ecosystems. J. Fungi 2021, 7, 648. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef]
- Alain, K.; Querellou, J. Cultivating the uncultured: Limits, advances and future challenges. Extremophiles 2009, 13, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Arfi, Y.; Buée, M.; Marchand, C.; Levasseur, A.; Record, E. Multiple markers pyrosequencing reveals highly diverse and host-specific fungal communities on the mangrove trees Avicennia marina and Rhizophora stylosa. FEMS Microbiol. Ecol. 2012, 79, 433–444. [Google Scholar] [CrossRef]
- Arfi, Y.; Marchand, C.; Wartel, M.; Record, E. Fungal diversity in anoxic-sulfidic sediments in a mangrove soil. Fungal Ecol. 2012, 5, 282–285. [Google Scholar] [CrossRef]
- Picard, K.T. Coastal marine habitats harbor novel early-diverging fungal diversity. Fungal Ecol. 2017, 25, 1–13. [Google Scholar] [CrossRef]
- Hassett, B.T.; Gradinger, R. Chytrids dominate arctic marine fungal communities. Environ. Microbiol. 2016, 18, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Hassett, B.T.; Ducluzeau, A.L.; Collins, R.E.; Gradinger, R. Spatial distribution of aquatic marine fungi across the western Arctic and sub-arctic. Environ. Microbiol. 2017, 19, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Kilias, E.S.; Junges, L.; Šupraha, L.; Leonard, G.; Metfies, K.; Richards, T.A. Chytrid fungi distribution and co-occurrence with diatoms correlate with sea ice melt in the Arctic Ocean. Commu. Biol. 2020, 3, 183. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.M.; Bian, X.M.; Guo, J.J.; Cai, L. A High-Level Fungal Diversity in the Intertidal Sediment of Chinese Seas Presents the Spatial Variation of Community Composition. Front. Microbiol. 2016, 7, 2098. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Fuhrman, J.A.; Cram, J.A.; Needham, D.M. Marine microbial community dynamics and their ecological interpretation. Nat. Rev. Microbiol. 2015, 13, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.D.; Cunliffe, M. Multi-year assessment of coastal planktonic fungi reveals environmental drivers of diversity and abundance. ISME J. 2016, 10, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Sen, B.; He, Y.D.; Xie, N.D.; Wang, G.Y. Spatiotemporal distribution and assemblages of planktonic fungi in the coastal waters of the Bohai Sea. Front. Microbiol. 2018, 9, 584. [Google Scholar] [CrossRef]
- Wang, M.M.; Ma, Y.Y.; Cai, L.; Tedersoo, L.; Bahram, M.; Burgaud, G.; Long, X.D.; Zhang, S.M.; Li, W. Seasonal dynamics of mycoplankton in the Yellow Sea reflect the combined effect of riverine inputs and hydrographic conditions. Mol. Ecol. 2021, 30, 3624–3637. [Google Scholar] [CrossRef]
- Gao, F.L.; Li, J.X.; Hu, J.; Sui, B.L.; Wang, C.X.; Sun, C.J.; Li, X.G.; Ju, P. The seasonal distribution characteristics of microplastics on bathing beaches along the coast of Qingdao, China. Sci. Total Environ. 2021, 783, 146969. [Google Scholar] [CrossRef]
- Pervez, R.; Lai, Z.P. Spatio-temporal variations of litter on Qingdao tourist beaches in China. Environ. Pollut. 2022, 303, 119060. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, L.L.; Li, J.L.; Huang, X.P. Long-term changes of nutrients and biocenoses indicating the anthropogenic influences on ecosystem in Jiaozhou Bay and Daya Bay, China. Mar. Pollut. Bull. 2021, 168, 112406. [Google Scholar] [CrossRef]
- González, M.D.C.; Herrera, T.; Ulloa, M.; Hanlin, R.T. Abundance and diversity of microfungi in three coastal beaches of Mexico. Mycoscience 1998, 39, 115–121. [Google Scholar] [CrossRef]
- Collins, R.E.; Rocap, G.; Deming, J.W. Persistence of bacterial and archaeal communities in sea ice through an Arctic winter. Environ. Microbiol. 2010, 12, 1828–1841. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.M.; Pan, H.Q.; Burgaud, G.; Liang, S.K.; Guo, J.J.; Luo, T.; Li, Z.X.; Zhang, S.M.; Cai, L. Highlighting patterns of fungal diversity and composition shaped by ocean currents using the East China Sea as a model. Mol. Ecol. 2018, 27, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Põlme, S.; Riit, T.; Liiv, I.; Kõljalg, U.; Kisand, V.; Nilsson, R.H.; Hildebrand, F.; et al. Shotgun metagenomes and multiple primer pair-barcode combinations of amplicons reveal biases in metabarcoding analyses of fungi. MycoKeys 2015, 10, 1–43. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Nilsson, R.H.; Tedersoo, L.; Abarenkov, K.; Carlsen, T.; Kjøller, R.; Kõljalg, U.; Pennanen, T.; Rosendahl, S.; Stenlid, J.; et al. Fungal community analysis by high-throughput sequencing of amplified markers—A user’s guide. New Phytol. 2013, 199, 288–299. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Ryberg, M.; Hartmann, M.; Branco, S.; Wang, Z.; Godhe, A.; De Wit, P.; Sanchez-Garcia, M.; Ebersberger, I.; Sousa, F.; et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol. Evol. 2013, 4, 914–919. [Google Scholar] [CrossRef]
- Fu, L.M.; Niu, B.F.; Zhu, Z.W.; Wu, S.T.; Li, W.Z. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef] [PubMed]
- Morgulis, A.; Coulouris, G.; Raytselis, Y.; Madden, T.L.; Agarwala, R.; Schäffer, A.A. Database indexing for production MegaBLAST searches. Bioinformatics 2008, 24, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Wang, Q.; Greenfield, P.; Charleston, M.; Porras-Alfaro, A.; Kuske, C.R.; Cole, J.R.; Midgley, D.J.; Tran-Dinh, N. Fungal identification using a Bayesian classifier and the Warcup training set of internal transcribed spacer sequences. Mycologia 2016, 108, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Firendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version, 2.5-3. 2018. Available online: https://CRAN.Rproject.org/package=vegan (accessed on 17 October 2018).
- Gao, Z.; Johnson, Z.I.; Wang, G.Y. Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters. ISME J. 2010, 4, 111–120. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Sen, K.; He, Y.; Xie, Y.X.; Wang, G.Y. Impact of environmental gradients on the abundance and diversity of planktonic fungi across coastal habitats of contrasting trophic status. Sci. Total. Environ. 2019, 683, 822–833. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.M.; Burgaud, G.; Yu, H.M.; Cai, L. Fungal community composition and potential depth-related driving factors impacting distribution pattern and trophic modes from epi- to abyssopelagic zones of the Western Pacific Ocean. Microb. Ecol. 2019, 78, 820–831. [Google Scholar] [CrossRef]
- Rédou Creff, V.; Ciobanu, M.C.; Pachiadaki, M.G.; Edgcomb, V.V.P.; Alain, K.; Barbier, G.; Burgaud, G. In-depth analyses of deep subsurface sediments using 454-pyrosequencing reveals a reservoir of buried fungal communities at record-breaking depths. FEMS Microb. Ecol. 2014, 90, 908–921. [Google Scholar] [CrossRef]
- Quémener, M.; Mara, P.; Schubotz, F.; Beaudoin, D.; Li, W.; Pachiadaki, M.; Sehein, T.R.; Sylvan, J.B.; Li, J.; Barbier, G.; et al. Meta-omics highlights the diversity, activity and adaptations of fungi in deep oceanic crust. Environ. Microbiol. 2020, 22, 3950–3967. [Google Scholar] [CrossRef]
- Smith, A.J.H.; Potvin, L.R.; Lilleskov, E.A. Fertility-dependent effects of ectomycorrhizal fungal communities on white spruce seedling nutrition. Mycorrhiza 2015, 25, 649–662. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; García-Romera, I.; Aranda, E. Potential of non-ligninolytic fungi in bioremediation of chlorinated and polycyclic aromatic hydrocarbons. New Biotechnol. 2015, 32, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Guo, Y.; Zhang, M.; Pan, W.; Wang, J.J. Stronger network connectivity with lower diversity of soil fungal community was presented in coastal marshes after sixteen years of freshwater restoration. Sci. Total Environ. 2020, 744, 140623. [Google Scholar] [CrossRef]
- Álvarez-Barragán, J.; Cravo-Laureau, C.; Wick, L.Y.; Duran, R. Fungi in PAH-contaminated marine sediments: Cultivable diversity and tolerance capacity towards PAH. Mar. Pollut. Bull. 2021, 164, 112082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hu, J.; Yang, S.; Xu, W.; Wang, Z.; Zhuang, P.; Grossart, H.P.; Luo, Z. Biodegradation of polyester polyurethane by the marine fungus Cladosporium halotolerans 6UPA1. J. Hazard. Mater. 2022, 437, 129406. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Liu, R.; Sun, C. A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J. Hazard. Mater. 2022, 431, 128617. [Google Scholar] [CrossRef]
- Richards, T.A.; Jones, M.D.M.; Leonard, G.; Bass, D. Marine fungi: Their ecology and molecular diversity. Ann. Rev. Mar. Sci. 2012, 4, 495–522. [Google Scholar] [CrossRef]
- Manohar, C.S.; Boekhout, T.; Müller, W.H.; Stoeck, T. Tritirachium candoliense sp. nov., a novel basidiomycetous fungus isolated from the anoxic zone of the Arabian Sea. Fungal Biol. 2014, 118, 139–149. [Google Scholar] [CrossRef]
- Bärlocher, F.; Boddy, L. Aquatic fungal ecology—How does it differ from terrestrial? Fungal Ecol. 2016, 19, 5–13. [Google Scholar] [CrossRef]
- Bartoli, M.; Nizzoli, D.; Zilius, M.; Bresciani, M.; Pusceddu, A.; Bianchelli, S.; Sundbäck, K.; Razinkovas-Baziukas, A.; Viaroli, P. Denitrification, nitrogen uptake, and organic matter quality undergo different seasonality in sandy and muddy sediments of a turbid estuary. Front. Microbiol. 2020, 11, 612700. [Google Scholar] [CrossRef]
- Gong, N.; Shao, K.S.; Shen, K.; Gu, Y.B.; Liu, Y.A.; Ye, J.Q.; Hu, C.M.; Shen, L.Y.; Chen, Y.L.; Li, D.W.; et al. Chemical control of overwintering green algae to mitigate green tide in the Yellow Sea. Mar. Pollut. Bull. 2021, 168, 112424. [Google Scholar] [CrossRef]
- Gleason, F.H.; Scholz, B.; Jephcott, T.G.; Van Ogtrop, F.F.; Henderson, L.; Lilje, O.; Kittelmann, S.; Macarthur, D.J. Key ecological roles for zoosporic true fungi in aquatic habirtats. Microbiol. Spectr. 2017, 5, 10–1128. [Google Scholar] [CrossRef]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef]
- Tisthammer, K.H.; Cobian, G.M.; Amend, A.S. Global biogeography of marine fungi is shaped by the environment. Fungal Ecol. 2016, 19, 39–46. [Google Scholar] [CrossRef]
- Castellani, C.; Altunbaş, Y. Seasonal change in acclimatised respiration rate of Temora longicornis (Müller). Mar. Ecol. Prog. Ser. 2013, 500, 83–101. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Karsani, S.A.; Alias, S.A. Fungal survival under temperature stress: A proteomic perspective. PeerJ 2020, 8, e10423. [Google Scholar] [CrossRef] [PubMed]
- Shearer, C.A.; Descals, E.; Kohlmeyer, B.; Kohlmeyer, J.; Marvanová, L.; Padgett, D.; Porter, D.; Raja, H.A.; Schmit, J.P.; Thorton, H.A.; et al. Fungal biodiversity in aquatic habitats. Biodivers. Conserv. 2007, 16, 49–67. [Google Scholar] [CrossRef]
- Jones, E.B.G. Marine fungi: Some factors influencing biodiversity. Fungal Divers. 2000, 4, 53–73. [Google Scholar]
- He, Q.; Bertness, M.D.; Bruno, J.F.; Li, B.; Chen, G.Q.; Coverdale, T.C.; Altieri, A.H.; Bai, J.H.; Sun, T.; Pennings, S.C.; et al. Economic development and coastal ecosystem change in China. Sci. Rep. 2014, 4, 5995. [Google Scholar] [CrossRef]
- Yang, F.X.; Wei, Q.S.; Chen, H.T.; Yao, Q.Z. Long-term variations and influence factors of nutrients in the western North Yellow Sea, China. Mar. Pollut. Bull. 2018, 135, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y. Status of marine biodiversity of the China seas. PLoS ONE 2013, 8, e50719. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, Q.; Pan, Z.; Burgaud, G.; Ma, H.; Zheng, Y.; Wang, M.; Cai, L. Seasonal and Spatial Dynamics of Fungal Diversity and Communities in the Intertidal Zones of Qingdao, China. J. Fungi 2023, 9, 1015. https://doi.org/10.3390/jof9101015
Li W, Li Q, Pan Z, Burgaud G, Ma H, Zheng Y, Wang M, Cai L. Seasonal and Spatial Dynamics of Fungal Diversity and Communities in the Intertidal Zones of Qingdao, China. Journal of Fungi. 2023; 9(10):1015. https://doi.org/10.3390/jof9101015
Chicago/Turabian StyleLi, Wei, Qi Li, Zhihui Pan, Gaëtan Burgaud, Hehe Ma, Yao Zheng, Mengmeng Wang, and Lei Cai. 2023. "Seasonal and Spatial Dynamics of Fungal Diversity and Communities in the Intertidal Zones of Qingdao, China" Journal of Fungi 9, no. 10: 1015. https://doi.org/10.3390/jof9101015
APA StyleLi, W., Li, Q., Pan, Z., Burgaud, G., Ma, H., Zheng, Y., Wang, M., & Cai, L. (2023). Seasonal and Spatial Dynamics of Fungal Diversity and Communities in the Intertidal Zones of Qingdao, China. Journal of Fungi, 9(10), 1015. https://doi.org/10.3390/jof9101015









