RNA-Seq Based Transcriptome Analysis of Aspergillus oryzae DSM 1863 Grown on Glucose, Acetate and an Aqueous Condensate from the Fast Pyrolysis of Wheat Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Media and Cultivation Conditions
2.3. Formation and Detoxification of the PAC
2.4. RNA Isolation, Library Preparation and Sequencing
2.5. RNA-Seq Data Analysis
2.6. Analytics
2.6.1. Quantification of the Fungal Cell Dry Weight (CDW)
2.6.2. Enzymatic Acetate Assay
2.6.3. HPLC Analysis
3. Results and Discussion
3.1. Characterization of the Exponential Growth Phase in A. oryzae Shake Flask Cultures
3.2. General Characteristics of the RNA-Seq Data
3.3. Differential Gene Expression during Growth on the Different C-Sources
3.4. Functional Enrichment and Pathway Analyses
3.4.1. Carbon Metabolism
Starch and Sucrose Metabolism
Glycolysis, Glyoxylate and the TCA Cycle
3.4.2. Biosynthesis of Amino Acids
Arginine and Proline Metabolism
Glycine, Serine and Threonine Metabolism
Cysteine and Its Role in Antioxidant Defense
Tryptophan Metabolism
3.4.3. Pyruvate Metabolism as a Key Pathway in the Comparison of Pure Acetate and PAC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Component | Concentration [g/L] | |
|---|---|---|
| Before Detoxification | After Detoxification | |
| acetic acid | 35.40 | 40.05 1 |
| propionic acid | 10.42 | 0 |
| possibly propanoic acid, ethenyl ester | 0.18 | 0 |
| ethylene glycol | 3.27 | 7.63 |
| hydroxy-acetaldehyde | 1.90 | 0 |
| 3-hydroxy propionaldehyde | 0.28 | 0 |
| 2-butenal | 0.58 | 0 |
| butandial (or propanal) | 0.34 | 0 |
| acetol (hydroxypropanone) | 51.46 | 38.42 |
| 2-butanone | 1.98 | 0.38 |
| 1-hydroxy-2-butanone | 6.71 | 3.86 |
| 2,3- butandione (diacetyl) | 2.40 | 0.37 |
| 3-hydroxy-2-butanone(acetoin) | 1.42 | 1.54 |
| 1-acetyloxy-propan-2-one | 1.07 | 0 |
| cyclopentanone | 1.12 | 0 |
| 2-cyclopenten-1-one | 3.35 | 0.33 |
| 2,3-dimethyl-2-cyclopenten-1-one | 0.22 | 0 |
| 2-methyl-2-cyclopenten-1-one | 1.35 | 0 |
| 3-methyl-2-cyclopenten-1-one | 0.68 | 0 |
| 2-cyclohexen-1-one | 0.11 | 0 |
| possibly 3-methyl-2-butanone | 0.09 | 0 |
| possibly 3-methyl-3-buten-2-one | 0.16 | 0 |
| 2-pentanone | 0.51 | 0 |
| 2,3-pentanedione | 0.63 | 0.17 |
| 3-penten-2-one | 0.44 | 0 |
| isomere of 3-methyl-2-Cyclopenten-1-one | 0.28 | 0 |
| possibly 2-ethyl-butanal | 0.31 | 0 |
| possibly 3,4-dimethyl-2-cyclopenten-1-one | 0.27 | 0 |
| isomere of 2,3-dimethyl-2-cyclopenten-1-one | 0.42 | 0 |
| 3-methyl-1,2-cyclopentanedione | 1.50 | 0 |
| 2,3,4-trimethyl-2-cyclopenten-1-one | 0.17 | 0 |
| 2(5H)-furanone | 0.64 | 0 |
| 2-furaldehyde | 2.84 | 0.16 |
| 5-methyl-2-furaldehyde | 0.13 | 0 |
| 1-(2-furanyl)-ethanone | 0.30 | 0 |
| 3-methyl-, (5H)-furan-2-one | 0.04 | 0 |
| γ-butyrolactone | 0.71 | 0.71 |
| 1-methoxy-4-methyl-benzene | 0.06 | 0 |
| indene | 0.08 | 0 |
| phenol | 0.63 | 0 |
| o-cresol | 0.46 | 0 |
| p-cresol | 0.17 | 0 |
| m-cresol | 0.21 | 0 |
| 2,5-dimethyl-phenol | 0.19 | 0 |
| 2,4-dimethyl-phenol | 0.08 | 0 |
| 2,6-dimethyl-phenol | 0.06 | 0 |
| 4-ethyl-phenol | 0.14 | 0 |
| guaiacol | 1.80 | 0 |
| 3-methyl-guaiacol | 0.16 | 0 |
| 4-methyl-guaiacol | 0.36 | 0 |
| 4-ethyl-guaiacol | 0.27 | 0 |
| 4-vinyl-guaiacol | 0.24 | 0 |
| syringol | 0.17 | 0 |
| D-limonene or isomere | 0.14 | 0 |
| 2-acetyl-5-norbornene | 0.06 | 0 |
| 2-methyl-1,3-dioxolane | 0.12 | 0 |
| possibly 2,3-dihydro-1,4-dioxin | 0.09 | 0 |
| Condition | DGE | KEGG Pathway ID | KEGG Pathway Name | Set Size | Adjusted p-Value |
|---|---|---|---|---|---|
| Glc vs. Ace | down | aor00330 | Arginine and proline metabolism | 14 | 0.06 |
| aor01212 | Fatty acid metabolism | 13 | 0.10 | ||
| aor00071 | Fatty acid degradation | 12 | 0.17 | ||
| aor00250 | Alanine, aspartate and glutamate metabolism | 14 | 0.17 | ||
| aor00260 | Glycine, serine and threonine metabolism | 12 | 0.20 | ||
| Glc vs. PAC | up | aor02010 | ABC transporters | 10 | 0.09 |
| aor00564 | Glycerophospholipid metabolism | 16 | 0.17 | ||
| down | aor00260 | Glycine, serine and threonine metabolism | 19 | 0.06 | |
| aor04146 | Peroxisome | 20 | 0.11 | ||
| aor00330 | Arginine and proline metabolism | 16 | 0.11 |
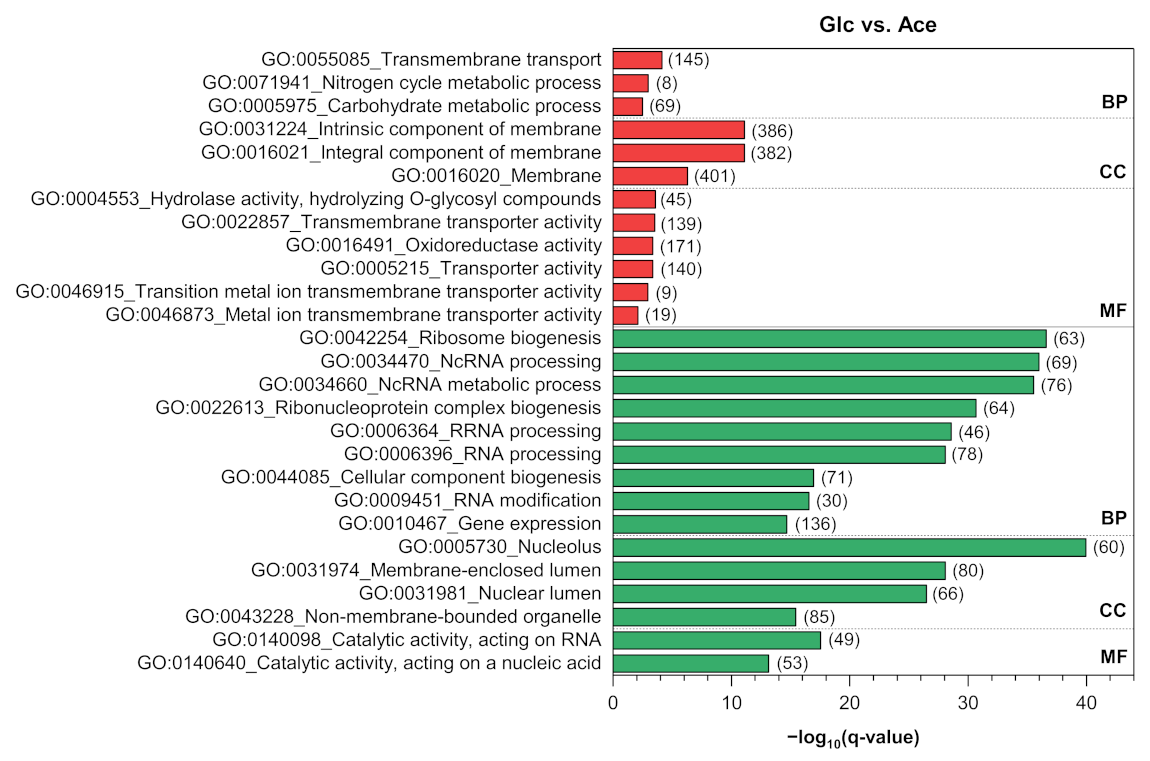
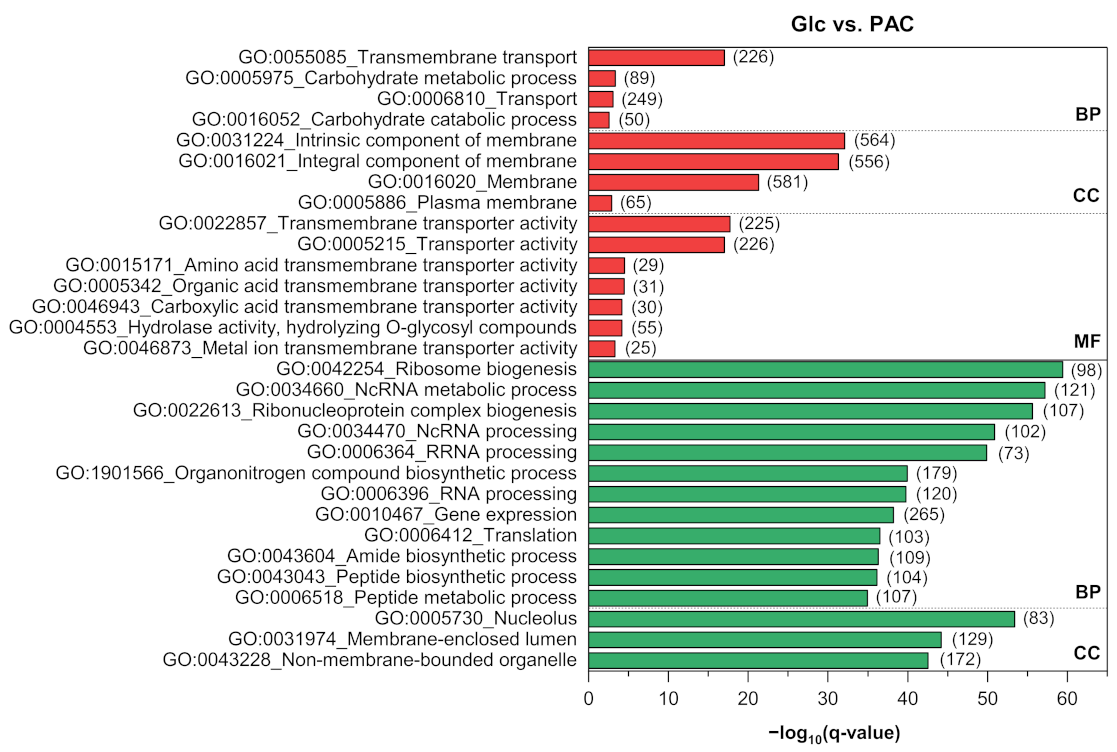
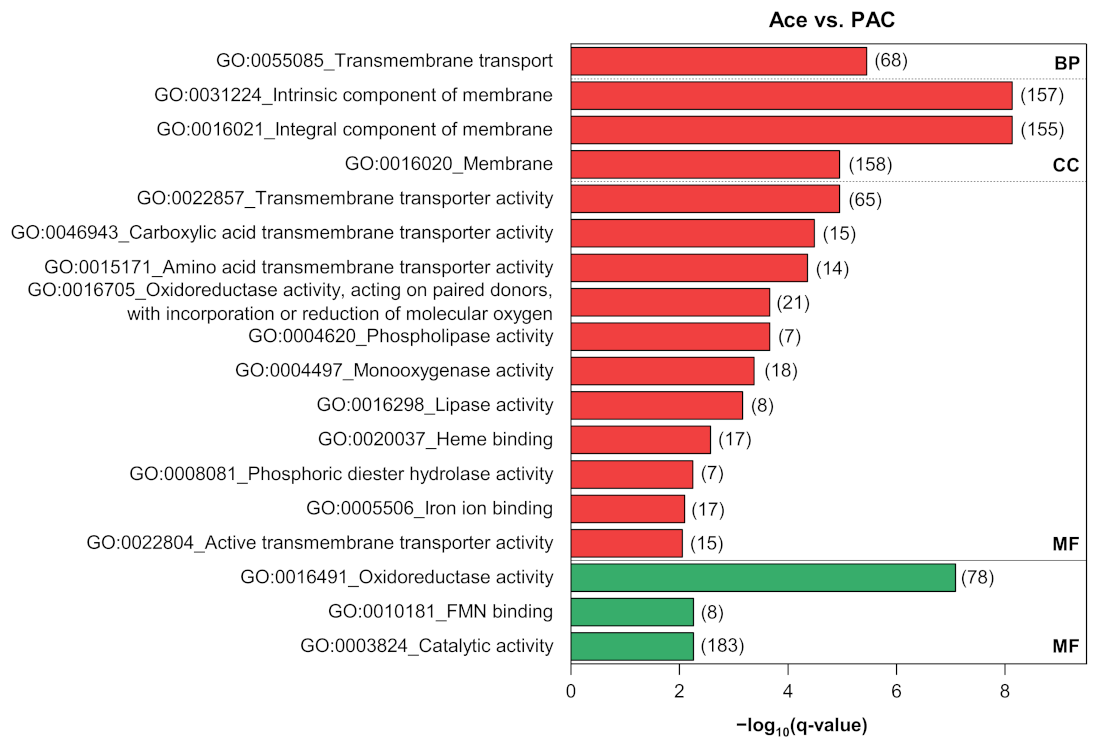
Appendix B
Differential Expression of Selected Secondary Metabolite Clusters
| Secondary Metabolite | Gene Name | Gene ID | Fold Change | ||
|---|---|---|---|---|---|
| Glc vs. Ace | Glc vs. PAC | Ace vs. PAC | |||
| heptelidic acid | hepA | AO090011000408 | 4.82 | 8.09 | 3.28 |
| hepB1 | - | - | - | - | |
| hepC | AO090011000410 | 6.05 | 8.52 | - | |
| hepD | AO090011000411 | 4.85 | 7.03 | - | |
| hepE | AO090011000412 | 5.51 | 7.27 | - | |
| hepF | AO090011000413 | 2.61 | 5.50 | 2.89 | |
| hepG (gpdB) | AO090011000414 | 2.03 | 3.46 | 1.43 | |
| hepH | AO090011000415 | 3.99 | 7.46 | 3.47 | |
| hepR | AO090011000416 | - | 1.29 | - | |
| hepS | AO090011000417 | - | 1.32 | - | |
| kojic acid | kojA | AO090113000136 | 1.67 | 2.11 | - |
| kojR | AO090113000137 | 2.13 | 2.95 | - | |
| kojT | AO090113000138 | - | 2.05 | - | |
| cyclopiazonic acid | cpaR1 | - | - | - | - |
| cpaA | AO090026000001 | - | 5.67 | 5.42 | |
| cpaD | AO090026000002 | - | 6.88 | 7.56 | |
| cpaO | AO090026000003 | - | 7.37 | 7.83 | |
| cpaH | AO090026000004 | 1.45 | 9.43 | 7.98 | |
| cpaM2/cpaT | AO090026000005 | - | 6.00 | 5.34 | |
References
- Pfitzer, C.; Dahmen, N.; Troger, N.; Weirich, F.; Sauer, J.; Gunther, A.; Muller-Hagedorn, M. Fast pyrolysis of wheat straw in the bioliq pilot plant. Energy Fuels 2016, 30, 8047–8054. [Google Scholar] [CrossRef]
- Niebel, A.; Funke, A.; Pfizer, C.; Dahmen, N.; Weih, N.; Richter, D.; Zimmerlin, B. Fast Pyrolysis of Wheat Straw—Improvements of Operational Stability in 10 Years of Bioliq Pilot Plant Operation. Energy Fuels 2021, 35, 11333–11345. [Google Scholar] [CrossRef]
- Kövilein, A.; Umpfenbach, J.; Ochsenreither, K. Acetate as substrate for l-malic acid production with Aspergillus oryzae DSM 1863. Biotechnol. Biofuels 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Kubisch, C.; Ochsenreither, K. Valorization of a Pyrolytic Aqueous Condensate and Its Main Components for L-Malic Acid Production with Aspergillus oryzae DSM 1863. Fermentation 2022, 8, 107. [Google Scholar] [CrossRef]
- Jin, B.; van Leeuwen, H.J.; Patel, B.; Yu, Q. Utilisation of starch processing wastewater for production of microbial biomass protein and fungal α-amylase by Aspergillus oryzae. Bioresour. Technol. 1998, 66, 201–206. [Google Scholar] [CrossRef]
- Shankar, S.K.; Mulimani, V.H. α-Galactosidase production by Aspergillus oryzae in solid-state fermentation. Bioresour. Technol. 2007, 98, 958–961. [Google Scholar] [CrossRef]
- Singh, B. Phytase production by Aspergillus oryzae in solid-state fermentation and its applicability in dephytinization of wheat ban. Appl. Biochem. Biotechnol. 2014, 173, 1885–1895. [Google Scholar]
- Knuf, C.; Nookaew, I.; Brown, S.H.; McCulloch, M.; Berry, A.; Nielsen, J. Investigation of malic acid production in Aspergillus oryzae under nitrogen starvation conditions. Appl. Environ. Microbiol. 2013, 79, 6050–6058. [Google Scholar] [CrossRef] [Green Version]
- Ochsenreither, K.; Fischer, C.; Neumann, A.; Syldatk, C. Process characterization and influence of alternative carbon sources and carbon-to-nitrogen ratio on organic acid production by Aspergillus oryzae DSM1863. Appl. Microbiol. Biotechnol. 2014, 98, 5449–5460. [Google Scholar] [CrossRef]
- Dörsam, S.; Kirchhoff, J.; Bigalke, M.; Dahmen, N.; Syldatk, C.; Ochsenreither, K. Evaluation of Pyrolysis Oil as Carbon Source for Fungal Fermentation. Front. Microbiol. 2016, 7, 2059. [Google Scholar] [CrossRef] [Green Version]
- Kubisch, C.; Ochsenreither, K. Detoxification of a pyrolytic aqueous condensate from wheat straw for utilization as substrate in Aspergillus oryzae DSM 1863 cultivations. Biotechnol. Biofuels 2022, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wang, Y.; Tu, G.; Zan, Z.; Wu, X. Adaptation and transcriptome analysis of Aureobasidium pullulans in corncob hydrolysate for increased inhibitor tolerance to malic acid production. PLoS ONE 2015, 10, e0121416. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Albers, E.; Olsson, L. Evolutionary engineering strategies to enhance tolerance of xylose utilizing recombinant yeast to inhibitors derived from spruce biomass. Biotechnol. Biofuels 2012, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Kitahara, Y.; Wang, Z.; Zhao, X.; Du, W.; Liu, D. Novel mutant strains of Rhodosporidium toruloides by plasma mutagenesis approach and their tolerance for inhibitors in lignocellulosic hydrolyzate. J. Chem. Technol. Biotechnol. 2014, 89, 735–742. [Google Scholar] [CrossRef]
- Hasunuma, T.; Ismail, K.S.K.; Nambu, Y.; Kondo, A. Co-expression of TAL1 and ADH1 in recombinant xylose-fermenting Saccharomyces cerevisiae improves ethanol production from lignocellulosic hydrolysates in the presence of furfural. J. Biosci. Bioeng. 2014, 117, 165–169. [Google Scholar] [CrossRef]
- Larsson, S.; Nilvebrant, N.-O.; Jönsson, L. Effect of overexpression of Saccharomyces cerevisiae Pad1p on the resistance to phenylacrylic acids and lignocellulose hydrolysates under aerobic and oxygen-limited conditions. Appl. Microbiol. Biotechnol. 2001, 57, 167–174. [Google Scholar] [CrossRef]
- Ottenheim, C.; Verdejo, C.; Zimmermann, W.; Wu, J.C. Hemicellulase production by Aspergillus niger DSM 26641 in hydrothermal palm oil empty fruit bunch hydrolysate and transcriptome analysis. J. Biosci. Bioeng. 2014, 118, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Cong, B.; Wang, N.; Liu, S.; Liu, F.; Yin, X.; Shen, J. Isolation, characterization and transcriptome analysis of a novel Antarctic Aspergillus sydowii strain MS-19 as a potential lignocellulosic enzyme source. BMC Microbiol. 2017, 17, 129. [Google Scholar] [CrossRef]
- De Gouvêa, P.F.; de Bernardi, A.V.; Gerolamo, L.E.; Souza Santos E de Riaño-Pachón, D.M.; Uyemura, S.A.; Dinamarco, T.M. Transcriptome and secretome analysis of Aspergillus fumigatus in the presence of sugarcane bagasse. BMC Genom. 2018, 19, 232. [Google Scholar] [CrossRef]
- Corrêa, C.L.; Midorikawa, G.E.O.; Noronha, E.F.; Alves, G.S.C.; Togawa, R.C.; Silva-Junior, O.B.; Costa Marcos Mota do Carmo Grynberg, P.; Miller, R.N.G. Transcriptome profiling-based analysis of carbohydrate-active enzymes in Aspergillus terreus involved in plant biomass degradation. Front. Bioeng. Biotechnol. 2020, 8, 1179. [Google Scholar] [CrossRef]
- Hill, T.W.; Kafer, E. Improved protocols for Aspergillus minimal medium: Trace element and minimal medium salt stock solutions. Fungal Genet. Rep. 2001, 48, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Appelt, J.; Strüven, J.O.; Eidam, P.; Meier, D. Hydrodeoxygenierung von ligninstämmigen Reststoffen für die Erzeugung von Intermediaten im FCC-Prozess. Holztechnologie 2019, 60, 32–40. [Google Scholar]
- Babraham Bioinformatics. FastQC: A Quality Control Tool for High throughput Sequence Data; Babraham Institute: Cambridge, UK, 2011. [Google Scholar]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.-I.; Arima, T.; Akita, O.; Kashiwagi, Y. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Gingeras, T.R. Mapping RNA-seq reads with STAR. Curr. Protoc. Bioinform. 2015, 51, 11.14.1–11.14.19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Pant, G.; Bhavnasi, Y.K.; Blanchard Jr, S.G.; Brouwer, C. Pathview Web: User friendly pathway visualization and data integration. Nucleic Acids Res. 2017, 45, W501–W508. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernández-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef] [Green Version]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Qi, W.; Yuan, Z.; Wang, Z. Comparative metabolomic analysis of furfural stress response in Aspergillus terreus. Cellulose 2019, 26, 8227–8236. [Google Scholar] [CrossRef]
- Rajesh, R.O.; Godan, T.K.; Rai, A.K.; Sahoo, D.; Pandey, A.; Binod, P. Biosynthesis of 2, 5-furan dicarboxylic acid by Aspergillus flavus APLS-1: Process optimization and intermediate product analysis. Bioresour. Technol. 2019, 284, 155–160. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hu, Z.; Ma, L.; Li, H.; Ai, M.; Han, J.; Zeng, B. Transcriptome analysis of different growth stages of Aspergillus oryzae reveals dynamic changes of distinct classes of genes during growth. BMC Microbiol. 2018, 18, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335. [Google Scholar] [CrossRef] [PubMed]
- Semighini, C.P.; Goldman, M.H.S.; Goldman, G.H. Multi-copy suppression of an Aspergillus nidulans mutant sensitive to camptothecin by a putative monocarboxylate transporter. Curr. Microbiol. 2004, 49, 229–233. [Google Scholar] [CrossRef]
- Semchyshyn, H.M.; Abrat, O.B.; Miedzobrodzki, J.; Inoue, Y.; Lushchak, V.I. Acetate but not propionate induces oxidative stress in bakers’ yeast Saccharomyces cerevisiae. Redox Rep. 2011, 16, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, P.K.; Ho, C.-Y.; Chan, C.-K.; Martin, V.J.J.; Trevors, J.T.; Lee, H. Transcriptional profiling of Saccharomyces cerevisiae T2 cells upon exposure to hardwood spent sulphite liquor: Comparison to acetic acid, furfural and hydroxymethylfurfural. Antonie Van Leeuwenhoek 2013, 103, 1281–1295. [Google Scholar] [CrossRef]
- Von Döhren, H. A survey of nonribosomal peptide synthetase (NRPS) genes in Aspergillus nidulans. Fungal Genet. Biol. 2009, 46, S45–S52. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; Zwiers, L.-H.; de Waard, M.A. Secretion of natural and synthetic toxic compounds from filamentous fungi by membrane transporters of the ATP-binding cassette and major facilitator superfamily. Eur. J. Plant Pathol. 2002, 108, 719–734. [Google Scholar] [CrossRef]
- Imamura, K.; Tsuyama, Y.; Hirata, T.; Shiraishi, S.; Sakamoto, K.; Yamada, O.; Akita, O.; Shimoi, H. Identification of a gene involved in the synthesis of a dipeptidyl peptidase IV inhibitor in Aspergillus oryzae. Appl. Environ. Microbiol. 2012, 78, 6996–7002. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, Y.; Tokuoka, M.; Koyama, Y. Functional analysis of the cyclopiazonic acid biosynthesis gene cluster in Aspergillus oryzae RIB 40. Biosci. Biotechnol. Biochem. 2011, 75, 1110042693. [Google Scholar] [CrossRef]
- Shinohara, Y.; Nishimura, I.; Koyama, Y. Identification of a gene cluster for biosynthesis of the sesquiterpene antibiotic, heptelidic acid, in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2019, 83, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Marui, J.; Yamane, N.; Ohashi-Kunihiro, S.; Ando, T.; Terabayashi, Y.; Sano, M.; Ohashi, S.; Ohshima, E.; Tachibana, K.; Higa, Y. Kojic acid biosynthesis in Aspergillus oryzae is regulated by a Zn (II) 2Cys6 transcriptional activator and induced by kojic acid at the transcriptional level. J. Biosci. Bioeng. 2011, 112, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Lebar, M.D.; Cary, J.W.; Majumdar, R.; Carter-Wientjes, C.H.; Mack, B.M.; Wei, Q.; Uka, V.; de Saeger, S.; Di Mavungu, J.D. Identification and functional analysis of the aspergillic acid gene cluster in Aspergillus flavus. Fungal Genet. Biol. 2018, 116, 14–23. [Google Scholar] [CrossRef]
- Bauer, B.E.; Rossington, D.; Mollapour, M.; Mamnun, Y.; Kuchler, K.; Piper, P.W. Weak organic acid stress inhibits aromatic amino acid uptake by yeast, causing a strong influence of amino acid auxotrophies on the phenotypes of membrane transporter mutants. Eur. J. Biochem. 2003, 270, 3189–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheu, C.W.; Konings, W.N.; Freese, E. Effects of acetate and other short-chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J. Bacteriol. 1972, 111, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ask, M.; Bettiga, M.; Mapelli, V.; Olsson, L. The influence of HMF and furfural on redox-balance and energy-state of xylose-utilizing Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Hao, Z.; Zhao, J.; Jin, Y.; Huang, J.; Zhou, R.; Wu, C. Comparative physiological and transcriptomic analyses reveal salt tolerance mechanisms of Zygosaccharomyces rouxii. Process Biochem. 2019, 82, 59–67. [Google Scholar] [CrossRef]
- Paes, B.G.; Steindorff, A.S.; Formighieri, E.F.; Pereira, I.S.; Almeida, J.R.M. Physiological characterization and transcriptome analysis of Pichia pastoris reveals its response to lignocellulose-derived inhibitors. AMB Express 2021, 11, 2. [Google Scholar] [CrossRef]
- Reczey, K.; Brumbauer, A.; Bollok, M.; Szengyel, Z.S.; Zacchi, G. Use of hemicellulose hydrolysate for β-glucosidase fermentation. Appl. Biochem. Biotechnol. 1998, 70, 225–235. [Google Scholar] [CrossRef]
- Tanaka, M.; Gomi, K. Induction and repression of hydrolase genes in Aspergillus oryzae. Front. Microbiol. 2021, 12, 1237. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, S.; Tanaka, M.; Shintani, T.; Gomi, K. Improved α-amylase production by Aspergillus oryzae after a double deletion of genes involved in carbon catabolite repression. Appl. Microbiol. Biotechnol. 2014, 98, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.; Nielsen, J. Influence of carbon source on α-amylase production by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2001, 57, 346–349. [Google Scholar] [CrossRef]
- Fillinger, S.; Chaveroche, M.-K.; van Dijck, P.; de Vries, R.; Ruijter, G.; Thevelein, J.; d’Enfert, C. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. The GenBank accession number for the sequence reported in this paper is AF043230. Microbiology 2001, 147, 1851–1862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Pupo, E.C.; Pérez-Llano, Y.; Tinoco-Valencia, J.R.; Sánchez, N.S.; Padilla-Garfias, F.; Calahorra, M.; Del Sánchez, N.C.; Sánchez-Reyes, A.; Del Rodríguez-Hernández, M.R.; Peña, A. Osmolyte Signatures for the Protection of Aspergillus sydowii Cells under Halophilic Conditions and Osmotic Shock. J. Fungi 2021, 7, 414. [Google Scholar] [CrossRef]
- Crowe, L.M.; Mouradian, R.; Crowe, J.H.; Jackson, S.A.; Womersley, C. Effects of carbohydrates on membrane stability at low water activities. Biochim. Biophys. Acta (BBA)-Biomembr. 1984, 769, 141–150. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Panneman, H.; Ruijter, G.J.G.; van den Broeck, H.C.; Visser, J. Cloning and biochemical characterisation of Aspergillus niger hexokinase: The enzyme is strongly inhibited by physiological concentrations of trehalose 6-phosphate. Eur. J. Biochem. 1998, 258, 223–232. [Google Scholar] [CrossRef]
- Puttikamonkul, S.; Willger, S.D.; Grahl, N.; Perfect, J.R.; Movahed, N.; Bothner, B.; Park, S.; Paderu, P.; Perlin, D.S.; Cramer, R.A., Jr. Trehalose 6-phosphate phosphatase is required for cell wall integrity and fungal virulence but not trehalose biosynthesis in the human fungal pathogen Aspergillus fumigatus. Mol. Microbiol. 2010, 77, 891–911. [Google Scholar] [CrossRef] [Green Version]
- Thammahong, A.; Caffrey-Card, A.K.; Dhingra, S.; Obar, J.J.; Cramer, R.A. Aspergillus fumigatus trehalose-regulatory subunit homolog moonlights to mediate cell wall homeostasis through modulation of chitin synthase activity. MBio 2017, 8, e00056-17. [Google Scholar] [CrossRef] [Green Version]
- Im Lee, J.; Choi, J.H.; Park, B.C.; Park, Y.H.; Lee, M.Y.; Park, H.-M.; Maeng, P.J. Differential expression of the chitin synthase genes of Aspergillus nidulans, chsA, chsB, and chsC, in response to developmental status and environmental factors. Fungal Genet. Biol. 2004, 41, 635–646. [Google Scholar] [CrossRef] [PubMed]
- You, K.M.; Rosenfield, C.-L.; Knipple, D.C. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 2003, 69, 1499–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl. Environ. Microbiol. 2007, 73, 110–116. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Ma, L.; Hu, Z.; Li, H.; Ai, M.; Long, C.; Zeng, B. Deep sequencing analysis of transcriptomes in Aspergillus oryzae in response to salinity stress. Appl. Microbiol. Biotechnol. 2018, 102, 897–906. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunihiro, S.; Sano, M.; Zhang, Y.; Eto, S.; Chang, Y.-C.; Suzuki, T.; Jigami, Y.; Machida, M. Comprehensive cloning and expression analysis of glycolytic genes from the filamentous fungus, Aspergillus oryzae. Curr. Genet. 2000, 37, 322–327. [Google Scholar] [CrossRef]
- Stemple, C.J.; Davis, M.A.; Hynes, M.J. The facC gene of Aspergillus nidulans encodes an acetate-inducible carnitine acetyltransferase. J. Bacteriol. 1998, 180, 6242–6251. [Google Scholar] [CrossRef] [PubMed]
- Armitt, S.; Roberts, C.F.; Kornberg, H.L. The role of isocitrate lyase in Aspergillus nidulans. FEBS Lett. 1970, 7, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wu, D.; Yang, X.; Hong, J. Transcriptomic analysis of thermotolerant yeast Kluyveromyces marxianus in multiple inhibitors tolerance. RSC Adv. 2018, 8, 14177–14192. [Google Scholar] [CrossRef] [Green Version]
- Horváth, I.S.; Sjöde, A.; Alriksson, B.; Jönsson, L.J.; Nilvebrant, N.-O. Critical Conditions for Improved Fermentability During Overliming of Acid Hydrolysates from Spruce. In ABAB Symposium, Proceedings of the Twenty-Sixth Symposium on Biotechnology for Biofuels and Chemicals; Chattanooga, TN, USA, 9–12 May 2004, Davison, B.H., Evans, B.R., Finkelstein, M., McMillan, J.D., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 1031–1044. [Google Scholar]
- Ma, L.; Fu, L.; Hu, Z.; Li, Y.; Zheng, X.; Zhang, Z.; Jiang, C.; Zeng, B. Modulation of fatty acid composition of Aspergillus oryzae in response to ethanol stress. Microorganisms 2019, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhou, J.; Liu, L.; Chen, J. Arginine: A novel compatible solute to protect Candida glabrata against hyperosmotic stress. Process Biochem. 2011, 46, 1230–1235. [Google Scholar] [CrossRef]
- Cheng, Y.; Du, Z.; Zhu, H.; Guo, X.; He, X. Protective effects of arginine on Saccharomyces cerevisiae against ethanol stress. Sci. Rep. 2016, 6, 31311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baynes, B.M.; Wang, D.I.C.; Trout, B.L. Role of arginine in the stabilization of proteins against aggregation. Biochemistry 2005, 44, 4919–4925. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boch, J.; Kempf, B.; Bremer, E. Osmoregulation in Bacillus subtilis: Synthesis of the osmoprotectant glycine betaine from exogenously provided choline. J. Bacteriol. 1994, 176, 5364–5371. [Google Scholar] [CrossRef] [Green Version]
- Kelavkar, U.P.; Chhatpar, H.S. Polyol concentrations in Aspergillus repens grown under salt stress. World J. Microbiol. Biotechnol. 1993, 9, 579–582. [Google Scholar] [CrossRef]
- Lambou, K.; Pennati, A.; Valsecchi, I.; Tada, R.; Sherman, S.; Sato, H.; Beau, R.; Gadda, G.; Latgé, J.-P. Pathway of glycine betaine biosynthesis in Aspergillus fumigatus. Eukaryot. Cell 2013, 12, 853–863. [Google Scholar] [CrossRef] [Green Version]
- Meijer, S.; Otero, J.; Olivares, R.; Andersen, M.R.; Olsson, L.; Nielsen, J. Overexpression of isocitrate lyase—Glyoxylate bypass influence on metabolism in Aspergillus niger. Metab. Eng. 2009, 11, 107–116. [Google Scholar] [CrossRef]
- Borges-Walmsley, M.I.; Turner, G.; Bailey, A.M.; Brown, J.; Lehmbeck, J.; Clausen, I.G. Isolation and characterisation of genes for sulphate activation and reduction in Aspergillus nidulans: Implications for evolution of an allosteric control region by gene duplication. Mol. Gen. Genet. MGG 1995, 247, 423–429. [Google Scholar] [CrossRef]
- Miller, E.N.; Jarboe, L.R.; Turner, P.C.; Pharkya, P.; Yomano, L.P.; York, S.W.; Nunn, D.; Shanmugam, K.T.; Ingram, L.O. Furfural inhibits growth by limiting sulfur assimilation in ethanologenic Escherichia coli strain LY180. Appl. Environ. Microbiol. 2009, 75, 6132–6141. [Google Scholar] [CrossRef] [Green Version]
- Costa, V.; Quintanilha, A.; Moradas-Ferreira, P. Protein oxidation, repair mechanisms and proteolysis in Saccharomyces cerevisiae. IUBMB Life 2007, 59, 293–298. [Google Scholar] [CrossRef]
- Hisada, H.; Hata, Y.; Kawato, A.; Abe, Y.; Akita, O. Cloning and expression analysis of two catalase genes from Aspergillus oryzae. J. Biosci. Bioeng. 2005, 99, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Müller, F.; Bernecker, K.; Dahmen, N.; Takors, R.; Blombach, B. Valorization of pyrolysis water: A biorefinery side stream, for 1, 2-propanediol production with engineered Corynebacterium glutamicum. Biotechnol. Biofuels 2017, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Du, G.; Chen, J. Salt-induced osmotic stress for glutathione overproduction in Candida utilis. Enzym. Microb. Technol. 2009, 45, 324–329. [Google Scholar] [CrossRef]
- Mulet, J.M.; Alemany, B.; Ros, R.; Calvete, J.J.; Serrano, R. Expression of a plant serine O-acetyltransferase in Saccharomyces cerevisiae confers osmotic tolerance and creates an alternative pathway for cysteine biosynthesis. Yeast 2004, 21, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Shimizu, M.; Hoshino, T.; Takaya, N. The glutathione system of Aspergillus nidulans involves a fungus-specific glutathione S-transferase. J. Biol. Chem. 2009, 284, 8042–8053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thön, M.; Al-Abdallah, Q.; Hortschansky, P.; Brakhage, A.A. The thioredoxin system of the filamentous fungus Aspergillus nidulans: Impact on development and oxidative stress response. J. Biol. Chem. 2007, 282, 27259–27269. [Google Scholar] [CrossRef] [Green Version]
- Misslinger, M.; Scheven, M.T.; Hortschansky, P.; López-Berges, M.S.; Heiss, K.; Beckmann, N.; Heigl, T.; Hermann, M.; Krüger, T.; Kniemeyer, O. The monothiol glutaredoxin GrxD is essential for sensing iron starvation in Aspergillus fumigatus. PLoS Genet. 2019, 15, e1008379. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Dong, Y.; Yu, Q.; Kai, Z.; Zhang, M.; Jia, C.; Xiao, C.; Zhang, B.; Zhang, B.; Li, M. Function of glutaredoxin 3 (Grx3) in oxidative stress response caused by iron homeostasis disorder in Candida albicans. Future Microbiol. 2017, 12, 1397–1412. [Google Scholar] [CrossRef]
- Rodríguez-Manzaneque, M.T.; Tamarit, J.; Bellí, G.; Ros, J.; Herrero, E. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol. Biol. Cell 2002, 13, 1109–1121. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.L.; Ma, M. Pathway-based signature transcriptional profiles as tolerance phenotypes for the adapted industrial yeast Saccharomyces cerevisiae resistant to furfural and HMF. Appl. Microbiol. Biotechnol. 2020, 104, 3473–3492. [Google Scholar] [CrossRef] [PubMed]
- Coschigano, P.W.; Magasanik, B. The URE2 gene product of Saccharomyces cerevisiae plays an important role in the cellular response to the nitrogen source and has homology to glutathione S-transferases. Mol. Cell. Biol. 1991, 11, 822–832. [Google Scholar]
- Saxena, M.; Allameh, A.; Mukerji, K.G.; Raj, H.G. Studies on glutathione S-transferases of Aspergillus flavus group in relation to aflatoxin production. J. Toxicol. Toxin Rev. 1989, 8, 319–328. [Google Scholar] [CrossRef]
- Li, B.-Z.; Yuan, Y.-J. Transcriptome shifts in response to furfural and acetic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2010, 86, 1915–1924. [Google Scholar] [CrossRef]
- Godin, S.K.; Lee, A.G.; Baird, J.M.; Herken, B.W.; Bernstein, K.A. Tryptophan biosynthesis is important for resistance to replicative stress in Saccharomyces cerevisiae. Yeast 2016, 33, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, L.; Ikui, A.E. Tryptophan confers resistance to SDS-associated cell membrane stress in Saccharomyces cerevisiae. PLoS ONE 2019, 14, e0199484. [Google Scholar]
- Jones, R.P.; Greenfield, P.F. Ethanol and the fluidity of the yeast plasma membrane. Yeast 1987, 3, 223–232. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Tanaka, T.; Furusawa, C.; Nagahisa, K.; Hirasawa, T.; Shimizu, H. Comprehensive phenotypic analysis for identification of genes affecting growth under ethanol stress in Saccharomyces cerevisiae. FEMS Yeast Res. 2009, 9, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Hirasawa, T.; Yoshikawa, K.; Nakakura, Y.; Nagahisa, K.; Furusawa, C.; Katakura, Y.; Shimizu, H.; Shioya, S. Identification of target genes conferring ethanol stress tolerance to Saccharomyces cerevisiae based on DNA microarray data analysis. J. Biotechnol. 2007, 131, 34–44. [Google Scholar] [CrossRef]
- Abe, F.; Horikoshi, K. Tryptophan permease gene TAT2 confers high-pressure growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 2000, 20, 8093–8102. [Google Scholar] [CrossRef]
- Lee, C.; Park, C. Bacterial responses to glyoxal and methylglyoxal: Reactive electrophilic species. Int. J. Mol. Sci. 2017, 18, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, J.P. Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 1993, 21, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A. Metabolism of methylglyoxal in microorganisms. Annu. Rev. Microbiol. 1984, 38, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, T. Analytical methods for trace levels of reactive carbonyl compounds formed in lipid peroxidation systems. J. Pharm. Biomed. Anal. 2006, 41, 12–25. [Google Scholar] [CrossRef]
- Murata, K.; Saikusa, T.; Fukuda, Y.; Watanabe, K.; Inoue, Y.; Shimosaka, M.; Kimura, A. Metabolism of 2-oxoaldehydes in yeasts: Possible role of glycolytic bypath as a detoxification system in l-threonine catabolism by Saccharomyces cerevisiae. Eur. J. Biochem. 1986, 157, 297–301. [Google Scholar] [CrossRef]
- Bennett, G.N.; San, K.-Y. Microbial formation, biotechnological production and applications of 1, 2-propanediol. Appl. Microbiol. Biotechnol. 2001, 55, 1–9. [Google Scholar] [CrossRef]
- Inoue, Y.; Rhee, H.-I.; Watanabe, K.; Murata, K.; Kimura, A. Metabolism of 2-ketoaldehydes in mold: Purification and characterization of glyoxalase I from Aspergillus niger. J. Biochem. 1987, 102, 583–589. [Google Scholar] [CrossRef]
- Inoue, Y.; Tsujimoto, Y.; Kimura, A. Expression of the glyoxalase I gene of Saccharomyces cerevisiae is regulated by high osmolarity glycerol mitogen-activated protein kinase pathway in osmotic stress response. J. Biol. Chem. 1998, 273, 2977–2983. [Google Scholar] [CrossRef] [Green Version]
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645. [Google Scholar] [CrossRef]
- Bankapalli, K.; Saladi, S.; Awadia, S.S.; Goswami, A.V.; Samaddar, M.; D’Silva, P. Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae. J. Biol. Chem. 2015, 290, 26491–26507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasim, S.; Hussin, N.A.; Alomar, F.; Bidasee, K.R.; Nickerson, K.W.; Wilson, M.A. A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J. Biol. Chem. 2014, 289, 1662–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, M.-K.; Ku, M.; Kang, S.-O. NAD+-linked alcohol dehydrogenase 1 regulates methylglyoxal concentration in Candida albicans. FEBS Lett. 2014, 588, 1144–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, K.; Banerjee, A.B.; Ray, S.; Ray, M. Reduction of methylglyoxal in Escherichia coli K12 by an aldehyde reductase and alcohol dehydrogenase. Mol. Cell. Biochem. 1996, 156, 117–124. [Google Scholar] [CrossRef]
- Petersson, A.; Almeida, J.R.M.; Modig, T.; Karhumaa, K.; Hahn-Hägerdal, B.; Gorwa-Grauslund, M.F.; Lidén, G. A 5-hydroxymethyl furfural reducing enzyme encoded by the Saccharomyces cerevisiae ADH6 gene conveys HMF tolerance. Yeast 2006, 23, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.R.M.; Röder, A.; Modig, T.; Laadan, B.; Lidén, G.; Gorwa-Grauslund, M.-F. NADH-vs NADPH-coupled reduction of 5-hydroxymethyl furfural (HMF) and its implications on product distribution in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2008, 78, 939–945. [Google Scholar] [CrossRef]
- Modig, T.; Liden, G.; Taherzadeh, M.J. Inhibition effects of furfural on alcohol dehydrogenase, aldehyde dehydrogenase and pyruvate dehydrogenase. Biochem. J. 2002, 363, 769–776. [Google Scholar] [CrossRef]
- Simpson-Lavy, K.; Kupiec, M. Carbon catabolite repression in yeast is not limited to glucose. Sci. Rep. 2019, 9, 6491. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, H.; Liu, J.; Li, S.; Guo, J.; Li, H.; Jia, X.; Huo, H.; Zheng, Z.; You, S. Old yellow enzymes: Structures and structure-guided engineering for stereocomplementary bioreduction. Appl. Microbiol. Biotechnol. 2020, 104, 8155–8170. [Google Scholar] [CrossRef]
- Terabayashi, Y.; Sano, M.; Yamane, N.; Marui, J.; Tamano, K.; Sagara, J.; Dohmoto, M.; Oda, K.; Ohshima, E.; Tachibana, K. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010, 47, 953–961. [Google Scholar] [CrossRef]
- Tamano, K.; Kuninaga, M.; Kojima, N.; Umemura, M.; Machida, M.; Koike, H. Use of the kojA promoter, involved in kojic acid biosynthesis, for polyketide production in Aspergillus oryzae: Implications for long-term production. BMC Biotechnol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, I.; Shinohara, Y.; Oguma, T.; Koyama, Y. Survival strategy of the salt-tolerant lactic acid bacterium, Tetragenococcus halophilus, to counteract koji mold, Aspergillus oryzae, in soy sauce brewing. Biosci. Biotechnol. Biochem. 2018, 82, 1437–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Zang, X.; Jamieson, C.S.; Lin, H.-C.; Houk, K.N.; Zhou, J.; Tang, Y. Biosynthesis of the fungal glyceraldehyde-3-phosphate dehydrogenase inhibitor heptelidic acid and mechanism of self-resistance. Chem. Sci. 2020, 11, 9554–9562. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Sakai, K.; Endo, A. Koningic acid (heptelidic acid) inhibition of glyceraldehyde-3-phosphate dehydrogenases from various sources. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1992, 1120, 113–116. [Google Scholar] [CrossRef]
- Kato, N.; Tokuoka, M.; Shinohara, Y.; Kawatani, M.; Uramoto, M.; Seshime, Y.; Fujii, I.; Kitamoto, K.; Takahashi, T.; Takahashi, S. Genetic safeguard against mycotoxin cyclopiazonic acid production in Aspergillus oryzae. ChemBioChem 2011, 12, 1376–1382. [Google Scholar] [CrossRef]
- Sugiyama, S. Selection of micro-organisms for use in the fermentation of soy sauce. Food Microbiol. 1984, 1, 339–347. [Google Scholar] [CrossRef]
- Chang, P.-K.; Ehrlich, K.C.; Fujii, I. Cyclopiazonic acid biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins 2009, 1, 74–99. [Google Scholar] [CrossRef] [Green Version]

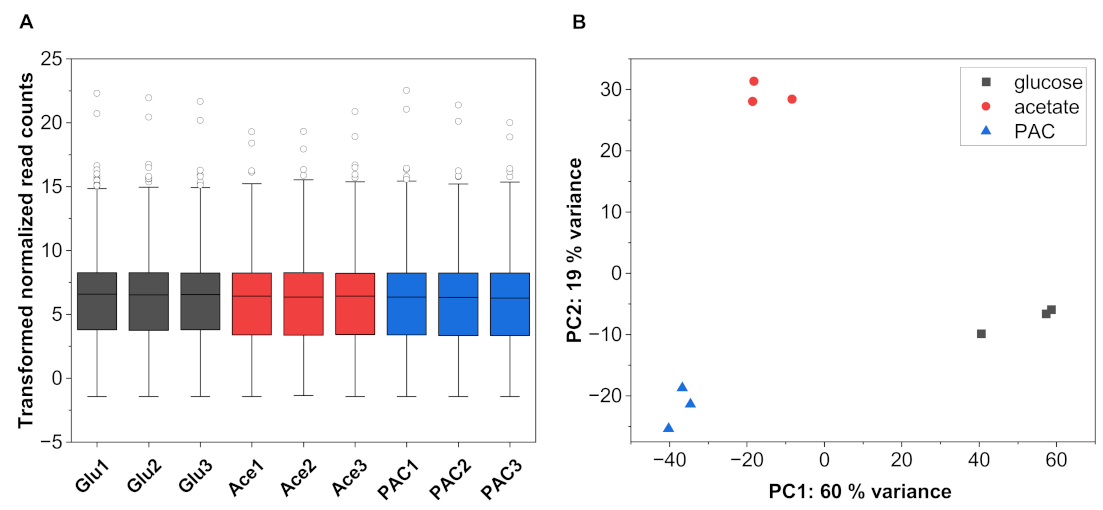
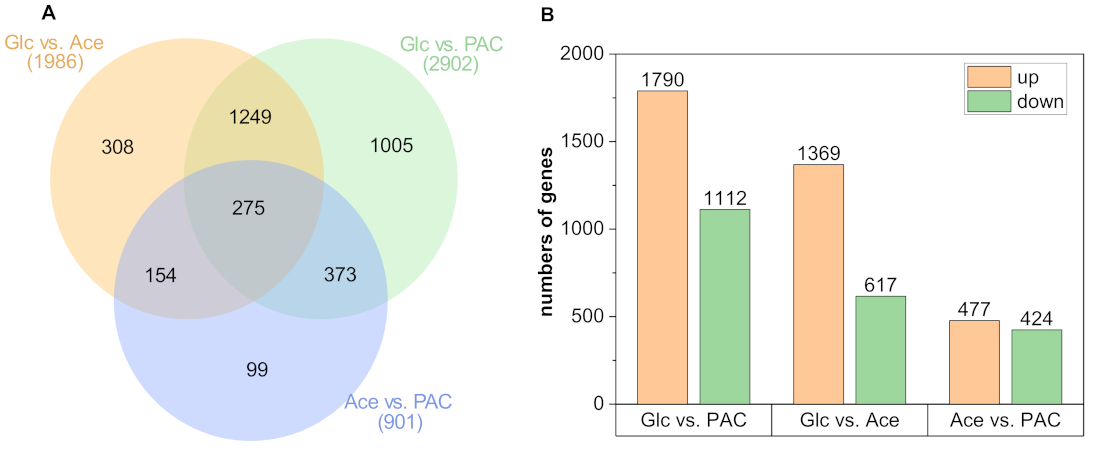
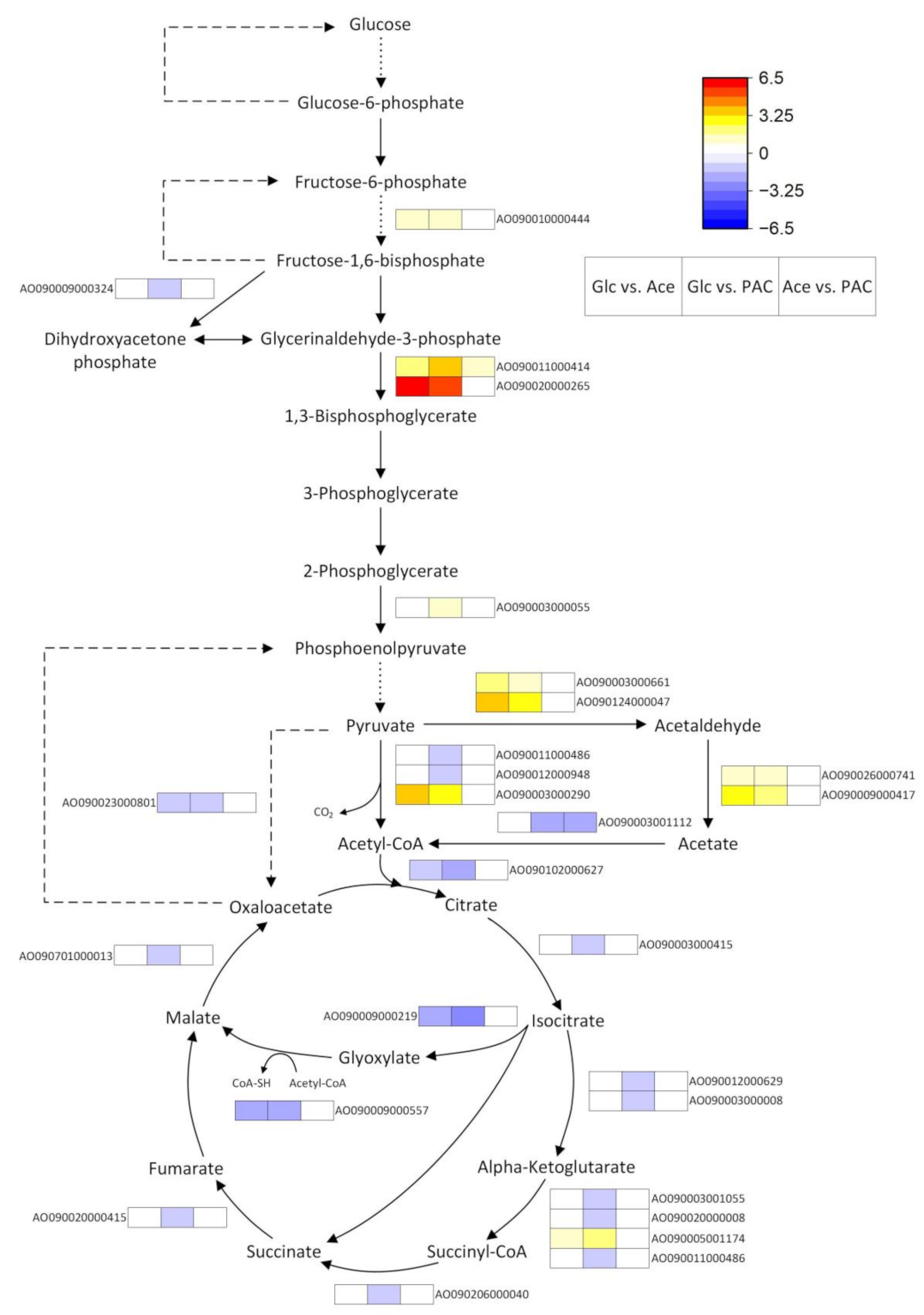
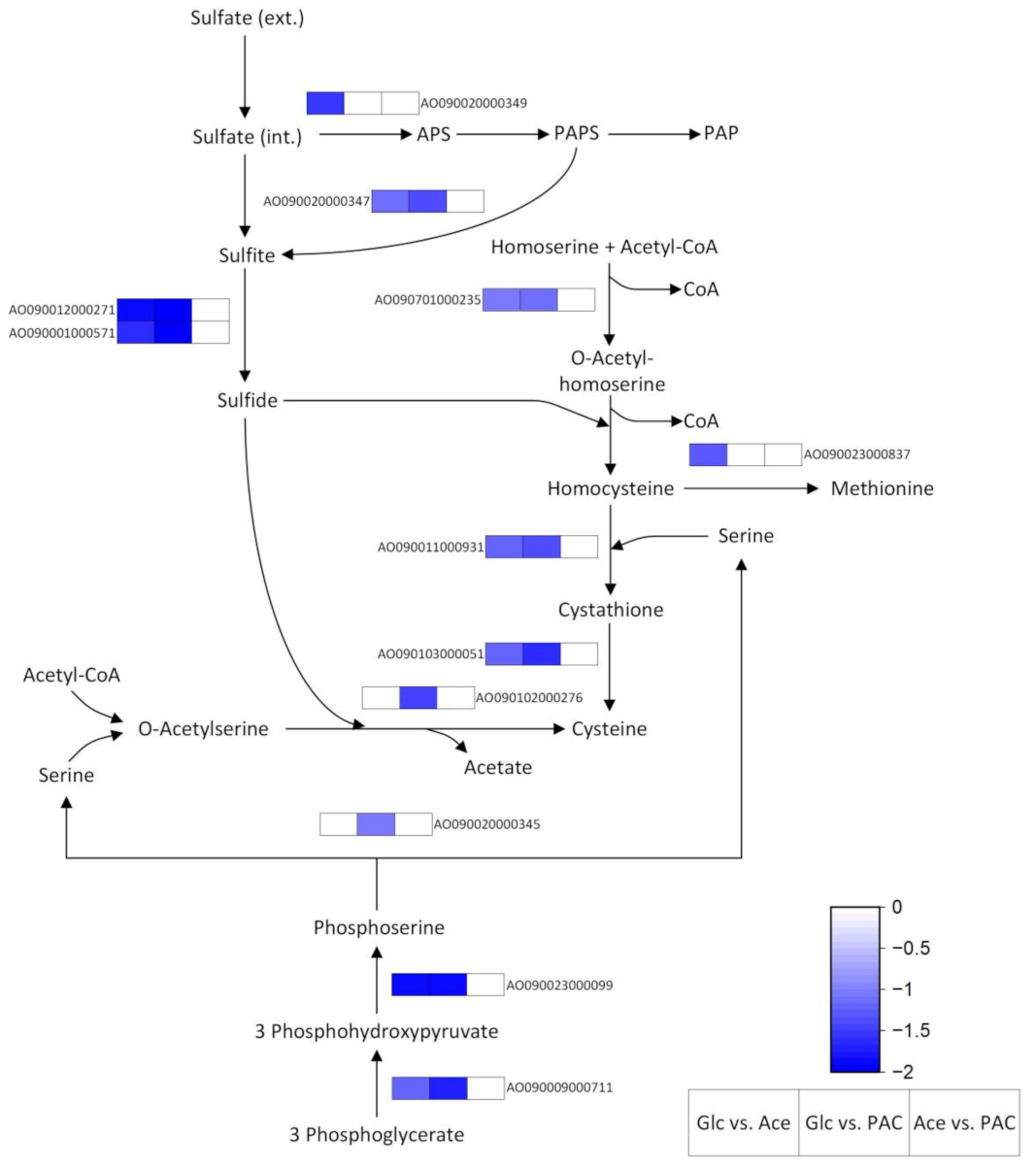
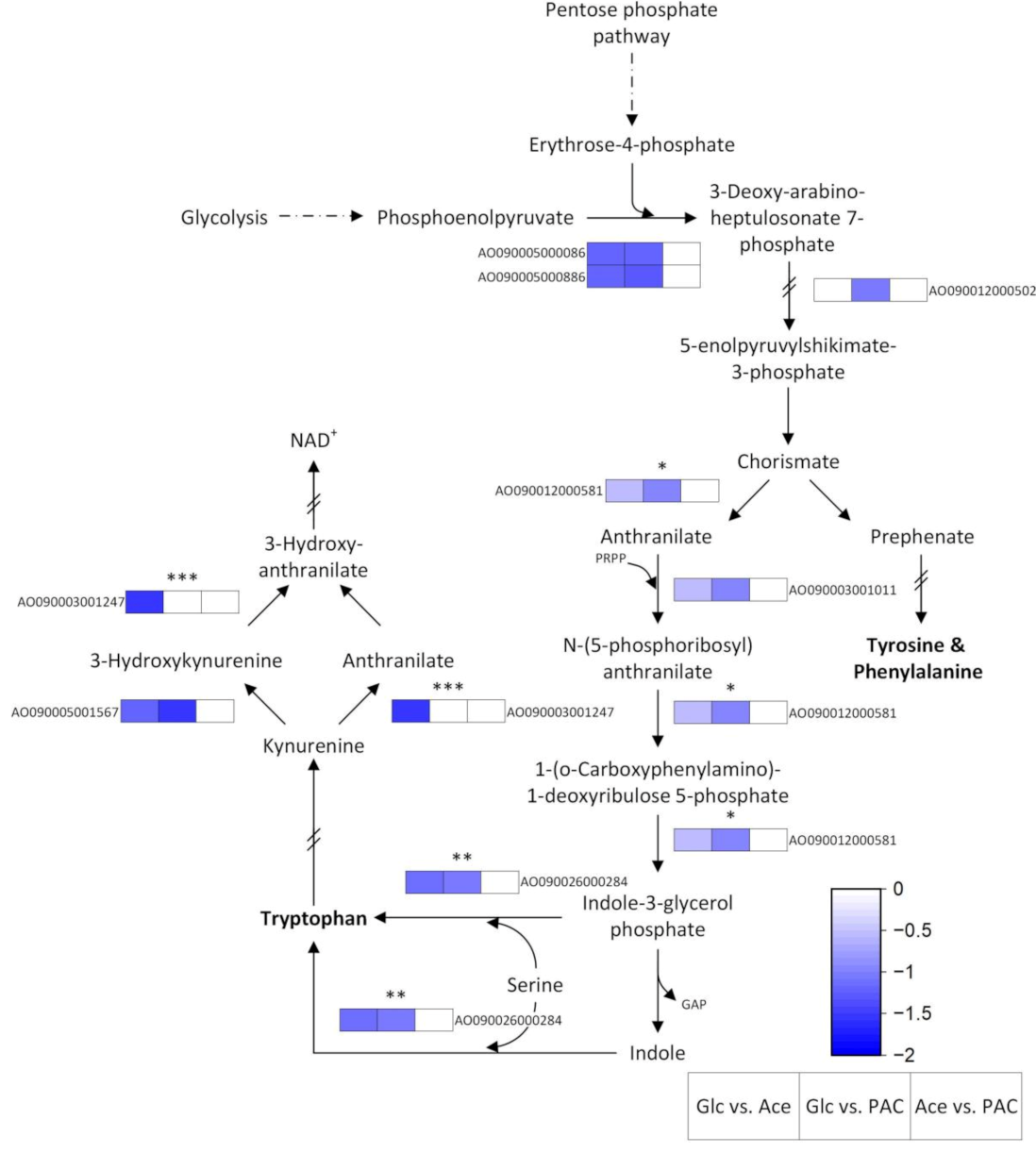

| Sample | Read Count | Alignment Ratio [%] | ||
|---|---|---|---|---|
| Raw | Clean | Uniquely Mapped | Unaligned | |
| Glc1 | 22,221,898 | 17,902,548 | 97 | 3 |
| Glc2 | 20,409,071 | 16,336,686 | 96 | 3 |
| Glc3 | 21,015,522 | 16,346,846 | 93 | 6 |
| Ace1 | 19,792,462 | 15,781,442 | 95 | 4 |
| Ace2 | 18,718,483 | 14,332,714 | 93 | 7 |
| Ace3 | 20,242,931 | 15,735,543 | 93 | 7 |
| PAC1 | 22,870,039 | 17,628,982 | 94 | 5 |
| PAC2 | 19,884,644 | 15,059,444 | 96 | 4 |
| PAC3 | 19,141,036 | 14,198,088 | 86 | 14 |
| Condition | DGE | KEGG Pathway ID | KEGG Pathway Name | Set Size | Adjusted p-Value |
|---|---|---|---|---|---|
| Glc vs. Ace | up | aor00500 | Starch and sucrose metabolism | 18 | 4.12 × 10−4 |
| aor00010 | Glycolysis/Gluconeogenesis | 14 | 2.61 × 10−2 | ||
| down | aor03008 | Ribosome biogenesis in eukaryotes | 27 | 7.72 × 10−14 | |
| aor01230 | Biosynthesis of amino acids | 39 | 4.10 × 10−8 | ||
| aor01210 | 2-oxocarboxylic acid metabolism | 12 | 4.12 × 10−4 | ||
| aor01200 | Carbon metabolism | 32 | 4.12 × 10−4 | ||
| aor01110 | Biosynthesis of secondary metabolites | 126 | 4.76 × 10−4 | ||
| aor00270 | Cysteine and methionine metabolism | 11 | 5.20 × 10−4 | ||
| aor01100 | Metabolic pathways | 253 | 1.23 × 10−3 | ||
| aor00380 | Tryptophan metabolism | 16 | 4.14 × 10−3 | ||
| aor00230 | Purine metabolism | 16 | 9.92 × 10−3 | ||
| aor00630 | Glyoxylate and dicarboxylate metabolism | 12 | 0.01 | ||
| aor00360 | Phenylalanine metabolism | 11 | 0.02 | ||
| Glc vs. PAC | up | aor00500 | Starch and sucrose metabolism | 25 | 1.50 × 10−4 |
| aor00520 | Amino sugar and nucleotide sugar metabolism | 19 | 4.40 × 10−3 | ||
| aor00100 | Steroid biosynthesis | 13 | 0.01 | ||
| down | aor03008 | Ribosome biogenesis in eukaryotes | 40 | 1.46 × 10−15 | |
| aor03010 | Ribosome | 39 | 8.16 × 10−14 | ||
| aor01230 | Biosynthesis of amino acids | 61 | 1.03 × 10−11 | ||
| aor01210 | 2-oxocarboxylic acid metabolism | 25 | 5.34 × 10−9 | ||
| aor03013 | RNA transport | 25 | 6.75 × 10−9 | ||
| aor01200 | Carbon metabolism | 51 | 1.16 × 10−7 | ||
| aor00270 | Cysteine and methionine metabolism | 19 | 1.50 × 10−6 | ||
| aor00970 | Aminoacyl-tRNA biosynthesis | 19 | 2.78 × 10−6 | ||
| aor03040 | Spliceosome | 16 | 3.68 × 10−6 | ||
| aor01110 | Biosynthesis of secondary metabolites | 182 | 1.22 × 10−5 | ||
| aor03020 | RNA polymerase | 12 | 1.31 × 10−5 | ||
| aor00190 | Oxidative phosphorylation | 16 | 2.23 × 10−5 | ||
| aor01100 | Metabolic pathways | 365 | 2.98 × 10−5 | ||
| aor03018 | RNA degradation | 18 | 5.57 × 10−5 | ||
| aor00020 | Citrate cycle (TCA cycle) | 16 | 8.90 × 10−5 | ||
| aor00290 | Valine, leucin and isoleucine biosynthesis | 11 | 1.22 × 10−3 | ||
| aor00630 | Glyoxylate and dicarboxylate metabolism | 20 | 1.27 × 10−3 | ||
| aor00680 | Methane metabolism | 11 | 2.18 × 10−3 | ||
| aor00250 | Alanine, aspartate and glutamate metabolism | 15 | 8.81 × 10−3 | ||
| aor00380 | Tryptophan metabolism | 20 | 9.44 × 10−3 | ||
| aor00620 | Pyruvate metabolism | 26 | 0.01 | ||
| aor00770 | Pantothenate and CoA biosynthesis | 15 | 0.02 | ||
| aor01212 | Fatty acid metabolism | 14 | 0.03 | ||
| aor00230 | Purine metabolism | 20 | 0.03 |
| Enzyme | E.C. | Regulation | Gene ID | Fold Change | |
|---|---|---|---|---|---|
| Glc vs. Ace | Glc vs. PAC | ||||
| β-fructofuranosidase | 3.2.1.26 | up | AO090020000640 | 1.71 | 2.71 |
| AO090701000038 | 1.51 | 1.50 | |||
| α-glucosidase | 3.2.1.20 | AO090003001209 | 4.18 | 3.93 | |
| trehalose 6-phosphate (T6P) synthase | 2.4.1.15 2.4.1.347 | AO090102000159 | 3.83 | 3.39 | |
| T6P synthase/phosphatase subunit | 2.4.1.15 3.1.3.12 | AO090005001531 | 1.40 | 1.46 | |
| glucan endo-1,3-β-D-glucosidase | 3.2.1.39 | AO090009000117 | 1.24 | 1.13 | |
| glucan 1,3-β-glucosidase | 3.2.1.58 | AO090011000362 | 1.39 | - | |
| AO090001000604 | - | 1.53 | |||
| AO090003000990 | - | 3.11 | |||
| β-glucosidase | 3.2.1.21 | AO090001000544 | 1.42 | 1.80 | |
| AO090038000425 | 2.37 | - | |||
| AO090003000497 | - | 1.97 | |||
| AO090001000266 | - | 3.66 | |||
| AO090701000274 | - | 1.12 | |||
| endoglucanase | 3.2.1.4 | AO090011000715 | 2.34 | 2.54 | |
| AO090003001342 | - | 3.70 | |||
| arabinogalactan endo-1,4-β-galactosidase | 3.2.1.89 | AO090001000492 | 2.04 | - | |
| cellulose 1,4-β-cellobiosidase | 3.2.1.91 | AO090001000348 | 2.16 | 3.95 | |
| glycogen debranching enzyme | 2.4.1.25 3.2.1.33 | AO090005000884 | 1.43 | 1.79 | |
| α-amylase | 3.2.1.1 | AO090003001497 | 4.19 | 2.50 | |
| AO090003001498 | 2.24 | 1.20 | |||
| AO090023000944 | 6.33 | 7.45 | |||
| AO090120000196 | 6.40 | 7.07 | |||
| glucoamylase | 3.2.1.3 | AO090010000746 | 6.63 | 9.13 | |
| 1,4-α-glucan branching enzyme | 2.4.1.18 | AO090010000483 | 1.02 | 1.66 | |
| 1,3-β-glucan synthase | 2.4.1.34 | AO090009000174 | - | 1.11 | |
| glucan 1,3-β-glucosidase | 3.2.1.58 | down | AO090038000279 | - | −1.04 |
| β-glucosidase | 3.2.1.21 | AO090701000841 | - | −3.24 | |
| Enzyme | EC | Gene ID | Fold Change | ||
|---|---|---|---|---|---|
| Glc vs. Ace | Glc vs. PAC | Ace vs. PAC | |||
| catalase | 1.11.1.6 | AO090701000158 | 2.38 | 2.76 | - |
| AO090120000068 | −1.15 | - | 1.07 | ||
| AO090020000389 | - | −6.43 | −4.00 | ||
| cytochrome c peroxidase | 1.11.1.5 | AO090023000654 | −1.26 | −1.48 | - |
| AO090103000329 | −2.95 | −2.67 | - | ||
| superoxide dismutase | 1.15.1.1 | AO090005001580 | −1.01 | −1.60 | - |
| AO090020000521 | −1.20 | −1.30 | |||
| glutathione synthase | 6.3.2.3 | AO090701000193 | - | −1.07 | −0.96 |
| glutaredoxin 3 | - | AO090009000473 | - | −1.17 | −0.95 |
| monothiol glutaredoxin | - | AO090023001002 | - | −1.47 | - |
| thioredoxin | AO090026000065 | - | - | −5.80 | |
| AO090026000708 | - | −1.28 | −1.40 | ||
| AO090020000504 | 2.05 | 1.78 | - | ||
| glutathione S-transferase | 2.5.1.18 | AO090005000973 | −1.36 | −1.16 | - |
| AO090003000631 | - | −2.87 | −3.31 | ||
| AO090103000485 | - | −2.73 | −2.37 | ||
| AO090012000378 | 2.34 | - | −2.51 | ||
| AO090010000447 | - | 1.18 | - | ||
| AO090103000134 | 1.49 | 1.79 | - | ||
| AO090103000149 | 9.85 | 5.37 | −4.50 | ||
| Condition | EC | Gene ID | Fold Change |
|---|---|---|---|
| Glc vs. PAC | 1.1.1.1 | AO090005000125 | 1.68 |
| AO090038000108 | 2.85 | ||
| AO090026000555 | 3.43 | ||
| 1.1.1.2 | AO090005001358 | −3.78 | |
| AO090010000668 | −6.14 | ||
| Glc vs. Ace | 1.1.1.1 | AO090009000634 | 1.47 |
| AO090038000108 | 4.19 | ||
| 1.1.1.2 | AO090038000575 | −1.62 | |
| Ace vs. PAC | 1.1.1.1 | AO090026000555 | 2.28 |
| 1.1.1.2 | AO090005001358 | −3.63 | |
| AO090023000460 | −3.39 | ||
| AO090010000668 | −8.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubisch, C.; Kövilein, A.; Aliyu, H.; Ochsenreither, K. RNA-Seq Based Transcriptome Analysis of Aspergillus oryzae DSM 1863 Grown on Glucose, Acetate and an Aqueous Condensate from the Fast Pyrolysis of Wheat Straw. J. Fungi 2022, 8, 765. https://doi.org/10.3390/jof8080765
Kubisch C, Kövilein A, Aliyu H, Ochsenreither K. RNA-Seq Based Transcriptome Analysis of Aspergillus oryzae DSM 1863 Grown on Glucose, Acetate and an Aqueous Condensate from the Fast Pyrolysis of Wheat Straw. Journal of Fungi. 2022; 8(8):765. https://doi.org/10.3390/jof8080765
Chicago/Turabian StyleKubisch, Christin, Aline Kövilein, Habibu Aliyu, and Katrin Ochsenreither. 2022. "RNA-Seq Based Transcriptome Analysis of Aspergillus oryzae DSM 1863 Grown on Glucose, Acetate and an Aqueous Condensate from the Fast Pyrolysis of Wheat Straw" Journal of Fungi 8, no. 8: 765. https://doi.org/10.3390/jof8080765
APA StyleKubisch, C., Kövilein, A., Aliyu, H., & Ochsenreither, K. (2022). RNA-Seq Based Transcriptome Analysis of Aspergillus oryzae DSM 1863 Grown on Glucose, Acetate and an Aqueous Condensate from the Fast Pyrolysis of Wheat Straw. Journal of Fungi, 8(8), 765. https://doi.org/10.3390/jof8080765






