Effect of Melanization on Thallus Microstructure in the Lichen Lobaria pulmonaria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
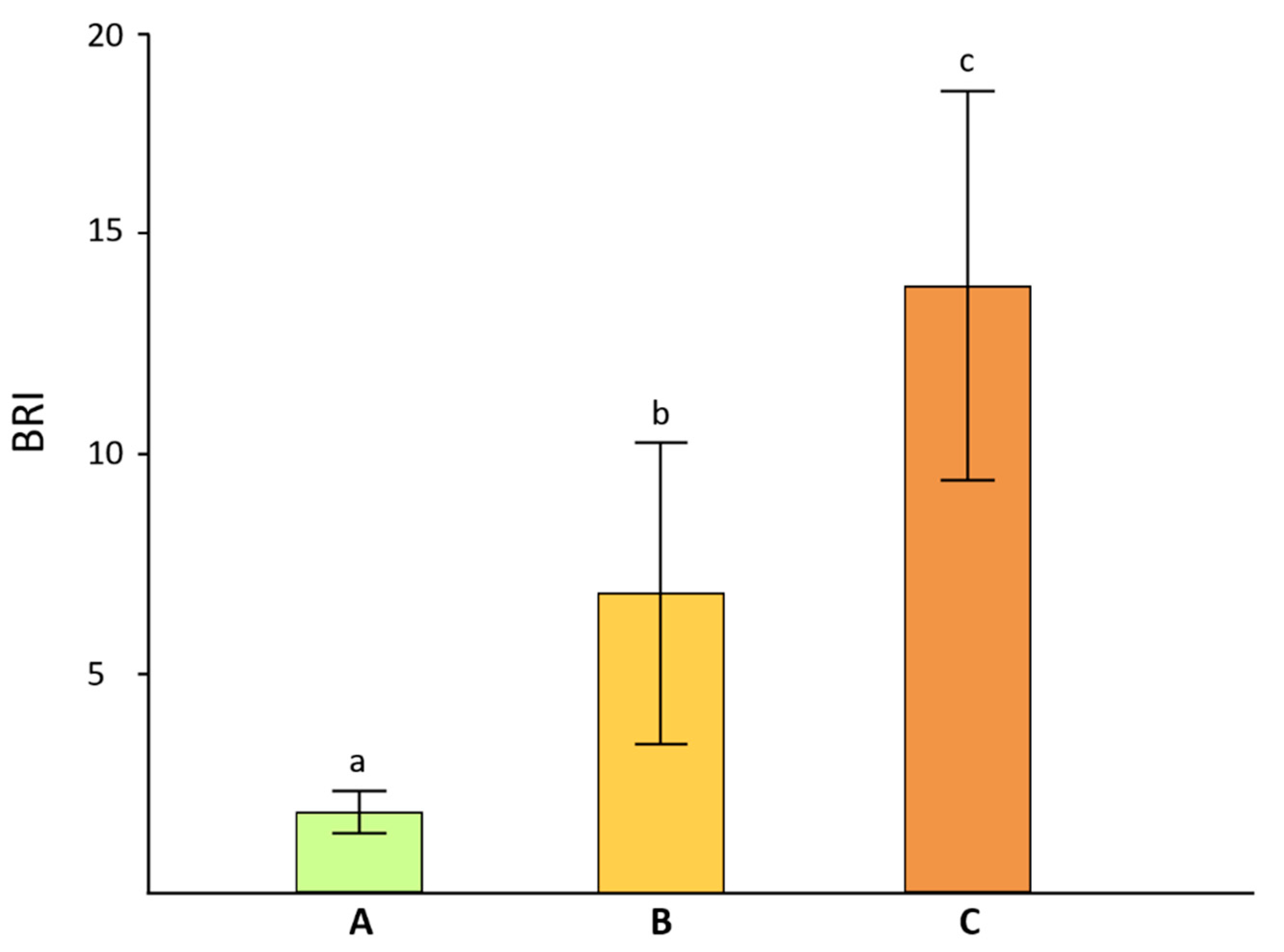
2.2.1. Reflectance Measurements
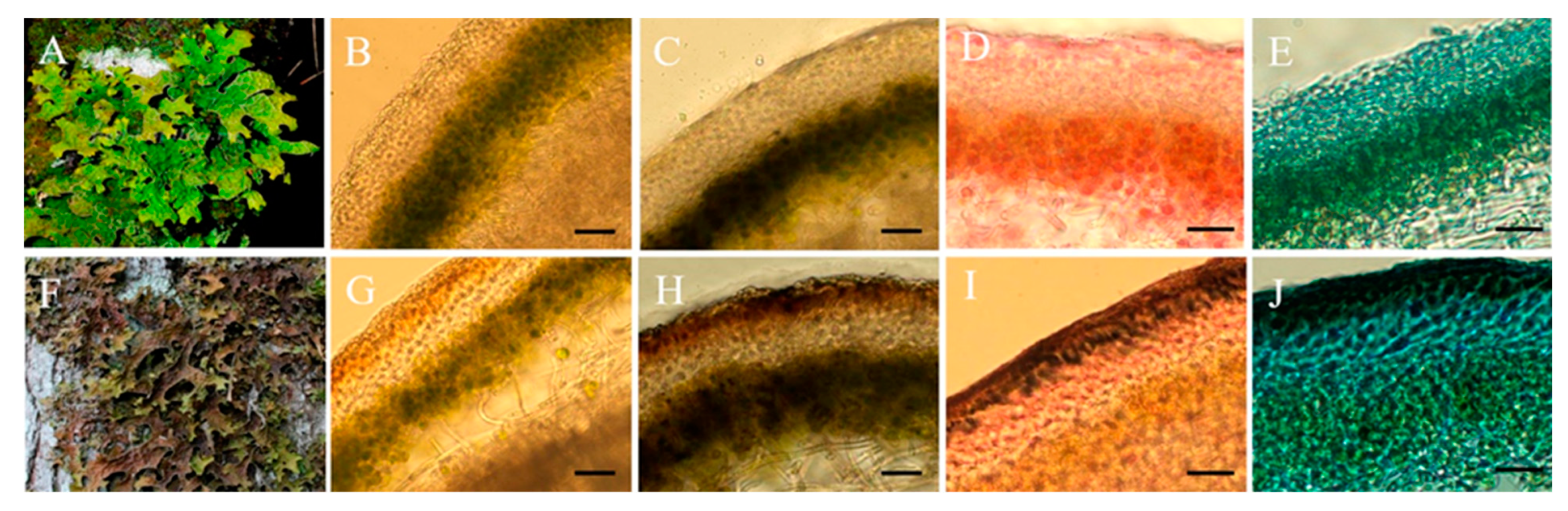
2.2.2. Light Microscopy
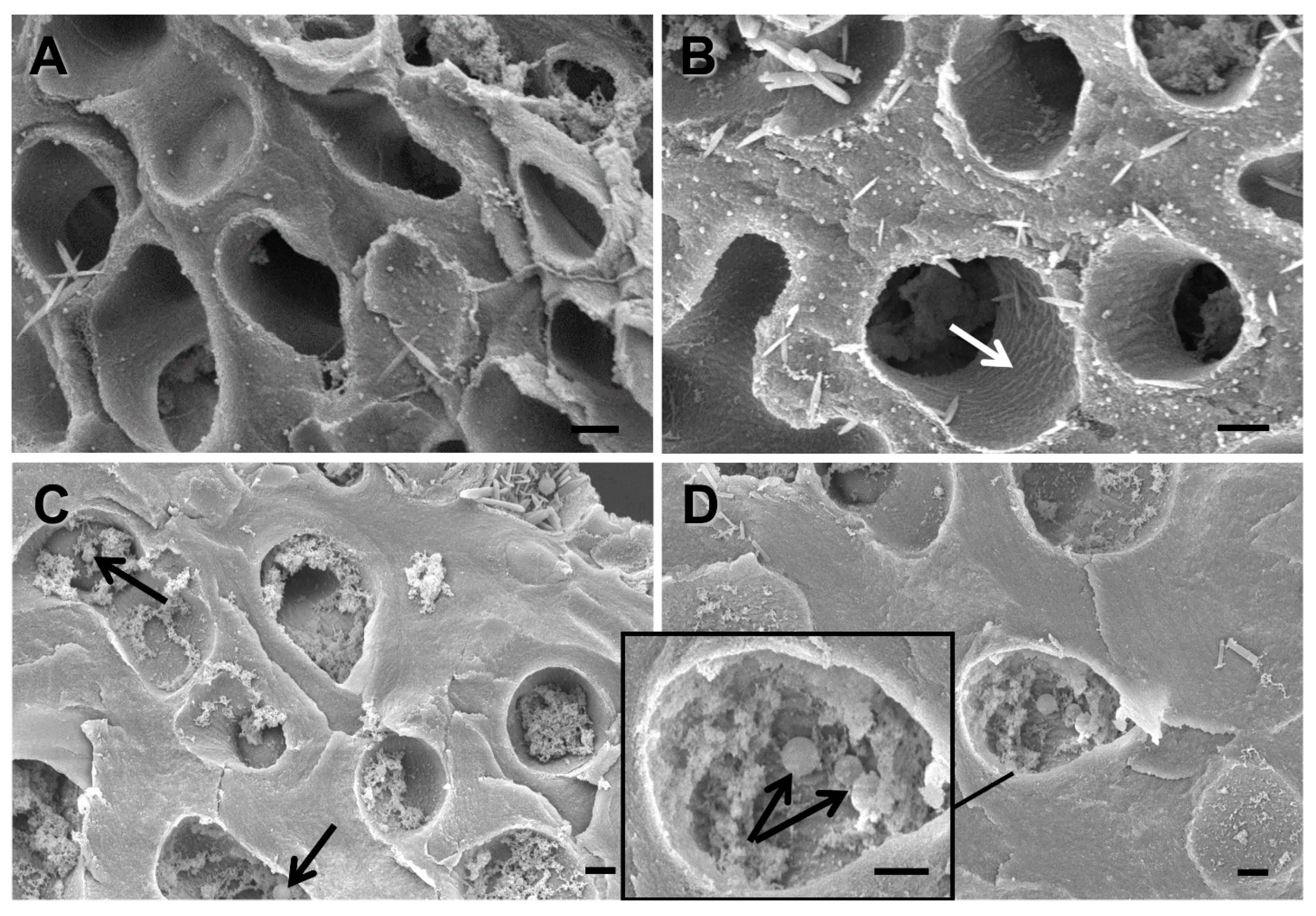
2.2.3. Scanning Electron Microscopy
2.2.4. Transmission Electron Microscopy
2.2.5. Extraction and Purification of Melanin
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nash, T.H., III (Ed.) Lichen Biology; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Eisenreich, W.; Knispel, N.; Beck, A. Advanced methods for the study of the chemistry and the metabolism of lichens. Phytochem. Rev. 2011, 10, 445–456. [Google Scholar] [CrossRef]
- Beckett, R.; Kranner, I.; Minibayeva, F. Stress physiology and the symbiosis. In Lichen Biology, 2nd ed.; Nash, T.H., III, Ed.; Cambridge University Press: Cambridge, UK, 2008; pp. 136–153. [Google Scholar]
- Mafole, T.C.; Solhaug, K.A.; Minibayeva, F.V.; Beckett, R.P. Occurrence and possible roles of melanic pigments in lichenized ascomycetes. Fungal Biol. Rev. 2019, 33, 159–165. [Google Scholar] [CrossRef]
- Singh, S.; Malhotra, A.G.; Pandey, A.; Pandey, K.M. Computational model for pathway reconstruction to unravel the evolutionary significance of melanin synthesis. Bioinformation 2013, 9, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Rassabina, A.E.; Gurjanov, O.P.; Beckett, R.P.; Minibayeva, F.V. Melanin from the lichens Cetraria islandica and Pseudevernia furfuracea: Structural features and physicochemical properties. Biochemistry 2020, 85, 623–628. [Google Scholar] [CrossRef]
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Solhaug, K.A.; Gauslaa, Y.; Nybakken, L.; Bilger, W. UV-induction of sun-screening pigments in lichens. New Phytol. 2003, 158, 91–100. [Google Scholar] [CrossRef]
- Mostert, A.B. Melanin, the what, the why and the how: An introductory review for materials scientists interested in flexible and versatile polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef]
- Cornejo, C.; Scheidegger, C. New morphological aspects of cephalodium formation in the lichen Lobaria pulmonaria (Lecanorales, Ascomycota. Lichenologist 2013, 45, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, E.; Benesperi, R.; Brunialti, G.; Nuzzo, L.D.; Fackovcová, Z.; Frati, L.; Giordani, P.; Nascimbene, J.; Ravera, S.; Vallese, C.; et al. Vitality and growth of the threatened lichen Lobaria pulmonaria (L.) Hoffm. in response to logging and implications for its conservation in mediterranean oak forests. Forests 2020, 11, 995. [Google Scholar] [CrossRef]
- McCune, B.; Geiser, L. Macrolichens of the Pacific Northwest; Oregon State University Press: Corvallis, OR, USA, 1997. [Google Scholar]
- Gauslaa, Y.; Solhaug, K.A. Fungal melanins as a sun screen for symbiotic green algae in the lichen Lobaria pulmonaria. Oecologia 2001, 126, 462–471. [Google Scholar] [CrossRef]
- Beckett, R.P.; Solhaug, K.A.; Gauslaa, Y.; Minibayeva, F. Improved photoprotection in melanized lichens is a result of fungal solar radiation screening rather than photobiont acclimation. Lichenologist 2019, 51, 483–491. [Google Scholar] [CrossRef]
- Chivkunova, O.B.; Solovchenko, A.E.; Sokolova, S.G.; Merzlyak, M.N.; Reshetnikova, I.V.; Gitelson, A.A. Reflectance spectral features and detection of superficial scald–induced browning in storing apple fruit. J. Russ. Phytopathol. Soc. 2001, 2, 73–77. [Google Scholar]
- Słominski, A.; Moellmann, G.; Kuklinska, E.; Bomirski, A.; Pawelek, J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J. Cell Sci. 1988, 89, 287–296. [Google Scholar] [CrossRef]
- Rejniak, J. New method of melanin staining in histological preparations. Pathol. Pol. 1956, 7, 101–103. [Google Scholar]
- Lillie, R.D. A Nile blue staining technic for the differentiation of melanin and lipofuscins. Stain Technol. 1956, 31, 151–153. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, I.B.; Araújo, G.R.S.; Brito-Santos, F.; Figueiredo-Carvalho, M.H.G.; Zancopé-Oliveira, R.M.; Frases, S.; Almeida-Paes, R. Comparative biophysical and ultrastructural analysis of melanins produced by clinical strains of different species from the Trichosporonaceae family. Front. Microbiol. 2022, 13, 876611. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mafole, T.C.; Chiang, C.; Solhaug, K.A.; Beckett, R.P. Melanisation in the old forest lichen Lobaria pulmonaria (L) Hoffm. reduces the efficiency of photosynthesis. Fungal Ecol. 2017, 29, 103–110. [Google Scholar] [CrossRef]
- Mafole, T.C.; Solhaug, K.A.; Minibayeva, F.V.; Beckett, R.P. Tolerance to photoinhibition within a lichen species is higher in melanised thalli. Photosynthetica 2019, 57, 96–102. [Google Scholar] [CrossRef]
- Gessler, N.N.; Egorova, A.S.; Belozerskaya, T.A. Melanin pigments of fungi under extreme environmental conditions. Appl. Biochem. Microbiol. 2014, 50, 105–113. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Pavan, M.E.; López, N.I.; Pettinari, M.J. Melanin biosynthesis in bacteria, regulation and production perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.L.; Double, K.L.; Gerber, J.P. Using Sepia melanin as a PD model to describe the binding characteristics of neuromelanin. J. Chem. Neuroanat. 2015, 64, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Fujikawa, K.; Zucca, F.A.; Zecca, L.; Ito, S. The structure of neuromelanin as studied by chemical degradative methods. J. Neurochem. 2003, 86, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.J.; Cunha, M.M.; Batista, E.J.; Seabra, S.H.; De Souza, W.; Rozental, S. Effects of tricyclazole (5-methyl-1, 2, 4-triazol [3, 4] benzothiazole), a specific DHN–melanin inhibitor, on the morphology of Fonsecaea pedrosoi conidia and sclerotic cells. Microsc. Res. Tech. 2006, 69, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; Raghav, V.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489. [Google Scholar] [CrossRef]
- Solano, F. Melanins: Skin pigments and much more—Types, structural models, biological functions, and formation routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef] [Green Version]
- Matee, L.P.; Beckett, R.P.; Solhaug, K.A.; Minibayeva, F.V. Characterization and role of tyrosinases in the lichen Lobaria pulmonaria (L.) Hoffm. Lichenologist 2016, 48, 311–322. [Google Scholar] [CrossRef]
- Beilinson, Y.; Rassabina, A.; Lunev, I.; Faizullin, D.; Greenbaum, A.; Salnikov, V.; Zuev, Y.; Minibayeva, F.; Feldman, Y. Dielectric response of hydrated water as a structural signature of nanoconfined lichen melanins. Phys. Chem. Chem. Phys. 2022; in press. [Google Scholar]
- Chatterjee, S.; Prados-Rosales, R.; Itin, B.; Casadevall, A.; Stark, R.E. Solid-state NMR reveals the carbon-based molecular architecture of Cryptococcus neoformans fungal eumelanins in the cell wall. J. Biol. Chem. 2015, 290, 13779–13790. [Google Scholar] [CrossRef] [Green Version]
- Nosanchuk, J.D.; Stark, R.E.; Casadevall, A. Fungal melanin: What do we know about structure? Front. Microbiol. 2015, 6, 1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, A.J.; Cunha, M.M.L.; Miranda, K.; Hentschel, J.; Plattner, H.; da Silva, M.B.; Salgado, C.G.; de Souza, W.; Rozental, S. Ultrastructural characterization of melanosomes of the human pathogenic fungus Fonsecaea pedrosoi. J. Struct. Biol. 2008, 162, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Nakouzi, A.; Crippa, P.R.; Eisner, M. Fungal melanins differ in planar stacking distances. PLoS ONE 2012, 7, e30299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
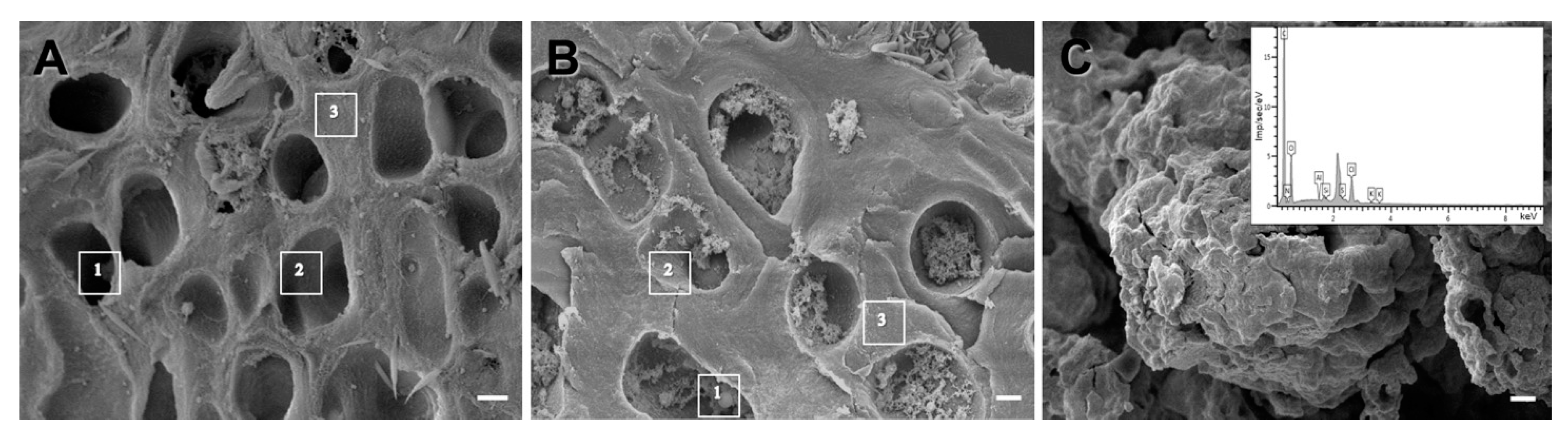

| Zones | C | N | O | Si | Al | Na | S | Cu |
|---|---|---|---|---|---|---|---|---|
| Non-melanized thallus of L. pulmonaria | ||||||||
| Lumen of hyphae (1) | 68.6 | 3.4 | 21.4 | 3.2 | 0.1 | 1.9 | 0.6 | 0.6 |
| Lumen of hyphae with smooth surface (2) | 65.7 | 4.9 | 24.8 | 3.0 | 0.2 | 1.4 | 0.6 | 1.0 |
| Hyphal cell walls (3) | 63.5 | 5.0 | 26.3 | 3.2 | 0.2 | 1.3 | 0.7 | 1.0 |
| Melanized thallus of L. pulmonaria | ||||||||
| Lumen of hyphae with melanin granules (1) | 60.8 | 5.5 | 27.6 | 4.1 | 0.1 | 0.9 | 0.3 | 0.7 |
| Lumen of hyphae with rough surface (2) | 64.2 | 4.3 | 26.3 | 3.8 | 0 | 0.8 | 0.3 | 0.4 |
| Hyphal cell walls (3) | 64.7 | 3.9 | 28.0 | 3.6 | 0.1 | 1.0 | 0.3 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daminova, A.G.; Rogov, A.M.; Rassabina, A.E.; Beckett, R.P.; Minibayeva, F.V. Effect of Melanization on Thallus Microstructure in the Lichen Lobaria pulmonaria. J. Fungi 2022, 8, 791. https://doi.org/10.3390/jof8080791
Daminova AG, Rogov AM, Rassabina AE, Beckett RP, Minibayeva FV. Effect of Melanization on Thallus Microstructure in the Lichen Lobaria pulmonaria. Journal of Fungi. 2022; 8(8):791. https://doi.org/10.3390/jof8080791
Chicago/Turabian StyleDaminova, Amina G., Alexey M. Rogov, Anna E. Rassabina, Richard P. Beckett, and Farida V. Minibayeva. 2022. "Effect of Melanization on Thallus Microstructure in the Lichen Lobaria pulmonaria" Journal of Fungi 8, no. 8: 791. https://doi.org/10.3390/jof8080791
APA StyleDaminova, A. G., Rogov, A. M., Rassabina, A. E., Beckett, R. P., & Minibayeva, F. V. (2022). Effect of Melanization on Thallus Microstructure in the Lichen Lobaria pulmonaria. Journal of Fungi, 8(8), 791. https://doi.org/10.3390/jof8080791






