Comparison of Pigment Production by Filamentous Fungal Strains under Submerged (SmF) and Surface Adhesion Fermentation (SAF)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains Selection
2.2. Fungal DNA Extraction, Amplification, and Sequencing
2.3. Fungal Identification and Phylogenetic Analysis
2.4. Inoculum Preparation
2.5. Production Medium Composition
2.6. Fermentation Systems
2.7. Extracellular Pigment and Biomass Recovery
2.8. Determination of Pigment Intensities (AU)
3. Results and Discussion
3.1. Molecular Identification and Phylogenetic Analysis of Pigment-Producing Fungal Strains
3.2. Pigments Spectra of Selected Fungal Strains
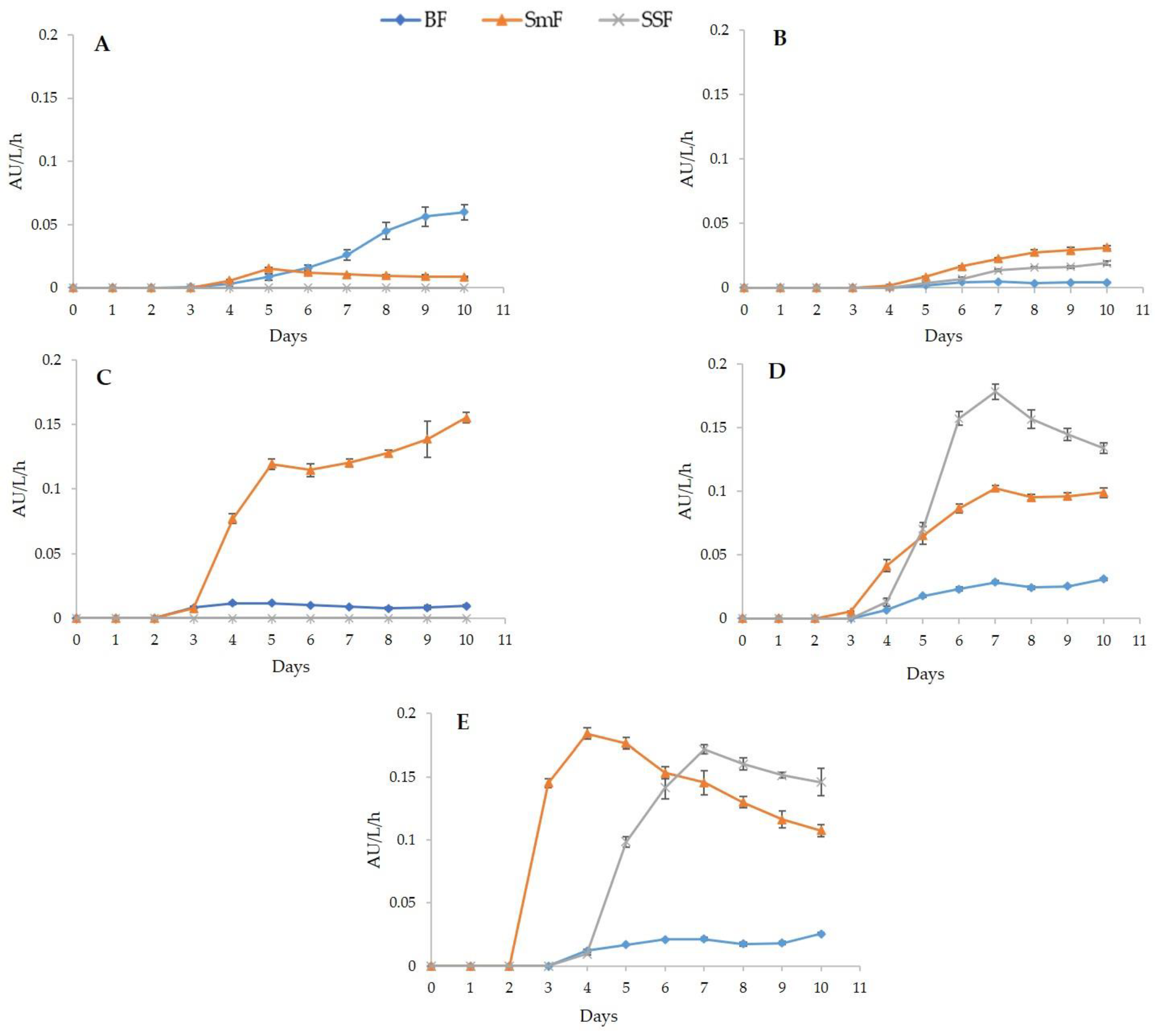
3.3. Pigments Productivity and Yields
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ramesh, C.; Dufossé, L. Ecological and biotechnological aspects of pigmented microbes: A way forward in development of food and pharmaceutical grade pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef]
- Global Natural Colorants Market Research Report 2022 (Status and Outlook). Available online: https://www.360researchreports.com/global-natural-colorants-market-21179188 (accessed on 10 August 2022).
- Downham, A.; Collins, P. Colouring our foods in the last and next millennium. Int. J. Food Sci. Technol. 2000, 35, 5–22. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-derived colorants for food, cosmetic and textile industries: A review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V. Microbial pigments as natural color sources: Current trends and future perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vishwakarma, H.S.; Sngh, J.; Dwivedi, S.; Kumar, M. Microbial pigments: Production and their applications in various industries. Int. J. Pharm. Chem. Biol. Sci. 2015, 5, 203–212. [Google Scholar]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Meruvu, H.; dos Santos, J.C. Colors of life: A review on fungal pigments. Crit. Rev. Biotechnol. 2021, 41, 1153–1177. [Google Scholar] [CrossRef]
- Narsing Rao, M.P.; Xiao, M.; Li, W.J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry-Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety evaluation of fungal pigments for food applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal Pigments: Potential Coloring Compounds for Wide Ranging Applications in Textile Dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Factories 2009, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.A.; Zaccarim, B.R.; de Lencastre Novaes, L.C.; Jozala, A.F.; dos Santos, C.A.; Teixeira, M.F.; Santos-Ebinuma, V.C. Natural colorants from filamentous fungi. Appl. Microbiol. Biotechnol. 2016, 100, 2511–2521. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Biotechnological approaches for the production of natural colorants by Talaromyces/Penicillium: A review. Biotechnol. Adv. 2020, 43, 107601. [Google Scholar] [CrossRef]
- Ramesh, C.; Prasastha, V.R.; Venkatachalam, M.; Dufossé, L. Natural Substrates and Culture Conditions to Produce Pigments from Potential Microbes in Submerged Fermentation. Fermentation 2022, 8, 460. [Google Scholar] [CrossRef]
- Gutiérrez-Correa, M.; Villena, G.K. Surface adhesion fermentation: A new fermentation category. Rev. Peru. De Biol. 2003, 10, 113–124. [Google Scholar] [CrossRef][Green Version]
- Gamarra, N.N.; Villena, G.K.; Gutiérrez-Correa, M. Cellulase production by Aspergillus niger in biofilm, solid-state, and submerged fermentations. Appl. Microbiol. Biotechnol. 2010, 87, 545–551. [Google Scholar] [CrossRef]
- Vega, K.; Villena, G.K.; Sarmiento, V.H.; Ludeña, Y.; Vera, N.; Gutiérrez-Correa, M. Production of Alkaline Cellulase by Fungi Isolated from an Undisturbed Rain Forest of Peru. Biotechnol. Res. Int. 2012, 2012, 934325. [Google Scholar] [CrossRef]
- Vega, K.; Sarmiento, V.; Ludeña, Y.; Vera, N.; Tamariz-Angeles, C.; Villena, G.K.; Gutiérrez-Correa, M. Alkaline Cellulase Production by Penicillium mallochii LMB-HP37 Isolated from Soils of a Peruvian Rainforest. Biotechnol. J. Int. 2015, 7, 160–168. [Google Scholar] [CrossRef]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef]
- Villena, G.K.; Gutiérrez-Correa, M. Production of cellulase by Aspergillus niger biofilms developed on polyester cloth. Lett. Appl. Microbiol. 2006, 43, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Sehnem, N.T.; de Bittencourt, L.R.; Camassola, M.; Dillon, A. Cellulase production by Penicillium echinulatum on lactose. Appl. Microbiol. Biotechnol. 2006, 72, 163–167. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Frisvad, J.C. Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front. Microbiol. 2015, 5, 773. [Google Scholar] [CrossRef]
- Rivera, K.G.; Díaz, J.; Chavarría-Díaz, F.; García, M.; Urb, M.; Thorn, R.G.; Louis-Seize, G.; Janzen, D.H.; Seifert, K.A. Penicillium mallochii and P. guanacastense, two new species isolated from Costa Rican caterpillars. Mycotaxon 2012, 119, 315–328. [Google Scholar] [CrossRef]
- Yilmaz, N.; Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of the genus Talaromyces. Stud. Mycol. 2014, 78, 175–341. [Google Scholar] [CrossRef]
- Park, M.S.; Chung, D.; Baek, K.; Lim, Y.W. Three Unrecorded Species Belonging to Penicillium Section Sclerotiora from Marine Environments in Korea. Mycobiology 2019, 47, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef] [PubMed]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and Colorants from Filamentous Fungi. In Fungal Metabolites. Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufossé, L.; Caro, Y. Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J. Fungi 2017, 3, 21. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Magalon, H.; Dufossé, L.; Fouillaud, M. Production of pigments from the tropical marine-derived fungi Talaromyces albobiverticillius: New resources for natural red-colored metabolites. J. Food Compos. Anal. 2018, 70, 35–48. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Zelena, M.; Cacciola, F.; Ceslova, L.; Girard-Valenciennes, E.; Clerc, P.; Dugo, P.; Mondello, L.; Fouillaud, M.; Rotondo, A.; et al. Partial characterization of the pigments produced by the marine-derived fungus Talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J. Food Compos. Anal. 2018, 67, 38–47. [Google Scholar] [CrossRef]
- Pimenta, L.; Gomes, D.C.; Cardoso, P.G.; Takahashi, J.A. Recent findings in azaphilone pigments. J. Fungi 2021, 7, 541. [Google Scholar] [CrossRef]
- Osmanova, N.; Schultze, W.; Ayoub, N. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 2010, 9, 315–342. [Google Scholar] [CrossRef]
- Chen, C.; Tao, H.; Chen, W.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Recent advances in the chemistry and biology of azaphilones. RSC Adv. 2020, 10, 10197–10220. [Google Scholar] [CrossRef]
- Trung, N.; Thong, N.; Cuong, D.; Manh, T.; Hoang, L.; Hien, N.; Nam, P.; Quang, D.; Mechler, A.; Vo, Q. Radical Scavenging Activity of Natural Anthraquinones: A Theoretical Insight. ACS Omega 2021, 6, 13391–13397. [Google Scholar] [CrossRef]
- Nakanishi, S.; Kakita, S.; Takahashi, I.; Kawahara, K.; Tsukuda, E.; Sano, T.; Yamada, K.; Yoshida, M.; Kase, H.; Matsuda, Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 1992, 267, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.M.; Kim, K.; Kwon, S.L.; Na, J.; Lee, H.; Jang, S.; Kim, C.H.; Jung, J.; Kim, J.-J. Investigation of filamentous fungi producing safe, functional water-soluble pigments. Mycobiology 2018, 46, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Pigments, microbial. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- dos Santos, P.O.; Ferraz, C.G.; Ribeiro, P.R.; Miranda, F.M.; da Silva, F.; de Souza, J.T.; Roque, M.R.D.A.; Soares, A.C. Antioxidant and antibacterial activities of the chlorine pigment sclerotiorin from Penicillium mallochii and its chemotaxonomic significance. Biochem. Syst. Ecol. 2019, 86, 103915. [Google Scholar] [CrossRef]
- Bouhri, Y.; Askun, T.; Tunca, B.; Deniz, G.; Aksoy, S.A.; Mutlu, M. The orange-red pigment from Penicillium mallochii: Pigment production, optimization, and pigment efficacy against Glioblastoma cell lines. Biocatal. Agric. Biotechnol. 2020, 23, 101451. [Google Scholar] [CrossRef]
- Bara, R.; Aly, A.H.; Pretsch, A.; Wray, V.; Wang, B.; Proksch, P.; Debbab, A. Antibiotically active metabolites from Talaromyces wortmannii, an endophyte of Aloe vera. J. Antibiot. 2013, 66, 491–493. [Google Scholar] [CrossRef]
- Kaur, S.; Arora, N.; Kaur, S. Characterization of Yellow Pigments Produced by Pencillium sp. under Solid State Cultivation. J. Biotechnol. Biomater. 2017, 7, 259. [Google Scholar] [CrossRef]
- Molelekoa, T.B.J.; Regnier, T.; da Silva, L.S.; Augustyn, W. Production of Pigments by Filamentous Fungi Cultured on Agro-Industrial by-Products Using Submerged and Solid-State Fermentation Methods. Fermentation 2021, 7, 295. [Google Scholar] [CrossRef]
- de Oliveira, F.; Pedrolli, D.B.; Teixeira, M.F.S.; Santos-Ebinuma, V.C. Water-soluble fluorescent red colorant production by Talaromyces amestolkiae. Appl. Microbiol. Biotechnol. 2019, 103, 6529–6541. [Google Scholar] [CrossRef]
- de Oliveira, F.; Lima, C.d.A.; Lopes, A.M.; Marques, D.D.A.V.; Druzian, J.I.; Pessoa Júnior, A.; Santos-Ebinuma, V.C. Microbial Colorants Production in Stirred-Tank Bioreactor and Their Incorporation in an Alternative Food Packaging Biomaterial. J. Fungi 2020, 6, 264. [Google Scholar] [CrossRef]
- Pandit, S.G.; Mekala Ramesh, K.P.; Puttananjaiah, M.H.; Dhale, M.A. Cicer arietinum (Bengal gram) husk as alternative for Talaromyces purpureogenus CFRM02 pigment production: Bioactivities and identification. LWT 2019, 116, 108499. [Google Scholar] [CrossRef]
- Zaccarim, B.R.; de Oliveira, F.; Passarini, M.; Duarte, A.; Sette, L.D.; Jozala, A.F.; Teixeira, M.F.S.; Santos-Ebinuma, V.C. Sequencing and phylogenetic analyses of Talaromyces amestolkiae from amazon: A producer of natural colorants. Biotechnol. Prog. 2019, 35, e2684. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, C.T.; Ogbonna, C.N.; Ogbonna, J.C.; Aoyagi, H. Production and stability of pigments by Talaromyces purpurogenus LC128689 in an alternating air phase-liquid phase cultivation system. Biotechnol. Appl. Biochem. 2021, 69, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Pan, T.; Zhang, W.; Wang, Z. Submerged culture of marine-derived Penicillium sclerotiorum FS50 to produce sclerotiorin. Process Biochem. 2019, 79, 28–31. [Google Scholar] [CrossRef]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A.A. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef]
- Mapari, S.A.; Meyer, A.S. Evaluation of Epicoccum nigrum for growth, morphology and production of natural colorants in liquid media and on a solid rice medium. Biotechnol. Lett. 2008, 30, 2183–2190. [Google Scholar] [CrossRef]
- Méndez, A.; Pérez, C.; Montañéz, J.C.; Martínez, G.; Aguilar, C.N. Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J. Zhejiang Univ. Sci. B 2011, 12, 961–968. [Google Scholar] [CrossRef]
- Manan, M.A.; Mohamad, R.; Ariff, A. Monascus spp.: A source of Natural Microbial Color through Fungal Biofermentation. J. Microbiol. Exp. 2017, 5, 00148. [Google Scholar] [CrossRef]
- Carlile, M.J.; Watkinson, S.C.; Gooday, G.W. The fungi as a major group of microorganisms. In The Fungi; Michael, J., Watkinson, C.S.C., Gooday, G.W., Eds.; Academic Press: Cambridge, MA, USA, 2001; pp. 1–9. [Google Scholar] [CrossRef]
- Kelecom, A. Secondary metabolites from marine microorganisms. An. Acad. Bras. Ciênc. 2002, 74, 151–170. [Google Scholar] [CrossRef]
- Lin, L.-J.; Tao, M.-H.; Chen, Y.-C.; Zheng, C.-X.; Huo, G.-H.; Zhang, W.-M. Secondary metabolites from the solid culture of marine fungal strain Penicillium sclerotiorum FS50. Mycosystema 2015, 34, 117–123. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and Derivatives from Marine-Derived Fungi: Structural Diversity and Selected Biological Activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef]
- Manan, M.A.; Webb, C. Design aspects of solid state fermentation as applied to microbial bioprocessing. Appl. Biotechnol. Bioeng. 2017, 4, 511–532. [Google Scholar] [CrossRef]
- Velázquez Arellano, M.E.; Benavente Valdés, J.R.; Morlett Chávez, J.A.; Aguilar González, C.N. Producción de pigmentos por Monascus spp. en medio sólido empleando residuos agroindustriales. Investig. Y Cienc. 2016, 24, 89–95. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Morales-Martínez, T.K.; Albergamo, A.; Salvo, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Medium design from corncob hydrolyzate for pigment production by Talaromyces atroroseus GH2: Kinetics modeling and pigments characterization. Biochem. Eng. J. 2020, 161, 107698. [Google Scholar] [CrossRef]
- Villena, G.K.; Gutiérrez-Correa, M. Morphological patterns of Aspergillus niger biofilms and pellets related to lignocellulolytic enzyme productivities. Lett. Appl. Microbiol. 2007, 45, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Villena, G.K.; Gutiérrez-Correa, M. Kinetic analysis of Aspergillus niger cellulase and xylanase production in biofilm and submerged fermentation. J. Microbiol. Biotechnol. Res. 2012, 2, 805–814. [Google Scholar]
- Villena, G.K.; Fujikawa, T.; Tsuyumu, S.; Gutiérrez-Correa, M. Differential gene expression of some lignocellulolytic enzymes in Aspergillus niger biofilms. Rev. Peru. De Biol. 2009, 15, 097–102. [Google Scholar]
- Lee, B.K.; Piao, H.Y.; Chung, W.J. Production of red pigments by Monascus purpureus in solid-state culture. Biotechnol. Bioprocess Eng. 2002, 7, 21–25. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X.; Wu, Z.; Qi, H.; Wang, Z. Export of intracellular Monascus pigments by two-stage microbial fermentation in nonionic surfactant micelle aqueous solution. J. Biotechnol. 2012, 162, 202–209. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X.; Wu, Z.; Qi, H.; Wang, Z. Perstraction of intracellular pigments by submerged cultivation of Monascus in nonionic surfactant micelle aqueous solution. Appl. Microbiol. Biotechnol. 2012, 94, 81–89. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, X.; Wu, Z.; Qi, H.; Wang, Z. Solubilization capacity of nonionic surfactant micelles exhibiting strong influence on export of intracellular pigments in Monascus fermentation. Microb. Biotechnol. 2013, 6, 540–550. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Oliveira, J.; Sousa-Gallagher, M. Perstraction of intracellular pigments through submerged fermentation of Talaromyces spp. in a surfactant rich media: A novel approach for enhanced pigment recovery. J. Fungi 2017, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Gill, M. The biosynthesis of pigments in Basidiomycetes. Aust. J. Chem. 2001, 54, 721–734. [Google Scholar] [CrossRef]
- Bergmann, P.; Frank, C.; Reinhardt, O.; Takenberg, M.; Werner, A.; Berger, R.G.; Zschätzsch, M. Pilot-Scale Production of the Natural Colorant Laetiporic Acid, Its Stability and Potential Applications. Fermentation 2022, 8, 684. [Google Scholar] [CrossRef]
- Bergmann, P.; Takenberg, M.; Frank, C.; Zschätzsch, M.; Werner, A.; Berger, R.G.; Ersoy, F. Cultivation of Inonotus hispidus in stirred tank and wave bag bioreactors to produce the natural colorant hispidin. Fermentation 2022, 8, 541. [Google Scholar] [CrossRef]
- Prabhu, G.; Bhat, D.; Bhat, R.M.; Selvaraj, S. A Critical Look at Bioproducts Co-cultured Under Solid State Fermentation and Their Challenges and Industrial Applications. Waste Biomass Valorization 2022, 13, 3095–3111. [Google Scholar] [CrossRef]
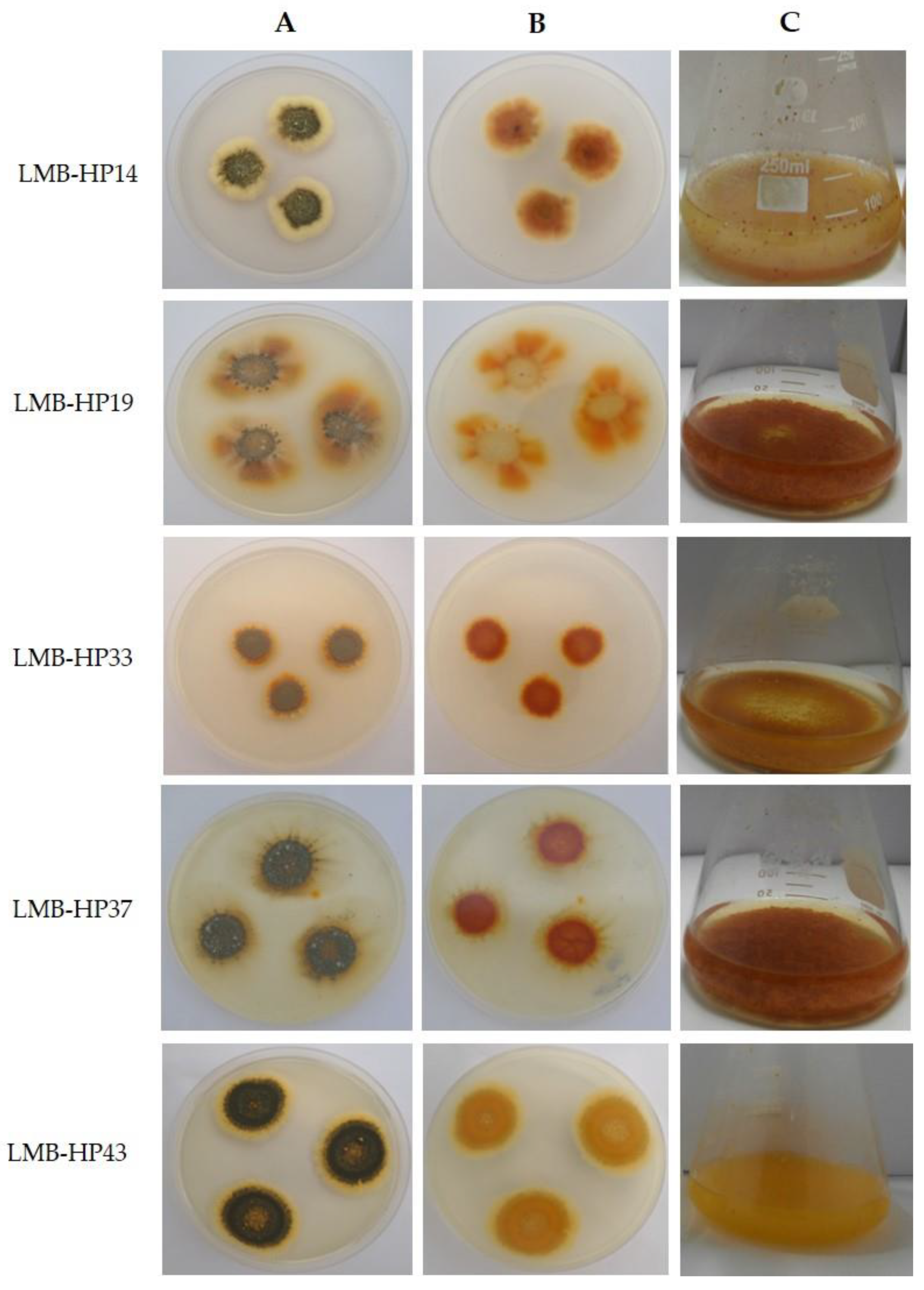

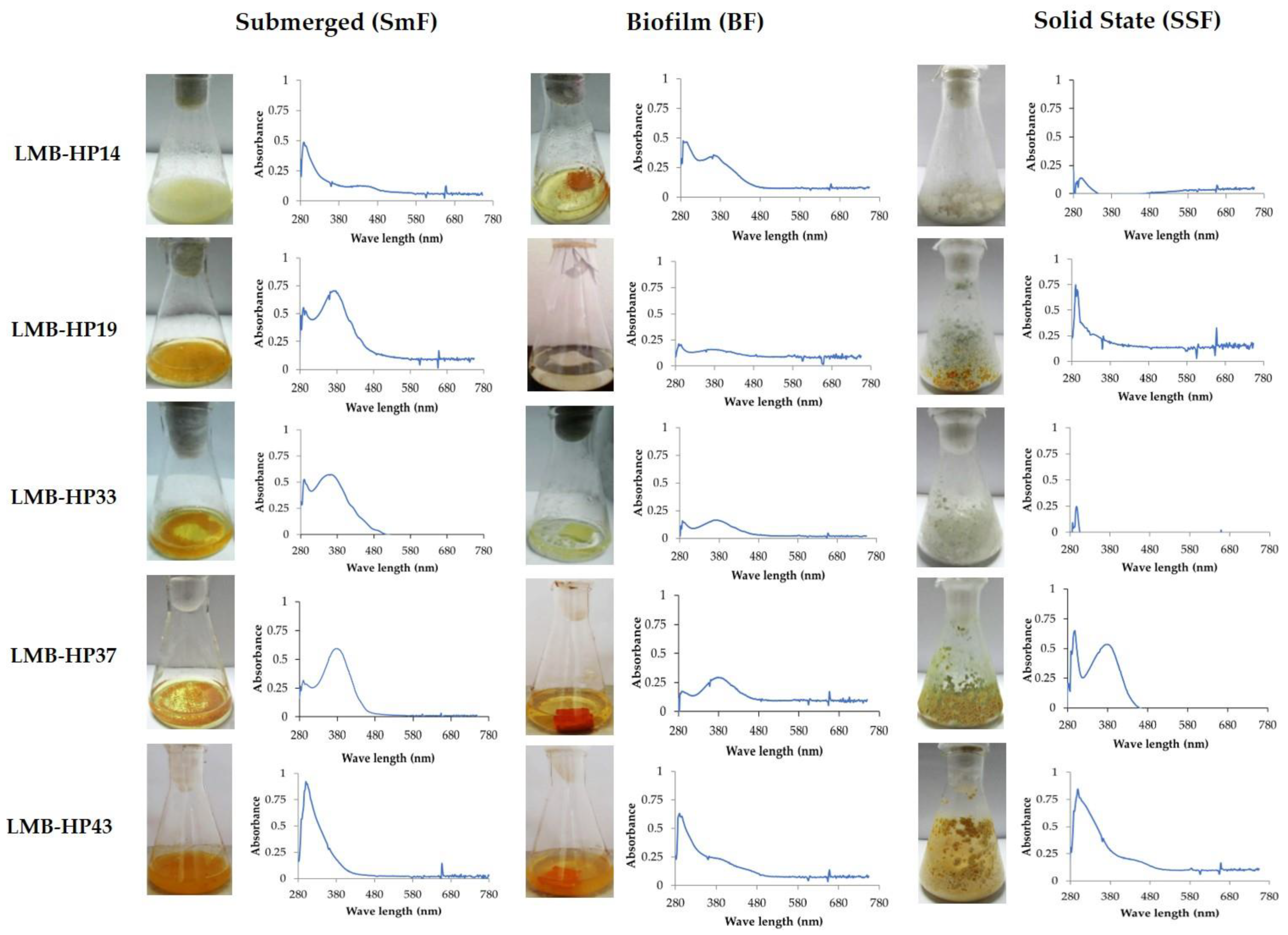
| Fungal Strain | Identity | Reference | Accession N° |
|---|---|---|---|
| LMB-HP14 | Talaromyces wortmannii | This work | OP035499 |
| LMB-HP19 | Penicillium mallochii | This work | OP035500 |
| LMB-HP33 | Penicillium maximae | This work | OP035501 |
| LMB-HP37 | Penicillium mallochii | Vega et al. [21] | OP035502 |
| LMB-HP43 | Talaromyces brunneus | This work | OP035503 |
| Strain | Yield (AU/g) | ||
|---|---|---|---|
| Fermentation Systems | |||
| Surface Adhesion Fermentation (SAF) | |||
| Submerged (SmF) | Biofilm (BF) | Solid-State (SSF) * | |
| LMB-HP14 | 0.05 ± 0.01 | 0.64 ± 0.02 | 0.00 ± 0.00 |
| LMB-HP19 | 0.20 ± 0.01 | 0.13 ± 0.01 | 0.78 ± 0.03 |
| LMB-HP33 | 0.29 ± 0.01 | 0.18 ± 0.03 | 0.00 ± 0.00 |
| LMB-HP37 | 0.62 ± 0.03 | 0.43 ± 0.03 | 9.05 ± 0.34 |
| LMB-HP43 | 1.54 ± 0.07 | 0.62 ± 0.14 | 14.57 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rengifo, L.R.; Rosas, P.; Méndez, N.; Ludeña, Y.; Sirvas, S.; Samolski, I.; Villena, G.K. Comparison of Pigment Production by Filamentous Fungal Strains under Submerged (SmF) and Surface Adhesion Fermentation (SAF). J. Fungi 2023, 9, 48. https://doi.org/10.3390/jof9010048
Rengifo LR, Rosas P, Méndez N, Ludeña Y, Sirvas S, Samolski I, Villena GK. Comparison of Pigment Production by Filamentous Fungal Strains under Submerged (SmF) and Surface Adhesion Fermentation (SAF). Journal of Fungi. 2023; 9(1):48. https://doi.org/10.3390/jof9010048
Chicago/Turabian StyleRengifo, Liliana R., Paola Rosas, Nicolás Méndez, Yvette Ludeña, Susana Sirvas, Ilanit Samolski, and Gretty K. Villena. 2023. "Comparison of Pigment Production by Filamentous Fungal Strains under Submerged (SmF) and Surface Adhesion Fermentation (SAF)" Journal of Fungi 9, no. 1: 48. https://doi.org/10.3390/jof9010048
APA StyleRengifo, L. R., Rosas, P., Méndez, N., Ludeña, Y., Sirvas, S., Samolski, I., & Villena, G. K. (2023). Comparison of Pigment Production by Filamentous Fungal Strains under Submerged (SmF) and Surface Adhesion Fermentation (SAF). Journal of Fungi, 9(1), 48. https://doi.org/10.3390/jof9010048






