Diversity of Ascomycota in Jilin: Introducing Novel Woody Litter Taxa in Cucurbitariaceae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Isolation
2.2. Morphological Observation
2.3. DNA Extraction, PCR Amplification and Sequencing
2.4. Phylogenetic Analysis
2.5. Analysis of Matrix Partitions by Assemble Species by Automatic Partitioning
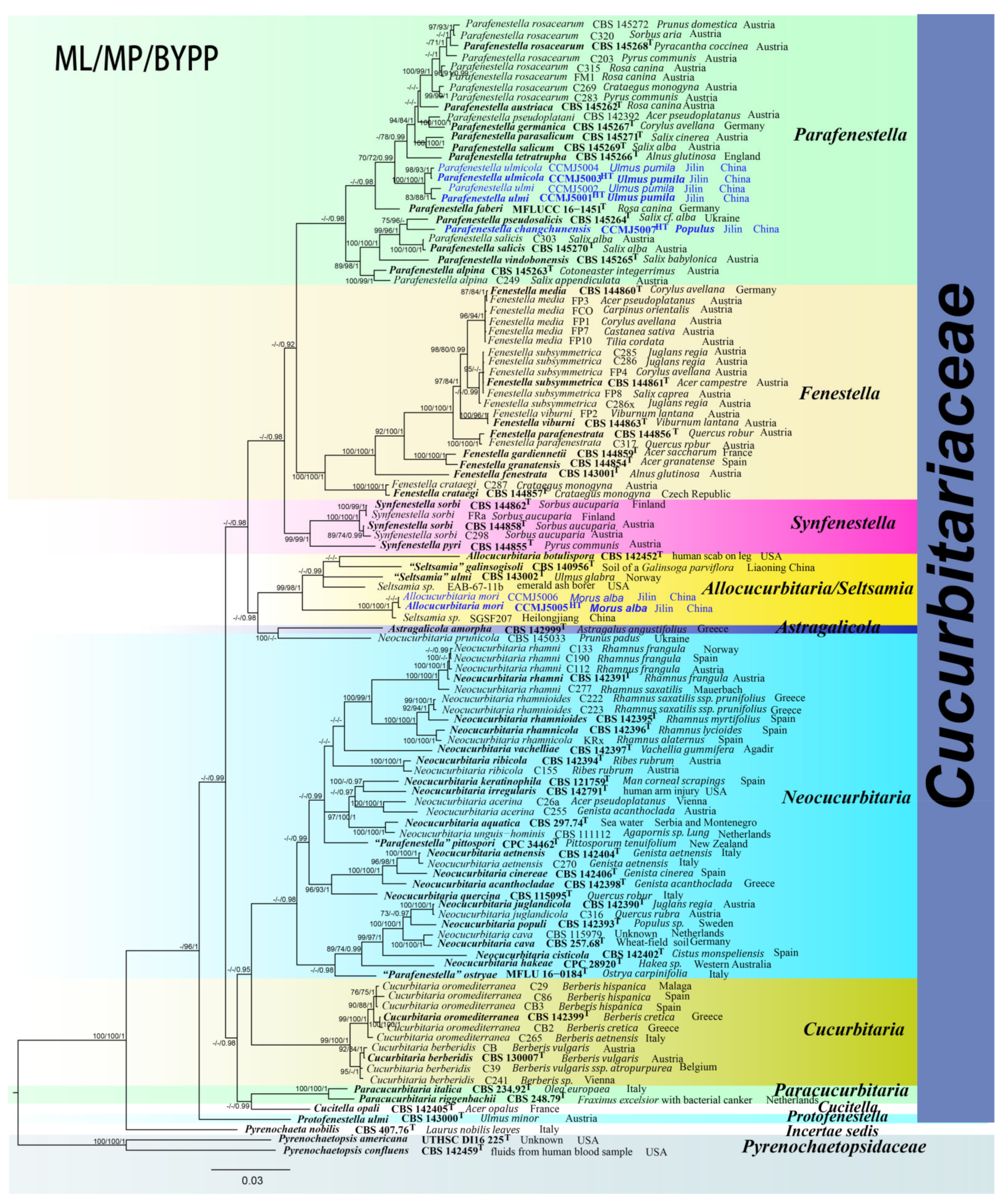
3. Results
3.1. Phylogenetic Analyses
3.2. ASAP: Assemble Species by Automatic Partitioning
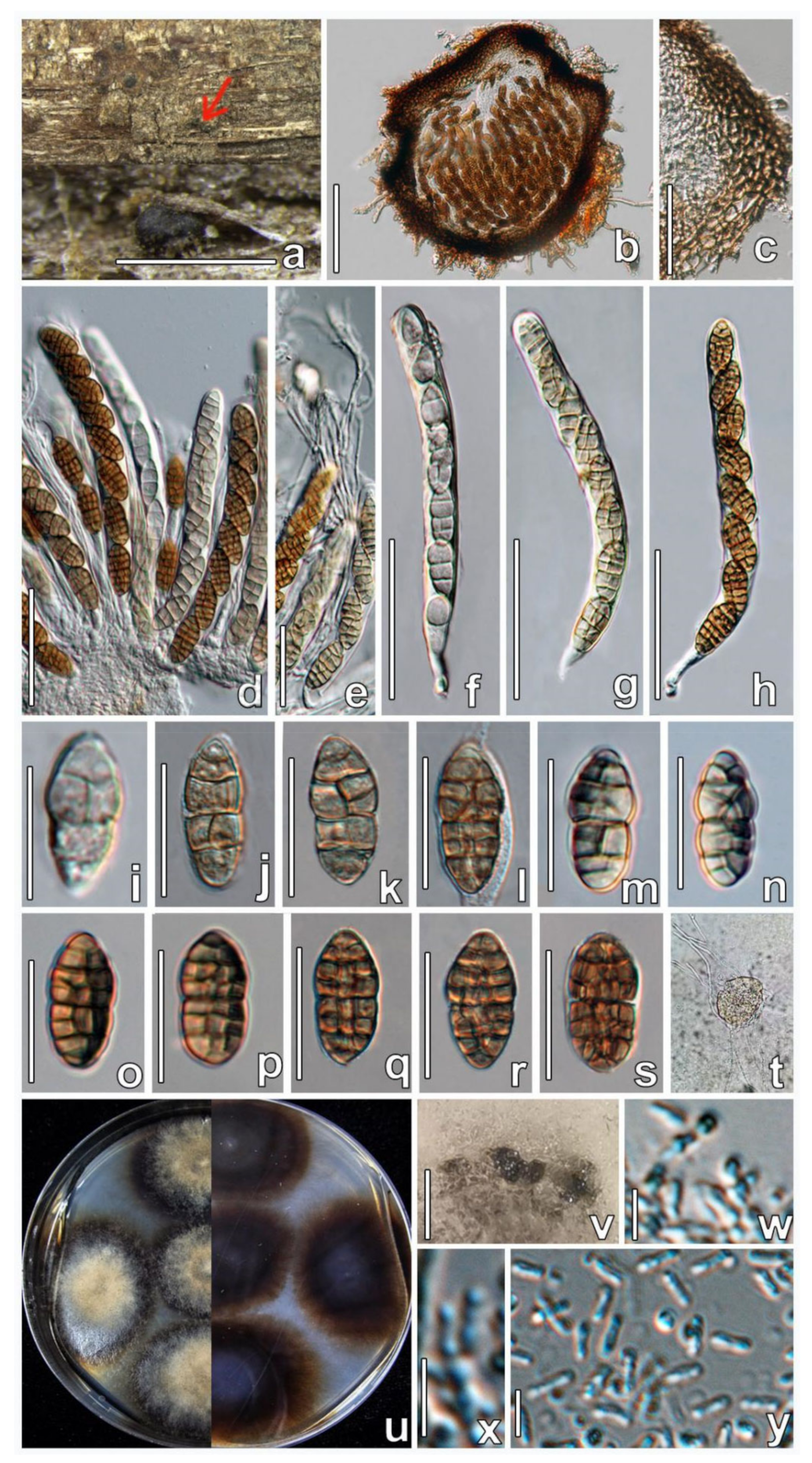
3.3. Taxonomy
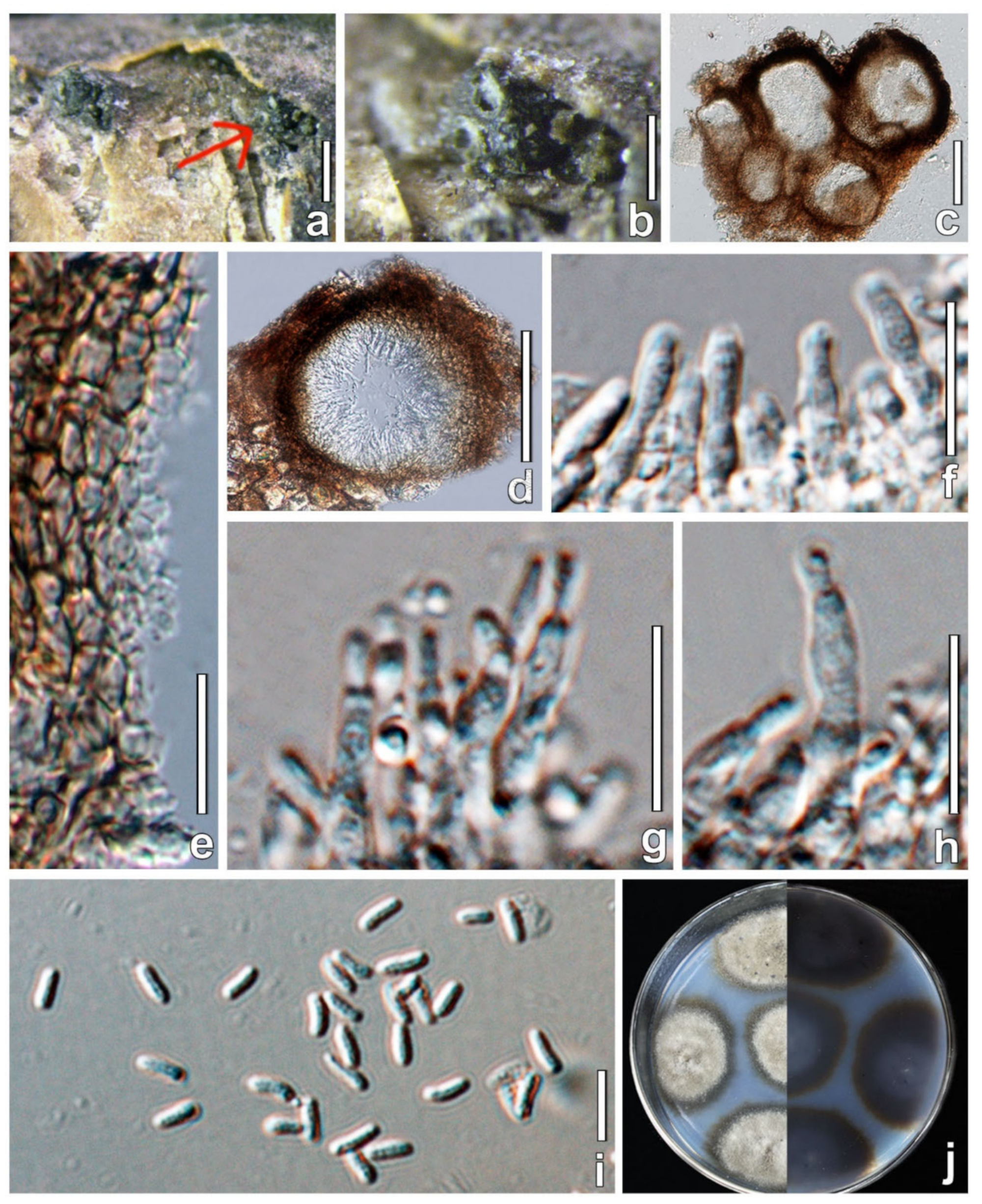
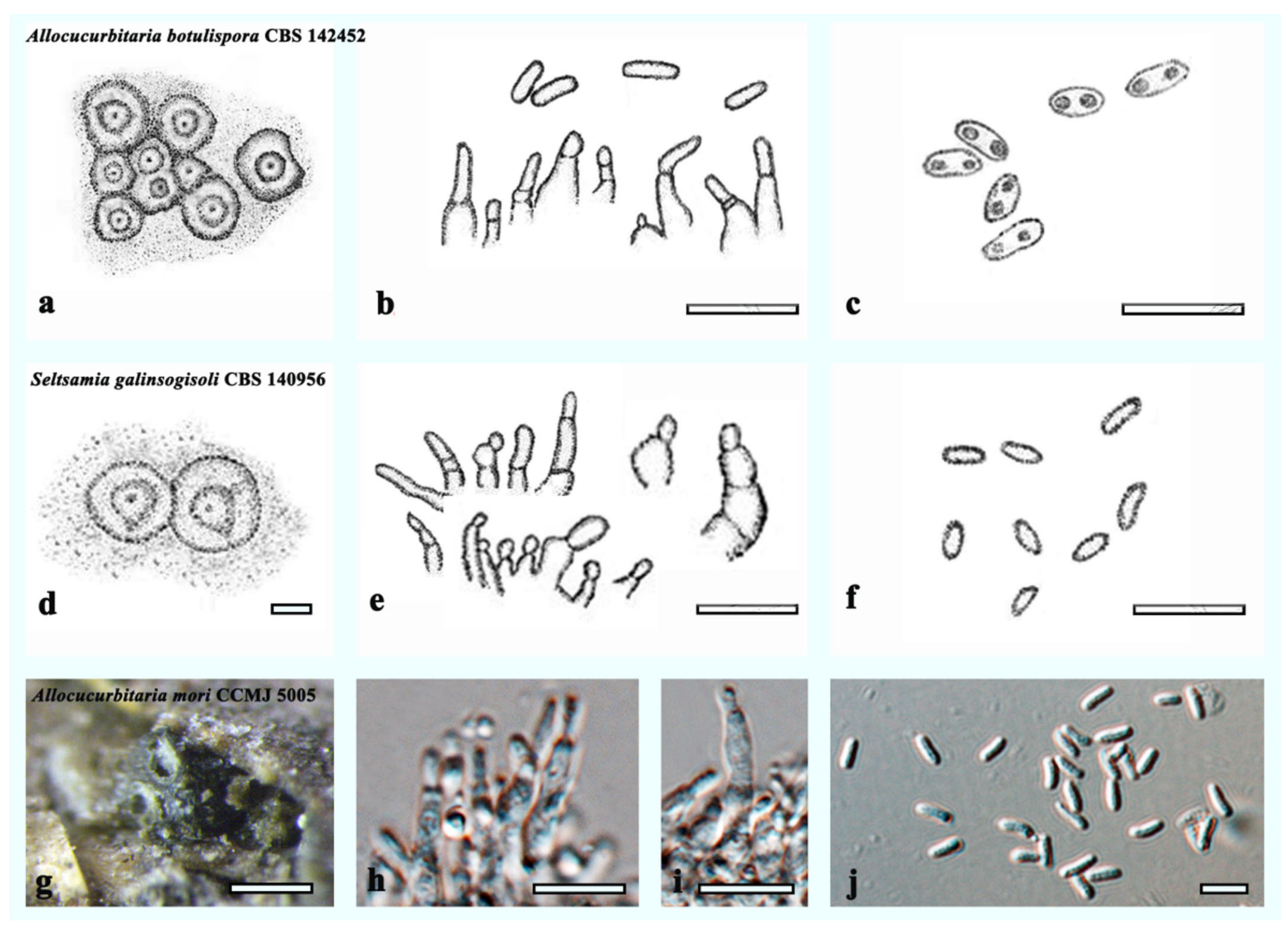
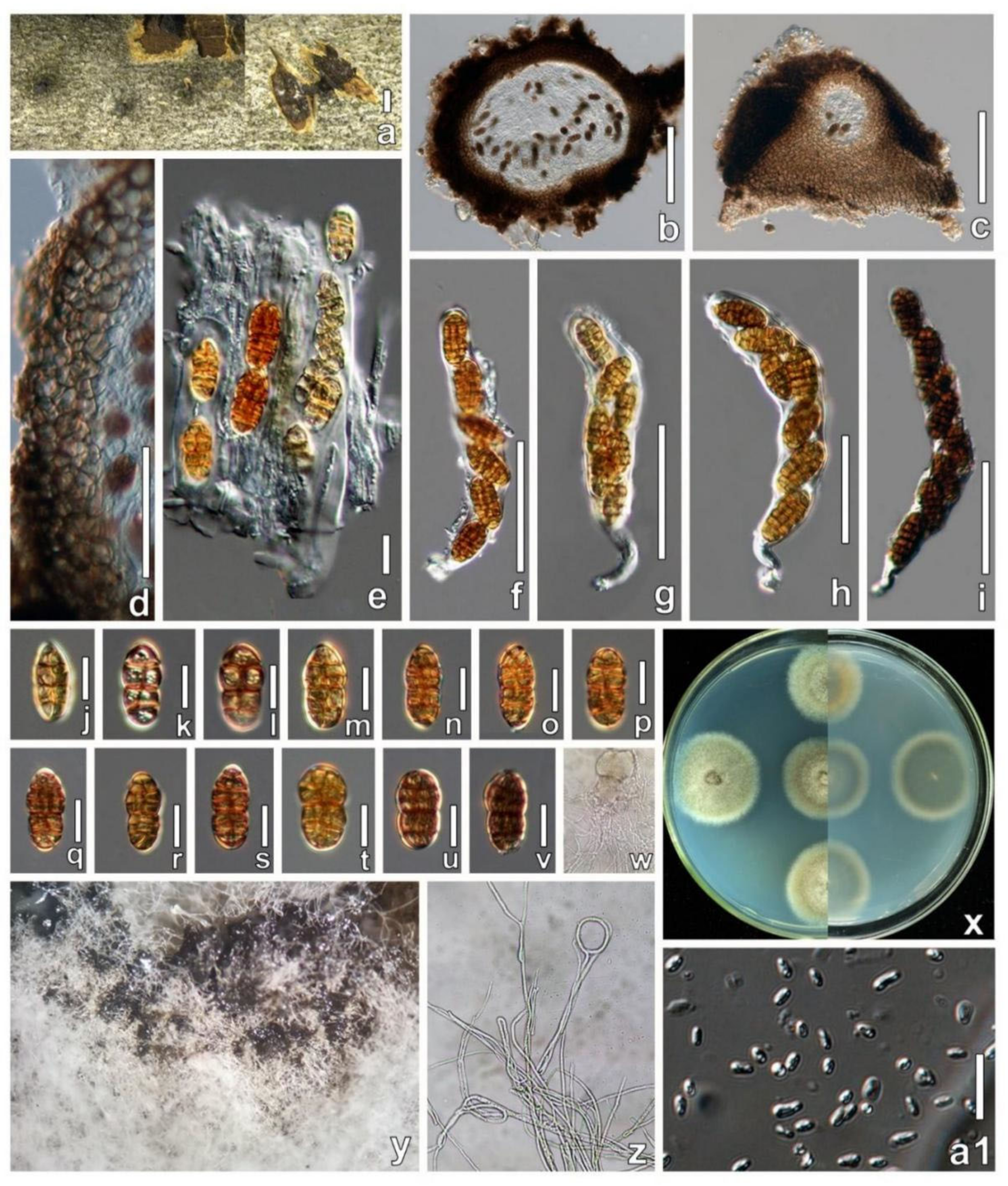

4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycological 2001, 105, 1422–1432. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Niskanen, T.; Suwannarach, N.; Wannathes, N.; Chen, Y.J.; McKenzie, E.H.; Maharachchikumbura, S.S.; Buyck, B.; Zhao, C.L.; Fan, Y.G.; et al. The numbers of fungi: Are the most speciose genera truly diverse? Fungal Divers. 2022, 27, 387–462. [Google Scholar] [CrossRef]
- Phukhamsakda, C.; Nilsson, R.H.; Bhunjun, C.S.; de Farias, A.R.; Sun, Y.R.; Wijesinghe, S.N.; Raza, M.; Bao, D.F.; Lu, L.; Tibpromma, S.; et al. The numbers of fungi: Contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Divers. 2022, 28, 327–386. [Google Scholar] [CrossRef]
- Wu, B.; Hussain, M.; Zhang, W.; Stadler, M.; Liu, X.; Xiang, M. Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 2019, 10, 127–140. [Google Scholar] [CrossRef]
- Liu, J.; Diamond, J. China’s environment in a globalizing world. Nature 2005, 435, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.L.; Hyde, K.D.; Liu, J.K.; Bhat, D.J.; Bao, D.F.; Li, W.L.; Su, H.Y. Lignicolous freshwater fungi from China II: Novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 2018, 9, 444–461. [Google Scholar] [CrossRef]
- Zheng, H.; Wan, Y.K.; Li, J.; Rafael, F.C.R.; Yu, Z.F. Phialolunulospora vermispora (Chaetosphaeriaceae, Sordariomycetes), a novel asexual genus and species from freshwater in southern China. MycoKeys 2020, 76, 17. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Wang, X.C.; Zhuang, W.Y.; Cheng, X.H.; Zhao, P. New species of Talaromyces (Fungi) isolated from soil in Southwestern China. Biology 2021, 10, 745. [Google Scholar] [CrossRef]
- Zheng, P. China’s Geography; China Intercontinental Press: Beijing, China, 2006. [Google Scholar]
- Zhang, X.; Wang, W.C.; Fang, X.Q.; Ye, Y. Vegetation of Northeast China during the late seventeenth to early twentieth century as revealed by historical documents. Reg. Environ. Change 2011, 11, 869–882. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Zhu, L.J.; Cherubini, P.; Li, Z.S.; Zhang, Y.D.; Wang, X.C. Species-specific indication of 13 tree species growth on climate warming in temperate forest community of northeast China. Ecol. Indic. 2021, 133, 108389. [Google Scholar] [CrossRef]
- Winter, H.G. Pilze—Ascomyceten. In GL Rabenhorst’s Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz; Verlag von Eduard Kummer: Leipzig, Germany, 1885; Volume 1, pp. 65–528. [Google Scholar]
- Wijayawardenem, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.L.; Aptroot, A.; Leontyev, D.V.; Saxena, R.K.; et al. Outline of fungi and funguslike taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Checa, J.; Blanco, M.N.; Olariaga, I.; Tello, S.; Voglmayr, H. A preliminary account of the Cucurbitariaceae. Stud. Mycol. 2018, 90, 71–118. [Google Scholar] [CrossRef] [PubMed]
- Jaklitsch, W.M.; Voglmayr, H. Fenestelloid clades of the Cucurbitariaceae. Persoonia 2020, 44, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Monkai, J.; Tibpromma, S.; Manowong, A.; Mapook, A.; Norphanphoun, C.; Hyde, K.D.; Promputtha, I. Discovery of three novel Cytospora species in Thailand and their antagonistic potential. Diversity 2021, 13, 488. [Google Scholar] [CrossRef]
- Index Fungorum. 2022. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 11 April 2022).
- Jayasiri, S.C.; Hyde, K.D.; Ariyawansa, H.; Bhat, D.J.; Buyck, B.; Cai, L.; Dai, Y.C.; Abd-Elsalam, K.A.; Ertz, D.; Hidayat, I.; et al. The faces of fungi database: Fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 2015, 74, 3–18. [Google Scholar] [CrossRef]
- De Hoog, G.S.; Gerrits van den Ende, A.H.G. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 1998, 41, 183–189. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dissanayake, L.S.; Wijesinghe, S.N.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guid. Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Voglmayr, H.; Akulov, O.Y.; Jaklitsch, W.M. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycol. Prog. 2016, 15, 921. [Google Scholar] [CrossRef] [PubMed]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Weber, N.S.; James, M.T. Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. J. Mol. Evol. 1997, 89, 48–65. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Hall, M.A. Correlation-Based feature selection for machine learning. PhD Thesis, The University of Waikato, Hamilton, New Zealand, 1999. [Google Scholar]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Voglmayr, H.; Jaklitsch, W.M. Corynespora, Exosporium and Helminthosporium revisited—New species and generic reclassification. Stud. Mycol. 2017, 87, 43–76. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4, Tree Figure Drawing Tool. 2014. Available online: http.ac.uk/software/figtree (accessed on 8 July 2022).
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol Ecol Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Phukhamsakda, C.; Tibpromma, S.; McKenzie, E.H.C.; Bhat, D.J.; Li, Y.; Hyde, K.D. Microfungi associated with dead leaves of Dasymaschalon obtusipetalum, Garcinia propinqua and Mammea harmandii, with the description of 20 new and rare species. Mycosphere 2022, in press. [Google Scholar]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 1–69. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Wu, Y.Y.; Zhang, M.Y.; Cheng, J.; Dube, B.; Yu, H.J.; Zhang, Y.X. New antimicrobial compounds produced by Seltsamia galinsogisoli sp. nov., isolated from Galinsoga parviflora as potential inhibitors of FtsZ. Sci. Rep. 2019, 9, 8319. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Sutton, D.A.; Cano-Lira, J.F.; Paredes, K.; Wiederhold, N.; Guarro, J.; Stchigel, A.M. Coelomycetous fungi in the clinical setting: Morphological convergence and cryptic diversity. Mol. Phylogenet. Evol. 2017, 55, 552–567. [Google Scholar] [CrossRef]
- Species Fungorum. 2022. Available online: http://www.speciesfungorum.org/Names/Names.asp (accessed on 20 May 2022).
- Wanasinghe, D.N.; Phookamsak, R.; Jeewon, R.; Wen, J.L.; Hyde, K.D.; Jones, E.B.G.; Camporesi, E.; Promputtha, I. A family level rDNA based phylogeny of Cucurbitariaceae and Fenestellaceae with descriptions of new Fenestella species and Neocucurbitaria gen. nov. Mycosphere 2017, 8, 397–414. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsa-ard, J.; Thangavel, R. Fungal Planet description sheets: 951–1041. Persoonia 2019, 43, 223. [Google Scholar] [CrossRef] [PubMed]
- Doilom, M.; Liu, J.K.; Jaklitsch, W.M.; Ariyawansa, H.; Wijayawardene, N.N.; Chukeatirote, E.; Zhang, M.; McKenzie, E.H.C.; Geml, J.; Voglmayr, H.; et al. An outline of the family Cucurbitariaceae. Sydowia 2013, 65, 167–192. [Google Scholar]
- Xu, R.; Su, W.X.; Tian, S.Q.; Bhunjun, C.S.; Tibpromma, S.; Hyde, K.D.; Li, Y.; Phukhamsakda, C. Synopsis of leptosphaeriaceae and introduction of three new taxa and one new record from China. J. Fungi 2022, 8, 416. [Google Scholar] [CrossRef]
- Bhunjun, C.S.; Dong, Y.; Jayawardena, R.S.; Jeewon, R.; Phukhamsakda, C.; Bundhun, D.; Hyde, K.D.; Sheng, J. A polyphasic approach to delineate species in Bipolaris. Fungal Divers. 2020, 102, 225–256. [Google Scholar] [CrossRef]
- Chethana, K.W.; Manawasinghe, I.S.; Hurdeal, V.G.; Bhunjun, C.S.; Appadoo, M.A.; Gentekaki, E.; Raspé, O.; Promputtha, I.; Hyde, K.D. What are fungal species and how to delineate them? Fungal Divers. 2021, 109, 1–25. [Google Scholar] [CrossRef]
- Pem, D.; Jeewon, R.; Chethana, K.W.T.; Hongsanan, S.; Doilom, M.; Suwannarach, N.; Hyde, K.D. Species concepts of Dothideomycetes: Classification, phylogenetic inconsistencies and taxonomic standardization. Fungal Divers. 2021, 109, 283–319. [Google Scholar] [CrossRef]
- Sharma, V.; Salwal, R. Molecular markers and their use in taxonomic characterization of Trichoderma spp. In Molecular Markers in Mycology; Singh, B.P., Gupta, V.K., Eds.; Springer: Cham, Switzerland, 2017; pp. 37–52. [Google Scholar]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Zhu, X.S.; Chen, Y.; Wu, Y.; Wang, Y.; Li, C.Y. Advances in research and application of DNA barcoding in plant pathogenic fungi. J. Agric. Sci. Technol. 2021, 49, 1247–1252. [Google Scholar]
- Niraikulam, A.; Hyungdon, Y.; Natarajan, S. Protein Coding Genes for Better Resolution of Phylogenetic Analysis. J. Biotechnol. 2010, 5, 74. [Google Scholar]
- Phukhamsakda, C.; McKenzie, E.H.C.; Phillips, A.J.L.; Jones, E.B.G.; Jayarama Bhat, D.J.; Stadler, M.; Bhunjun, C.S.; Wanasinghe, D.N.; Thongbai, B.; Camporesi, E.; et al. Microfungi associated with Clematis (Ranunculaceae) with an integrated approach to delimiting species boundaries. Fungal Divers. 2020, 102, 1–203. [Google Scholar] [CrossRef]
- Chen, H.R. Structural and Functional Relationship between JWA and α-Tubulin. Master’s Thesis, Nanjing Medical University, Nanjing, China, 2004. [Google Scholar]
- Samson, R.A.; Seifert, K.A.; Kuijpers, A.F.A.; Houbraken, J.A.M.P.; Frisvadet, J.C. Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud Mycol. 2004, 49, 175–200. [Google Scholar]
- Magaña-Dueñas, V.; Stchigel, A.M.; Cano-Lira, J.F. New Coelomycetous fungi from freshwater in Spain. J. Fungi 2021, 7, 368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Xu, T.L.; Veresoglou, S.D.; Hu, H.W.; Hao, Z.P.; Hu, Y.J.; Liu, L.; Deng, Y.; Rillig, M.C.; Chen, B.D. Plant diversity represents the prevalent determinant of soil fungal community structure across temperate grasslands in northern China. Soil Biol. Biochem. 2017, 110, 12–21. [Google Scholar] [CrossRef]
- De Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 2010, 102, 1066–1081. [Google Scholar] [CrossRef] [PubMed]

| Amplification Loci (Primer Pair Forward/Reverse) | PCR Conditions | References |
|---|---|---|
| ITS (ITS5/ITS4) | An initial denaturation step of 5 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 30 s at 56 °C and 90 s at 72 °C, and a final extension step of 10 min at 72 °C, and 10 °C for holding temperature | White et al. [21] |
| rpb2 (fRPB2-5F/fRPB2-7cR) | Vilgalys et al. [23] | |
| tef1-α (2218F/983R) | Carbone and Kohn [24] Rehner and Buckley [25] | |
| LSU (LROR/LR5) | An initial denaturation step of 5 min at 94 °C, followed by 35 cycles of 30 s at 94 °C, 45 s at 53 °C and 90 s at 72 °C, and a final extension step of 10 min at 72 °C, and 10 °C for holding temperature | Vilgalys and Hester [22] |
| Β-tub (T1/Bt2b) | O’Donnell and Cigelnik [26] | |
| Taxon | Strain | Host/Substrate | Typification Status | GenBank Accession Numbers | ||||
|---|---|---|---|---|---|---|---|---|
| ITS | LSU | rpb2 | tef1-α | β-tub | ||||
| Allocucurbitaria botulispora | CBS 142452 | human scab on leg | Holotype | LT592932 | LN907416 | LT593070 | – | LT593001 |
| Allocucurbitaria mori | HMJAU 60183 | Morus alba | Holotype | OL996120 | OL897171 | OL944505 | OL944601 | OL898725 |
| Allocucurbitaria mori | HMJAU 60184 | Morus alba | Isotype | OL996121 | OL897172 | OL944506 | OL944602 | OL898720 |
| Astragalicola amorpha | CBS 142999 | Astragalus angustifolius | Holotype | MF795753 | MF795753 | MF795795 | MF795842 | MF795883 |
| Cucitella opali | CBS 142405 | Acer opalus | Holotype | MF795754 | MF795754 | MF795796 | MF795843 | MF795884 |
| Cucurbitaria berberidis | C39 | Berberis vulgaris subsp. atropurpurea | – | MF795755 | MF795755 | MF795797 | MF795844 | MF795885 |
| Cucurbitaria berberidis | CB | Berberis vulgaris | – | MF795757 | MF795757 | MF795799 | MF795846 | MF795887 |
| Cucurbitaria berberidis | CBS 130007 = CB1 | Berberis vulgaris | Epitype | MF795758 | MF795758 | MF795800 | – | – |
| Cucurbitaria berberidis | CBS 142401 = C241 | Berberis sp. | – | MF795756 | MF795756 | MF795798 | MF795845 | MF795886 |
| Cucurbitaria oromediterranea | C265 | Berberis aetnensis | – | MF795762 | MF795762 | MF795804 | MF795850 | MF795891 |
| Cucurbitaria oromediterranea | C29 | Berberis hispanica | – | MF795759 | MF795759 | MF795801 | MF795847 | MF795888 |
| Cucurbitaria oromediterranea | C86 | Berberis hispanica | – | MF795760 | MF795760 | MF795802 | MF795848 | MF795889 |
| Cucurbitaria oromediterranea | CB2 | Berberis cretica | – | MF795763 | MF795763 | MF795805 | MF795851 | MF795892 |
| Cucurbitaria oromediterranea | CB3 | Berberis hispanica | – | MF795764 | MF795764 | MF795806 | MF795852 | – |
| Cucurbitaria oromediterranea | CBS 142399 = C229 | Berberis cretica | Holotype | MF795761 | MF795761 | MF795803 | MF795849 | MF795890 |
| Fenestella crataegi | C287 | Crataegus monogyna | – | MK356281 | MK356281 | – | MK357554 | MK357598 |
| Fenestella crataegi | CBS 144857 = C314 | Crataegus monogyna | Epitype | MK356282 | MK356282 | MK357512 | MK357555 | MK357599 |
| Fenestella fenestrata | CBS 143001 = FP9 | Alnus glutinosa | Epitype | MF795765 | MF795765 | MF795807 | MF795853 | MF795893 |
| Fenestella gardiennetii | CBS 144859 = FM | Acer saccharum | Holotype | MK356283 | MK356283 | MK357513 | MK357556 | MK357600 |
| Fenestella granatensis | CBS 144854 = C279 | Acer granatense | Holotype | MK356284 | MK356284 | MK357514 | MK357557 | MK357601 |
| Fenestella media | CBS 144860 = FP | Corylus avellana | Epitype | MK356285 | MK356285 | MK357515 | MK357558 | MK357602 |
| Fenestella media | FCO | Carpinus orientalis | – | MK356286 | MK356286 | MK357516 | MK357559 | – |
| Fenestella media | FP1 | Corylus avellana | – | MK356287 | MK356287 | MK357517 | MK357560 | MK357603 |
| Fenestella media | FP3 | Acer pseudoplatanus | – | MK356288 | MK356288 | MK357518 | MK357561 | MK357604 |
| Fenestella media | FP7 | Castanea sativa | – | MK356289 | MK356289 | MK357519 | MK357562 | MK357605 |
| Fenestella media | FP10 | Tilia cordata | – | MK356290 | MK356290 | MK357520 | MK357563 | MK357606 |
| Fenestella parafenestrata | CBS 144856 = C306 | Quercus robur | Holotype | MK356291 | MK356291 | MK357521 | MK357564 | MK357607 |
| Fenestella parafenestrata | C317 | Salix sp. | – | MK356292 | MK356292 | MK357522 | MK357565 | MK357608 |
| Fenestella subsymmetrica | CBS 144861 = FP6 | Acer campestre | Holotype | MK356297 | MK356297 | MK357525 | MK357569 | MK357610 |
| Fenestella subsymmetrica | C285 | Juglans regia | – | MK356293 | MK356293 | MK357523 | MK357566 | – |
| Fenestella subsymmetrica | C286 | Juglans regia | – | MK356294 | MK356294 | – | MK357567 | – |
| Fenestella subsymmetrica | C286x | Juglans regia | – | MK356295 | MK356295 | – | – | – |
| Fenestella subsymmetrica | FP4 | Corylus avellana | – | MK356296 | MK356296 | MK357524 | MK357568 | MK357609 |
| Fenestella subsymmetrica | FP8 | Salix caprea | – | MK356298 | MK356298 | MK357526 | MK357570 | MK357611 |
| Fenestella viburni | CBS 144863 = FVL | Viburnum lantana | Holotype | MK356300 | MK356300 | MK357528 | MK357572 | MK357613 |
| Fenestella viburni | FP2 | Viburnum lantana | – | MK356299 | MK356299 | MK357527 | MK357571 | MK357612 |
| Neocucurbitaria acanthocladae | CBS 142398 = C225 | Genista acanthoclada | Holotype | MF795766 | MF795766 | MF795808 | MF795854 | MF795894 |
| Neocucurbitaria acerina | C26a | Acer pseudoplatanus | – | MF795767 | MF795767 | MF795809 | MF795855 | MF795895 |
| Neocucurbitaria acerina | CBS 142403 = C255 | Acer pseudoplatanus | – | MF795768 | MF795768 | MF795810 | MF795856 | MF795896 |
| Neocucurbitaria aetnensis | CBS 142404 = C261 | Genista aetnensis | Holotype | MF795769 | MF795769 | MF795811 | MF795857 | MF795897 |
| Neocucurbitaria aetnensis | C270 | Genista aetnensis | – | MF795770 | MF795770 | MF795812 | MF795858 | MF795898 |
| Neocucurbitaria aquatica | CBS 297.74 | Sea water | Holotype | LT623221 | EU754177 | LT623278 | – | LT623238 |
| Neocucurbitaria cava | CBS 115979 | – | – | AY853248 | EU754198 | LT623273 | – | LT623234 |
| Neocucurbitaria cava | CBS 257.68 | Wheat-field soil | Epitype | JF740260 | EU754199 | LT717681 | – | KT389844 |
| Neocucurbitaria cinereae | CBS 142406 = KU9 | Genista cinerea | Holotype | MF795771 | MF795771 | MF795813 | MF795859 | MF795899 |
| Neocucurbitaria cisticola | CBS 142402 = C244 | Cistus monspeliensis | Holotype | MF795772 | MF795772 | MF795814 | MF795860 | MF795900 |
| Neocucurbitaria hakeae | CBS 142109 = CPC 28920 | Hakeasp. | Holotype | KY173436 | KY173526 | KY173593 | – | KY173613 |
| Neocucurbitaria irregularis | CBS 142791 | Subcutaneous tissue from injured human arm | Holotype | LT592916 | LN907372 | LT593054 | – | LT592985 |
| Neocucurbitaria juglandicola | C316 | Quercus rubra | – | MK356301 | MK356301 | MK357529 | MK357573 | MK357614 |
| Neocucurbitaria juglandicola | CBS 142390 = BW6 | Juglans regia | Holotype | MF795773 | MF795773 | MF795815 | MF795861 | MF795901 |
| Neocucurbitaria keratinophila | CBS 121759 | From human corneal scrapings (keratitis) | Holotype | EU885415 | LT623215 | LT623275 | – | LT623236 |
| Neocucurbitaria populi | CBS 142393 = C28 | Populussp. | Holotype | MF795774 | MF795774 | MF795816 | MF795862 | MF795902 |
| Neocucurbitaria prunicola | CBS 145033 | Prunus padus | – | MK442594 | MK442534 | MK442668 | – | MK442737 |
| Neocucurbitaria quercina | CBS 115095 | Quercus robur | Neotype | LT623220 | GQ387619 | LT623277 | – | LT623237 |
| Neocucurbitaria rhamni | CBS 142391 = C1 | Rhamnus frangula | Epitype | MF795775 | MF795775 | MF795817 | MF795863 | – |
| Neocucurbitaria rhamni | C112 | Rhamnus frangula | – | MF795776 | MF795776 | MF795818 | MF795864 | MF795903 |
| Neocucurbitaria rhamni | C133 | Rhamnus frangula | – | MF795777 | MF795777 | MF795819 | MF795865 | MF795904 |
| Neocucurbitaria rhamni | C190 | Rhamnus frangula | – | MF795778 | MF795778 | MF795820 | MF795866 | – |
| Neocucurbitaria rhamni | C277 | Rhamnus saxatilis | – | MF795779 | MF795779 | MF795821 | MF795867 | MF795905 |
| Neocucurbitaria rhamnicola | CBS 142396 = C185 | Rhamnus lycioides | Holotype | MF795780 | MF795780 | MF795822 | MF795868 | MF795906 |
| Neocucurbitaria rhamnicola | KRx | Rhamnus alaternus | – | MF795781 | MF795781 | MF795823 | MF795869 | MF795907 |
| Neocucurbitaria rhamnioides | C222 | Rhamnus saxatilis subsp. prunifolius | – | MF795783 | MF795783 | MF795825 | MF795871 | MF795909 |
| Neocucurbitaria rhamnioides | C223 | Rhamnus saxatilis subsp. prunifolius | – | MF795784 | MF795784 | MF795826 | MF795872 | MF795910 |
| Neocucurbitaria rhamnioides | CBS 142395 = C118 | Rhamnus myrtifolius | Holotype | MF795782 | MF795782 | MF795824 | MF795870 | MF795908 |
| Neocucurbitaria ribicola | CBS 142394 = C55 | Ribes rubrum | Holotype | MF795785 | MF795785 | MF795827 | MF795873 | MF795911 |
| Neocucurbitaria ribicola | C155 | Ribes rubrum | – | MF795786 | MF795786 | MF795828 | MF795874 | MF795912 |
| Neocucurbitaria unguis-hominis | CBS 111112 | Agapornis sp. | – | LT623222 | GQ387623 | LT623279 | – | LT623239 |
| Neocucurbitaria vachelliae | CBS 142397 = C192 | Vachellia gummifera | Holotype | MF795787 | MF795787 | MF795829 | MF795875 | MF795913 |
| Paracucurbitaria italica | CBS 234.92 | Olea europaea | Holotype | LT623219 | EU754176 | LT623274 | – | LT623235 |
| Paracucurbitaria riggenbachii | CBS 248.79 | Fraxinus excelsiorwith bacterial canker | Holotype | LT903672 | GQ387608 | LT903673 | – | LT900365 |
| Parafenestella alpina | CBS 145263 = C198 | Cotoneaster integerrimus | Holotype | MK356302 | MK356302 | MK357530 | MK357574 | MK357615 |
| Parafenestella alpina | C249 | Salix appendiculata | – | MK356303 | MK356303 | MK357531 | MK357575 | MK357616 |
| Parafenestella austriaca | CBS 145262 = C152 | Rosa canina | Holotype | MK356304 | MK356304 | MK357532 | MK357576 | MK357617 |
| Parafenestella changchunensis | HMJAU 60182 | PopulusL. | Holotype | OL996119 | OL897170 | – | OL944600 | OL898719 |
| Parafenestella faberi | MFLUCC 16-1451 | Rosa canina | Holotype | KY563071 | KY563074 | – | – | – |
| Parafenestella germanica | CBS 145267 = C307 | Corylus avellana | Holotype | MK356305 | MK356305 | MK357533 | MK357577 | MK357618 |
| Parafenestella ostryae | MFLU 16-0184 | Ostrya carpinifolia | – | KY563072 | KY563075 | – | – | – |
| Parafenestella pittospori | CPC 34462 | Pittosporum tenuifolium | Holotype | MN562098 | MN567606 | – | – | – |
| Parafenestella pseudoplatani | CBS 142392 = C26 | Acer pseudoplatanus | Holotype | MF795788 | MF795788 | MF795830 | MF795876 | MF795914 |
| Parafenestella pseudosalicis | CBS 145264 = C301 | Salixcf. alba | Holotype | MK356307 | MK356307 | MK357535 | MK357579 | MK357620 |
| Parafenestella rosacearum | CBS 145268 = C309 | Pyracantha coccinea | Holotype | MK356311 | MK356311 | MK357539 | MK357583 | MK357624 |
| Parafenestella rosacearum | C203 | Pyrus communis | – | MK356308 | MK356308 | MK357536 | MK357580 | MK357621 |
| Parafenestella rosacearum | C269 | Crataegus monogyna | – | MK356309 | MK356309 | MK357537 | MK357581 | MK357622 |
| Parafenestella rosacearum | C283 | Pyrus communis | – | MK356310 | MK356310 | MK357538 | MK357582 | MK357623 |
| Parafenestella rosacearum | C315 | Rosa canina | – | MK356312 | MK356312 | MK357540 | MK357584 | MK357625 |
| Parafenestella rosacearum | C320 | Sorbus aria | – | MK356315 | MK356315 | MK357543 | MK357587 | – |
| Parafenestella rosacearum | CBS 145272 = FP11 | Prunus domestica | – | MK356314 | MK356314 | MK357542 | MK357586 | MK357627 |
| Parafenestella rosacearum | FM1 | Rosa canina | – | MK356313 | MK356313 | MK357541 | MK357585 | MK357626 |
| Parafenestella salicis | CBS 145270 = C313 | Salix alba | Neotype | MK356317 | MK356317 | MK357545 | MK357589 | MK357629 |
| Parafenestella salicis | C303 | Salix alba | – | MK356316 | MK356316 | MK357544 | MK357588 | MK357628 |
| Parafenestella salicum | CBS 145269 = C311 | Salix alba | Holotype | MK356318 | MK356318 | MK357546 | MK357590 | MK357630 |
| Parafenestella tetratrupha | CBS 145266 = C304 | Alnus glutinosa | Epitype | MK356319 | MK356319 | MK357547 | MK357591 | MK357631 |
| Parafenestella ulmi | HMJAU 60178 | Ulmus pumilaL. | Holotype | OL996115 | OL897166 | OL944501 | OL944596 | OL898723 |
| Parafenestella ulmi | HMJAU 60179 | Ulmus pumilaL. | Isotype | OL996116 | OL897167 | OL944502 | OL944597 | OL898717 |
| Parafenestella ulmicola | HMJAU 60180 | Ulmus pumilaL. | Holotype | OL996117 | OL897168 | OL944503 | OL944598 | OL898724 |
| Parafenestella ulmicola | HMJAU 60181 | Ulmus pumilaL. | Isotype | OL996118 | OL897169 | OL944504 | OL944599 | OL898718 |
| Parafenestella vindobonensis | CBS 145265 = C302 | Salix babylonica | Holotype | MK356320 | MK356320 | MK357548 | MK357592 | MK357632 |
| Protofenestella ulmi | CBS 143000 = FP5 | Ulmus minor | Holotype | MF795791 | MF795791 | MF795833 | MF795879 | MF795915 |
| Pyrenochaeta nobilis | CBS 407.76 = AFTOL-ID 1856 | Laurus nobilis leaves | Neotype | MF795792 | MF795792 | MF795834 | MF795880 | MF795916 |
| Pyrenochaetopsis americana | UTHSC DI16-225 | – | Holotype | LT592912 | LN907368 | LT593050 | – | LT592981 |
| Pyrenochaetopsis confluens | CBS 142459 | Deep tissue/ fluids from human blood sample | Holotype | LT592950 | LN907446 | LT593089 | – | LT593019 |
| Seltsamia galinsogisoli | CBS 140956 = CGMCC 3.17981 =SYPF 7336 | Soil of a Galinsoga parviflora | Epitype | KU759584 | KU759581 | – | – | – |
| Seltsamia sp. | EAB-67-11b | Emerald ash borer | – | MT777389 | – | – | – | – |
| Seltsamia sp. | SGSF207 | – | – | MK192899 | – | – | – | – |
| Seltsamia ulmi | CBS 143002 = L150 | Ulmus glabra | Holotype | MF795794 | MF795794 | MF795836 | MF795882 | MF795918 |
| Synfenestella pyri | CBS 144855 = C297 | Pyrus communis | Holotype | MK356321 | MK356321 | MK357549 | MK357593 | MK357633 |
| Synfenestella sorbi | C298 | Sorbus aucuparia | – | MK356325 | MK356325 | MK357553 | MK357597 | MK357636 |
| Synfenestella sorbi | CBS 144858 = C196 | Sorbus aucuparia | Holotype | MK356324 | MK356324 | MK357552 | MK357596 | MK357635 |
| Synfenestella sorbi | CBS 144862 = FR | Sorbus aucuparia | Epitype | MK356322 | MK356322 | MK357550 | MK357594 | MK357634 |
| Synfenestella sorbi | FRa | Sorbus aucuparia | – | MK356323 | MK356323 | MK357551 | MK357595 | – |
| Taxon | Sexual Morph | ||
|---|---|---|---|
| Ascomata | Asci | Ascospores | |
| P. alpina | 240–375 μm diam, globose, subglobose or pyriform, usually tightly aggregated in bark on a perithecial host fungus in small numbers, with brown to black, subicular hyphae. | 170–208 × 18.5–21.5 μm, cylindrical to oblong, a short stipe and simple or knob-like base, containing 6–8 ascospores in uniseriate arrangement. | 24–30.5 × 12–14 μm, typically ellipsoid to fusoid often inequilateral, pale or yellowish-brown, eventually dark brown, with 7–15 transverse and 2–4 longitudinal septa. |
| P. austriaca | 283–431 μm diam, subglobose to pyriform, scattered or aggregated, basally and laterally surrounded by subhyaline to dark brown subicular hyphae. | 159–205 × 16–19.5 μm, cylindrical, with a short stipe and simple or knob-like base, containing 4–8 ascospores in uniseriate arrangement. | 27–32.5 × 13–15 µm, broadly ellipsoid, symmetric, dark brown or dark reddish-brown, with 9–14 distantly spaced transverse and 3–5 longitudinal septa. |
| P. changchunensis | 280 × 353 μm, globose to depressed globose, solitary or aggregated forming visible black bumps submerged under bark. | 95–138 × 16–21 μm, broad cylindrical, short-pedicellate, curved, some curved, 6–8 spores ocular chamber is not visible at maturity, uniseriate arrangement. | 18–25 × 8–13 μm, fusiform to oval, light yellow to dark brown, developing 2 main septa, 4–6 transverse septa, 2–3 longitudinal septa. |
| P. faberi | 300–500 μm diam, tightly or loosely aggregated in small numbers, with ostiolar, partly erumpent through bark fissures, maxing with Cytospora species. | 135–180 × 18.5–23.5 μm, cylindrical to oblong or narrowly clavate, a short stipe and simple or knob-like base, 4–8 ascospores in uniseriate to partly biseriate arrangement. | 28.5–36 × 12.5–16 µm, variable in shape, pale or yellowish-brown to dark brown, with 1–4 main septa, 7–14 transverse and 1–5 longitudinal septa. |
| P. germanica | 230–450 μm diam, black, solitary or in small groups on inner bark or on the ostiolar level of old Diaporthe decedens. | 140–173 × 17.5–22 μm, cylindrical to oblong, with a short stipe and simple or knob-like base, containing 2–8 ascospores (obliquely or overlapping), uniseriate arrangement. | 29–39.5 × 13–16.5 μm, ellipsoid to broadly fusoid, turning yellow to yellow-brown to dark brown, with 1–3 main septa, 8–15 transverse and 3–6 longitudinal septa. |
| P. parasalicum | 270–400 μm diam, immersed in bark, globose, subglobose or pyriform, forming groups, maxing with Cytospora species. | 185–219 × 22–27 μm, cylindrical to oblong, with a short stipe and simple or knob-like base, containing 4–8 ascospores (overlapping, obliquely), uniseriate to partly biseriate arrangement. | 36–44 × 15.8–19.3 μm, fusoid or ellipsoid, yellow-brown to dark brown, with 2 main septa, 11–16 distinct transverse septa and 3–5 longitudinal septa. |
| P. pseudosalicis | 300–400 diam, subglobose to subpyriform, immersed in bark or on ascomata of an effete perithecial fungus, often with concave apex, covered with subicular hyphae. | 186–215 × 17.5–19 μm, cylindrical to oblong, with a short stipe and simple or knob-like base, containing 4–8 ascospores in uniseriate arrangement. | 25–29 × 12–14 μm, ellipsoid, yellow-brown to dark brown, with 1–3 main septa, 7–11 transverse and 2–4 longitudinal septa, with minute guttules. |
| P. rosacearum | 285–432 μm diam, globose, subglobose to subpyriform, immersed on often blackened inner bark, scattered or in small groups, erumpent through bark fissures. | 181–240 × 19–22 μm, cylindrical to oblong, with a short-contorted stipe and simple or knob-like base, containing 2–8 ascospores in uniseriate, rarely partly biseriate arrangement. | 28–35 × 13.5–16.5 μm, ellipsoid, symmetric to inequilateral, yellow-brown to dark brown, with 1–3 main septa, 7–15 transverse and 2–5 longitudinal septa. |
| P. salicis | 275–442 μm diam, globose, subglobose to pyriform or subconical, immersed below the epidermis on inner bark, partly erumpent through bark fissures. | 141–188 × 16–19 μm, cylindrical to oblong, with a short stipe and simple or knob-like base, containing 1–8 ascospores in (obliquely) uniseriate to partly biseriate arrangement. | 23–29 × 11–13.5 μm, ellipsoid to fusoid, symmetric, golden yellow-brown (when fresh) to dark brown, with 1–3 main septa, 5–11 transverse and 1–3 longitudinal septa. |
| P. salicum | 270–420 diam, globose, subglobose or pyriform, immersed in bark, the inner bark layers connected to the host, scattered or aggregate, cover with subicular hyphae. | 181–228 × 19.5–24 μm, cylindrical, with a short stipe and simple or knob-like base, containing 6–8 ascospores in (overlapping) uniseriate arrangement. | 27–33 × 12.5–16 μm, broadly ellipsoid to broadly fusoid, first 2-celled and hyaline, turning golden yellow to dark brown or dark reddish-brown, with 9 –14 transverse and 3–4 longitudinal septa. |
| P. tetratrupha | 300–500 μm diam, globose, subglobose or pyriform, immersed, tightly or loosely aggregated in whitish to dark brown subiculum, erumpent through fissures. | 154–229 × 18.5–22.2 μm, cylindrical to oblong, with a short stipe and simple or knob-like base, containing 2–8 ascospores in uniseriate arrangement. | 26.5–33.5 × 13–16.5 μm, ellipsoid, yellow-brown to reddish-brown to dark brown, with 1–3 main septa, 8–17 distinct transverse and 2–4 longitudinal septa. |
| P. ulmi | 170–225 × 194–260 μm, globose to ellipsoid, immersed under the host epidermis, visible as black spots or having a convex surface. | 115–181 × 11–15 μm, cylindrical, mostly curved, short-pedicellate, containing 8 ascospores, uniseriate to partially overlapping. | 18–24 × 8–12 μm, broadly ellipsoid, yellowish to brown, with 5–8 transversely septate, 1–2 longitudinal septa. |
| P. ulmicola | 242–434 × 310–462 μm, globose to subglobose, on the surface, semi-immersed, visible as a convex hemisphere, with a papilla. | 105–153 × 11–14 μm, broad cylindrical, some curved, short-pedicellate, containing 8 ascospores, short-pedicellate, uniseriate, rarely overlapping. | 17–22 × 8–12 μm, broadly oval, yellowish to brown, with 4–8 transversely septate and 1–3 vertical septate. |
| P. vindobonensis | 308–425 μm diam, globose, subglobose or pyriform, immersed in bark, partially erumpent, tightly aggregated in small groups on inner bark mixing with pseudostromata of a Cytospora sp. | 179–214 × 13.5–15.5 μm, cylindrical, with a short stipe and simple or knob-like base, containing 4–8 ascospores in uniseriate arrangement. | 24.5–30.5 × 9.5–11 μm, oblong, fusoid or narrowly ellipsoid, turning yellowish to medium brown, 1–6 main septa, when mature with 7–11 thick transverse and 1–3 septa, containing minute droplets. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.; Xu, R.; Bhunjun, C.S.; Tian, S.; Dai, Y.; Li, Y.; Phukhamsakda, C. Diversity of Ascomycota in Jilin: Introducing Novel Woody Litter Taxa in Cucurbitariaceae. J. Fungi 2022, 8, 905. https://doi.org/10.3390/jof8090905
Su W, Xu R, Bhunjun CS, Tian S, Dai Y, Li Y, Phukhamsakda C. Diversity of Ascomycota in Jilin: Introducing Novel Woody Litter Taxa in Cucurbitariaceae. Journal of Fungi. 2022; 8(9):905. https://doi.org/10.3390/jof8090905
Chicago/Turabian StyleSu, Wenxin, Rong Xu, Chitrabhanu S. Bhunjun, Shangqing Tian, Yueting Dai, Yu Li, and Chayanard Phukhamsakda. 2022. "Diversity of Ascomycota in Jilin: Introducing Novel Woody Litter Taxa in Cucurbitariaceae" Journal of Fungi 8, no. 9: 905. https://doi.org/10.3390/jof8090905
APA StyleSu, W., Xu, R., Bhunjun, C. S., Tian, S., Dai, Y., Li, Y., & Phukhamsakda, C. (2022). Diversity of Ascomycota in Jilin: Introducing Novel Woody Litter Taxa in Cucurbitariaceae. Journal of Fungi, 8(9), 905. https://doi.org/10.3390/jof8090905








