Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection, Examination, Isolation, and Conservation
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Sequence Alignment and Phylogenetic Analyses
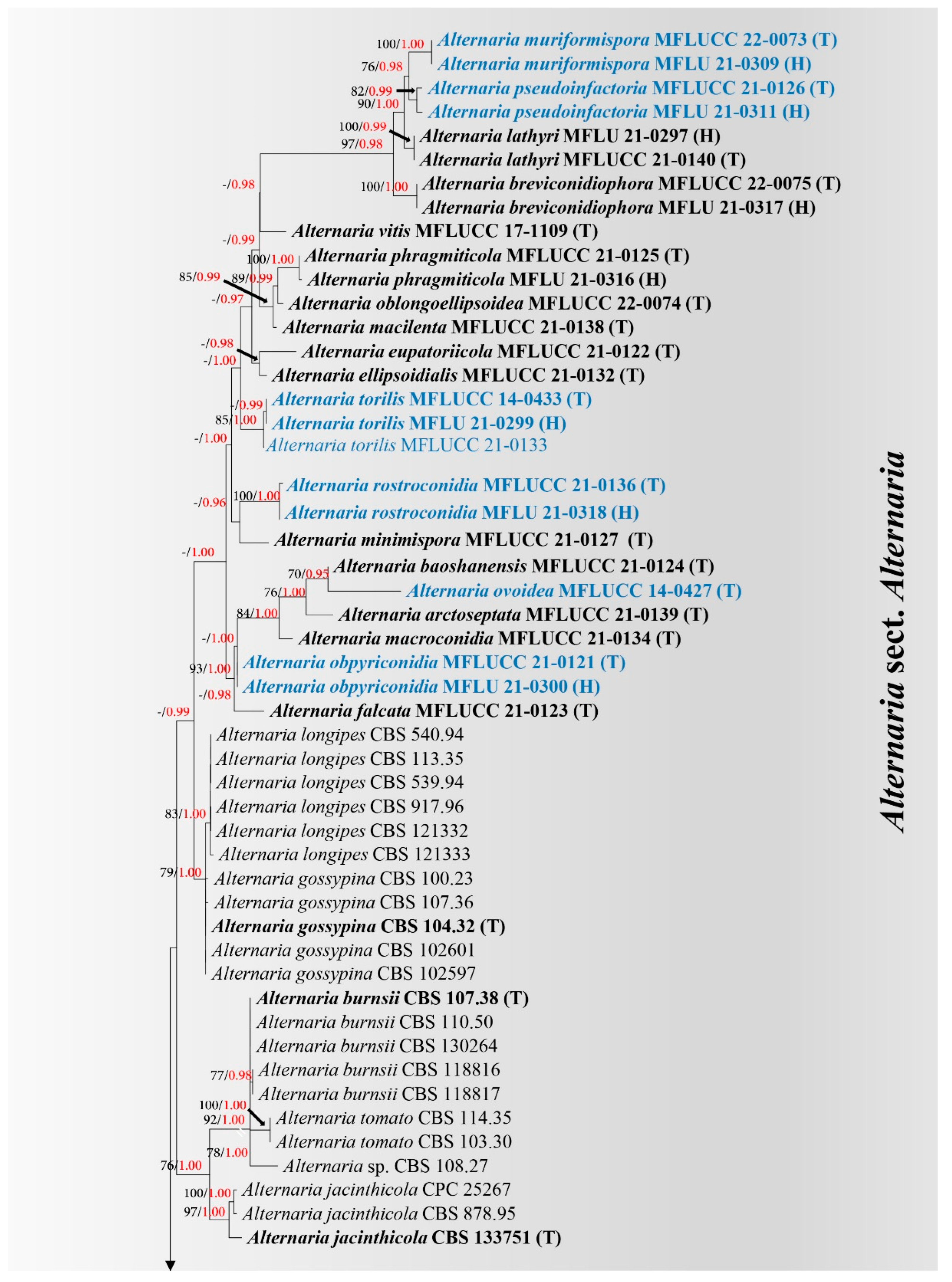
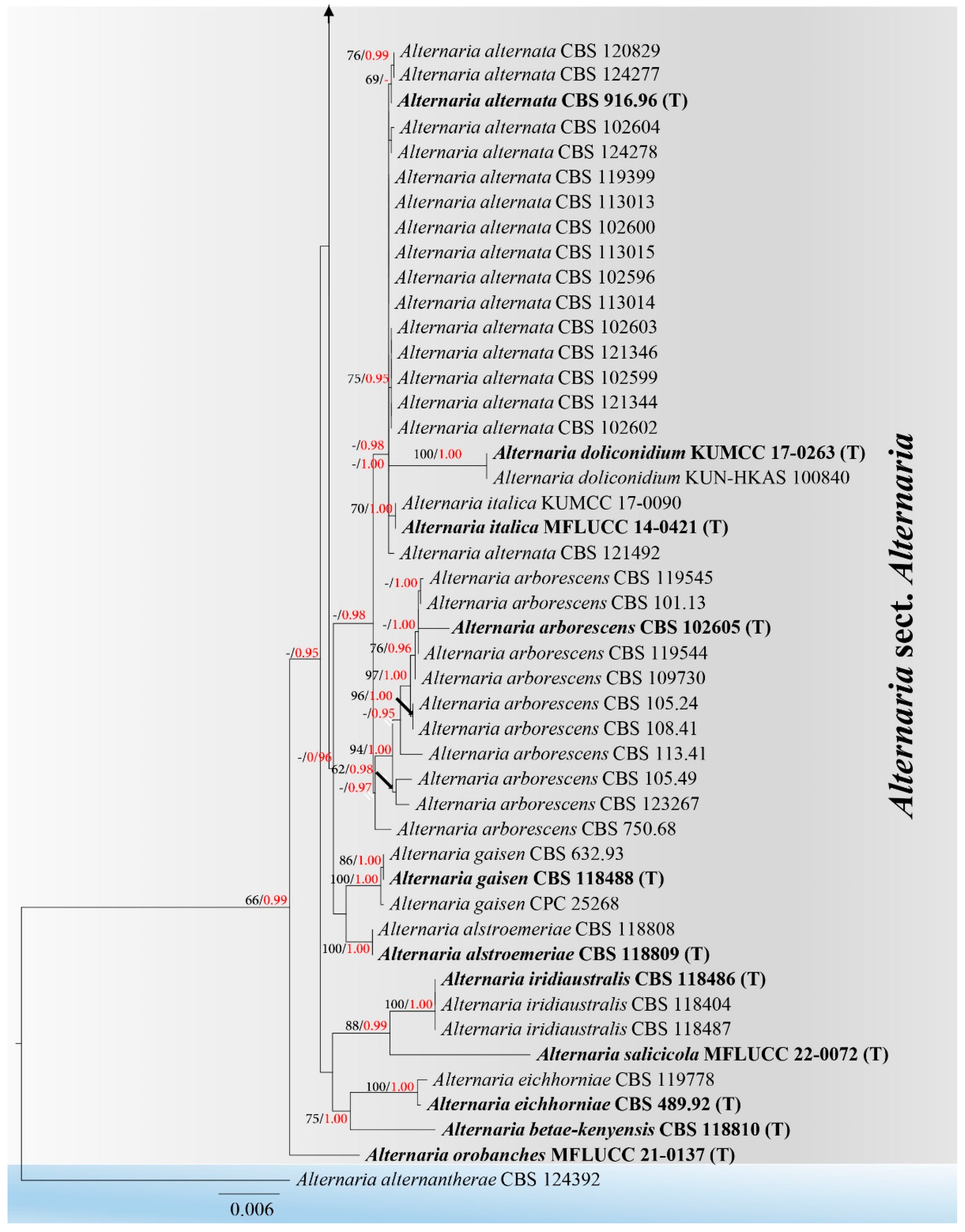
3. Results
3.1. Phylogeny
3.2. Taxonomy
- Index Fungorum number: IF 559795
- Etymology: Named after its muriform conidia.
- Holotype: MFLU 21-0309
- Index Fungorum number: IF 559797
- Etymology: Named after its obpyriform conidia.
- Holotype: MFLU 21-0300
- Index Fungorum number: IF 559798
- Etymology: Referring to its ovoid (droplets-like) conidia.
- Holotype: MFLU 21-0298
- Index Fungorum number: IF 559799
- Etymology: Referring to the conidial structures resemble Alternaria section infectoriae.
- Holotype: MFLU 21-0311
- Index Fungorum number: IF 559800
- Etymology: Referring to the rostrate conidia.
- Holotype: MFLU 21-0318
- Index Fungorum number: IF 559801
- Etymology: Named after the host genus “Torilis”.
- Holotype: MFLU 21-0299
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hongsanan, S.; Hyde, K.D.; Phookamsak, R.; Wanasinghe, D.N.; McKenzie, E.H.C.; Sarma, V.V.; Boonmee, S.; Lücking, R.; Bhat, J.D.; Liu, N.; et al. Refined families of Dothideomycetes: Dothideomycetidae and Pleosporomycetidae. Mycosphere 2020, 11, 1553–2107. [Google Scholar] [CrossRef]
- Li, J.-F.; Jiang, H.-B.; Jeewon, R.; Hongsanan, S.; Bhat, D.J.; Tang, S.-M.; Lumyong, S.; Mortimer, P.E.; Xu, J.-C.; Camporesi, E.; et al. Alternaria: Update on species limits, evolution, multi-locus phylogeny, and classification. Stud. Fungi, 2022; under review. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa–2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Species Fungorum. Available online: http://www.speciesfungorum.org/Names/Names.asp (accessed on 4 July 2022).
- Ahmadpour, A.; Ghosta, Y.; Poursafar, A. Novel species of Alternaria section Nimbya from Iran as revealed by morphological and molecular data. Mycologia 2021, 113, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Cheng, H.; Htun, A.A.; Ge, H.; Xia, Z.Z.; Guo, J.W.; Deng, J.X.; Du, T. Phylogeny and taxonomy of two new Alternaria (Ascomycota: Pleosporaceae) species in section Gypsophilae from China. Mycol. Prog. 2021, 20, 355–363. [Google Scholar] [CrossRef]
- He, L.; Cheng, H.; Zhao, L.; Htun, A.A.; Yu, Z.H.; Deng, J.-X.; Qi, L.-L. Morphological and molecular identification of two new Alternaria species (Ascomycota, Pleosporaceae) in section Radicina from China. MycoKeys 2021, 78, 187–198. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [Green Version]
- Woudenberg, J.H.C.; Truter, M.; Groenewald, J.Z.; Crous, P.W. Large-spored Alternaria pathogens in section Porri disentangled. Stud. Mycol. 2014, 79, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.P.; Rotondo, F.; Gannibal, P.B. Biodiversity and taxonomy of the pleomorphic genus Alternaria. Mycol. Prog. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Hyde, K.D.; Jeewon, R.; Ghobad-Nejhad, M.; Wanasinghe, D.N.; Liu, N.; Phillips, A.J.L.; Oliveira-Filho, J.R.C.; da Silva, G.A.; Gibertoni, T.B.; et al. One stop shop II: Taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50. Fungal Divers. 2019, 94, 41–129. [Google Scholar] [CrossRef]
- Thomma, B.P. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Farr, D.F.; Rossman, A.Y. Fungal databases, US National Fungal Collections, ARS USDA. Available online: http://nt.ars-grin.gov/fungaldatabases (accessed on 21 May 2022).
- Lawrence, D.P.; Gannibal, P.B.; Peever, T.L.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannibal, P.B. Distribution of Alternaria species among sections. 2. Section Alternaria. Mycotaxon 2016, 130, 941–949. [Google Scholar] [CrossRef]
- Ariyawansa, H.; Thambugala, K.M.; Manamgoda, D.S.; Jayawardena, R.; Camporesi, E.; Boonmee, S.; Wanasinghe, D.; Phookamsak, R.; Hongsanan, S.; Singtripop, C.; et al. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers. 2015, 71, 85–139. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Biodiversity Series Volume: 6; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2007; 780p. [Google Scholar]
- Gannibal, P.B.; Lawrence, D.P. Distribution of Alternaria species among sections. 5. Species producing conidia with many longitudinal septa. Mycotaxon 2018, 133, 285–291. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Phukhamsakda, C.; Hyde, K.D.; Jeewon, R.; Lee, H.B.; Jones, E.B.G.; Tibpromma, S.; Tennakoon, D.S.; Dissanayake, A.J.; Jayasiri, S.C.; et al. Fungal diversity notes 709–839: Taxonomic and phylogenetic contributions to fungal taxa with an emphasis on fungi on Rosaceae. Fungal Divers. 2018, 89, 1–236. [Google Scholar] [CrossRef]
- Jayawardena, R.S.; Purahong, W.; Zhang, W.; Wubet, T.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J.; Yan, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 2018, 90, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, J.; Nakashima, C. Japanese species of Alternaria and their species boundaries based on host range. Fungal Syst. Evol. 2020, 5, 197–281. [Google Scholar] [CrossRef]
- Pryor, B.M.; Gilbertson, R.L. Molecular phylogenetic relationships amongst Alternaria species and related fungi based upon analysis of nuclear ITS and mt SSU rDNA sequences. Mycol. Res. 2000, 104, 1312–1321. [Google Scholar] [CrossRef] [Green Version]
- Peever, T.L.; Su, G.; Carpenter-Boggs, L.; Timmer, L.W. Molecular systematics of citrus-associated Alternaria species. Mycologia 2004, 96, 119–134. [Google Scholar] [CrossRef]
- Hong, S.G.; Cramer, R.A.; Lawrence, C.B.; Pryor, B.M. Alt a 1 allergen homologs from Alternaria and related taxa: Analysis of phylogenetic content and secondary structure. Fungal Genet. Biol. 2005, 42, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Peever, T.L.; Pryor, B.M. An expanded multi-locus phylogeny does not resolve species among the small-spored Alternaria species complex. Mycologia 2009, 101, 95–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, A.D.; Barbara, D.J.; Harrison, R.J.; Lane, C.R.; Sreenivasaprasad, S.; Woodhall, J.W.; Clarkson, J.P. Discrete lineages within Alternaria alternata species group: Identification using new highly variable loci and support from morphological characters. Fungal Biol. 2015, 119, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Armitage, A.D.; Cockerton, H.M.; Sreenivasaprasad, S.; Woodhall, J.; Lane, C.R.; Harrison, R.J.; Clarkson, J.P. Genomics evolutionary history and diagnostics of the Alternaria alternata species group including apple and Asian pear pathotypes. Front. Microbiol. 2020, 10, 3124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafri, A.A.; Maharachchikumbura, S.S.N.; Hyde, K.D.; Al-Saady, N.A.; Al-Sadi, A.M. A new section and a new species of Alternaria from Oman. Phytotaxa 2019, 405, 279–289. [Google Scholar] [CrossRef]
- Runa, F.; Park, M.S.; Pryor, B.M. Ulocladium systematics revisited: Phylogeny and taxonomic status. Mycol. Prog. 2009, 8, 35–47. [Google Scholar] [CrossRef]
- Senanayake, I.C.; Rathnayaka, A.R.; Marasinghe, D.S.; Calabon, M.S.; Gentekaki, E.; Lee, H.B.; Hurdeal, V.G.; Pem, D.; Dis-sanayake, L.S.; Wijesinghe, S.N.; et al. Morphological approaches in studying fungi: Collection, examination, isolation, sporulation and preservation. Mycosphere 2020, 11, 2678–2754. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Berbee, M.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl. Acids. Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. Mafft online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, D.; Michalak, I. raxmlGUI: A graphical front-end for RAxML. Org. Divers. Evol. 2012, 12, 335–337. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest 2.0. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef]
- Zhaxybayeva, O.; Gogarten, J.P. Bootstrap, Bayesian probability and maximum likelihood mapping: Exploring new tools for comparative genome analyses. BMC Genom. 2002, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A. FigTree. Tree Figure Drawing Tool Version 1.4.0; Institute of Evolutionary Biology, University of Edinburgh: Scotland, UK, 2012; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 25 May 2022).
- Pryor, B.; Michailides, T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria late blight of pistachio. Phytopathology 2002, 92, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Jeewon, R.; Hyde, K.D. Establishing species boundaries and new taxa among fungi: Recommendations to resolve taxonomic ambiguities. Mycosphere 2016, 7, 1669–1677. [Google Scholar] [CrossRef]






| Species Name | Strains/Voucher No. | GenBank Accession Numbers | ||||||
|---|---|---|---|---|---|---|---|---|
| SSU | LSU | rpb2 | ITS | gapdh | tef1-α | Alt-a1 | ||
| Alternaria alstroemeriae | CBS 118808 | KP124917 | KP124447 | KP124764 | KP124296 | KP124153 | KP125071 | KP123845 |
| Alternaria alstroemeriae | CBS 118809 T | NG063029 | NG069882 | KP124765 | NR163686 | KP124154 | KP125072 | MH084526 |
| Alternaria alternantherae | CBS 124392 | KC584506 | KC584251 | KC584374 | KC584179 | KC584096 | KC584633 | KP123846 |
| Alternaria alternata | CBS 102596 | KP124950 | MH874392 | KP124796 | MH862796 | KP124183 | KP125104 | KP123877 |
| Alternaria alternata | CBS 102599 | KP124952 | MH874395 | KP124798 | MH862799 | KP124185 | KP125106 | KP123879 |
| Alternaria alternata | CBS 102600 | KP124953 | MH874396 | KP124799 | MH862800 | KP124186 | KP125107 | KP123880 |
| Alternaria alternata | CBS 102602 | KP124954 | MH877754 | KP124800 | KP124332 | KP124187 | KP125108 | KP123881 |
| Alternaria alternata | CBS 102603 | KP124955 | KP124485 | KP124801 | KP124333 | KP124188 | KP125109 | KP123882 |
| Alternaria alternata | CBS 102604 | KP124956 | MH874399 | KP124802 | MH862803 | - | KP125110 | - |
| Alternaria alternata | CBS 113013 | KP124963 | KP124493 | KP124809 | KP124341 | KP124195 | KP125117 | KP123889 |
| Alternaria alternata | CBS 113014 | KP124964 | KP124494 | KP124810 | KP124342 | KP124196 | KP125118 | KP123890 |
| Alternaria alternata | CBS 113015 | KP124965 | KP124495 | KP124811 | KP124343 | KP124197 | KP125119 | KP123891 |
| Alternaria alternata | CBS 119399 | KP124983 | KP124513 | KP124829 | KP124361 | - | KP125137 | KP123910 |
| Alternaria alternata | CBS 120829 | KP124986 | KP124516 | KP124832 | KP124364 | KP124216 | KP125140 | KP123912 |
| Alternaria alternata | CBS 121344 | KP124988 | KP124518 | KP124834 | KP124365 | KP124217 | KP125142 | KP123913 |
| Alternaria alternata | CBS 121346 | KP124989 | KP124519 | KP124835 | KP124366 | KP124218 | KP125143 | KP123914 |
| Alternaria alternata | CBS 121492 | KP124994 | KP124524 | KP124840 | KP124370 | KP124222 | KP125148 | KP123918 |
| Alternaria alternata | CBS 124277 | KP124997 | KP124527 | KP124843 | KP124373 | KP124225 | KP125151 | KP123921 |
| Alternaria alternata | CBS 124278 | KP124998 | KP124528 | KP124844 | KP124374 | KP124226 | KP125152 | KP123922 |
| Alternaria alternata | CBS 916.96 T | KC584507 | DQ678082 | KC584375 | AF347031 | AY278808 | KC584634 | - |
| Alternaria arborescens | CBS 101.13 | KP125016 | KP124546 | KP124862 | KP124392 | KP125170 | KP124244 | KP123940 |
| Alternaria arborescens | CBS 105.24 | KP125017 | KP12454 | KP124863 | KP124393 | KP125171 | KP124245 | KP123941 |
| Alternaria arborescens | CBS 105.49 | KP125020 | KP124550 | KP124866 | KP124396 | KP125174 | KP124248 | KP123944 |
| Alternaria arborescens | CBS 108.41 | KP125018 | KP124548 | KP124864 | KP124394 | KP125172 | KP124246 | KP123942 |
| Alternaria arborescens | CBS 113.41 | KP125019 | KP124549 | KP124865 | KP124395 | KP125173 | KP124247 | KP123943 |
| Alternaria arborescens | CBS 750.68 | KP125021 | KP124551 | KP124868 | KP124398 | KP125176 | KP124250 | KP123945 |
| Alternaria arborescens | CBS 102605 T | KC584509 | KC584253 | KC584377 | AF347033 | AY278810 | KC584636 | AY563303 |
| Alternaria arborescens | CBS 109730 | KP125022 | KP124552 | KP124869 | KP124399 | KP125177 | KP124251 | KP123946 |
| Alternaria arborescens | CBS 119544 | NG063030 | NG_069254 | KP124878 | MH863062 | KP125186 | JQ646321 | KP123955 |
| Alternaria arborescens | CBS 119545 | KP125032 | KP124562 | KY392798 | KP124409 | KP125187 | KP124260 | KP123956 |
| Alternaria arborescens | CBS 123267 | KP125035 | KP124565 | KP124882 | KP124412 | KP125190 | KP124263 | KP123959 |
| Alternaria arctoseptata | MFLUCC 21-0139 T | MZ621874 | MZ621948 | OK236655 | - | 0K236608 | OK236702 | OK236755 |
| Alternaria betae-kenyensis | CBS 118810 T | NG_063032 | NG_069256 | JQ905180 | NR136118 | JQ905161 | KP125197 | JQ905104 |
| Alternaria baoshanensis | MFLUCC 21-0124 T | MZ621878 | MZ621952 | OK236659 | MZ622003 | OK236613 | OK236706 | OK236760 |
| Alternaria breviconidiophora | MFLUCC 22-0075 T | MZ621870 | MZ621944 | OK236651 | MZ621997 | OK236604 | OK236698 | OK236751 |
| Alternaria breviconidiophora | MFLU 21-0317 | MZ621871 | MZ621945 | OK236652 | MZ621998 | OK236605 | OK236699 | OK236752 |
| Alternaria burnsii | CBS 107.38 T | NG063033 | N069257 | JQ646457 | NR136119 | JQ646305 | KP125198 | JQ646388 |
| Alternaria burnsii | CBS 110.50 | KP125044 | KP124574 | KP124890 | KP124421 | KP124271 | KP125199 | KP123968 |
| Alternaria burnsii | CBS 118816 | KP125046 | KP124576 | KP124892 | KP124423 | KP124273 | KP125201 | KP123970 |
| Alternaria burnsii | CBS 118817 | KP125047 | KP124577 | KP124893 | KP124424 | KP124274 | KP125202 | KP123971 |
| Alternaria burnsii | CBS 130264 | KP125048 | KP124578 | KP124894 | KP124425 | KP124275 | KP125203 | KP123972 |
| Alternaria doliconidium | KUN-HKAS 100840T | NG065142 | NG069551 | - | NR158361 | - | - | - |
| Alternaria doliconidium | KUMCC 17-0263 T | MG829094 | MG828980 | - | MG828864 | - | - | - |
| Alternaria eichhorniae | CBS 119778 | KP125050 | KP124580 | KP124896 | KP124426 | KP124277 | KP125205 | - |
| Alternaria eichhorniae | CBS 489.92 T | NG063034 | KP124579 | KP124895 | - | KP124276 | KP125204 | KP123973 |
| Alternaria ellipsoidialis | MFLUCC 21-0132 T | MZ621862 | MZ621936 | OK236643 | MZ621989 | OK236596 | OK236690 | OK236743 |
| Alternaria eupatoriicola | MFLUCC 21-0122 T | MZ621855 | MZ621929 | OK236636 | MZ621982 | OK236589 | OK236683 | OK236736 |
| Alternaria falcata | MFLUCC 21-0123 T | MZ621865 | MZ62139 | OK236649 | MZ621992 | OK236599 | OK236693 | OK236746 |
| Alternaria gaisen | CBS 118488 T | KP125051 | KP124581 | KP124897 | KP124427 | KP124278 | KP125206 | KP123975 |
| Alternaria gaisen | CBS 632.93 | KC584531 | KC584275 | KC584399 | KC584197 | KC584116 | KC584658 | KP123974 |
| Alternaria gaisen | CPC 25268 | KP125052 | KP124582 | KP124898 | KP124428 | KP124279 | KP125207 | KP123976 |
| Alternaria gossypina | CBS 100.23 | KP125053 | KP124583 | KP124899 | KP124429 | KP124280 | KP125208 | KP123977 |
| Alternaria gossypina | CBS 104.32 T | KP125054 | KP124584 | KP124900 | KP124430 | JQ646312 | KP125209 | JQ646395 |
| Alternaria gossypina | CBS 107.36 | KP125055 | KP124585 | KP124901 | KP124431 | - | KP125210 | - |
| Alternaria gossypina | CBS 102597 | KP125056 | MH874393 | KP124902 | MH862797 | KP124281 | KP125211 | KP123978 |
| Alternaria gossypina | CBS 102601 | KP125057 | MH874397 | KP124903 | MH862801 | KP124282 | KP125212 | KP123979 |
| Alternaria iridiaustralis | CBS 118486 T | NG_063035 | NG_069258 | KP124905 | NR_136120 | KP124284 | KP125214 | KP123981 |
| Alternaria iridiaustralis | CBS 118487 | KP125060 | KP124590 | KP124906 | KP124436 | KP124285 | KP125215 | KP123982 |
| Alternaria iridiaustralis | CBS 118404 | KP125058 | KP124588 | KP124904 | KP124434 | KP124283 | KP125213 | KP123980 |
| Alternaria italica | KUMCC 17-0090 | - | - | - | MG764018 | - | - | - |
| Alternaria italica | MFLUCC 14-0421 T | - | MG818319 | MG859737 | MG764017 | - | - | - |
| Alternaria jacinthicola | CBS 133751 T | KP125062 | KP124592 | KP124908 | KP124438 | KP124287 | KP125217 | KP123984 |
| Alternaria jacinthicola | CBS 878.95 | KP125061 | KP124591 | KP124907 | KP124437 | KP124286 | KP125216 | KP123983 |
| Alternaria jacinthicola | CPC 25267 | KP125063 | KP124593 | KP124909 | KP124439 | KP124288 | KP125218 | KP123985 |
| Alternaria lathyri | MFLUCC 21-0140 T | MZ621847 | MZ621921 | OK236628 | MZ621974 | OK236581 | OK236675 | OK236728 |
| Alternaria lathyri | MFLU 21-0297 | MZ621848 | MZ621922 | OK236629 | MZ621975 | OK236582 | OK236676 | OK236729 |
| Alternaria longipes | CBS 113.35 | KP125064 | KP124594 | KP124910 | KP124440 | KP124289 | KP125219 | KP123986 |
| Alternaria longipes | CBS 121332 | KP125067 | KP124597 | KP124913 | KP124443 | KP124292 | KP125222 | KP123989 |
| Alternaria longipes | CBS 121333 | KP125068 | KP124598 | KP124914 | KP124444 | KP124293 | KP125223 | KP123990 |
| Alternaria longipes | CBS 539.94 | KP125065 | KP124595 | KP124911 | KP124441 | KP124290 | KP125220 | KP123987 |
| Alternaria longipes | CBS 540.94 | KC584541 | KC584285 | KC584409 | - | - | KC584667 | - |
| Alternaria longipes | CBS 917.96 | KP125066 | KP124596 | KP124912 | KP124442 | KP124291 | KP125221 | KP123988 |
| Alternaria macilenta | MFLUCC 21-0138 T | MZ621845 | MZ621919 | OK236626 | MZ621972 | OK236579 | OK236673 | OK236726 |
| Alternaria macroconidia | MFLUCC 21-0134 T | MZ621876 | MZ621950 | OK236657 | MZ622001 | OK236610 | OK236704 | OK236757 |
| Alternaria minimispora | MFLUCC 21-0127 T | MZ621853 | MZ621927 | OK236634 | MZ621980 | OK236587 | OK236681 | OK236734 |
| Alternaria muriformispora | MFLUCC 22-0073 T | MZ621849 | MZ621923 | OK236630 | MZ621976 | OK236583 | OK236677 | OK236730 |
| Alternaria muriformispora | MFLU 21-0309 | MZ621850 | MZ621924 | OK236631 | MZ621977 | OK236584 | OK236678 | OK236731 |
| Alternaria oblongoellipsoidea | MFLUCC 22-0074 T | MZ621840 | MZ621914 | OK236621 | MZ621967 | OK236574 | OK236668 | OK236721 |
| Alternaria obpyriconidia | MFLUCC 21-0121 T | MZ621851 | MZ621925 | OK236633 | MZ621978 | OK236585 | OK236680 | OK236732 |
| Alternaria obpyriconidia | MFLU 21-0300 | MZ621852 | MZ621926 | OK236632 | MZ621979 | OK236586 | OK236679 | OK236733 |
| Alternaria orobanches | MFLUCC 21-0137T | MZ621882 | MZ621956 | - | MZ622007 | - | OK236710 | OK236763 |
| Alternaria ovoidea | MFLUCC 14-0427T | MZ621880 | MZ621954 | OK236661 | MZ622005 | OK236614 | OK236708 | OK236761 |
| Alternaria phragmiticola | MFLUCC 21-0125 T | MZ621867 | MZ621941 | OK236649 | MZ621994 | OK236602 | OK236696 | OK236749 |
| Alternaria phragmiticola | MFLU 21-0316 | MZ621868 | MZ621942 | OK236650 | MZ621995 | OK236603 | OK236697 | OK236750 |
| Alternaria pseudoinfectoria | MFLUCC 21-0126 T | MZ621857 | MZ621931 | OK236638 | MZ621984 | OK236591 | OK236685 | OK236738 |
| Alternaria pseudoinfectoria | MFLU 21-0311 | MZ621858 | MZ621932 | OK236639 | MZ621985 | OK236592 | OK236686 | OK236739 |
| Alternaria rostroconidia | MFLUCC 21-0136 T | MZ621842 | MZ621916 | OK236623 | MZ621969 | OK236576 | OK236670 | OK236723 |
| Alternaria rostroconidia | MFLU 21-0318 | MZ621843 | MZ621917 | OK236624 | MZ621970 | OK236577 | OK236671 | OK236724 |
| Alternaria salicicola | MFLUCC 22-0072 T | MZ621872 | MZ621946 | OK236653 | MZ621999 | OK236606 | OK236700 | OK236753 |
| Alternaria sp. | CBS 108.27 | KC584601 | KC584343 | KC584468 | KC584236 | KC584162 | KC584727 | - |
| Alternaria tomato | CBS 103.30 | KP125069 | KP124599 | KP124915 | KP124445 | KP124294 | KP125224 | KP123991 |
| Alternaria tomato | CBS 114.35 | KP125070 | KP124600 | KP124916 | KP124446 | KP124295 | KP125225 | KP123992 |
| Alternaria torilis | MFLUCC 21-0133 | MZ621859 | MZ621933 | OK236640 | MZ621986 | OK236593 | OK236687 | OK236740 |
| Alternaria torilis | MFLU 21-0299 | MZ621860 | MZ621934 | OK236642 | MZ621987 | OK236595 | OK236689 | OK236742 |
| Alternaria torilis | MFLUCC 14-0433 T | MZ621861 | MZ621935 | OK236641 | MZ621988 | OK236594 | OK236688 | OK236741 |
| Alternaria vitis | MFLUCC 17-1109 T | - | - | - | MG764007 | - | - | - |
| Species | Nucleotide Base Difference of Each Informative Gene Regions | ||||
|---|---|---|---|---|---|
| Alt-a1 | gapdh | ITS | rpb2 | tef1-α | |
| Alternaria arctoseptata | 11/476 bp (2.3%) | 15/570 bp (2.6%) | - | 39/560 bp (7.0%) | 4/240 bp (1.7%) |
| A. baoshanensis | 8/474 bp (1.7%) | 15/568 bp (2.6%) | 5/515 bp (1%) | 40/559 bp (7.2%) | 3/240 bp (1.3%) |
| A. falcata | 10/474 bp (2.1%) | 12/568 bp (2.1%) | 5/515 bp (1%) | 37/559 bp (6.6%) | 4/240 bp (1.7%) |
| A. macroconidia | 11/474 bp (2.3%) | 11/567 bp (1.9%) | 4/515 bp (0.8%) | 54/560 bp (9.6%) | 4/240 bp (1.7%) |
| A. ovoidea | 16/470 bp (3.4%) | 14/568 bp (2.5%) | 4/515 bp (0.8%) | 42/559 bp (7.5%) | 3/240 bp (1.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Phookamsak, R.; Jiang, H.; Bhat, D.J.; Camporesi, E.; Lumyong, S.; Kumla, J.; Hongsanan, S.; Mortimer, P.E.; Xu, J.; et al. Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy. J. Fungi 2022, 8, 898. https://doi.org/10.3390/jof8090898
Li J, Phookamsak R, Jiang H, Bhat DJ, Camporesi E, Lumyong S, Kumla J, Hongsanan S, Mortimer PE, Xu J, et al. Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy. Journal of Fungi. 2022; 8(9):898. https://doi.org/10.3390/jof8090898
Chicago/Turabian StyleLi, Junfu, Rungtiwa Phookamsak, Hongbo Jiang, Darbhe Jayarama Bhat, Erio Camporesi, Saisamorn Lumyong, Jaturong Kumla, Sinang Hongsanan, Peter E. Mortimer, Jianchu Xu, and et al. 2022. "Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy" Journal of Fungi 8, no. 9: 898. https://doi.org/10.3390/jof8090898
APA StyleLi, J., Phookamsak, R., Jiang, H., Bhat, D. J., Camporesi, E., Lumyong, S., Kumla, J., Hongsanan, S., Mortimer, P. E., Xu, J., & Suwannarach, N. (2022). Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy. Journal of Fungi, 8(9), 898. https://doi.org/10.3390/jof8090898








