Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Cryptococcus neoformans
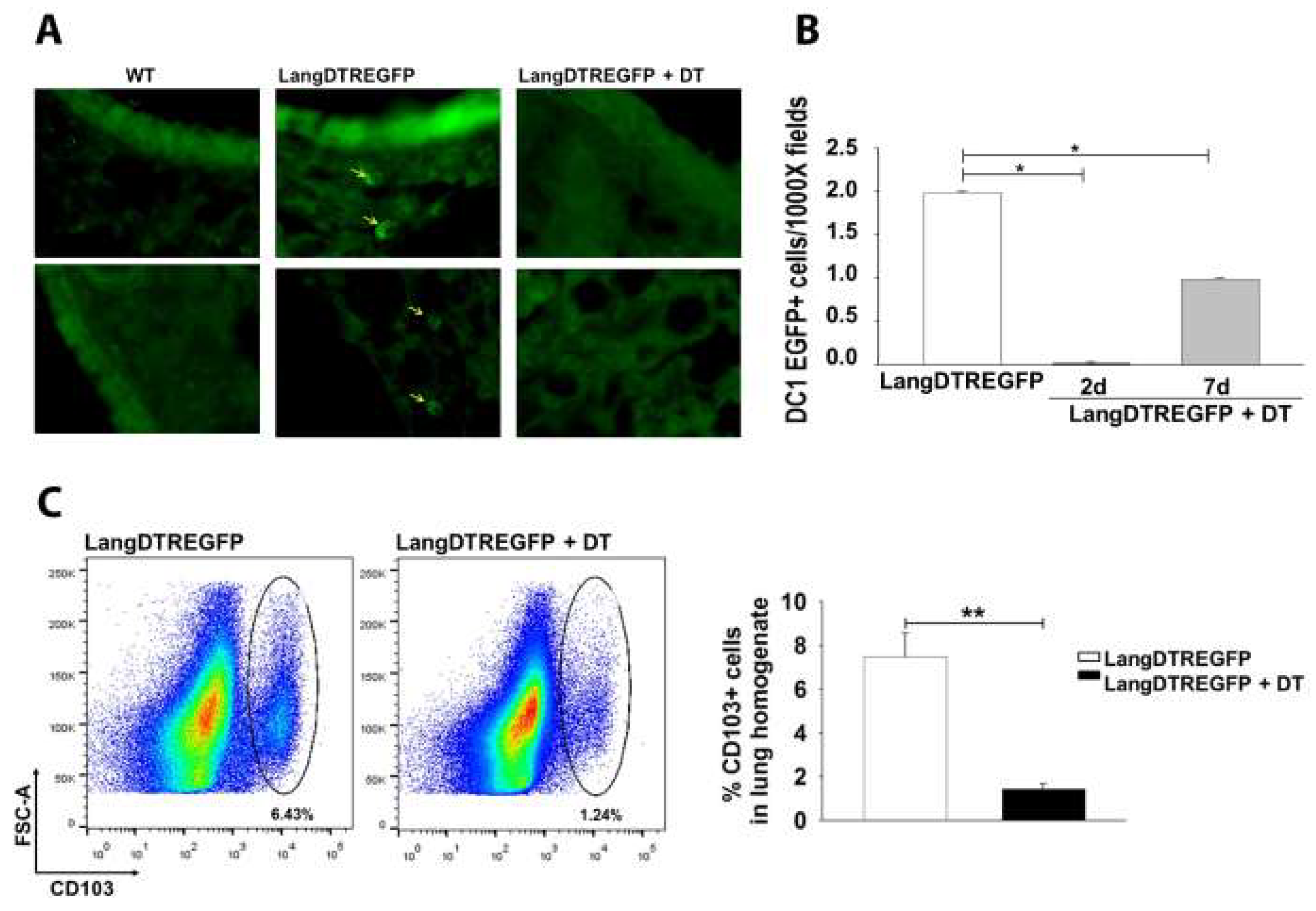
2.3. Pulmonary Infection and Langerin-Expressing Cell Depletion in LangEGFPDTR Mice
2.4. Histology
2.5. Fungal Burden
2.6. Cultures of Pulmonary Cells
2.7. Flow Cytometry Analysis
2.8. Cytokine Production
2.9. Fungal Growth Inhibition by Lung Cells
2.10. Arginase Activity Assay
2.11. Immunofluorescence
2.12. Statistical Analysis
3. Results
3.1. LangDC1 Depletion Favors the Pulmonary Clearance of C. neoformans at the Early Stage of Infection
3.2. LangDC1 Downregulate Cytokine Production and Antifungal Activity of Lung Cells during the Early Stage of Cryptococcal Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firacative, C.; Lizarazo, J.; Illnait-Zaragozí, M.T.; Castañeda, E. The Status of Cryptococcosis in Latin America. Mem. Inst. Oswaldo Cruz 2018, 113, e170554. [Google Scholar] [CrossRef] [Green Version]
- Kindermann, M.; Knipfer, L.; Obermeyer, S.; Müller, U.; Alber, G.; Bogdan, C.; Schleicher, U.; Neurath, M.F.; Wirtz, S. Group 2 Innate Lymphoid Cells (ILC2) Suppress Beneficial Type 1 Immune Responses During Pulmonary Cryptococcosis. Front. Immunol. 2020, 11, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, B.N.; Hawkins, A.N.; Wozniak, K.L. Pulmonary Macrophage and Dendritic Cell Responses to Cryptococcus neoformans. Front. Cell. Infect. Microbiol. 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginhoux, F.; Liu, K.; Helft, J.; Bogunovic, M.; Greter, M.; Hashimoto, D.; Price, J.; Yin, N.; Bromberg, J.; Lira, S.A.; et al. The Origin and Development of Nonlymphoid Tissue CD103+ DCs. J. Exp. Med. 2009, 206, 3115–3130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Laar, L.; Guilliams, M.; Tavernier, S. Isolation of Conventional Dendritic Cells from Mouse Lungs. In Dendritic Cell Protocols; Segura, E., Onai, N., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1423, pp. 139–152. ISBN 978-1-4939-3604-5. [Google Scholar]
- Ardain, A.; Marakalala, M.J.; Leslie, A. Tissue-resident Innate Immunity in the Lung. Immunology 2020, 159, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Sung, S.-S.J.; Fu, S.M.; Rose, C.E.; Gaskin, F.; Ju, S.-T.; Beaty, S.R. A Major Lung CD103 (α E )-β 7 Integrin-Positive Epithelial Dendritic Cell Population Expressing Langerin and Tight Junction Proteins. J. Immunol. 2006, 176, 2161–2172. [Google Scholar] [CrossRef] [Green Version]
- GeurtsvanKessel, C.H.; Willart, M.A.M.; van Rijt, L.S.; Muskens, F.; Kool, M.; Baas, C.; Thielemans, K.; Bennett, C.; Clausen, B.E.; Hoogsteden, H.C.; et al. Clearance of Influenza Virus from the Lung Depends on Migratory Langerin+CD11b− but Not Plasmacytoid Dendritic Cells. J. Exp. Med. 2008, 205, 1621–1634. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, F.M.; Berger, J.L.; Lingel, I.; Laumonnier, Y.; Lewkowich, I.P.; Schmudde, I.; König, P. Distribution and Interaction of Murine Pulmonary Phagocytes in the Naive and Allergic Lung. Front. Immunol. 2018, 9, 1046. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, K. Interactions of Cryptococcus with Dendritic Cells. JoF 2018, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, K.L.; Ravi, S.; Macias, S.; Young, M.L.; Olszewski, M.A.; Steele, C.; Wormley, F.L. Insights into the Mechanisms of Protective Immunity against Cryptococcus neoformans Infection Using a Mouse Model of Pulmonary Cryptococcosis. PLoS ONE 2009, 4, e6854. [Google Scholar] [CrossRef]
- Osterholzer, J.J.; Chen, G.-H.; Olszewski, M.A.; Curtis, J.L.; Huffnagle, G.B.; Toews, G.B. Accumulation of CD11b + Lung Dendritic Cells in Response to Fungal Infection Results from the CCR2-Mediated Recruitment and Differentiation of Ly-6C high Monocytes. J. Immunol. 2009, 183, 8044–8053. [Google Scholar] [CrossRef] [Green Version]
- Osterholzer, J.J.; Milam, J.E.; Chen, G.-H.; Toews, G.B.; Huffnagle, G.B.; Olszewski, M.A. Role of Dendritic Cells and Alveolar Macrophages in Regulating Early Host Defense against Pulmonary Infection with Cryptococcus neoformans. Infect. Immun. 2009, 77, 3749–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hole, C.R.; Wager, C.M.L.; Castro-Lopez, N.; Campuzano, A.; Cai, H.; Wozniak, K.L.; Wang, Y.; Wormley, F.L. Induction of Memory-like Dendritic Cell Responses In vivo. Nat. Commun. 2019, 10, 2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastman, A.J.; Osterholzer, J.J.; Olszewski, M.A. Role of Dendritic Cell–Pathogen Interactions in the Immune Response to Pulmonary Cryptococcal Infection. Future Microbiol. 2015, 10, 1837–1857. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, A.N.; Determann, B.F.; Nelson, B.N.; Wozniak, K.L. Transcriptional Changes in Pulmonary Phagocyte Subsets Dictate the Outcome Following Interaction with The Fungal Pathogen Cryptococcus neoformans. Front. Immunol. 2021, 12, 722500. [Google Scholar] [CrossRef]
- Kissenpfennig, A.; Henri, S.; Dubois, B.; Laplace-Builhé, C.; Perrin, P.; Romani, N.; Tripp, C.H.; Douillard, P.; Leserman, L.; Kaiserlian, D.; et al. Dynamics and Function of Langerhans Cells In Vivo. Immunity 2005, 22, 643–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helft, J.; Manicassamy, B.; Guermonprez, P.; Hashimoto, D.; Silvin, A.; Agudo, J.; Brown, B.D.; Schmolke, M.; Miller, J.C.; Leboeuf, M.; et al. Cross-Presenting CD103+ Dendritic Cells Are Protected from Influenza Virus Infection. J. Clin. Investig. 2012, 122, 4037–4047. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Stern, A.; Kanda, A.; Mionnet, C.; Cazareth, J.; Lazzari, A.; Fleury, S.; Dombrowicz, D.; Glaichenhaus, N.; Julia, V. Langerin+ Dendritic Cells Are Responsible for LPS-Induced Reactivation of Allergen-Specific Th2 Responses in Postasthmatic Mice. Mucosal Immunol. 2011, 4, 343–353. [Google Scholar] [CrossRef]
- Khare, A.; Krishnamoorthy, N.; Oriss, T.B.; Fei, M.; Ray, P.; Ray, A. Cutting Edge: Inhaled Antigen Upregulates Retinaldehyde Dehydrogenase in Lung CD103 + but Not Plasmacytoid Dendritic Cells to Induce Foxp3 De Novo in CD4 + T Cells and Promote Airway Tolerance. J. Immunol. 2013, 209, 1300193. [Google Scholar] [CrossRef] [Green Version]
- Revelli, D.A.; Boylan, J.A.; Gherardini, F.C. A Non-Invasive Intratracheal Inoculation Method for the Study of Pulmonary Melioidosis. Front. Cell. Infect. Microbiol. 2012, 2, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unkel, B.; Hoegner, K.; Clausen, B.E.; Lewe-Schlosser, P.; Bodner, J.; Gattenloehner, S.; Janßen, H.; Seeger, W.; Lohmeyer, J.; Herold, S. Alveolar Epithelial Cells Orchestrate DC Function in Murine Viral Pneumonia. J. Clin. Investig. 2012, 122, 3652–3664. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Ealey, K.N.; Kabata, H.; Koyasu, S. Isolation and Analysis of Group 2 Innate Lymphoid Cells in Mice. Nat. Protoc. 2015, 10, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-H.; Teitz-Tennenbaum, S.; Neal, L.M.; Murdock, B.J.; Malachowski, A.N.; Dils, A.J.; Olszewski, M.A.; Osterholzer, J.J. Local GM-CSF–Dependent Differentiation and Activation of Pulmonary Dendritic Cells and Macrophages Protect against Progressive Cryptococcal Lung Infection in Mice. J. Immunol. 2016, 196, 1810–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corraliza, I.M.; Campo, M.L.; Soler, G.; Modolell, M. Determination of Arginase Activity in Macrophages: A Micromethod. J. Immunol. Methods 1994, 174, 231–235. [Google Scholar] [CrossRef]
- Jain, A.V.; Zhang, Y.; Fields, W.B.; McNamara, D.A.; Choe, M.Y.; Chen, G.; Erb-Downward, J.; Osterholzer, J.J.; Toews, G.B.; Huffnagle, G.B.; et al. Th2 but Not Th1 Immune Bias Results in Altered Lung Functions in a Murine Model of Pulmonary Cryptococcus neoformans Infection. Infect. Immun. 2009, 77, 5389–5399. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, Y.; Arora, S.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Distinct Roles for IL-4 and IL-10 in Regulating T2 Immunity during Allergic Bronchopulmonary Mycosis. J. Immunol. 2005, 174, 1027–1036. [Google Scholar] [CrossRef]
- Davis, M.J.; Tsang, T.M.; Qiu, Y.; Dayrit, J.K.; Freij, J.B.; Huffnagle, G.B.; Olszewski, M.A. Macrophage M1/M2 Polarization Dynamically Adapts to Changes in Cytokine Microenvironments in Cryptococcus neoformans Infection. mBio 2013, 4, e00264-13. [Google Scholar] [CrossRef] [Green Version]
- Firacative, C.; Gressler, A.E.; Schubert, K.; Schulze, B.; Müller, U.; Brombacher, F.; von Bergen, M.; Alber, G. Identification of T Helper (Th)1- and Th2-Associated Antigens of Cryptococcus neoformans in a Murine Model of Pulmonary Infection. Sci. Rep. 2018, 8, 2681. [Google Scholar] [CrossRef]
- Price, M.S.; Perfect, J.R. Host Defenses Against Cryptococcosis. Immunol. Investig. 2011, 40, 786–808. [Google Scholar] [CrossRef]
- Hoag, K.A.; Street, N.E.; Huffnagle, G.B.; Lipscomb, M.F. Early Cytokine Production in Pulmonary Cryptococcus neoformans Infections Distinguishes Susceptible and Resistant Mice. Am. J. Respir. Cell Mol. Biol. 1995, 13, 487–495. [Google Scholar] [CrossRef]
- Huffnagle, G.B.; Boyd, M.B.; Street, N.E.; Lipscomb, M.F. IL-5 Is Required for Eosinophil Recruitment, Crystal Deposition, and Mononuclear Cell Recruitment during a Pulmonary Cryptococcus neoformans Infection in Genetically Susceptible Mice (C57BL/6). J. Immunol. 1998, 160, 2393–2400. [Google Scholar] [PubMed]
- Arora, S.; Hernandez, Y.; Erb-Downward, J.R.; McDonald, R.A.; Toews, G.B.; Huffnagle, G.B. Role of IFN-γ in Regulating T2 Immunity and the Development of Alternatively Activated Macrophages during Allergic Bronchopulmonary Mycosis. J. Immunol. 2005, 174, 6346–6356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Prooyen, N.; Henderson, C.A.; Hocking Murray, D.; Sil, A. CD103+ Conventional Dendritic Cells Are Critical for TLR7/9-Dependent Host Defense against Histoplasma Capsulatum, an Endemic Fungal Pathogen of Humans. PLoS Pathog. 2016, 12, e1005749. [Google Scholar] [CrossRef] [PubMed]
- Peron, G.; Oliveira, J.; Fernandes, F.F.; Verinaud, L. Lung CD103+ Dendritic Cells of Mice Infected with Paracoccidioides Brasiliensis Contribute to Treg Differentiation. Microb. Pathog. 2021, 150, 104696. [Google Scholar] [CrossRef]
- Zelante, T.; Wong, A.Y.W.; Ping, T.J.; Chen, J.; Sumatoh, H.R.; Viganò, E.; Hong Bing, Y.; Lee, B.; Zolezzi, F.; Fric, J.; et al. CD103+ Dendritic Cells Control Th17 Cell Function in the Lung. Cell Rep. 2015, 12, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Lacy-Hulbert, A.; Anderton, S.; Haslett, C.; Savill, J. Apoptotic Cell–Directed Resolution of Lung Inflammation Requires Myeloid Av Integrin–Mediated Induction of Regulatory T Lymphocytes. Am. J. Pathol. 2020, 190, 1224–1235. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, K. Regulation by Innate Immune T Lymphocytes in the Host Defense against Pulmonary Infection with Cryptococcus neoformans. Jpn. J. Infect. Dis. 2004, 57, 137–145. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guasconi, L.; Beccacece, I.; Volpini, X.; Burstein, V.L.; Mena, C.J.; Silvane, L.; Almeida, M.A.; Musri, M.M.; Cervi, L.; Chiapello, L.S. Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice. J. Fungi 2022, 8, 792. https://doi.org/10.3390/jof8080792
Guasconi L, Beccacece I, Volpini X, Burstein VL, Mena CJ, Silvane L, Almeida MA, Musri MM, Cervi L, Chiapello LS. Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice. Journal of Fungi. 2022; 8(8):792. https://doi.org/10.3390/jof8080792
Chicago/Turabian StyleGuasconi, Lorena, Ignacio Beccacece, Ximena Volpini, Verónica L. Burstein, Cristian J. Mena, Leonardo Silvane, Mariel A. Almeida, Melina Mara Musri, Laura Cervi, and Laura S. Chiapello. 2022. "Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice" Journal of Fungi 8, no. 8: 792. https://doi.org/10.3390/jof8080792
APA StyleGuasconi, L., Beccacece, I., Volpini, X., Burstein, V. L., Mena, C. J., Silvane, L., Almeida, M. A., Musri, M. M., Cervi, L., & Chiapello, L. S. (2022). Pulmonary Conventional Type 1 Langerin-Expressing Dendritic Cells Play a Role in Impairing Early Protective Immune Response against Cryptococcus neoformans Infection in Mice. Journal of Fungi, 8(8), 792. https://doi.org/10.3390/jof8080792




