Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Molecular Identification of Fungal Isolates
2.2. Antifungal Susceptibility Testing
2.3. Detection of cyp51A Mutations in Azole-Resistant Isolates
2.4. Ethics Statement
3. Results
3.1. Fungal Isolates
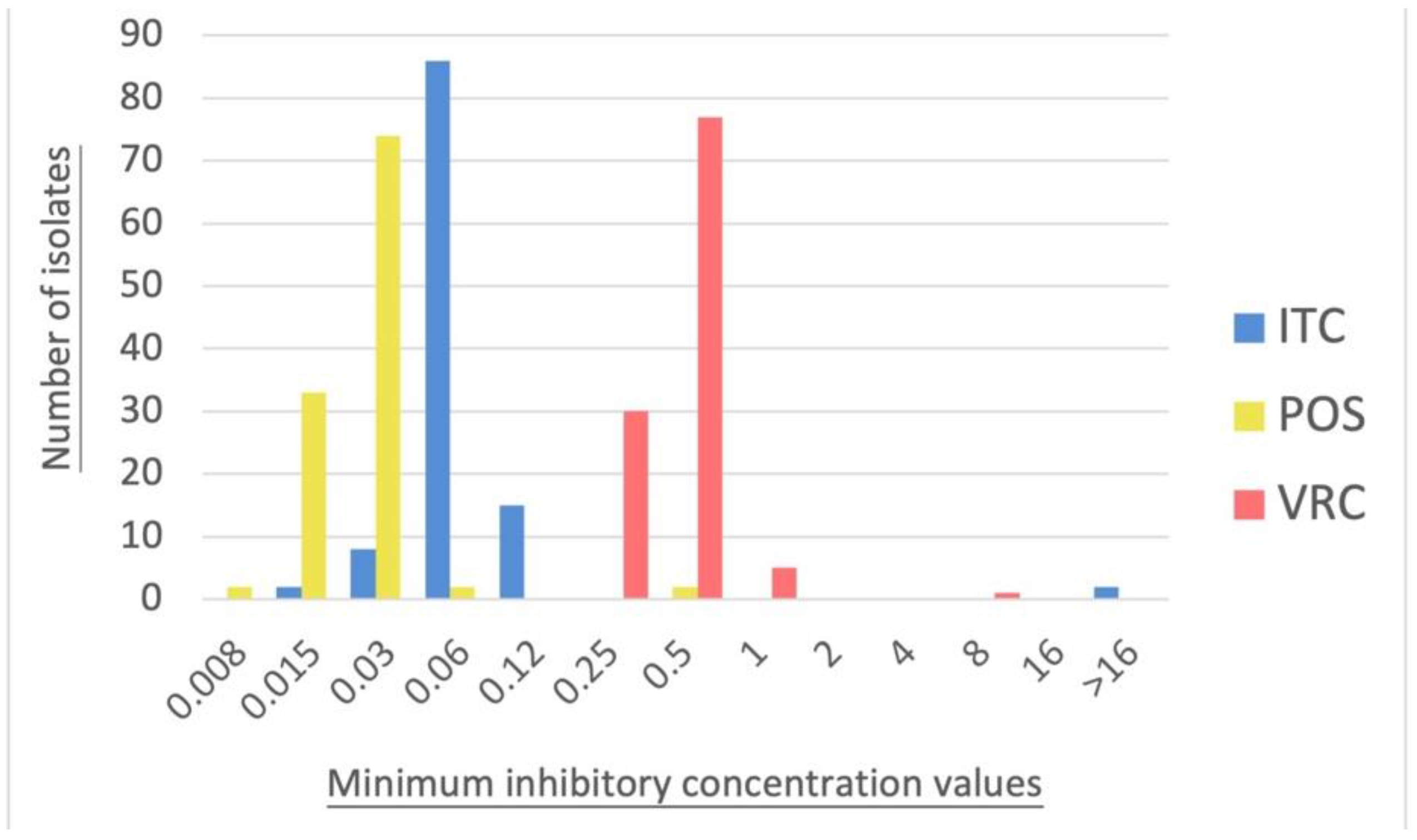
3.2. Antifungal Susceptibility Testing
3.3. Detection of cyp51A Mutations in Azole-Resistant Isolates
3.4. Clinical Profiles of Patients from Whom the Azole-Resistant A. fumigatus Were Isolated
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, C.; Shah, A. Allergic Aspergillus sinusitis and its association with allergic bronchopulmonary aspergillosis. Asia Pac. Allergy 2011, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F. Aspergillus fumigatus-Related Species in Clinical Practice. Front. Microbiol. 2016, 7, 683. [Google Scholar] [CrossRef]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Sugui, J.A. Aspergillus fumigatus—What makes the species a ubiquitous human fungal pathogen? PLoS Pathog. 2013, 9, e1003743. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Verweij, P.E. Aspergillus fumigatus and pan-azole resistance: Who should be concerned? Curr. Opin. Infect. Dis. 2020, 33, 290–297. [Google Scholar] [CrossRef]
- Rhodes, J.; Abdolrasouli, A.; Dunne, K.; Sewell, T.R.; Zhang, Y.; Ballard, E.; Brackin, A.P.; van Rhijn, N.; Chown, H.; Tsitsopoulou, A.; et al. Population genomics confirms acquisition of drug-resistant Aspergillus fumigatus infection by humans from the environment. Nat. Microbiol. 2022, 7, 663–674. [Google Scholar] [CrossRef]
- Burks, C.; Darby, A.; Gómez Londoño, L.; Momany, M.; Brewer, M.T. Azole-resistant Aspergillus fumigatus in the environment: Identifying key reservoirs and hotspots of antifungal resistance. PLoS Pathog. 2021, 17, e1009711. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; Alcazar-Fuoli, L.; Monteiro, M.C.; Monzon, S.; Cuesta, I.; Pelaez, T.; Mellado, E. Insight into the Significance of Aspergillus fumigatus cyp51A Polymorphisms. Antimicrob. Agents Chemother. 2018, 62, e00241-18. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.; Karawajczyk, A.; Rijs, A.J.; Zoll, J.; Verweij, P.E.; Melchers, W.J. Genotype-phenotype complexity of the TR46/Y121F/T289A cyp51A azole resistance mechanism in Aspergillus fumigatus. Fungal. Genet. Biol. 2015, 82, 129–135. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Alastruey-Izquierdo, A.; Mellado, E.; Cuenca-Estrella, M. Triazole Resistance in Aspergillus spp.: A Worldwide Problem? J. Fungi 2016, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lopez Jimenez, L.; Snelders, E.; Debets, A.J.M.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E.; Schoustra, S.E. Dynamics of Aspergillus fumigatus in Azole Fungicide-Containing Plant Waste in the Netherlands (2016–2017). Appl. Environ. Microbiol. 2021, 87, e02295-20. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rubio, R.; Cuenca-Estrella, M.; Mellado, E. Triazole Resistance in Aspergillus Species: An Emerging Problem. Drugs 2017, 77, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Liu, J.; Zeng, M.; Sang, H. Exploring the molecular mechanism of azole resistance in Aspergillus fumigatus. J. Mycol. Med. 2020, 30, 100915. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Kathuria, S.; Hagen, F.; Meis, J.F. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J. Antimicrob. Chemother. 2013, 69, 555–557. [Google Scholar] [CrossRef]
- Wu, C.J.; Wang, H.C.; Lee, J.C.; Lo, H.J.; Dai, C.T.; Chou, P.H.; Ko, W.C.; Chen, Y.C. Azole-resistant Aspergillus fumigatus isolates carrying TR₃₄/L98H mutations in Taiwan. Mycoses 2015, 58, 544–549. [Google Scholar] [CrossRef]
- Wu, C.J.; Liu, W.L.; Lai, C.C.; Chao, C.M.; Ko, W.C.; Wang, H.C.; Dai, C.T.; Hsieh, M.I.; Choi, P.C.; Yang, J.L.; et al. Multicenter Study of Azole-Resistant Aspergillus fumigatus Clinical Isolates, Taiwan. Emerg. Infect. Dis. 2020, 26, 804–806. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.; Innis, M.; Gelfand, D.; Sninsky, J. PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Tam, E.W.T.; Chen, J.H.K.; Lau, E.C.L.; Ngan, A.H.Y.; Fung, K.S.C.; Lee, K.-C.; Lam, C.-W.; Yuen, K.-Y.; Lau, S.K.P.; Woo, P.C.Y. Misidentification of Aspergillus nomius and Aspergillus tamarii as Aspergillus flavus: Characterization by internal transcribed spacer, β-Tubulin, and calmodulin gene sequencing, metabolic fingerprinting, and matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2014, 52, 1153–1160. [Google Scholar] [CrossRef]
- Alexander, B.D. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2017. [Google Scholar]
- Chen, J.; Li, H.; Li, R.; Bu, D.; Wan, Z. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J. Antimicrob. Chemother. 2005, 55, 31–37. [Google Scholar] [CrossRef]
- Weber, M.; Schaer, J.; Walther, G.; Kaerger, K.; Steinmann, J.; Rath, P.M.; Spiess, B.; Buchheidt, D.; Hamprecht, A.; Kurzai, O. FunResDB-A web resource for genotypic susceptibility testing of Aspergillus fumigatus. Med. Mycol. 2018, 56, 117–120. [Google Scholar] [CrossRef]
- Procop, G.W. Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi. M61; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and Outcome of Invasive Fungal Infection in Adult Hematopoietic Stem Cell Transplant Recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance Registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Cadena, J.; Thompson, G.R., 3rd; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.K.; Tiwari, S.; Shankar, J. Resistance mechanism and proteins in Aspergillus species against antifungal agents. Mycology 2019, 10, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Watanabe, A.; Kamei, K.; Goldman, G.H. Epidemiological and Genomic Landscape of Azole Resistance Mechanisms in Aspergillus Fungi. Front. Microbiol. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Reichert-Lima, F.; Lyra, L.; Pontes, L.; Moretti, M.L.; Pham, C.D.; Lockhart, S.R.; Schreiber, A.Z. Surveillance for azoles resistance in Aspergillus spp. highlights a high number of amphotericin B-resistant isolates. Mycoses 2018, 61, 360–365. [Google Scholar] [CrossRef]
- Ashu, E.E.; Korfanty, G.A.; Samarasinghe, H.; Pum, N.; You, M.; Yamamura, D.; Xu, J. Widespread amphotericin B-resistant strains of Aspergillus fumigatus in Hamilton, Canada. Infect. Drug Resist. 2018, 11, 1549–1555. [Google Scholar] [CrossRef]
- Blum, G.; Hörtnagl, C.; Jukic, E.; Erbeznik, T.; Pümpel, T.; Dietrich, H.; Nagl, M.; Speth, C.; Rambach, G.; Lass-Flörl, C. New insight into amphotericin B resistance in Aspergillus terreus. Antimicrob. Agents Chemother. 2013, 57, 1583–1588. [Google Scholar] [CrossRef]
- Jiménez-Ortigosa, C.; Moore, C.; Denning, D.W.; Perlin, D.S. Emergence of Echinocandin Resistance Due to a Point Mutation in the fks1 Gene of Aspergillus fumigatus in a Patient with Chronic Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2017, 61, e01277-17. [Google Scholar] [CrossRef]
- Satish, S.; Perlin, D.S. Echinocandin Resistance in Aspergillus fumigatus Has Broad Implications for Membrane Lipid Perturbations That Influence Drug-Target Interactions. Microbiol. Insights 2019, 12, 1178636119897034. [Google Scholar] [CrossRef]
- e Silva, A.P.; Miranda, I.M.; Branco, J.; Oliveira, P.; Faria-Ramos, I.; Silva, R.M.; Rodrigues, A.G.; Costa-de-Oliveira, S. FKS1 mutation associated with decreased echinocandin susceptibility of Aspergillus fumigatus following anidulafungin exposure. Sci. Rep. 2020, 10, 11976. [Google Scholar] [CrossRef]
- Procop, G.W. Epidemiological Cutoff Values for Antifungal Susceptibility Testing; Clinical and Laboratory Standard Institute (CLSI): Wayne, PA, USA, 2020. [Google Scholar]
- Guinea, J. Updated EUCAST Clinical Breakpoints against Aspergillus, Implications for the Clinical Microbiology Laboratory. J. Fungi 2020, 6, 343. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Kuo, S.F.; Wang, H.C.; Wu, C.J.; Lin, Y.S.; Li, W.S.; Lee, C.H. Azole resistance in Aspergillus species in Southern Taiwan: An epidemiological surveillance study. Mycoses 2019, 62, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Rivelli Zea, S.M.; Toyotome, T. Azole-resistant Aspergillus fumigatus as an emerging worldwide pathogen. Microbiol. Immunol. 2022, 66, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bueid, A.; Howard, S.J.; Moore, C.B.; Richardson, M.D.; Harrison, E.; Bowyer, P.; Denning, D.W. Azole antifungal resistance in Aspergillus fumigatus: 2008 and 2009. J. Antimicrob. Chemother. 2010, 65, 2116–2118. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, R.; Zhang, L.; Li, D.; Lv, G.; Shen, Y.; Zheng, H.; Zhang, Q.; Zhao, J.; Zheng, N.; et al. Multiple cyp51A-based mechanisms identified in azole-resistant isolates of Aspergillus fumigatus from China. Antimicrob. Agents Chemother. 2015, 59, 4321–4325. [Google Scholar] [CrossRef]
- Takeda, K.; Suzuki, J.; Watanabe, A.; Arai, T.; Koiwa, T.; Shinfuku, K.; Narumoto, O.; Kawashima, M.; Fukami, T.; Tamura, A.; et al. High detection rate of azole-resistant Aspergillus fumigatus after treatment with azole antifungal drugs among patients with chronic pulmonary aspergillosis in a single hospital setting with low azole resistance. Med. Mycol. 2020, 59, 327–334. [Google Scholar] [CrossRef]
- Jeanvoine, A.; Rocchi, S.; Bellanger, A.P.; Reboux, G.; Millon, L. Azole-resistant Aspergillus fumigatus: A global phenomenon originating in the environment? Med. Mal. Infect. 2020, 50, 389–395. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, B.; Mahto, K.K.; Meis, J.F.; Chowdhary, A. High-Frequency Direct Detection of Triazole Resistance in Aspergillus fumigatus from Patients with Chronic Pulmonary Fungal Diseases in India. J. Fungi 2020, 6, 67. [Google Scholar] [CrossRef]
- Resendiz Sharpe, A.; Lagrou, K.; Meis, J.F.; Chowdhary, A.; Lockhart, S.R.; Verweij, P.E. Triazole resistance surveillance in Aspergillus fumigatus. Med. Mycol. 2018, 56, 83–92. [Google Scholar] [CrossRef]
- van der Linden, J.W.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Kuijper, E.J.; Van Tiel, F.H.; Melchers, W.J.; Verweij, P.E. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef]
- van Paassen, J.; Russcher, A.; In ‘t Veld-van Wingerden, A.W.; Verweij, P.E.; Kuijper, E.J. Emerging aspergillosis by azole-resistant Aspergillus fumigatus at an intensive care unit in the Netherlands, 2010 to 2013. Euro. Surveill. 2016, 21, 30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Hsieh, M.I.; Choi, P.C.; Wu, C.J. Comparison of the Sensititre YeastOne and CLSI M38-A2 Microdilution Methods in Determining the Activity of Amphotericin B, Itraconazole, Voriconazole, and Posaconazole against Aspergillus Species. J. Clin. Microbiol. 2018, 56, e00780-18. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.L.; Johansen, H.K.; Fuursted, K.; Knudsen, J.D.; Gahrn-Hansen, B.; Jensen, R.H.; Howard, S.J.; Arendrup, M.C. A prospective survey of Aspergillus spp. in respiratory tract samples: Prevalence, clinical impact and antifungal susceptibility. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Lee, D.-G.; Kim, W.-B.; Chun, H.-S.; Park, C.; Myong, J.-P.; Park, Y.-J.; Choi, J.-K.; Lee, H.-J.; Kim, S.-H.; et al. Epidemiology and Antifungal Susceptibility Profile of Aspergillus Species: Comparison between Environmental and Clinical Isolates from Patients with Hematologic Malignancies. J. Clin. Microbiol. 2019, 57, e02023-18. [Google Scholar] [CrossRef]
- Pinto, E.; Monteiro, C.; Maia, M.; Faria, M.A.; Lopes, V.; Lameiras, C.; Pinheiro, D. Aspergillus Species and Antifungals Susceptibility in Clinical Setting in the North of Portugal: Cryptic Species and Emerging Azoles Resistance in A. fumigatus. Front. Microbiol. 2018, 9, 1656. [Google Scholar] [CrossRef]
- Rhijn, N.V.; Bromley, M.; Richardson, M.; Bowyer, P.; Steinbach, W.J.; Pietro, A.D. CYP51 Paralogue Structure Is Associated with Intrinsic Azole Resistance in Fungi. mBio 2021, 12, e01945-21. [Google Scholar] [CrossRef]
- van der Linden, J.W.; Arendrup, M.C.; Warris, A.; Lagrou, K.; Pelloux, H.; Hauser, P.M.; Chryssanthou, E.; Mellado, E.; Kidd, S.E.; Tortorano, A.M.; et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg. Infect. Dis. 2015, 21, 1041–1044. [Google Scholar] [CrossRef]
| AFG | MFG | CAS | 5FC | POS | VRC | ITC | FLC | AMB | |
|---|---|---|---|---|---|---|---|---|---|
| MIC range | ≤0.015 | ≤0.008 | ≤0.008–0.06 | 8–>64 | ≤0.008–0.5 | 0.25–8 | ≤0.015–>16 | 32–>256 | 1–4 |
| MIC50 | ≤0.015 | ≤0.008 | ≤0.008 | >64 | 0.03 | 0.5 | 0.06 | >256 | 2 |
| MIC90 | ≤0.015 | ≤0.008 | 0.015 | >64 | 0.03 | 0.5 | 0.12 | >256 | 2 |
| Strain No. | AFST Method | ISA | POS | VRC | ITC | FLC | AMB |
|---|---|---|---|---|---|---|---|
| CGMHD 1497 | YeastOne | ND | 0.03 | 0.5 | 0.06 | >256 | 4 |
| CLSI | 0.25 | 0.125 | 0.25 | 0.25 | >64 | 0.5 | |
| CGMHD 1524 | YeastOne | ND | 0.03 | 0.5 | 0.06 | >256 | 4 |
| CLSI | 0.5 | 0.125 | 0.25 | 0.5 | >64 | 0.5 | |
| CGMHD 2417 | YeastOne | ND | 0.03 | 0.5 | 0.06 | >256 | 4 |
| CLSI | 0.25 | 0.125 | 0.25 | 0.125 | >64 | 1 | |
| CGMHD 0641 | YeastOne | ND | 0.03 | 1 | 0.12 | >256 | 1 |
| CLSI | 0.5 | 0.125 | 0.5 | 0.25 | >64 | 0.25 | |
| CGMHD 0744 | YeastOne | ND | 0.06 | 1 | 0.12 | >256 | 2 |
| CLSI | 1 | 0.25 | 1 | 0.5 | >64 | 0.5 | |
| CGMHD 1652 | YeastOne | ND | 0.5 | 8 | >16 | >256 | 2 |
| CLSI | 4 | 0.5 | 4 | >16 | >64 | 0.25 | |
| CGMHD 2261 | YeastOne | ND | 0.5 | 1 | >16 | >256 | 2 |
| CLSI | >4 | 0.5 | 2 | >16 | >64 | 1 |
| Case No. | Age/ Gender | Specimen | Clinical Profile | Strain | cyp51A Mutation | MIC (μg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| POS | VRC | ITC | ISA | ||||||
| Case 1 | 50/ female | BAL | Clinical diagnosis: Hemoptysis due to bronchiectasis and bronchiolitis Treatment course: Symptomatic treatment Outcome: The patient had a favorable outcome and visited as a regular outpatient for more than 3 years. | CGMHD 1652 | TR34/L98H | 0.5 | 4 | >16 | 4 |
| Case 2 | 82/male | BAL | Clinical diagnosis: Invasive fungal tracheobronchitis. GM index in serum was 0.82. Treatment course: Voriconazole 300mg q12h loading day 1 then voriconazole 200mg q12h Outcome: After 19 days of voriconazole treatment, the patient died of respiratory failure with multi-organ failure. | CGMHD 2261 | TR34/L98H/S297T/F495I | 0.5 | 2 | >16 | >4 |
| Case 3 | 80/ female | BAL | Clinical diagnosis: Necrotizing pneumonia caused by carbapenem-resistant Klebsiella pneumoniae, and invasive aspergillosis. GM index in serum was 5.55. Treatment course: Caspofungin 35 mg qd due to liver failure Outcome: The patient died of profound shock, respiratory failure, and multi-organ failure after 10 days of treatment. | CGMHD 0744 | F46Y/M172V/N248T/D255E/E427K | 0.25 | 1 | 0.5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-H.; Huang, P.-Y.; Fan, Y.-C.; Sun, P.-L. Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan. J. Fungi 2022, 8, 908. https://doi.org/10.3390/jof8090908
Hsu T-H, Huang P-Y, Fan Y-C, Sun P-L. Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan. Journal of Fungi. 2022; 8(9):908. https://doi.org/10.3390/jof8090908
Chicago/Turabian StyleHsu, Tsun-Hao, Po-Yen Huang, Yun-Chen Fan, and Pei-Lun Sun. 2022. "Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan" Journal of Fungi 8, no. 9: 908. https://doi.org/10.3390/jof8090908
APA StyleHsu, T.-H., Huang, P.-Y., Fan, Y.-C., & Sun, P.-L. (2022). Azole Resistance and cyp51A Mutation of Aspergillus fumigatus in a Tertiary Referral Hospital in Taiwan. Journal of Fungi, 8(9), 908. https://doi.org/10.3390/jof8090908






