Distribution Types of Lichens in Hungary That Indicate Changing Environmental Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
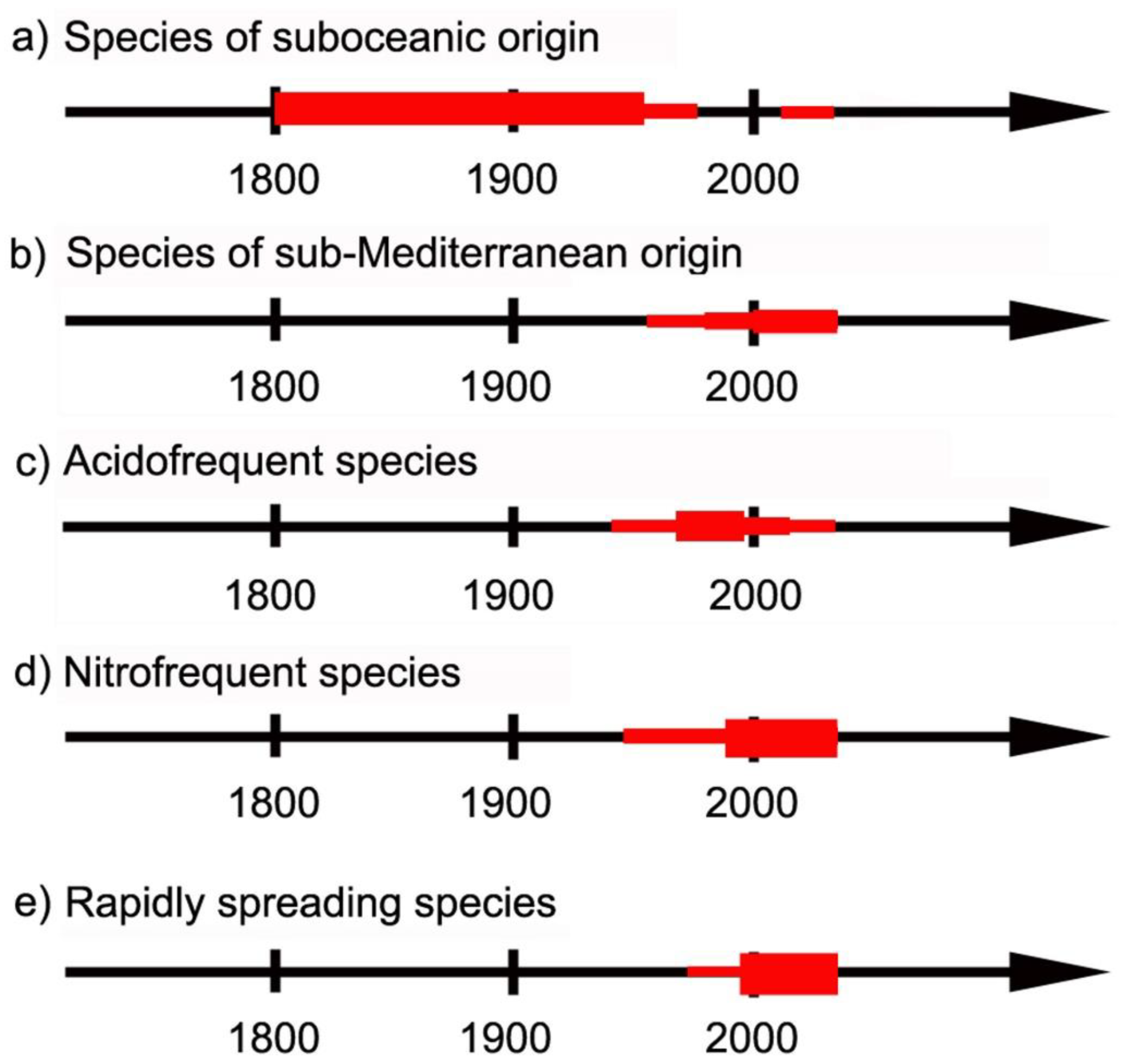
- (a)
- species with decreasing occurrences;
- (b)
- species with no or few former records but with increasing occurrences in recent decades;
- (c)
- species with increasing, then decreasing occurrences;
- (d)
- species with widely increasing occurrences in recent decades;
- (e)
- species with rapidly increasing occurrences.
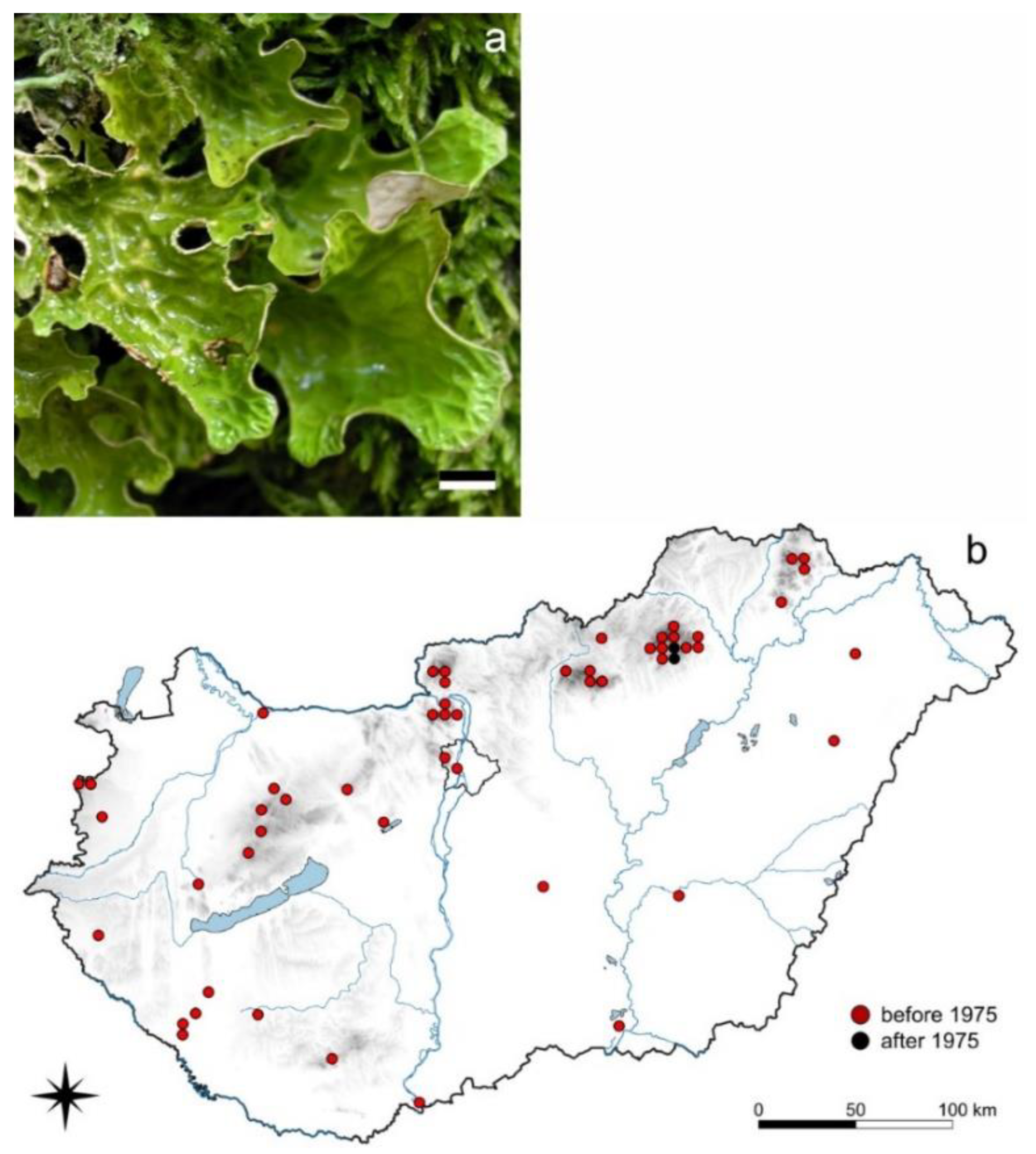
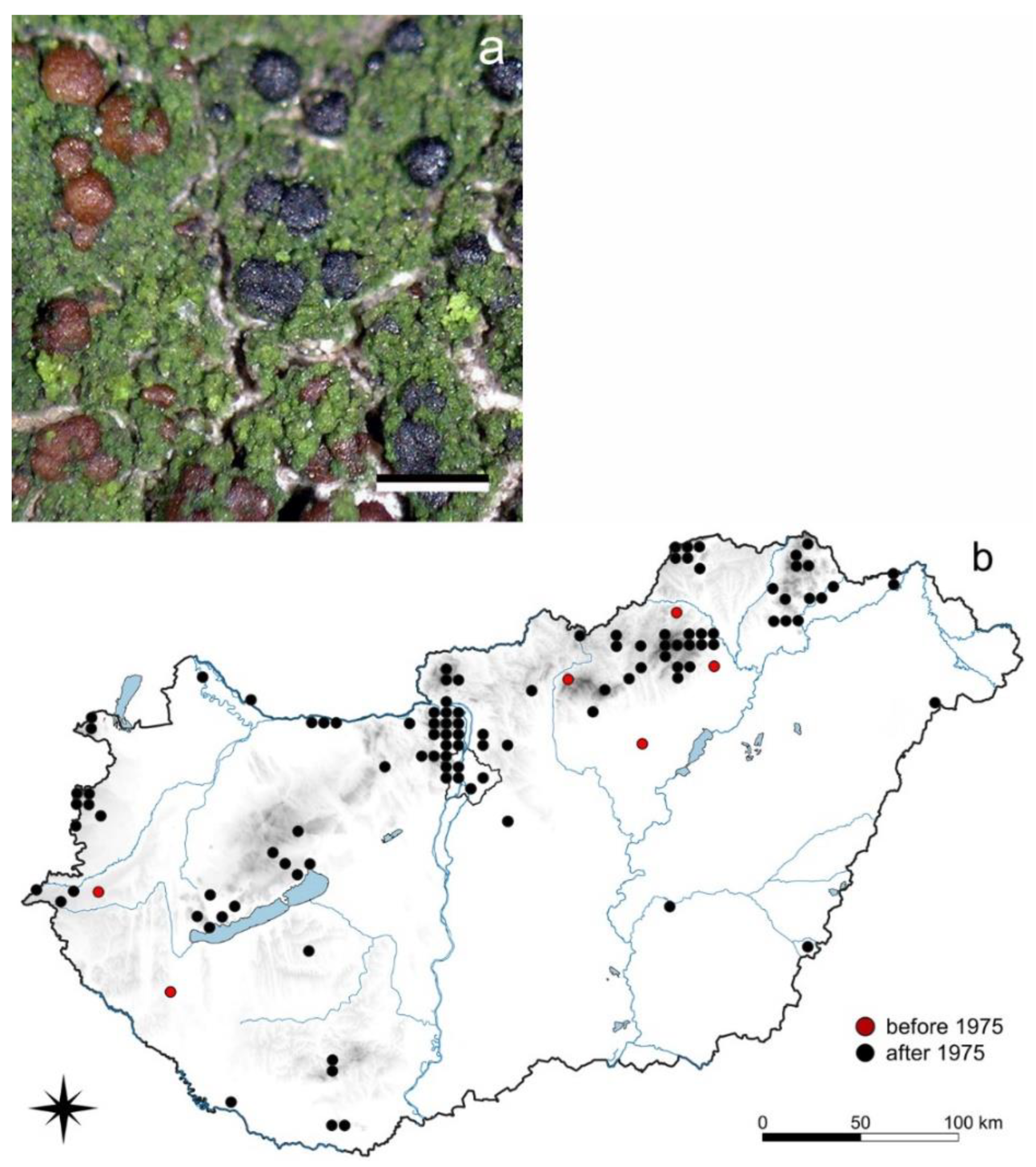
3.1. Species of Decreasing Occurrences
3.1.1. Lobaria pulmonaria L.
3.1.2. Menegazzia terebrata (Hoffm.) A. Massal.
3.2. Species with No or Few Former Records but with Increasing Occurrences in Recent Decades
3.2.1. Flavoparmelia soredians (Nyl.) Hale
3.2.2. Hyperphyscia adglutinata (Flörke) H. Mayrhofer et Poelt
3.2.3. Solenopsora candicans (Dicks.) J. Steiner
3.3. Species with Increasing, then Decreasing Occurrences
3.3.1. Scoliciosporum chlorococcum (Stenh.) Vězda
3.3.2. Straminella conizaeoides (Cromb.) S. Y. Kondr., Lőkös et Farkas
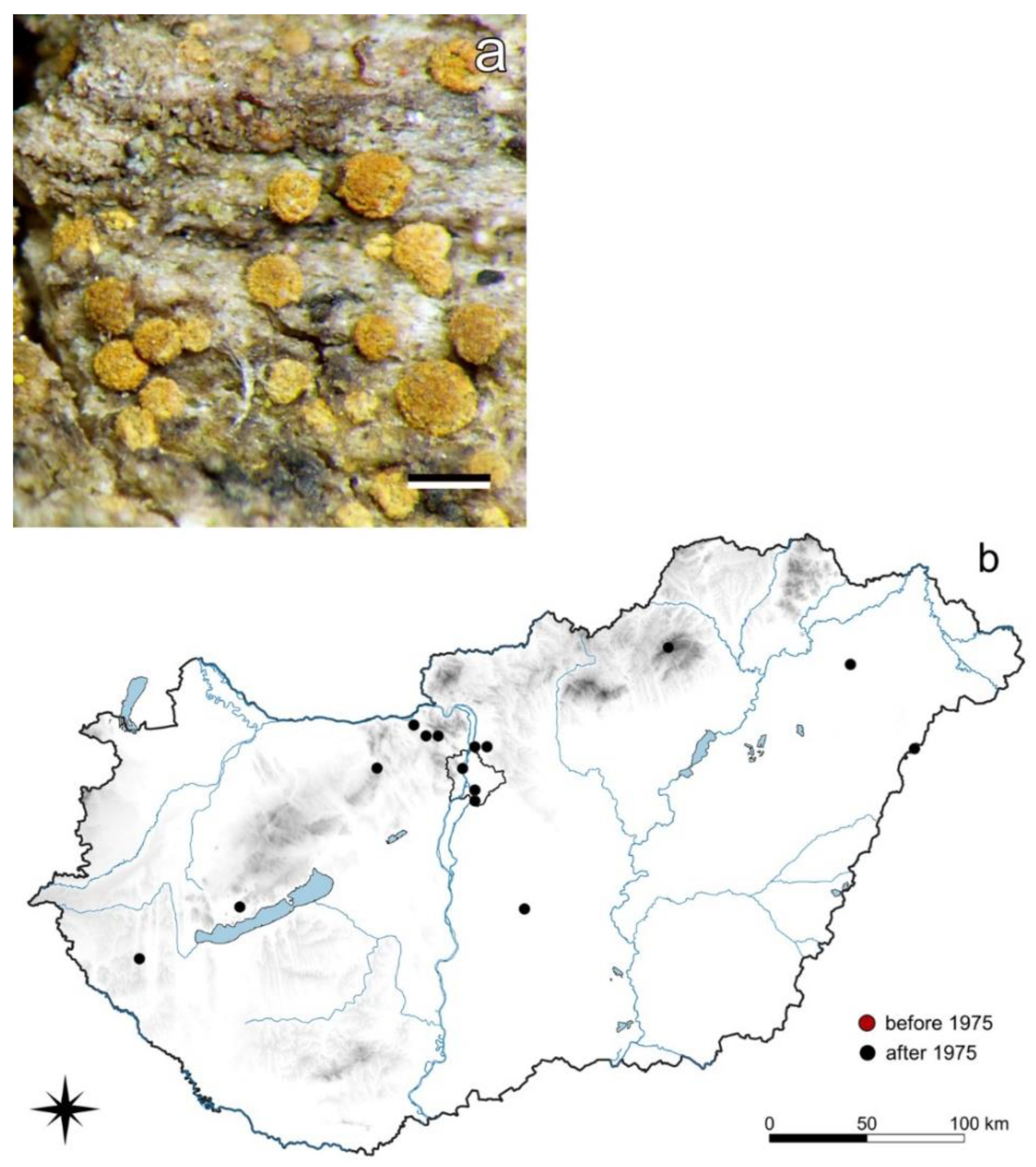
3.4. Species with Widely Increasing Occurrences
3.4.1. Physcia aipolioides (Nádv.) Breuss et Türk
3.4.2. Piccolia ochrophora (Nyl.) Hafellner
3.4.3. Xanthoria parietina (L.) Th. Fr.
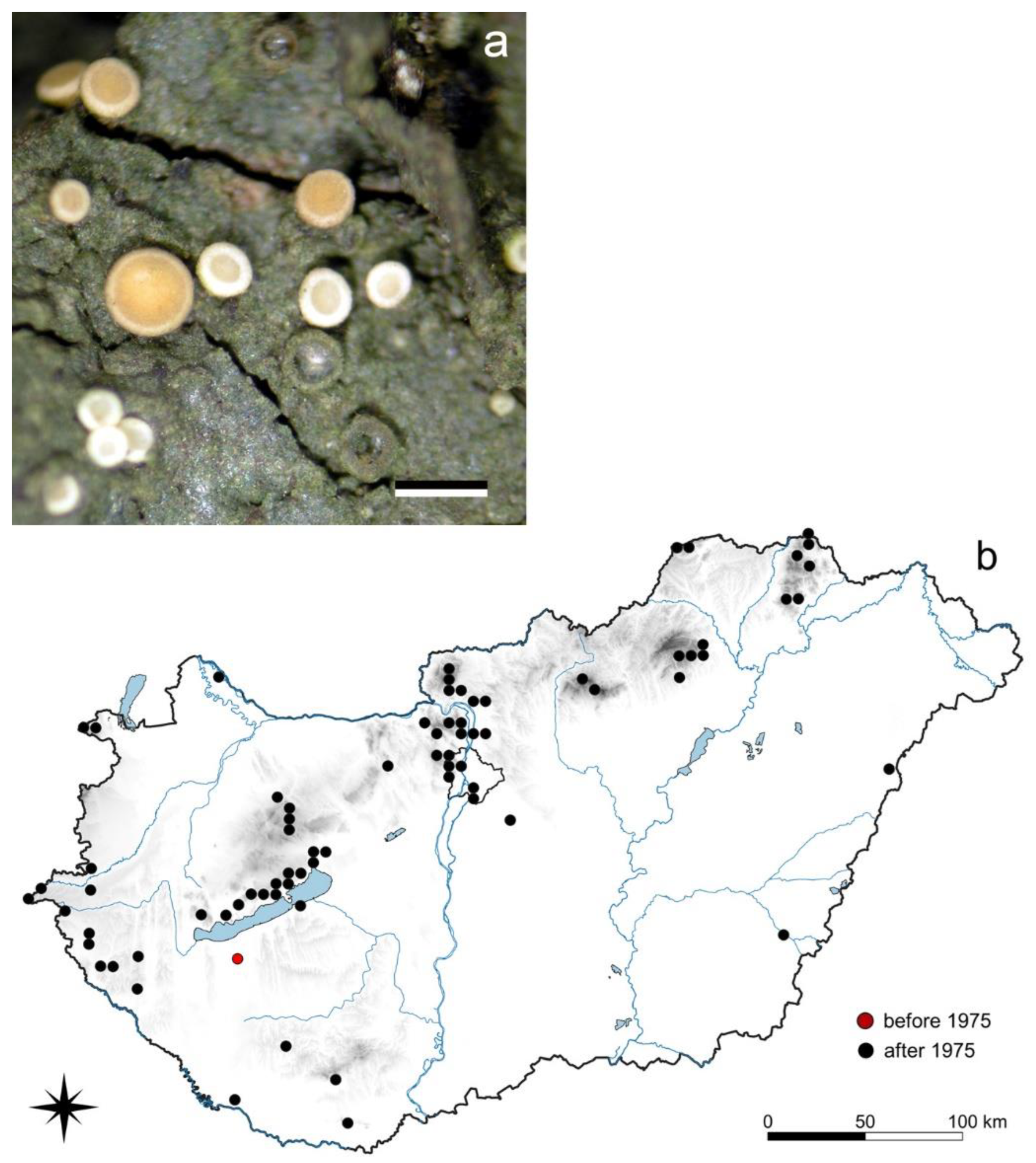
3.5. Species with Rapidly Increasing Occurrences
3.5.1. Absconditella lignicola Vězda et Pišút
3.5.2. Coenogonium pineti (Ach.) Lücking et Lumbsch
3.5.3. Evernia divaricata (L.) Ach.
4. Discussion
4.1. Species of Suboceanic Origin
4.2. Species of Sub-Mediterranean Requirements
4.3. Acidofrequent Species
4.4. Nitrofrequent Species
4.5. Rapidly Spreading Species of Uncertain Reason
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nash, T.H., III. Lichen Biology, 2nd ed.; University Press: Cambridge, UK, 2008; pp. 1–486. [Google Scholar]
- Seaward, M.R.D. (Ed.) Lichen Ecology; Academic Press: London, UK, 1977; pp. 1–550. [Google Scholar]
- Hawksworth, D.L.; Honegger, R. The lichen thallus: A symbiotic phenotype of nutritionally specialized fungi and and its response to gall producers. In Plant Galls. Organisms, Interactions, Populations; The Systematics Association Special Volume; Williams, M.A.J., Ed.; Clarendon Press: Oxford, UK, 1994; pp. 77–98. [Google Scholar]
- Spribille, T.; Tuovinen, V.; Resl, P.; Vanderpool, D.; Wolinski, H.; Aime, M.C.; Schneider, K.; Stabentheiner, E.; Toome-Heller, M.; Thor, G.; et al. Basidiomycete yeasts in the cortex of ascomycete macrolichens. Science 2016, 353, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, I.A.; Cardinale, M.; Berg, G.; Grube, M. Microbial cargo: Do bacteria on symbiotic propagules reinforce the microbiome of lichens? Environ. Microbiol. 2014, 16, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Grube, M. Lichens redefined as complex ecosystems. New Phytol. 2020, 227, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Borrer, W. Specimen of a Lichenographia Britannica, or Attempt at a History of the British Lichens; C. Sloman: Yarmouth, UK, 1839; pp. 1–240. [Google Scholar]
- Sernander, R. Stockholms Natur; Almquist & Wiksells: Uppsala, Sweden, 1926; pp. 1–189. [Google Scholar]
- Farkas, E. Légszennyeződési Vizsgálatok Budapest Területén Zuzmó-Bioindikátorokkal. (Air Pollution Studies by Lichen Bioidindicators in BUDAPEST). Master’s Thesis, ELTE TTK Növényrendszertani és -ökológiai Tanszék, Budapest, Hungary, 1982. Manuscript in Hungarian. pp. 1–91. [Google Scholar]
- Farkas, E.; Lőkös, L.; Verseghy, K. Lichens as indicators of air pollution in the Budapest Agglomeration. I. Air pollution map based on floristic data and heavy metal concentration measurements. Acta Bot. Hung. 1985, 31, 45–68. [Google Scholar]
- Hawksworth, D.L.; Rose, F. Lichens as Pollution Monitors; Edward Arnold: London, UK, 1976; pp. 1–60. [Google Scholar]
- Ferry, B.W.; Baddeley, M.S. Air Pollution and Lichens; Hawksworth, D.L., Ed.; The Athlon Press of the University of London: London, UK, 1973; pp. 1–389. [Google Scholar]
- Gilbert, O.L. Lichens and air pollution. In The Lichens; Ahmadjian, V., Hale, M.E., Eds.; Academic Press: New York, NY, USA; London, UK, 1973; pp. 443–472. [Google Scholar]
- Gries, C. Lichens as indicators of air pollution. In Lichen Biology; Nash, T.H., Ed.; University Press: Cambridge, UK, 1996; pp. 240–254. [Google Scholar]
- Białońska, D.; Dayan, F.E. Chemistry of the lichen hypogymnia physodes transplanted to an industrial region. J. Chem. Ecol. 2005, 31, 2975–2991. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; McManus, P.M. Changes in the lichen flora on trees in Epping forest through periods of increasing and then ameliorating sulphur dioxide air pollution. Essex Nat. 1992, 11, 92–101. [Google Scholar]
- Rose, C.I.; Hawksworth, D.L. Lichen recolonization in London’s cleaner air. Nature 1981, 289, 289–292. [Google Scholar] [CrossRef]
- Van Herk, C.M. Mapping of ammonia pollution with epiphytic lichens in the Netherlands. Lichenologist 1999, 31, 9–20. [Google Scholar] [CrossRef]
- Van Herk, C.M. Epiphytes on wayside trees as an indicator of eutrophication in the Netherlands. In Monitoring with Lichens—Monitoring Lichens; Nato Science Series. IV. Earth and Environmental Sciences; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 285–289. [Google Scholar]
- Van Dobben, H.F.; de Bakker, A.J. Re-mapping epiphytic lichen biodiversity in the Netherlands: Effects of decreasing SO2 and increasing NH3. Acta Bot. Neerl. 1996, 45, 55–71. [Google Scholar] [CrossRef]
- Ruoss, E. How agriculture affects lichen vegetation in central Switzerland. Lichenologist 1999, 31, 63–73. [Google Scholar] [CrossRef]
- Wolseley, P.; James, P. Using lichens as biomonitors of ammonia concentrations in Norfolk and Devon. Br. Lich. Soc. Bull. 2002, 91, 1–5. [Google Scholar]
- Bates, J.W.; Farmer, A.M. (Eds.) Bryophytes and Lichens in a Changing Environment; Clarendon Presss: Oxford, UK, 1992; pp. 1–404. [Google Scholar]
- Vagts, I.; Kinder, M. The response of different Cladonia species after treatment with fertilizer or lime in heathland. Lichenologist 1999, 31, 75–83. [Google Scholar] [CrossRef]
- Sparrius, L.B. Monitoring van Ammoniak Met Korstmossen in Friesland in 2003; BIO DIV; Adviesbureau voor Biodiversiteitsonderzoek: Gouda, The Netherlands, 2003; pp. 1–101. [Google Scholar]
- Bergamini, A.; Scheidegger, C.; Stofer, S.; Carvalho, P.; Davey, S.; Dietrich, M.; Dubs, F.; Farkas, E.; Groner, U.; Kärkkäinen, K.; et al. Performance of macrolichens and lichen genera as indicators of lichen species richness and composition. Cons. Biol. 2005, 19, 1051–1062. [Google Scholar] [CrossRef]
- Boch, S.; Saiz, H.; Allan, E.; Schall, P.; Prati, D.; Schulze, E.-D.; Hessenmöller, D.; Sparrius, L.B.; Fischer, M. Direct and indirect effects of management intensity and environmental factors on the functional diversity of lichens in Central European forests. Microorganisms 2021, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Stofer, S.; Bergamini, A.; Aragón, G.; Carvalho, P.; Coppins, B.J.; Davey, S.; Dietrich, M.; Farkas, E.; Kärkkäinen, K.; Keller, C.; et al. Species richness of lichen functional groups in relation to land use intensity. Lichenologist 2006, 38, 331–353. [Google Scholar] [CrossRef]
- Ellis, C.J.; Asplund, J.; Benesperi, R.; Branquinho, C.; Di Nuzzo, L.; Hurtado, P.; Martínez, I.; Matos, P.; Nascimbene, J.; Pinho, P.; et al. Functional traits in lichen ecology: A review of challenge and opportunity. Microorganisms 2021, 9, 766. [Google Scholar] [CrossRef]
- Adamska, E.; Juśkiewicz, W. Visualisation of the influence of habitat on lichen occurrence, Toruñ, Poland. J. Maps 2018, 14, 9–16. [Google Scholar] [CrossRef]
- Caruso, A.; Rudolphi, J. Influence of substrate age and quality on species diversity of lichens and bryophytes on stumps. Bryologist 2009, 112, 520–531. [Google Scholar] [CrossRef]
- Degtjarenko, P.; Matos, P.; Marmor, L.; Branquinho, C.; Randlane, T. Functional traits of epiphytic lichens respond to alkaline dust pollution. Fungal Ecol. 2018, 36, 81–88. [Google Scholar] [CrossRef]
- Dymytrova, L.; Brändli, U.-B.; Ginzler, C.; Scheidegger, C. Forest history and epiphytic lichens: Testing indicators for assessing forest autochthony in Switzerland. Ecol. Indic. 2017, 84, 847–857. [Google Scholar] [CrossRef]
- Łubek, A.; Kukwa, M.; Jaroszewisz, B.; Czortek, P. Shifts in lichen species and functional diversity in a primeval forest ecosystem as a response to environmental changes. Forests 2021, 12, 686. [Google Scholar] [CrossRef]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P.A. (Eds.) Monitoring with Lichens—Monitoring Lichens; NATO Science Series. IV. Earth and Environmental Sciences, 7; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 1–408. [Google Scholar]
- Woodruff, D.S. Declines of biomes and biotas and the future of evolution. Proc. Natl. Acad. Sci. USA 2001, 98, 5471–5476. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Vida, G. Helyünk a Bioszférában. (Our Place in the Biosphere); Typotex Kiadó: Budapest, Hungary, 2008; pp. 1–128. [Google Scholar]
- Lumbsch, H.T.; Ahti, T.; Altermann, S.; Amo de Paz, G.; Aptroot, A.; Arup, U.; Barcenas Pena, A.; Bawingan, P.A.; Benatti, M.N.; Betancourt, L.; et al. One hundred new species of lichenized fungi: A signature of undiscovered global diversity. Phytotaxa 2011, 18, 1–127. [Google Scholar] [CrossRef]
- Wirth, V. Einheimisch oder eingewandert? Über die Einschätzung von Neufunden von Flechten. (Indigenous or immigrated? How to evaluate new reports of lichens). Bibl. Lichenol. 1997, 67, 277–288. [Google Scholar]
- Vondrák, J.; Liška, J. Changes in distribution and substrate preferences of selected threatened lichens in the Czech Republic. Biologia 2010, 65, 595–602. [Google Scholar] [CrossRef]
- Hurtado, P.; Prieto, M.; de Bello, F.; Aragón, G.; López-Angulo, J.; Giorandi, P.; Díaz-Peña, E.M.; Vicente, R.; Merinero, S.; Košuthová, A.; et al. Contrasting environmental drivers determine biodiversity patterns in epiphytic lichen communities along a European gradient. Microorganisms 2020, 8, 1913. [Google Scholar] [CrossRef]
- National Atlas of Hungary: Volume 2. Natural Environment; Kocsis, K., Gercsák, G., Horváth, G., Keresztesi, Z., Nemerkényi, Z., Eds.; Geographical Institute, Research Centre for Astronomy and Earth Sciences: Budapest, Hungary, 2018; pp. 1–183. [Google Scholar]
- Gábris, G.; Pécsi, M.; Schweitzer, F.; Telbisz, T. Relief. In National Atlas of Hungary—Natural Environment; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; pp. 42–57. [Google Scholar]
- Molnár, Z.; Király, G.; Fekete, G.; Aszalós, R.; Barina, Z.; Bartha, D.; Biró, M.; Borhidi, A.; Bölöni, J.; Czúcz, B.; et al. Vegetation. In National Atlas of Hungary—Natural Environment; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; pp. 94–103. [Google Scholar]
- Bihari, Z.; Babolcsai, G.; Bartholy, J.; Ferenczi, Z.; Gerhátné Kerényi, J.; Haszpra, L.; Homoki-Ujváry, K.; Kovács, T.; Lakatos, M.; Németh, Á.; et al. Climate. In National Atlas of Hungary—Natural Environment; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; pp. 58–71. [Google Scholar]
- Kerényi, A.; Bihari, Z.; Fazekas, I.; Pásztor, L.; Tahy, Á.; Túri, Z.; Várallyay, G.; Zagyva, T.A. Environmental protection. In National Atlas of Hungary—Natural Environment; Kocsis, K., Ed.; MTA CSFK Geographical Institute: Budapest, Hungary, 2018; pp. 130–143. [Google Scholar]
- KSH, Központi Statisztikai Hivatal 2022. 5.3.14. Nemzetgazdasági Ágak és Háztartások Ammónia (Nh3) Kibocsátása (1990–2017). Available online: https://www.ksh.hu/stadat_eves_5 (accessed on 12 March 2022).
- Verseghy, K. Magyarország Zuzmóflórájának Kézikönyve; Magyar Természettudományi Múzeum: Budapest, Hungary, 1994; pp. 1–415. [Google Scholar]
- Lőkös, L.; Farkas, E. Revised Checklist of the Hungarian Lichen-Forming and Lichenicolous Fungi. (Magyarországi Zuzmók és Zuzmólakó Mikrogombák Revideált Fajlistája). 2009. Available online: https://ecolres.hu/Farkas.Edit (accessed on 27 September 2021).
- Smith, C.W.; Aptroot, A.; Coppins, B.J.; Fletcher, A.; Gilbert, O.L.; James, P.W.; Wolseley, P.A. (Eds.) The Lichens of Great Britain and Ireland; British Lichen Society: London, UK, 2009; pp. 1–1046. [Google Scholar]
- Wirth, V.; Hauck, M.; Schultz, M. Die Flechten Deutschlands; Ulmer Verlag: Stuttgart, Germany, 2013; pp. 1–1244. [Google Scholar]
- Arup, U.; Ekman, S.; Lindblom, L.; Mattsson, J.-E. High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist 1993, 25, 61–71. [Google Scholar] [CrossRef]
- Molnár, K.; Farkas, E. Depsides and depsidones in populations of the lichen Hypogymnia physodes and its genetic diversity. Ann. Bot. Fenn. 2011, 48, 473–482. [Google Scholar] [CrossRef]
- IF (Index Fungorum Partnership). Available online: http://www.indexfungorum.org/ (accessed on 27 September 2021).
- Thiers, B. (Continuously Updated). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 27 September 2021).
- Niklfeld, H. Bericht über die Kartierung der Flora Mitteleuropa. Taxon 1971, 20, 545–571. [Google Scholar] [CrossRef]
- Borhidi, A. Role of mapping the flora of Europe in nature conservation. Norrlinia 1984, 2, 87–98. [Google Scholar]
- Moberg, R.; Wirth, V. Meeting on lichen mapping in Europe. Agreements and further proposals. Stuttg. Beitr. Nat. Ser. A 1990, 456, 149–150. [Google Scholar]
- Wirth, V. Initiation of a European lichen mapping project. Proposals and considerations. Stuttg. Beitr. Nat. Ser. A 1990, 456, 147–148. [Google Scholar]
- Søchting, U. Lichen mapping project in Europe. Graph. Scr. 1991, 3, 72. [Google Scholar]
- Farkas, E.; Lőkös, L. A Peltigera leucophlebia magyarországon. (Peltigera leucophlebia in Hungary). Kitaibelia 2008, 13, 159. [Google Scholar]
- Farkas, E.; Lőkös, L. Lobaria pulmonaria (lichen-forming fungi) in Hungary. (A tüdőzuzmó (Lobaria pulmonaria) elterjedése Magyarországon). Mikol. Közlem. Clusiana 2009, 48, 11–18. [Google Scholar]
- Boros, Á. A visszacsatolt területek gyógynövénykincsei. (Herb assets of regions returned to Hungary). Herba 1940, 15, 1–7. [Google Scholar]
- Farkas, E.; Lajtha-Tabajdi, Á.; Lőkös, L.; Molnár, K.; Paczkó, L.; Sinigla, M. Flavoparmelia soredians (Parmeliaceae, lichenised Ascomycetes), a spreading lichen species in Hungary. Studia Bot. Hung. 2016, 47, 5–12. [Google Scholar] [CrossRef]
- Farkas, E.; Lőkös, L. Zuzmók biodiverzitás-vizsgálata Gyűrűfű környékén. (Biodiversity studies on lichen-forming fungi at Gyűrűfű (SW Hungary)). Mikol. Közlem. Clusiana 2010, 48, 145–153. [Google Scholar]
- Farkas, E.; Lőkös, L.; Sinigla, M.; Varga, N. A Mogyorós-hegy (Litér) és az Ugri-hegy (Királyszentistván) zuzmóflórája. (The lichen flora of the hills “Mogyorós-hegy” (Litér, Hungary) and “Ugri-hegy” (Királyszentistván, Hungary)). Folia Mus. Hist.-Nat. Bakony 2014, 31, 7–24. [Google Scholar]
- Varga, N.; Lőkös, L.; Farkas, E. The lichen-forming and lichenicolous fungi of the Soroksár Botanical Garden (Szent István University, Budapest, Hungary). Studia Bot. Hung. 2016, 47, 13–28. [Google Scholar] [CrossRef]
- Farkas, E.; Guttová, A.; Lőkös, L.; Molnár, K. Distribution of Solenopsora candicans (lichen-forming fungi, Catillariaceae) in Hungary. Acta Bot. Hung. 2011, 53, 305–311. [Google Scholar] [CrossRef]
- Lőkös, L. A Bacidia s. l. Zuzmónemzetség Hazai Fajainak Taxonómiai Revíziója. [Taxonomic Revision of the Bacidia s. l. Species in Hungary]. PhD Thesis, PTE, Pécs, Hungary, 2005; pp. 1–158. [Google Scholar]
- Lisická, E.; Lackovičová, A.; Liška, J.; Lőkös, L.; Lisický, M.J. Physcia aipolioides—Ein Beispiel einer invasiven Flechte oder einer unterschätzen Verbreitung? (Physcia aipolioides—An example of an invasive lichen or an underestimated distribution?). Sauteria 2008, 15, 303–318. [Google Scholar]
- Farkas, E. Notes and schedae to Lichenes Delicati Exsiccati Editae in memoriam Antonín Vězda (1920–2008), fasc. 4. Acta Bot. Hung. 2014, 56, 305–317. [Google Scholar] [CrossRef]
- Farkas, E.; Lőkös, L. Distribution of Absconditella lignicola (Stictidaceae, lichenized Ascomycetes) in Hungary. Studia Bot. Hung. 2021, 52, 115–124. [Google Scholar] [CrossRef]
- Farkas, E.; Lőkös, L. Dimerella pineti (lichen-forming fungus) in Hungary. Acta Microbiol. Immunol. Hung. 2005, 52, 228. [Google Scholar]
- Wirth, V. Ökologische Zeigerwerte von Flechten—erweiterte und aktualisierte Fassung. [Ecological indicator values of lichens —Enlarged and updated species list]. Herzogia 2010, 23, 229–248. [Google Scholar] [CrossRef]
- Hawksworth, D.L.; Rose, F. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 1970, 227, 145–148. [Google Scholar] [CrossRef]
- Wagner, H.H.; Werth, S.; Kalwij, J.M.; Bolli, J.C.; Scheidegger, C. Modelling forest colonization by an epiphytic lichen using a landscape genetic approach. Landsc. Ecol. 2006, 21, 849–865. [Google Scholar] [CrossRef]
- Walser, J.C. Molecular evidence for limited dispersal of vegetative propagules in the epiphytic lichen Lobaria pulmonaria. Amer. J. Bot. 2004, 91, 1273–1276. [Google Scholar] [CrossRef]
- Walser, J.C.; Zoller, S.; Büchler, U.; Scheidegger, C. Species-specific detection of Lobaria pulmonaria (lichenized ascomycete) diaspores in litter samples trapped in snow cover. Mol. Ecol. 2001, 10, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Walser, J.C.; Sperisen, C.; Soliva, M.; Scheidegger, C. Fungus-specific microsatellite primers of lichens: Application for the assessment of genetic variation on different spatial scales in Lobaria pulmonaria. Fungal Genet. Biol. 2003, 40, 72–82. [Google Scholar] [CrossRef]
- Walser, J.C.; Gugerli, F.; Holderegger, R.; Kuonen, D.; Scheidegger, C. Recombination and clonal propagation in different populations of the lichen Lobaria pulmonaria. Heredity 2004, 93, 322–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walser, J.C.; Holderegger, R.; Gugerli, F.; Hoebee, E. Microsatellites reveal regional population differentiation and isolation in Lobaria pulmonaria, an epiphytic lichen. Mol. Ecol. 2005, 14, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Werth, S.; Wagner, H.H.; Gugerli, F.; Holderegger, R.; Csencsics, D.; Kalwij, J.M.; Scheidegger, C. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 2006, 87, 2037–2046. [Google Scholar] [CrossRef]
- Werth, S.; Gugerli, F.; Holderegger, R.; Wagner, H.H.; Csencsics, D.; Scheidegger, C. Landscape-level gene flow in Lobaria pulmonaria, an epiphytic lichen. Mol. Ecol. 2007, 16, 2807–2815. [Google Scholar] [CrossRef]
- Rose, F. Lichenological indicators of age and environmental continuity in woodlands. In Lichenology: Progress and Problems; Brown, D.H., Hawksworth, D.L., Bailey, R.H., Eds.; Academic Press: London, UK, 1976; pp. 279–307. [Google Scholar]
- Kondratyuk, S.Y.; Coppins, B.J. (Eds.) Lobarion lichens as indicators of the primeval forests of the Eastern Carpathians. In Proceedings of the Darwin International Workshop: Honored to the 100-Years Anniversary of a Famous Ukrainian Lichenologist Professor Alfred M. Oxner (1898–1973), Kostrino, Ukraine, 25–30 May 1998; Phytosociocentre: Kiev, Ukraine, 1998; pp. 1–192. [Google Scholar]
- Farkas, E.; Lőkös, L.; Molnár, K. Az Ochrolechia arborea zuzmófaj megjelenése Magyarországon. (Ochrolechia arborea (lichen-forming fungi) in Hungary). Mikol. Közlem. Clusiana 2009, 48, 19–24. [Google Scholar]
- Matus, G.; Szepesi, J.; Rózsa, P.; Lőkös, L.; Varga, N.; Farkas, E. Xanthoparmelia mougeotii (Parmeliaceae, lichenised Ascomycetes) new to the lichen flora of Hungary. Studia Bot. Hung. 2017, 48, 89–104. [Google Scholar] [CrossRef][Green Version]
- Calchera, A.; Dal Grande, F.; Bode, H.B.; Schmitt, I. Biosynthetic gene content of the ‘perfume lichens’ Evernia prunastri and Pseudevernia furfuracea. Molecules 2019, 24, 203. [Google Scholar] [CrossRef]
- Carniel, F.C.; Gerdol, M.; Montagner, A.; Banchi, E.; de Moro, G.; Manfrin, C.; Muggia, L.; Pallavicini, A.; Tretiach, M. New features of desiccation tolerance in the lichen photobiont Trebouxia gelatinosa are revealed by a transcriptomic approach. Plant Mol. Biol. 2016, 91, 319–339. [Google Scholar] [CrossRef]
- Hell, A.F.; Gasulla, F.; González-Houcarde, M.; Pelegrino, M.T.; Seabra, A.B.; Campo, E.M.; Casano, L.M.; Centeno, D.C. Polyols-related gene expression is affected by cyclic desiccation in lichen microalgae. Environ. Exp. Bot. 2021, 185, 104397. [Google Scholar] [CrossRef]
- Steinhäuser, S.S.; Andrésson, O.S.; Pálsson, A.; Werth, S. Fungal and cyanobacterial gene expression in a lichen symbiosis: Effect of temperature and location. Fungal Biol. 2016, 120, 1194–1208. [Google Scholar] [CrossRef]
- Seaward, M.R.D.; Coppins, B.J. Lichens and hypertrophication. Bibl. Lichenol. 2004, 88, 561–572. [Google Scholar]
- Nygaard, E.; Tønsberg, T. Flavoparmelia soredians from Stavanger, new to Norway. Blyttia 2015, 73, 161–166. [Google Scholar]
- Jørgensen, P.M. The oceanic element in the Scandinavian lichen flora revisited. Acta Univ. Symb. Bot. Ups. 1996, 31, 297–317. [Google Scholar]
- Guttová, A.; Lőkös, L. Leptogium ferax (lichen-forming fungi, Collemataceae) new to Hungary. Acta Bot. Hung. 2011, 53, 321–324. [Google Scholar] [CrossRef]
- Farkas, E.; Lőkös, L. Parmelia submontana Hale (Parmeliaceae). Studia Bot. hung. 2020, 51, 82–84. [Google Scholar]
- Verseghy, K.; Farkas, E. Untersuchungen der Luftverunreinigung im Gebiet von Budapest mit Hilfe der Flechtenkartierung als Indikatoren. Ann. Univ. Sci. Budapest. Sect. Biol. 1984, 24–26, 163–184. [Google Scholar]
- Liška, J.; Pišút, I. Invázne lisajníky. (Invasive lichens). Zivot. Prostr. 2001, 35, 98–99. [Google Scholar]
- Kiszely-Vámosi, A. A Mátra hegység zuzmóflórája I. (The lichen flora of the Mátra Mountains, I). Folia Hist.-Nat. Musei Matra 1980, 6, 51–70. [Google Scholar]
- Thor, G. Some lichens from Hungary. Graph. Scr. 1988, 2, 67–71. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, E.; Varga, N.; Veres, K.; Matus, G.; Sinigla, M.; Lőkös, L. Distribution Types of Lichens in Hungary That Indicate Changing Environmental Conditions. J. Fungi 2022, 8, 600. https://doi.org/10.3390/jof8060600
Farkas E, Varga N, Veres K, Matus G, Sinigla M, Lőkös L. Distribution Types of Lichens in Hungary That Indicate Changing Environmental Conditions. Journal of Fungi. 2022; 8(6):600. https://doi.org/10.3390/jof8060600
Chicago/Turabian StyleFarkas, Edit, Nóra Varga, Katalin Veres, Gábor Matus, Mónika Sinigla, and László Lőkös. 2022. "Distribution Types of Lichens in Hungary That Indicate Changing Environmental Conditions" Journal of Fungi 8, no. 6: 600. https://doi.org/10.3390/jof8060600
APA StyleFarkas, E., Varga, N., Veres, K., Matus, G., Sinigla, M., & Lőkös, L. (2022). Distribution Types of Lichens in Hungary That Indicate Changing Environmental Conditions. Journal of Fungi, 8(6), 600. https://doi.org/10.3390/jof8060600






