Genome-Wide Screening and Stability Verification of the Robust Internal Control Genes for RT-qPCR in Filamentous Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Screening for Novel ICGs
2.3. Function Analysis of ICGs Candidates
2.4. Total RNA Extraction and RT-qPCR
2.5. Data Processing and Statistical Analysis
3. Results
3.1. Several Traditional Housekeeping Genes Frequently Show Instability
3.2. Stable ICGs Identified in the Literature Are Not Universally Applicable
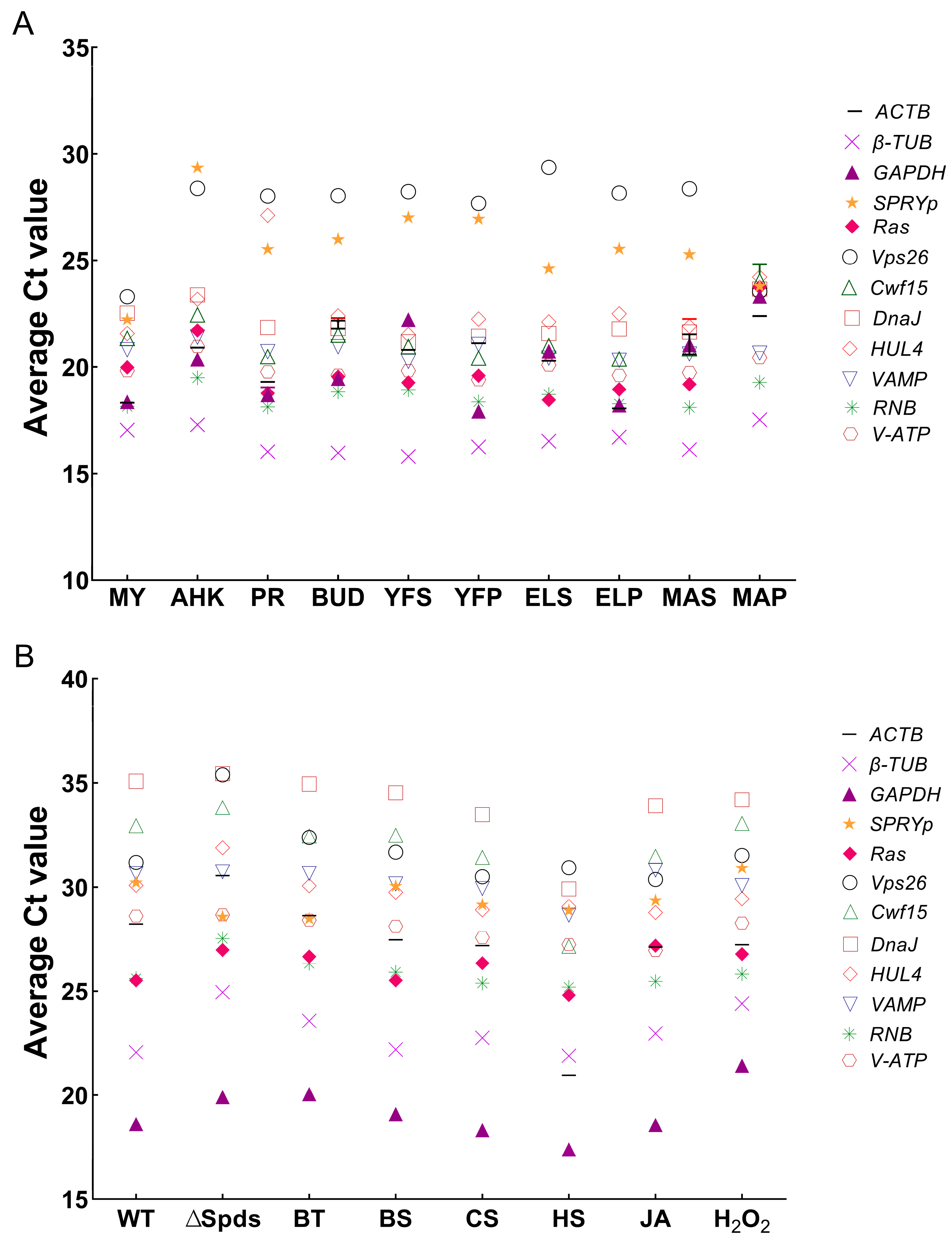
3.3. Screening of Novel Stable ICGs in Multiple Species
3.4. Identification and Functional Analysis of Novel Screened Stable ICGs
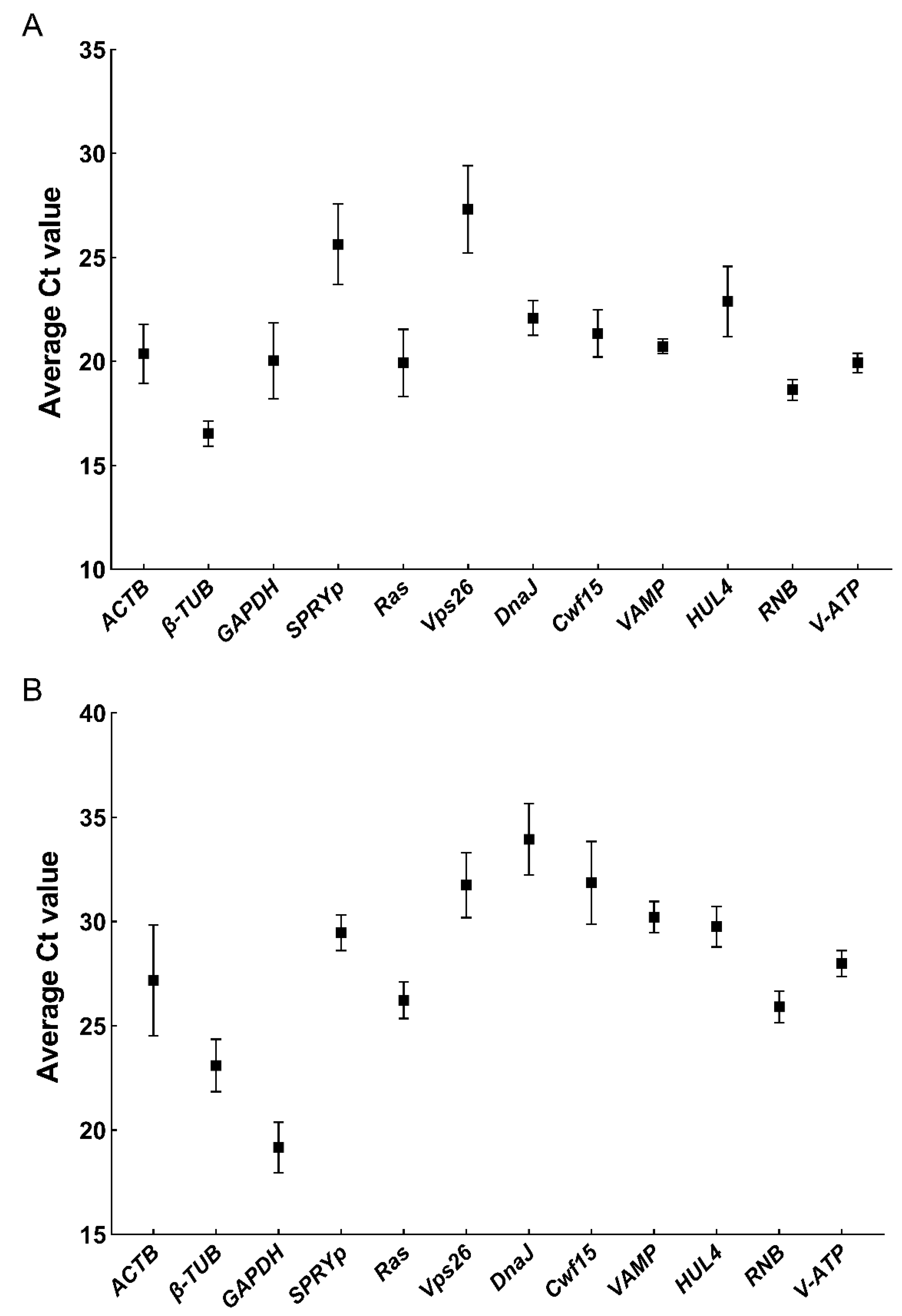
3.5. Mean and Dispersion of Ct values of Novel Screened ICGs in F. filiformis and N. crassa
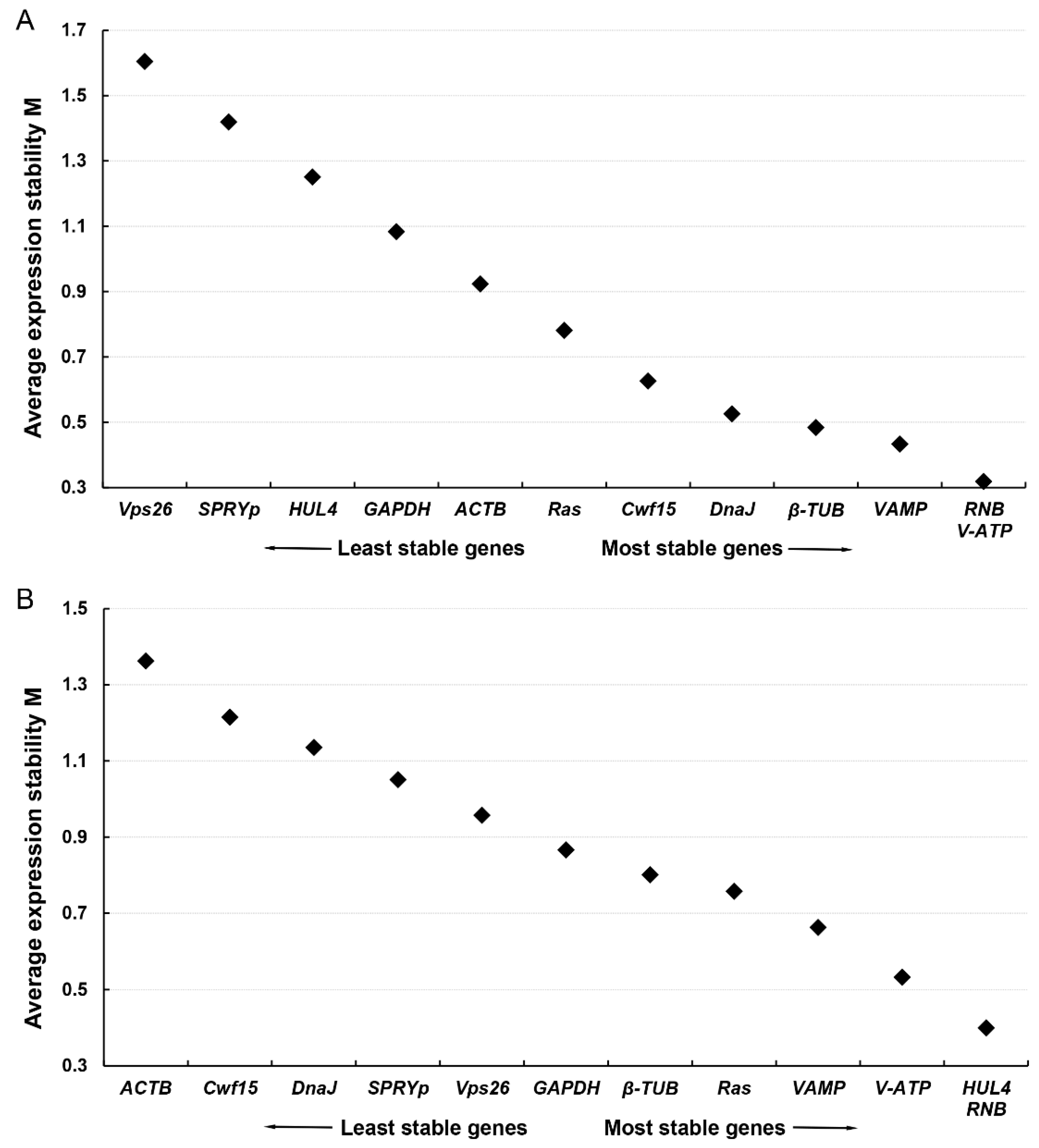
3.6. Stability Evaluation of Novel Screened Stable ICGs in F. filiformis and N. crassa
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gadkar, V.; Filion, M. New Developments in Quantitative Real-time Polymerase Chain Reaction Technology. Curr. Issues Mol. Biol. 2014, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Chen, J.; Li, L.; Li, J.; Li, D.; Wang, J.; Liu, J.; Liu, J. Reference gene selection for quantitative RT-PCR in Miscanthus sacchariflorus under abiotic stress conditions. Mol. Biol. Rep. 2022, 49, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Teste, M.-A.; Duquenne, M.; François, J.M.; Parrou, J.-L. Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol. Biol. 2009, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Wang, Z.; Li, M.; Cao, J.; Niu, G.; Xia, L.; Zou, D.; Wang, F.; Xu, X.; Han, X.; et al. ICG: A wiki-driven knowledgebase of internal control genes for RT-qPCR normalization. Nucleic Acids Res. 2017, 46, D121–D126. [Google Scholar] [CrossRef] [PubMed]
- Rego, E.C.S.; Pinheiro, T.D.M.; Antonino, J.D.; Alves, G.S.C.; Cotta, M.G.; Fonseca, F.C.D.A.; Miller, R.N.G. Stable reference genes for RT-qPCR analysis of gene expression in the Musa acuminata-Pseudocercospora musae interaction. Sci. Rep. 2019, 9, 14592. [Google Scholar] [CrossRef]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L.; et al. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef]
- Fu, N.; Li, J.; Wang, M.; Ren, L.; Zong, S.; Luo, Y. Identification and Validation of Reference Genes for Gene Expression Analysis in Different Development Stages of Amylostereum areolatum. Front. Microbiol. 2022, 12, 827241. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, Y.; Guo, Q. Expression stability of internal reference gene in response to Trichoderma polysporum infection in Avena fatua L. Curr. Genet. 2021, 67, 909–918. [Google Scholar] [CrossRef]
- Chapman, J.R.; Waldenström, J. With Reference to Reference Genes: A Systematic Review of Endogenous Controls in Gene Expression Studies. PLoS ONE 2015, 10, e0141853. [Google Scholar] [CrossRef]
- Chen, M.-D.; Wang, B.; Li, Y.-P.; Zeng, M.-J.; Liu, J.-T.; Ye, X.-R.; Zhu, H.-S.; Wen, Q.-F. Reference gene selection for qRT-PCR analyses of luffa (Luffa cylindrica) plants under abiotic stress conditions. Sci. Rep. 2021, 11, 3161. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, F.; Long, G.; Qin, L.; Deng, Z. Selection of reference genes for quantitative real-time RT-PCR analysis in citrus. Mol. Biol. Rep. 2011, 39, 1831–1838. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-M.; Tao, Z.; Shen, C.; Qian, D.; Wang, C.-L.; Zhou, Q.-C.; Jin, S. β-actin gene expression is variable among individuals and not suitable for normalizing mRNA levels in Portunus trituberculatus. Fish Shellfish Immunol. 2018, 81, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Huggett, J.F.; Dheda, K.; Bustin, S.; Zumla, A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005, 6, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Kozera, B.; Rapacz, M. Reference genes in real-time PCR. J. Appl. Genet. 2013, 54, 391–406. [Google Scholar] [CrossRef]
- Tao, Y.; van Peer, A.; Huang, Q.; Shao, Y.; Zhang, L.; Xie, B.; Jiang, Y.; Zhu, J.; Xie, B. Identification of novel and robust internal control genes from Volvariella volvacea that are suitable for RT-qPCR in filamentous fungi. Sci. Rep. 2016, 6, 29236. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, X.; Song, L.; Hang, M.; Liu, J.; Deng, B.; Meng, J. Screening of Reference Genes for qRT-PCR Amplification in Flammulina filiformis. Acta Edulis Fungi 2021, 28, 30–39. [Google Scholar] [CrossRef]
- Shen, C.-H.; Peng, L.-J.; Zhang, Y.-X.; Zeng, H.-R.; Yu, H.-F.; Jin, L.; Li, G.-Q. Reference Genes for Expression Analyses by qRT-PCR in Phthorimaea operculella (Lepidoptera: Gelechiidae). Insects 2022, 13, 140. [Google Scholar] [CrossRef]
- Liu, Y.-N.; Lu, X.-X.; Ren, A.; Shi, L.; Jiang, A.-L.; Yu, H.-S.; Zhao, M.-W. Identification of Reference Genes and Analysis of Heat Shock Protein Gene Expression in Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum, after Exposure to Heat Stress. Int. J. Med. Mushrooms 2017, 19, 1029–1040. [Google Scholar] [CrossRef]
- Lian, T.; Yang, T.; Liu, G.; Sun, J.; Dong, C. Reliable reference gene selection for Cordyceps militaris gene expression studies under different developmental stages and media. FEMS Microbiol. Lett. 2014, 356, 97–104. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, G.; Wang, C.; Gong, Y.; Bian, Y.; Zhou, Y. Selection and Validation of Reference Genes for qRT-PCR in Lentinula edodes under Different Experimental Conditions. Genes 2019, 10, 647. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, W.; Cai, Y.; Lan, A.-F.; Bian, Y. Validation of Internal Control Genes for Quantitative Real-Time PCR Gene Expression Analysis in Morchella. Molecules 2018, 23, 2331. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. A convenient growth medium for Neurospora crassa. Microb. Genet. Bull. 1956, 13, 42–47. [Google Scholar]
- Tao, Y.; Chen, R.; Yan, J.; Long, Y.; Tong, Z.; Song, H.; Xie, B. A hydrophobin gene, Hyd9, plays an important role in the formation of aerial hyphae and primordia in Flammulina filiformis. Gene 2019, 706, 84–90. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Tang, J.; Warburton, M. Reference Gene Selection for RT-qPCR Analysis in Maize Kernels Inoculated with Aspergillus flavus. Toxins 2021, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Singh, S.; Chakrabarti, A.; Rudramurthy, S.M.; Ghosh, A.K. Selection and evaluation of appropriate reference genes for RT-qPCR based expression analysis in Candida tropicalis following azole treatment. Sci. Rep. 2020, 10, 1972. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, H.; Chen, M.; Song, X.; Yu, C.; Zhao, Y.; Wu, Y. Reference Gene Selection for Quantitative Real-Time PCR of Mycelia from Lentinula edodes under High-Temperature Stress. BioMed Res. Int. 2018, 2018, 1670328. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rodríguez, M.; Córdoba, J.J.; Andrade, M.J. Design of Primers and Probes for Quantitative Real-Time PCR Methods. Methods Mol. Biol. 2015, 1275, 31–56. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Bustin, S.A.; Wittwer, C.T. MIQE: A Step Toward More Robust and Reproducible Quantitative PCR. Clin. Chem. 2017, 63, 1537–1538. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jonge, H.J.M.; Fehrmann, R.; De Bont, E.S.J.M.; Hofstra, R.; Gerbens, F.; Kamps, W.A.; de Vries, E.; Van Der Zee, A.G.J.; Meerman, G.J.T.; Ter Elst, A. Evidence Based Selection of Housekeeping Genes. PLoS ONE 2007, 2, e898. [Google Scholar] [CrossRef] [PubMed]
- Grubbs, F.E. Sample Criteria for Testing Outlying Observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- Ling, D.; Salvaterra, P.M. Robust RT-qPCR Data Normalization: Validation and Selection of Internal Reference Genes during Post-Experimental Data Analysis. PLoS ONE 2011, 6, e17762. [Google Scholar] [CrossRef]
- Cao, H.; Cao, F.; Roussel, A.-M.; Anderson, R.A. Quantitative PCR for glucose transporter and tristetraprolin family gene expression in cultured mouse adipocytes and macrophages. Vitr. Cell. Dev. Biol.-Anim. 2013, 49, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, E.; Sun, Y.; Wang, J.; Zhou, Q.; Ning, B.; Dial, S.L.; Guo, L.; Yang, J. Cross-platform comparison of SYBR® Green real-time PCR with TaqMan PCR, microarrays and other gene expression measurement technologies evaluated in the MicroArray Quality Control (MAQC) study. BMC Genom. 2008, 9, 328. [Google Scholar] [CrossRef] [Green Version]
| Sample Type | Species | GSE No. | Traditional Housekeeping Genes | Reference Genes from Vv # | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTB | β-TUB | GAPDH | SPRYp | Ras | Vps26 | |||||||||
| MFC | RPKM | MFC | RPKM | MFC | RPKM | MFC | RPKM | MFC | RPKM | MFC | RPKM | |||
| Different nutritional resources | Neurospora crassa | GSE60004 | 1.1 | 528.7 | 1.9 | 244.1 | 6.0 | 1367.8 | 2.1 | 57.2 | 1.5 | 53.8 | 1.2 | 33.9 |
| GSE35227 | 3.7 | 3537.8 | 3.6 | 4393.9 | 5.1 | 35,633.7 | 5.9 | 358.8 | 3.7 | 469.0 | 1.9 | 459.6 | ||
| GSE44673 | 1.3 | 907.7 | 1.6 | 603.9 | 2.4 | 3931.2 | 2.7 | 68.4 | 1.3 | 62.4 | 1.4 | 64.3 | ||
| GSE52316 | 1.1 | 1040.2 | 2.1 | 498.9 | 2.2 | 2725.7 | 1.4 | 85.5 | 1.8 | 39.1 | 1.2 | 43.8 | ||
| GSE60986 | 2.7 | 1082.7 | 3.7 | 614.5 | 3.4 | 2309.1 | 3.4 | 23.4 | 2.2 | 39.3 | 1.4 | 34.8 | ||
| GSE68517 | 2.0 | 972.5 | 2.2 | 474.4 | 4.1 | 4434.0 | 4.1 | 62.4 | 3.5 | 50.5 | 3.0 | 42.3 | ||
| GSE36719 | 2.3 | 9441.1 | 3.0 | 4126.0 | 1.7 | 47,828.7 | 3.2 | 485.6 | 1.2 | 239.6 | 2.0 | 285.9 | ||
| GSE42692 | 2.1 | 2886.0 | 2.6 | 4401.9 | 3.9 | 29,295.9 | 2.1 | 328.9 | 1.1 | 332.2 | 1.2 | 395.7 | ||
| GSE51091 | 1.7 | 1068.4 | 1.6 | 675.1 | 3.5 | 3795.7 | 1.2 | 36.6 | 1.1 | 72.5 | 1.2 | 40.0 | ||
| Trichoderma reesei | GSE53629 | 1.1 | 19,940.5 | 1.5 | 3357.9 | 2.8 | 43,090.2 | 2.0 | 3869.0 | 1.2 | 1293.1 | 1.3 | 1464.1 | |
| Aspergillus nidulans | GSE44100 | 1.1 | 663.7 | 1.5 | 287.6 | 1.4 | 881.7 | 1.1 | 81.2 | 1.7 | 92.3 | 1.1 | 41.3 | |
| Different development stages | Flammulina filiformis | Wang et al., 2015 | 2.8 | 1803.0 | 2.6 | 2067.8 | 19.7 | 1616.2 | 2.0 | 98.4 | 3.3 | 228.7 | 2.0 | 39.9 |
| Agaricus bisporus | GSE39569 | 2.5 | 3298.3 | 4.5 | 821.0 | 5.5 | 81,562.8 | 10.9 | 72.5 | 3.7 | 55,015.0 | 2.2 | 12,820.5 | |
| Neurospora crassa | GSE41484 | 2.4 | 1434.4 | 1.6 | 681.5 | 5.3 | 4659.3 | 3.2 | 115.6 | 1.8 | 284.8 | 2.5 | 92.8 | |
| Fusarium graminearum | GSE61865 | 2.7 | 849.2 | 5.3 | 230.2 | 4.3 | 2048.4 | 3.6 | 72.0 | 4.3 | 56.5 | 1.7 | 66.3 | |
| Different stresses | Neurospora crassa | GSE53013 | 1.6 | 1354.9 | 1.6 | 781.9 | 2.0 | 4027.3 | 2.7 | 18.1 | 2.5 | 47.2 | 1.9 | 41.4 |
| GSE52153 | 1.3 | 1493.3 | 1.6 | 792.9 | 1.5 | 4637.7 | 3.3 | 26.8 | 1.8 | 58.4 | 2.2 | 57.8 | ||
| GSE53534 | 1.8 | 591.9 | 2.5 | 357.0 | 2.4 | 16,553.0 | 2.0 | 22.0 | 2.8 | 15.5 | 1.7 | 16.1 | ||
| Magnaporthe oryzae | GSE57146 | 1.5 | 659.2 | 1.4 | 253.6 | 1.5 | 7283.7 | 1.2 | 41.4 | 1.8 | 61.5 | 1.4 | 28.4 | |
| Different strains | Flammulina filiformis | Wang et al., 2016 | 1.6 | 2234.2 | 1.8 | 1594.7 | 2.5 | 7846.6 | 1.6 | 107.8 | 3.1 | 164.7 | 1.4 | 24.9 |
| Volvariella volvacea | GSE43019 | 1.9 | 1840.7 | 1.7 | 9.7 | 1.7 | 5063.7 | 1.0 | 66.3 | 1.5 | 8.0 | 1.4 | 77.1 | |
| Aspergillus nidulans | GSE63672 | 1.4 | 1343.5 | 2.3 | 764.4 | 7.9 | 4399.4 | 1.5 | 149.4 | 1.5 | 159.3 | 1.5 | 71.5 | |
| The reference range of RPKM value * (Average RPKM) | 500–3550 (1479.5) | 200–4500 (1274.2) | 800–50,000 (11,115.7) | 18–150 (66.9) | 8–500 (126.8) | 16–100 (48.0) | ||||||||
| Sample Type | Species | GSE No. | Novel Internal Control Genes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DnaJ | Cwf15 | HUL4 | VAMP | RNB | V-ATP | |||||||||
| MFC | RPKM | MFC | RPKM | MFC | RPKM | MFC | RPKM | MFC | RPKM | MFC | RPKM | |||
| Different nutritional resources | Neurospora crassa | GSE60004 | 1.4 | 31.8 | 2.0 | 37.8 | 1.2 | 75.9 | 2.1 | 76.3 | 1.4 | 166.8 | 1.6 | 138.9 |
| GSE35227 | 2.0 | 250.2 | 2.0 | 216.4 | 1.8 | 592.0 | 2.2 | 581.9 | 1.7 | 1226.0 | 1.9 | 1861.9 | ||
| GSE44673 | 1.4 | 46.4 | 1.2 | 35.8 | 1.1 | 140.1 | 1.3 | 98.1 | 1.5 | 308.2 | 1.3 | 294.2 | ||
| GSE52316 | 1.2 | 36.1 | 1.3 | 33.1 | 1.4 | 95.0 | 1.4 | 86.7 | 1.4 | 275.2 | 1.3 | 292.7 | ||
| GSE60986 | 1.5 | 27.6 | 2.1 | 19.8 | 1.8 | 55.4 | 1.4 | 76.1 | 1.8 | 118.0 | 2.1 | 125.8 | ||
| GSE68517 | 1.5 | 32.0 | 1.5 | 28.1 | 1.7 | 79.6 | 2.3 | 85.1 | 3.0 | 157.6 | 1.7 | 245.8 | ||
| GSE36719 | 1.3 | 226.8 | 1.2 | 185.1 | 1.7 | 571.6 | 1.2 | 593.8 | 1.3 | 1184.6 | 1.4 | 1521.0 | ||
| GSE42692 | 1.1 | 220.7 | 1.2 | 206.4 | 1.5 | 545.7 | 1.1 | 522.1 | 1.6 | 1312.8 | 1.6 | 1685.7 | ||
| GSE51091 | 1.2 | 38.4 | 1.2 | 30.0 | 1.3 | 70.3 | 1.1 | 98.8 | 1.1 | 138.7 | 1.3 | 111.5 | ||
| Trichoderma reesei | GSE53629 | 1.2 | 1935.6 | 1.2 | 288.4 | 1.3 | 4480.3 | 1.2 | 2305.2 | 1.4 | 14,098.2 | 1.3 | 5809.2 | |
| Aspergillus nidulans | GSE44100 | 1.6 | 66.9 | 1.5 | 50.5 | 1.1 | 98.0 | 1.5 | 135.7 | 1.1 | 211.7 | 1.1 | 88.1 | |
| Different development stages | Flammulina filiformis | Wang et al., 2015 | 1.7 | 24.5 | 1.8 | 49.8 | 2.0 | 54.2 | 1.8 | 274.8 | 1.5 | 182.5 | 1.5 | 198.0 |
| Agaricus bisporus | GSE39569 | 1.4 | 4334.8 | 3.0 | 11,422.0 | 1.1 | 6239.8 | 2.4 | 2175.3 | 1.7 | 5820.0 | 1.4 | 10,573.8 | |
| Neurospora crassa | GSE41484 | 1.3 | 59.9 | 1.3 | 40.5 | 1.3 | 91.1 | 1.7 | 265.1 | 1.6 | 213.3 | 1.7 | 263.8 | |
| Fusarium graminearum | GSE61865 | 1.5 | 87.6 | 2.4 | 73.2 | 2.3 | 168.6 | 1.8 | 160.6 | 2.9 | 69.5 | 1.5 | 103.4 | |
| Different stresses | Neurospora crassa | GSE53013 | 1.6 | 25.2 | 1.6 | 30.8 | 2.2 | 49.5 | 1.5 | 81.1 | 1.9 | 87.1 | 2.1 | 101.9 |
| GSE52153 | 1.6 | 32.1 | 1.9 | 33.4 | 1.5 | 64.6 | 1.5 | 93.7 | 2.2 | 129.7 | 1.6 | 144.8 | ||
| GSE53534 | 1.4 | 22.2 | 1.5 | 30.2 | 1.7 | 30.9 | 1.5 | 46.2 | 1.7 | 53.6 | 1.6 | 79.5 | ||
| Magnaporthe oryzae | GSE57146 | 1.1 | 30.9 | 2.2 | 28.2 | 1.8 | 56.3 | 1.2 | 119.5 | 1.2 | 56.5 | 2.8 | 104.5 | |
| Different strains | Flammulina filiformis | Wang et al., 2016 | 1.9 | 24.1 | 1.6 | 44.6 | 1.5 | 70.3 | 1.2 | 217.1 | 1.8 | 163.2 | 1.3 | 151.4 |
| Volvariella volvacea | GSE43019 | 2.0 | 48.2 | 1.4 | 0.4 | 1.3 | 2.6 | 1.8 | 2.8 | 2.4 | 8.6 | 2.5 | 54.9 | |
| Aspergillus nidulans | GSE63672 | 1.3 | 109.2 | 1.3 | 67.1 | 1.9 | 293.5 | 1.5 | 165.8 | 2.0 | 467.9 | 1.4 | 79.8 | |
| The reference range of RPKM value * (Average RPKM) | 20–260 (72.0) | 15–300 (72.8) | 30–600 (160.3) | 45–600 (189.1) | 50–1350 (326.6) | 50–1900 (382.4) | ||||||||
| Gene Symbol | Gene Name | Pfam Annotation | Function |
|---|---|---|---|
| ACTB | β-actin | Actin (PF00022) | Polymerize into microfilament and constitute major component of the cytoskeleton |
| β-TUB | β-tubulin | Tubulin/FtsZ family, GTPase domain (PF00091) | Be involved in cell division and constitutes the main component of microtubules |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | Glyceraldehyde 3-phosphate dehydrogenase (PF02800) | Be involved in glycolysis and gluconeogenesis |
| SPRYp | SPRY-domain-containing protein | SPRY domain (PF00622) | Be involved in RNA processing and histone H3 methylation regulatory signaling pathways and regulates nutrient transport |
| Ras | Ras-2 protein | Ras family (PF00071) | Regulate cytoskeletal integrity, proliferation, apoptosis and cell migration |
| Vps26 | Vacuolar protein sorting protein 26 | Vacuolar protein sorting-associated protein 26 (PF03643) | Be involved in protein trafficking and regulate vesicular protein sorting |
| Cwf15 | Pre-mRNA-splicing factor cwc15 | Cwf15/Cwc15 cell cycle control protein (PF04889) | Be involved in pre-mRNA splicing |
| DnaJ | ER associated DnaJ chaperone | DnaJ domain (PF00226) | Be involved in folding of nascent proteins and regulate the responses to stress |
| HUL4 | E3 ubiquitin-protein ligase NEDD4 | HECT-domain (ubiquitin-transferase) (PF00632) | Constitute ubiquitin-protein ligases and participate in protein ubiquitination hydrolysis |
| VAMP | ATP-binding cassette, subfamily B (MDR/TAP), member 1 | MSP (Major sperm protein) domain (PF00635) | Constitute cell cytoskeleton, with related vesicle-associated membrane protein and participate in vesicle fusion |
| RNB | Exosome complex exonuclease DIS3/RRP44 | RNB domain (PF00773) | Constitute ribonuclease II and involved in the mRNA degradation pathway |
| V-ATP | V-type H+-transporting ATPase subunit A | ATP synthase alpha/beta family, nucleo-tide-binding domain (PF00006) | Driven proton pump and involved in protein transport, active transport of metabolites and homeostasis |
| Sample Sets | F.filiformis Samples Set (A) | N. crassa Samples Set (B) | Comprehensive Analysis A and B | |||
|---|---|---|---|---|---|---|
| Rank | Gene Name | Stability Value | Gene Name | Stability Value | Gene Name | Stability Value |
| 1 | RNB | 0.110 | RNB | 0.387 | V-ATP | 0.175 |
| 2 | V-ATP | 0.114 | VAMP | 0.398 | RNB | 0.261 |
| 3 | VAMP | 0.353 | V-ATP | 0.454 | β-TUB | 0.266 |
| 4 | DnaJ | 0.427 | HUL4 | 0.501 | VAMP | 0.348 |
| 5 | β-TUB | 0.440 | β-TUB | 0.512 | Ras | 0.546 |
| 6 | Cwf15 | 0.536 | Ras | 0.542 | GAPDH | 0.681 |
| 7 | ACTB | 0.745 | GAPDH | 0.563 | HUL4 | 0.699 |
| 8 | Ras | 0.909 | DnaJ | 0.639 | DnaJ | 1.098 |
| 9 | GAPDH | 1.104 | Cwf15 | 0.828 | Cwf15 | 1.350 |
| 10 | HUL4 | 1.185 | Vps26 | 0.835 | SPRYp | 1.426 |
| 11 | SPRYp | 1.251 | SPRYp | 0.948 | ACTB | 1.501 |
| 12 | Vps26 | 1.610 | ACTB | 1.365 | Vps26 | 1.507 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Xu, X.; Jing, Z.; Ye, J.; Li, H.; Li, X.; Shi, L.; Chen, M.; Wang, T.; Xie, B.; et al. Genome-Wide Screening and Stability Verification of the Robust Internal Control Genes for RT-qPCR in Filamentous Fungi. J. Fungi 2022, 8, 952. https://doi.org/10.3390/jof8090952
Yang Y, Xu X, Jing Z, Ye J, Li H, Li X, Shi L, Chen M, Wang T, Xie B, et al. Genome-Wide Screening and Stability Verification of the Robust Internal Control Genes for RT-qPCR in Filamentous Fungi. Journal of Fungi. 2022; 8(9):952. https://doi.org/10.3390/jof8090952
Chicago/Turabian StyleYang, Yayong, Xinyu Xu, Zhuohan Jing, Jun Ye, Hui Li, Xiaoyu Li, Lei Shi, Mengyu Chen, Tengyun Wang, Baogui Xie, and et al. 2022. "Genome-Wide Screening and Stability Verification of the Robust Internal Control Genes for RT-qPCR in Filamentous Fungi" Journal of Fungi 8, no. 9: 952. https://doi.org/10.3390/jof8090952
APA StyleYang, Y., Xu, X., Jing, Z., Ye, J., Li, H., Li, X., Shi, L., Chen, M., Wang, T., Xie, B., & Tao, Y. (2022). Genome-Wide Screening and Stability Verification of the Robust Internal Control Genes for RT-qPCR in Filamentous Fungi. Journal of Fungi, 8(9), 952. https://doi.org/10.3390/jof8090952





