Fungal Tracheobronchitis in Lung Transplant Recipients: Incidence and Utility of Diagnostic Markers
Abstract
1. Introduction
2. Materials and Methods
3. Results
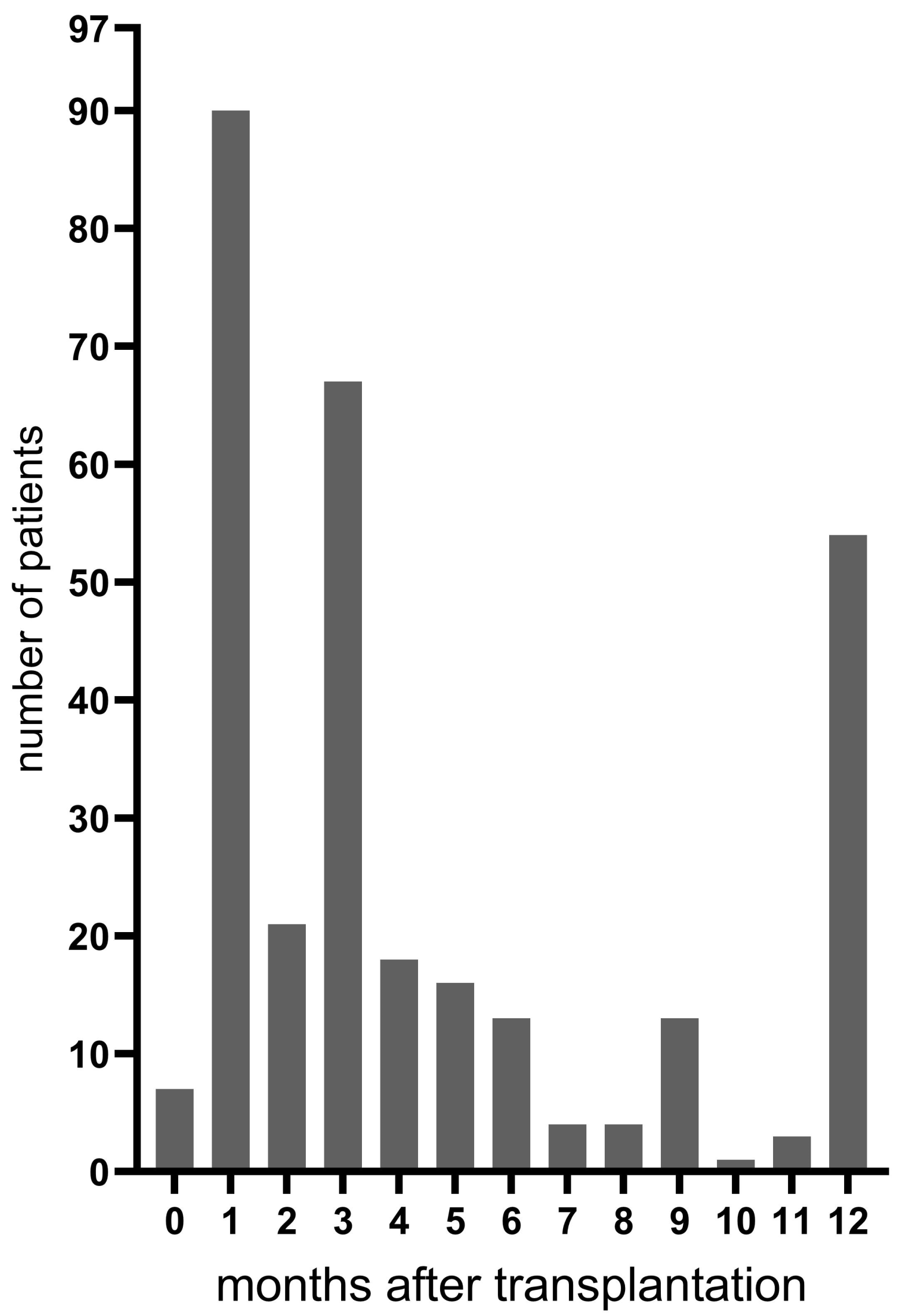
3.1. Patients
3.2. Fungal Tracheobronchitis and Colonization
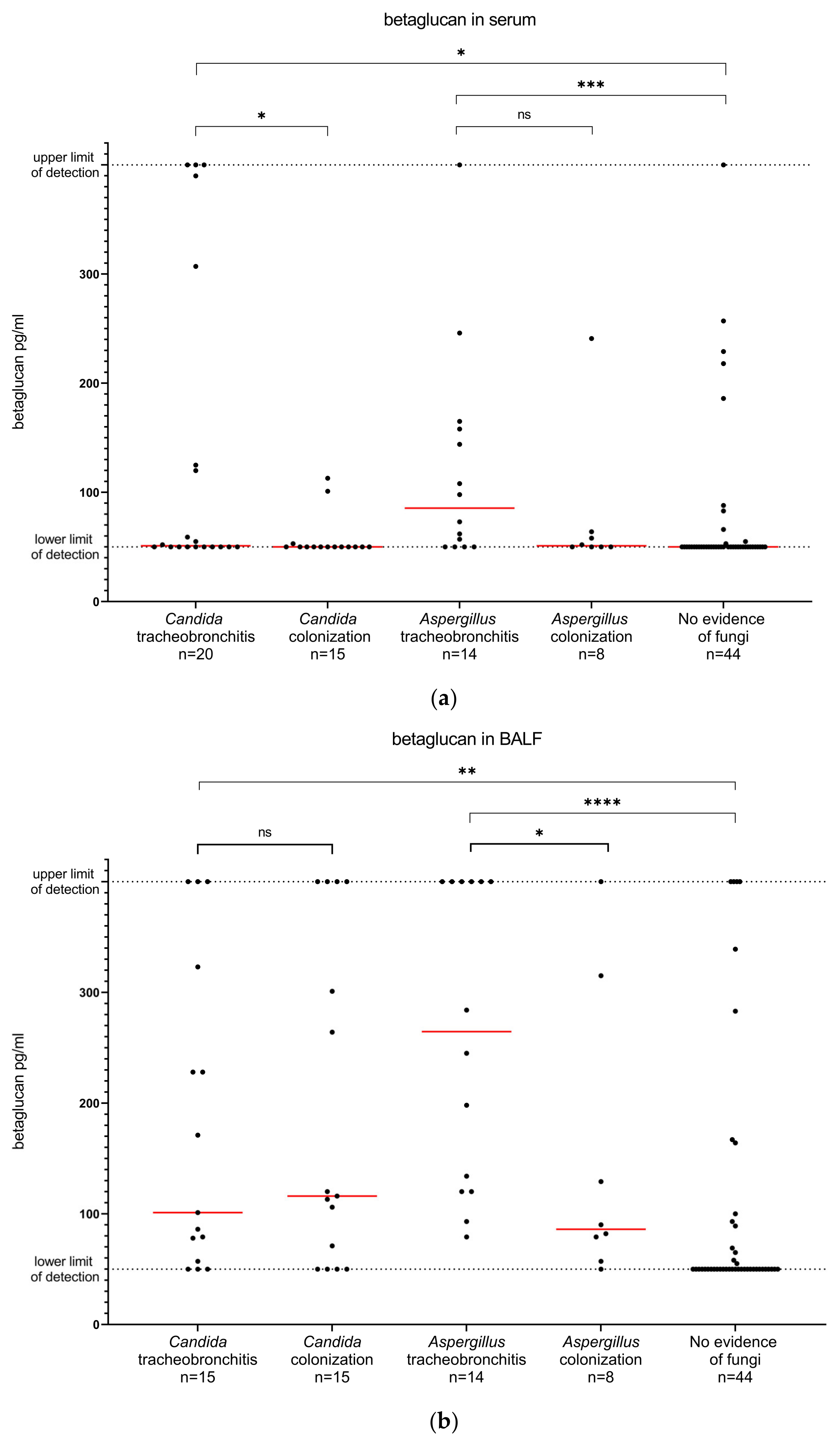
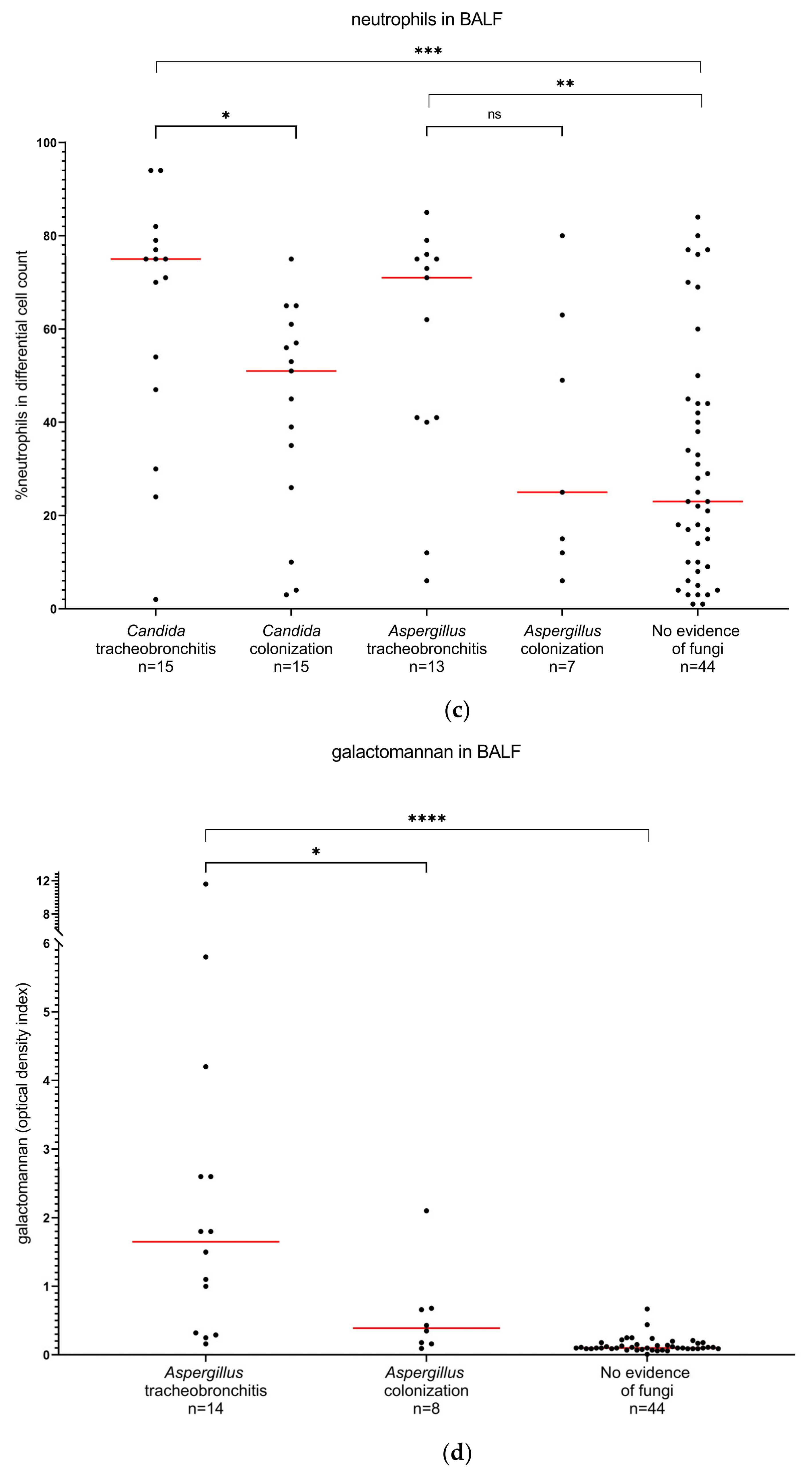
3.3. Diagnostic Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Moghaddam, S.M.; Ouédraogo, A.; Naylor, K.L.; Bota, S.E.; Husain, S.; Nash, D.M.; Paterson, J.M. Incidence and outcomes of invasive fungal infection among solid organ transplant recipients: A population-based cohort study. Transpl. Infect. Dis. 2020, 22, e13250. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.W.; Maziarz, E.K.; Arnold, C.J.; Johnson, M.D.; Workman, A.D.; Reynolds, J.M.; Perfect, J.R.; Alexander, B.D. Invasive Fungal Infection After Lung Transplantation: Epidemiology in the Setting of Antifungal Prophylaxis. Clin. Infect. Dis. 2020, 70, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Sole, A.; Alexander, B.D.; Aslam, S.; Avery, R.; Benden, C.; Billaud, E.M.; Chambers, D.; Danziger-Isakov, L.; Fedson, S.; et al. The 2015 International Society for Heart and Lung Transplantation Guidelines for the management of fungal infections in mechanical circulatory support and cardiothoracic organ transplant recipients: Executive summary. J. Heart Lung. Transplant. 2016, 35, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Clancy, C.J.; Nguyen, M.H. Fungal infections in lung transplantation. J. Thorac. Dis. 2021, 13, 6695–6707. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Husain, S. Aspergillus infections after lung transplantation: Clinical differences in type of transplant and implications for management. J. Heart Lung Transplant. 2003, 22, 258–266. [Google Scholar] [CrossRef]
- Kennedy, C.C.; Razonable, R.R. Fungal Infections After Lung Transplantation. Clin. Chest Med. 2017, 38, 511–520. [Google Scholar] [CrossRef]
- Pappas, P.G.; Silveira, F.P. Candida in solid organ transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. 4), S173–S179. [Google Scholar] [CrossRef]
- Palmer, S.M.; Perfect, J.R.; Howell, D.N.; Lawrence, C.M.; Miralles, A.P.; Davis, R.D.; Tapson, V.F. Candidal anastomotic infection in lung transplant recipients: Successful treatment with a combination of systemic and inhaled antifungal agents. J. Heart Lung Transplant. 1998, 17, 1029–1033. [Google Scholar]
- Husain, S.; Mooney, M.L.; Danziger-Isakov, L.; Mattner, F.; Singh, N.; Avery, R.; Ison, M.; Humar, A.; Padera, R.F.; Lawler, L.P.; et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J. Heart Lung Transplant. 2011, 30, 361–374. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2019, 71, 1367–1376. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. S1), e1–e38. [Google Scholar] [CrossRef]
- Aspelund, A.S.; Hammarstrom, H.; Inghammar, M.; Larsson, H.; Hansson, L.; Riise, G.C.; Friman, V.; Christensson, B.; Pahlman, L.I. Microbiological findings in bronchoalveolar lavage fluid from lung transplant patients in Sweden. Transpl. Infect. Dis. 2018, 20, e12973. [Google Scholar] [CrossRef]
- Atchade, E.; Desmard, M.; Kantor, E.; Genève, C.; Tebano, G.; De Tymowski, C.; Tran-Dinh, A.; Zappella, N.; Houzé, S.; Mal, H.; et al. Fungal Isolation in Respiratory Tract After Lung Transplantation: Epidemiology, Clinical Consequences, and Associated Factors. Transplant. Proc. 2020, 52, 326–332. [Google Scholar] [CrossRef]
- Stolz, D.; Stulz, A.; Müller, B.; Gratwohl, A.; Tamm, M. BAL neutrophils, serum procalcitonin, and C-reactive protein to predict bacterial infection in the immunocompromised host. Chest 2007, 132, 504–514. [Google Scholar] [CrossRef]
- Reynaud-Gaubert, M.; Thomas, P.; Gregoire, R.; Badier, M.; Cau, P.; Sampol, J.; Giudicelli, R.; Fuentes, P. Clinical utility of bronchoalveolar lavage cell phenotype analyses in the postoperative monitoring of lung transplant recipients. Eur. J. Cardio-Thorac. Surg. 2002, 21, 60–66. [Google Scholar] [CrossRef]
- Bhaskaran, A.; Kabbani, D.; Singer, L.G.; Prochnow, T.; Bhimji, A.; Rotstein, C.; Finkelman, M.A.; Keshavjee, S.; Husain, S. (1,3) beta-D-Glucan in Bronchoalveolar Lavage of Lung Transplant Recipients for the Diagnosis of Invasive Pulmonary Aspergillosis. Med. Mycol. 2016, 55, 173–179. [Google Scholar] [CrossRef]
- Shi, X.Y.; Liu, Y.; Gu, X.M.; Hao, S.Y.; Wang, Y.H.; Yan, D.; Jiang, S.J. Diagnostic value of (1 → 3)-beta-D-glucan in bronchoalveolar lavage fluid for invasive fungal disease: A meta-analysis. Respir. Med. 2016, 117, 48–53. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar]
- Stewart, S.; Fishbein, M.C.; Snell, G.I.; Berry, G.J.; Boehler, A.; Burke, M.M.; Glanville, A.; Gould, F.K.; Magro, C.; Marboe, C.C.; et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J. Heart Lung Transplant. 2007, 26, 1229–1242. [Google Scholar] [CrossRef]
- Gavalda, J.; Meije, Y.; Fortun, J.; Roilides, E.; Saliba, F.; Lortholary, O.; Munoz, P.; Grossi, P.; Cuenca-Estrella, M. Invasive fungal infections in solid organ transplant recipients. Clin. Microbiol. Infect. 2014, 20 (Suppl. 7), 27–48. [Google Scholar] [CrossRef]
- Nunley, D.R.; Gal, A.A.; Vega, J.D.; Perlino, C.; Smith, P.; Lawrence, E.C. Saprophytic fungal infections and complications involving the bronchial anastomosis following human lung transplantation. Chest 2002, 122, 1185–1191. [Google Scholar] [CrossRef][Green Version]
- Sole, A.; Morant, P.; Salavert, M.; Peman, J.; Morales, P. Aspergillus infections in lung transplant recipients: Risk factors and outcome. Clin. Microbiol. Infect. 2005, 11, 359–365. [Google Scholar] [CrossRef]
- Felton, T.W.; Roberts, S.A.; Isalska, B.; Brennan, S.; Philips, A.; Whiteside, S.; Doran, H.M.; Leonard, C.; Al-Aloul, M.; Yonan, N.; et al. Isolation of Aspergillus species from the airway of lung transplant recipients is associated with excess mortality. J. Infect. 2012, 65, 350–356. [Google Scholar] [CrossRef]
- Law, N.; Hamandi, B.; Fegbeutel, C.; Silveira, F.P.; Verschuuren, E.A.; Ussetti, P.; Chin-Hong, P.V.; Sole, A.; Holmes-Liew, C.L.; Billaud, E.M.; et al. Lack of association of Aspergillus colonization with the development of bronchiolitis obliterans syndrome in lung transplant recipients: An international cohort study. J. Heart Lung Transplant. 2019, 38, 963–971. [Google Scholar] [CrossRef]
- Pióro, A.; Latos, M.; Urlik, M.; Stącel, T.; Gawęda, M.; Pandel, A.; Przybyłowski, P.; Knapik, P.; Ochman, M. Antifungal Prophylaxis and Treatment Among Lung Transplant Recipients in Early Postoperative Stage: A Single-Center Study. Transplant. Proc. 2022, 54, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Husain, S. Early diagnosis of fungal infections in lung transplant recipients, colonization versus invasive disease? Curr. Opin. Organ Transplant. 2018, 23, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Speck, N.E.; Schuurmans, M.M.; Murer, C.; Benden, C.; Huber, L.C. Diagnostic value of plasma and bronchoalveolar lavage samples in acute lung allograft rejection: Differential cytology. Respir. Res. 2016, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Prattes, J.; Spiess, B.; Wagner, J.; Prueller, F.; Raggam, R.B.; Posch, V.; Duettmann, W.; Hoenigl, K.; Wolfler, A.; et al. Performance of galactomannan, beta-d-glucan, Aspergillus lateral-flow device, conventional culture, and PCR tests with bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 2014, 52, 2039–2045. [Google Scholar] [CrossRef]
- Prattes, J.; Flick, H.; Pruller, F.; Koidl, C.; Raggam, R.B.; Palfner, M.; Eigl, S.; Buzina, W.; Zollner-Schwetz, I.; Thornton, C.R.; et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 2014, 190, 922–929. [Google Scholar] [CrossRef]
- Rose, S.R.; Vallabhajosyula, S.; Velez, M.G.; Fedorko, D.P.; VanRaden, M.J.; Gea-Banacloche, J.C.; Lionakis, M.S. The utility of bronchoalveolar lavage beta-D-glucan testing for the diagnosis of invasive fungal infections. J. Infect. 2014, 69, 278–283. [Google Scholar] [CrossRef]
- Linder, K.A.; Kauffman, C.A.; Zhou, S.; Richards, B.J.; Kleiboeker, S.; Miceli, M.H. Performance of the (1,3)-Beta-D-Glucan Assay on Bronchoalveolar Lavage Fluid for the Diagnosis of Invasive Pulmonary Aspergillosis. Mycopathologia 2020, 185, 925–929. [Google Scholar] [CrossRef]
- Weinbergerova, B.; Kabut, T.; Kocmanova, I.; Lengerova, M.; Pospisil, Z.; Kral, Z.; Mayer, J. Bronchoalveolar lavage fluid and serum 1,3-β-D-glucan testing for invasive pulmonary aspergillosis diagnosis in hematological patients: The role of factors affecting assay performance. Sci. Rep. 2020, 10, 17963. [Google Scholar] [CrossRef]
- Mutschlechner, W.; Risslegger, B.; Willinger, B.; Hoenigl, M.; Bucher, B.; Eschertzhuber, S.; Lass-Florl, C. Bronchoalveolar Lavage Fluid (1,3)beta-D-Glucan for the Diagnosis of Invasive Fungal Infections in Solid Organ Transplantation: A Prospective Multicenter Study. Transplantation 2015, 99, e140–e144. [Google Scholar] [CrossRef]
- Alexander, B.D.; Smith, P.B.; Davis, R.D.; Perfect, J.R.; Reller, L.B. The (1,3){beta}-D-glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J. Clin. Microbiol. 2010, 48, 4083–4088. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Pasqualotto, A.C.; Xavier, M.O.; Sánchez, L.B.; de Oliveira Costa, C.D.; Schio, S.M.; Camargo, S.M.; Camargo, J.J.; Sukiennik, T.C.; Severo, L.C. Diagnosis of invasive aspergillosis in lung transplant recipients by detection of galactomannan in the bronchoalveolar lavage fluid. Transplantation 2010, 90, 306–311. [Google Scholar] [CrossRef]
- Husain, S.; Paterson, D.L.; Studer, S.M.; Crespo, M.; Pilewski, J.; Durkin, M.; Wheat, J.L.; Johnson, B.; McLaughlin, L.; Bentsen, C.; et al. Aspergillus galactomannan antigen in the bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in lung transplant recipients. Transplantation 2007, 83, 1330–1336. [Google Scholar] [CrossRef]
- Luong, M.L.; Clancy, C.J.; Vadnerkar, A.; Kwak, E.J.; Silveira, F.P.; Wissel, M.C.; Grantham, K.J.; Shields, R.K.; Crespo, M.; Pilewski, J.; et al. Comparison of an Aspergillus real-time polymerase chain reaction assay with galactomannan testing of bronchoalvelolar lavage fluid for the diagnosis of invasive pulmonary aspergillosis in lung transplant recipients. Clin. Infect. Dis. 2011, 52, 1218–1226. [Google Scholar] [CrossRef]
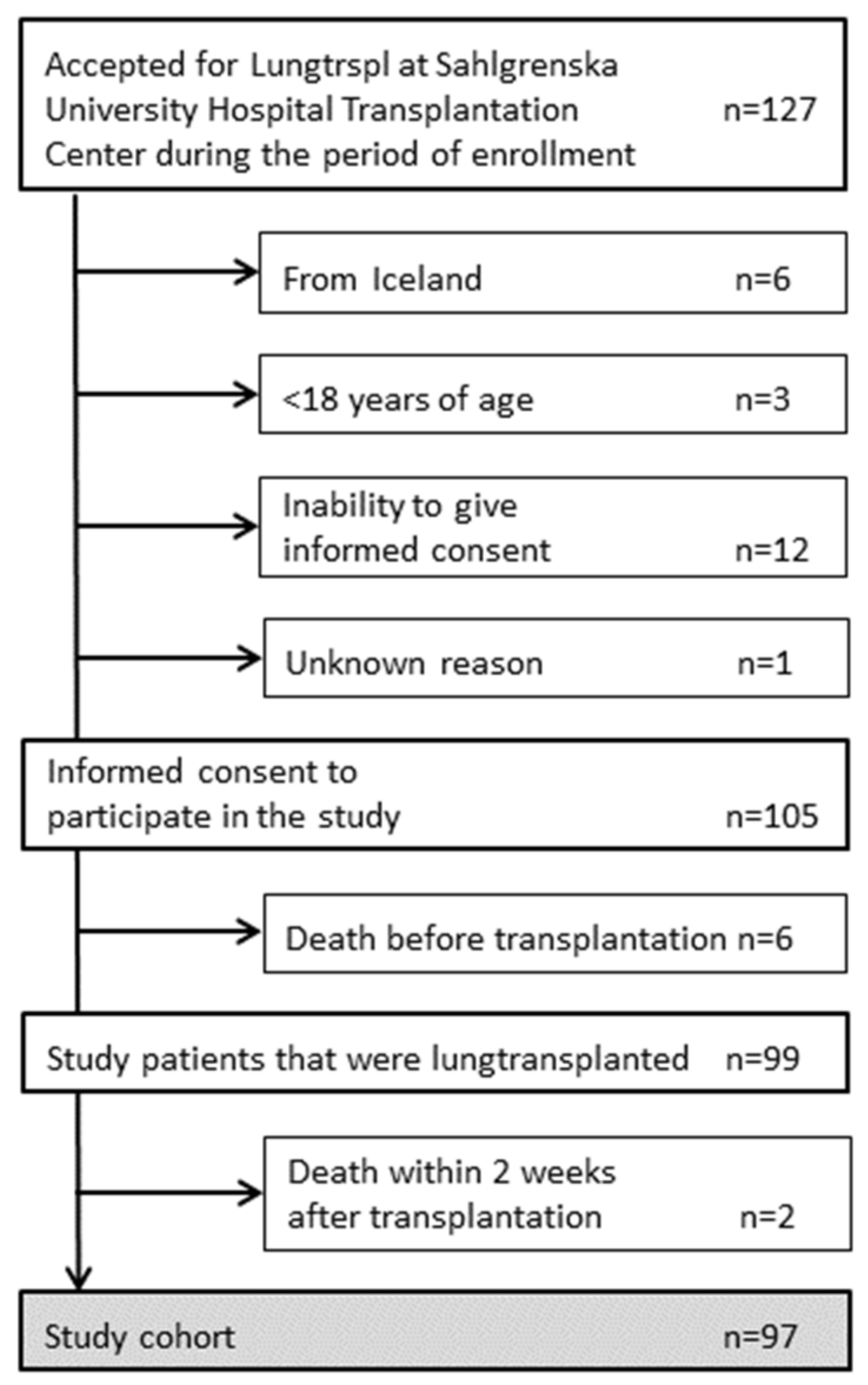
| n = 97 | |
|---|---|
| Male, n (%) | 45 (46) |
| Recipient age, years median (range) | 59 (18–70) |
| Donor age, years median (range) | 54 (14–76) |
| Type of lung transplantation, n (%) | |
| Single | 7 (7) |
| Double | 82 (85) |
| Re-transplantation | 8 (8) |
| Cold ischemia time, hours median (range) a | 7.1 (1.8–13.8) |
| Pretransplant underlying condition, n (%) | |
| Chronic obstructive pulmonary disease | 29 (30) |
| Pulmonary fibrosis | 25 (26) |
| Cystic fibrosis | 11 (11) |
| Alpha-1 antitrypsin deficiency | 12 (13) |
| Pulmonary arterial hypertension | 4 (4) |
| Bronchiolitis obliterans syndrome | 7 (7) |
| Sarcoidosis | 1 (1) |
| Bronchiectasis | 1 (1) |
| Other b | 7 (7) |
| Calcineurin inhibitor during the first postoperative year c, n (%) | |
| Cyclosporine | 78 (80) |
| Tacrolimus | 19 (20) |
| Bronchoscopies per patient during the first postoperative year, n median (range) | 3 (1–10) |
| Death during the first postoperative year, n (%) | 13 (13) |
| within one month after transplantation d | 1 |
| one to three months after transplantation | 2 |
| three to twelve months after transplantation | 10 |
| Total number of patients, n | 97 |
| Candida | |
| Colonization only, n (%) | 16 (16) |
| Time after transplantation a, median months (range) | 0.8 (0.6–3.1) |
| Tracheobronchitis, n (%) | 22 (23) |
| Time after transplantation b, median months (range) | 0.8 (0.2–12) |
| Aspergillus | |
| Colonization only, n (%) | 8 (8) |
| Time after transplantation b, median months (range) | 3 (0.8–12.0) |
| Tracheobronchitis, n (%) | 15 (15) |
| Time after transplantation, median months (range) | 3.4 (0.7–9.6) |
| No evidence of fungal colonization nor tracheobronchitis, n (%) | 44 (45) |
| Diagnostic Markers in BALF | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|
| Candida tracheobronchitis | ||
| neutrophils > 75% | 33.3 (15.2–58.3) | 91.5 (81.7–96.3) |
| Aspergillus tracheobronchitis | ||
| betaglucan > 110 pg/mL | 85.7 (60.1–97.5) | 78.8 (66.0–87.8) |
| betaglucan > 370 pg/mL | 42.9 (21.4–67.4) | 90.4 (79.4–95.8) |
| galactomannan > 0.25 | 90.9 (62.2–99.5) | 82.7 (70.3–90.6) |
| galactomannan > 0.5 | 63.6 (35.4–84.8) | 90.4 (79.4–95.8) |
| galactomannan > 1.0 | 63.6 (35.4–84.8) | 98.1 (89.9–99.9) |
| neutrophils > 75% | 23.1 (8.2–50.3) | 88.2 (76.6–94.5) |
| betaglucan > 110 pg/mL AND galactomannan > 0.5 | 54.6 (23.4–83.3) | 96.2 (86.8–99.5) |
| betaglucan > 110 pg/mL OR galactomannan > 0.5 | 100 (71.5–100) | 75 (61.1–86) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammarström, H.; Magnusson, J.; Stjärne Aspelund, A.; Stenmark, J.; Isaksson, J.; Kondori, N.; Riise, G.C.; Wennerås, C.; Friman, V. Fungal Tracheobronchitis in Lung Transplant Recipients: Incidence and Utility of Diagnostic Markers. J. Fungi 2023, 9, 3. https://doi.org/10.3390/jof9010003
Hammarström H, Magnusson J, Stjärne Aspelund A, Stenmark J, Isaksson J, Kondori N, Riise GC, Wennerås C, Friman V. Fungal Tracheobronchitis in Lung Transplant Recipients: Incidence and Utility of Diagnostic Markers. Journal of Fungi. 2023; 9(1):3. https://doi.org/10.3390/jof9010003
Chicago/Turabian StyleHammarström, Helena, Jesper Magnusson, Anna Stjärne Aspelund, Jakob Stenmark, Jenny Isaksson, Nahid Kondori, Gerdt C Riise, Christine Wennerås, and Vanda Friman. 2023. "Fungal Tracheobronchitis in Lung Transplant Recipients: Incidence and Utility of Diagnostic Markers" Journal of Fungi 9, no. 1: 3. https://doi.org/10.3390/jof9010003
APA StyleHammarström, H., Magnusson, J., Stjärne Aspelund, A., Stenmark, J., Isaksson, J., Kondori, N., Riise, G. C., Wennerås, C., & Friman, V. (2023). Fungal Tracheobronchitis in Lung Transplant Recipients: Incidence and Utility of Diagnostic Markers. Journal of Fungi, 9(1), 3. https://doi.org/10.3390/jof9010003






