Together Apart: Evaluating Lichen-Phorophyte Specificity in the Canarian Laurel Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Plot Selection and Sampling
2.3. Specimens Identification
2.4. Morphological Traits and Functional Groups
2.5. Data Analysis
3. Results
3.1. Lichen Diversity
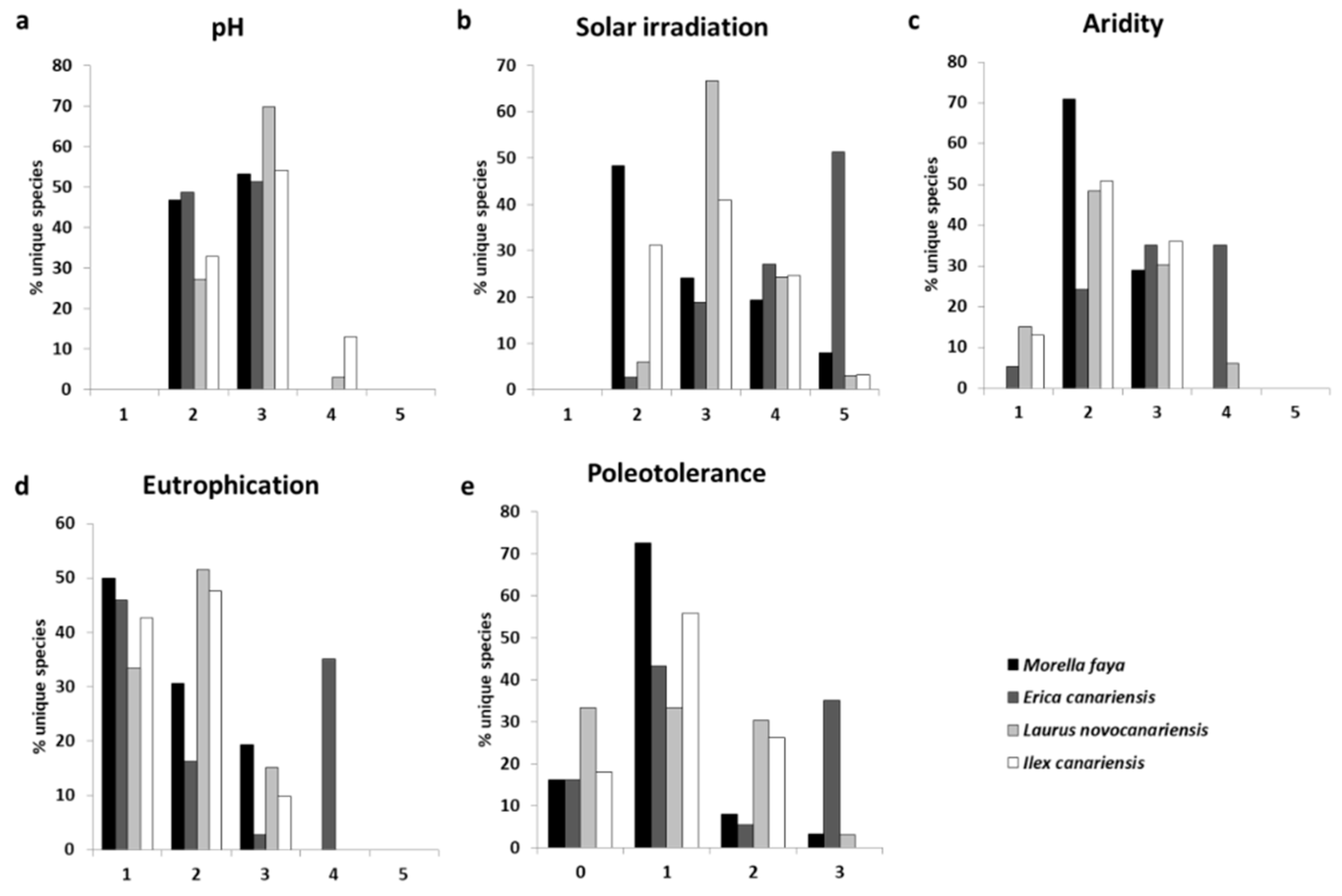
3.2. Traits and Lichen Functional Groups
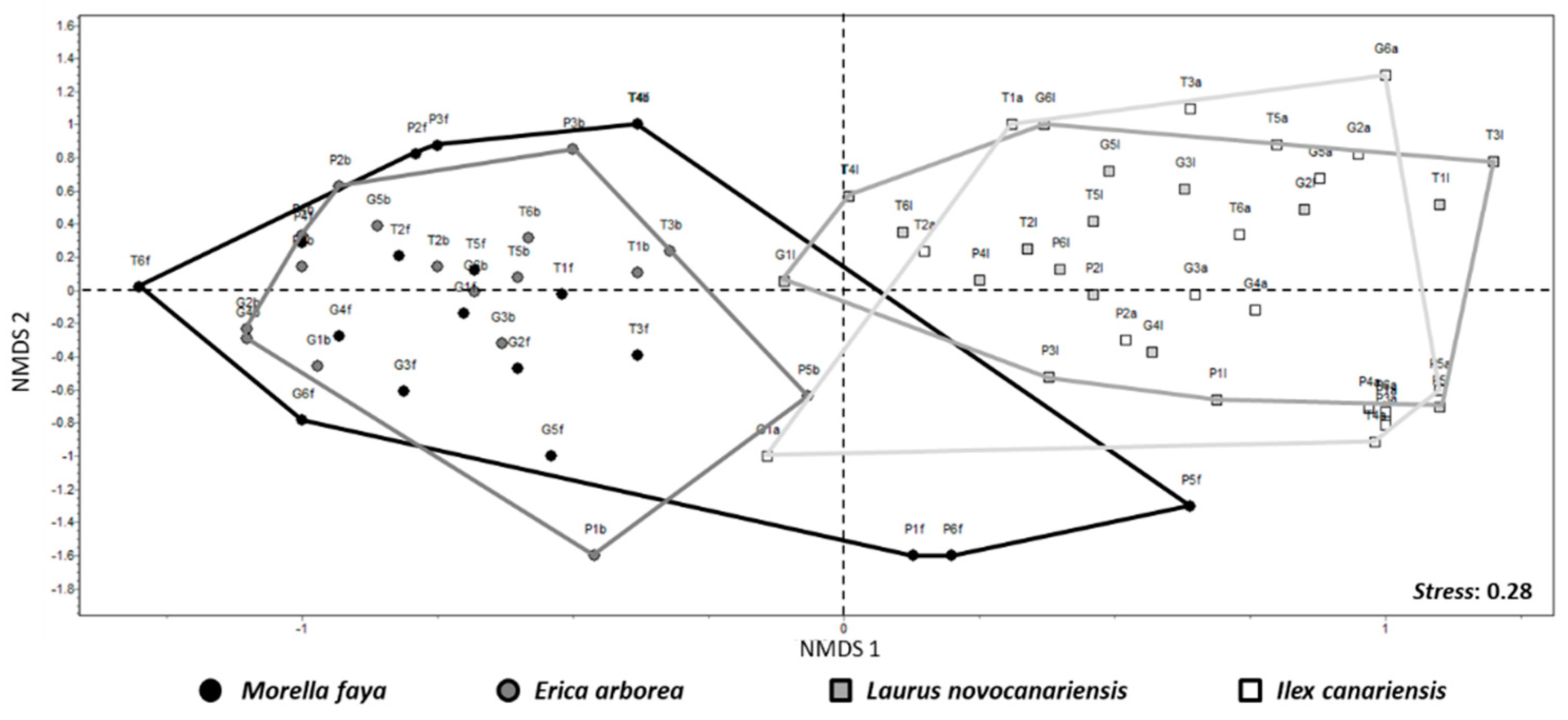
3.3. Species Composition
4. Discussion
4.1. Lichen Diversity
4.2. Functional Traits
4.3. Lichen Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Betzin, A. The Laurel Forest, an Example for Biodiversity Hotspots Threatened by Human Impact and Global Change. Ph.D. Thesis, University of Heidelberg, Heidelberg, Germany, 2014. [Google Scholar]
- Guimarães, A.; Olmeda, C. Management of Natura 2000 Habitat. 9360 *Macaronesian Laurel Forests (Laurus, Ocotea); European Commission: Brussels, Belgium, 2008.
- Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1992L0043:20070101:EN:PDF (accessed on 1 February 2021).
- Fernández-Palacios, J.M.; Whittaker, R.J. The Canaries: An important biogeographical meeting place. J. Biogeogr. 2008, 35, 379–387. [Google Scholar] [CrossRef]
- Del Arco, M.J.; González-González, R.; Garzón-Machado, V.; Pizarro-Hernández, B. Actual and potential natural vegetation on the Canary Islands and its conservation status. Biodivers. Conserv. 2010, 19, 3089–3140. [Google Scholar] [CrossRef]
- Foster, P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth Sci. Rev. 2001, 55, 73–106. [Google Scholar] [CrossRef]
- Sperling, F.G.; Washington, R.; Whittaker, R.J. Future climate change of the subtropical north Atlantic: Implications for the cloud forests of Tenerife. Clim. Chang. 2004, 65, 103–123. [Google Scholar] [CrossRef]
- Ritter, A.; Regalado, C.A.; Guerra, J.C. The impact of climate change on water fluxes in a Macaronesian cloud forest. Hydrol. Process. 2019, 33, 2828–2846. [Google Scholar] [CrossRef]
- Boch, S.; Prati, D.; Hessenmöller, D.; Schulze, E.D.; Fischer, M. Richness of lichen species, especially of threatened ones, is promoted by management methods furthering stand continuity. PLoS ONE 2013, 8, e55461. [Google Scholar] [CrossRef]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash III, T.H. Desiccation-Tolerance in Lichens: A Review. Bryologist 2009, 111, 576–593. [Google Scholar] [CrossRef]
- Biazrov, L. Lichens as indicators of radioactive contamination. J. Radioecol. 1993, 1, 15–20. [Google Scholar]
- Agnan, Y.; Séjalon-Delmas, N.; Claustres, A.; Probst, A. Investigation of spatial and temporal metal atmospheric deposition in France through lichen and moss bioaccumulation over one century. Sci. Total Environ. 2015, 529, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, J.; Hosek, J.; Brabec, M.; Hermy, M.; Dvorák, D.; Fellner, R.; Malícek, J.; Palice, Z.; Tencík, A.; Holá, E.; et al. Shared affinity of various forest-dwelling taxa point to the continuity of temperate forests. Ecol. Indic. 2019, 101, 904–912. [Google Scholar] [CrossRef]
- Abbott, I.; Williams, M.R. Silvicultural impacts in jarrah forest of Western Australia: Synthesis, evaluation, and policy implications of the forestcheck monitoring project of 2001–2006. Austral. For. 2011, 74, 350–360. [Google Scholar] [CrossRef]
- Stefańska-Krzaczek, E.; Staniaszek-Kik, M.; Szczepańska, K.; Szymura, T.H. Species diversity patterns in managed Scots pine stands in ancient forest sites. PLoS ONE 2019, 14, e0219620. [Google Scholar] [CrossRef] [PubMed]
- Sipman, H.; Harris, R.C. Lichens. Ecosyst. World 1989, 14, 303–309. [Google Scholar] [CrossRef]
- Marini, L.; Nascimbene, J.; Nimis, P.L. Large-scale patterns of epiphytic lichen species richness: Photobiont-dependent response to climate and forest structure. Sci. Total Environ. 2011, 409, 4381–4386. [Google Scholar] [CrossRef]
- Rundel, P.W. Ecological relationships of desert fog zone lichens. Bryologist 1978, 81, 277–293. [Google Scholar] [CrossRef]
- Hernández-Padrón, C.E. Flora y vegetación liquénica de las Islas Canarias. In Flora y Vegetación del Archipiélago Canario, Tratado Florístico de Canarias; Kunkel, G., Ed.; Primera Parte; Edirca Publisher: Las Palmas de Gran Canaria, Spain, 1992; pp. 151–170. [Google Scholar]
- Hernández-Padrón, C.E.; Pérez de Paz, P.L.; Sicilia, D.; Pérez-Vargas, I. Los Líquenes. In Parque Nacional de Garajonay. Patrimonio Mundial; Fernández, A.B., Ed.; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2008; pp. 178–189. [Google Scholar]
- Woda, C.; Huber, A.; Dohrenbusch, A. Vegetación epífita y captación de neblina en bosques siempreverdes en la Cordillera Pelada, sur de Chile. Bosque 2006, 27, 231–240. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potencial indicators of environmental conditions in forest ecosystems. Ecol. Ind. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Santamaría, J.M.; Martín, A. Tree bark as a bioindicator of air pollution in Navarra, Spain. Water Air Soil Pollut. 1997, 98, 381–387. [Google Scholar] [CrossRef]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and chemical composition of Pinus pinea L. bark. Ann. For. Sci. 1999, 56, 479–484. [Google Scholar] [CrossRef]
- Kennedy, P.G.; Izzo, A.D.; Bruns, T.D. There is high potential for the formation of common mycorrhizal networks between under- storey and canopy trees in a mixed evergreen forest. J. Ecol. 2003, 91, 1071–1080. [Google Scholar] [CrossRef]
- Käffer, M.I.; Ganade, G.; Marcelli, M.P. Lichen diversity and composition in Araucaria forests and tree monocultures in southern Brazil. Biodivers. Conserv. 2009, 18, 3543–3561. [Google Scholar] [CrossRef]
- Simijaca, D.; Moncada, B.; Lücking, R. Bosque de roble o plantación de coníferas, ¿qué prefieren los líquenes epífitos? [Oak forest or conifer plantation, what do epiphytic lichens prefer?]. Colomb. For. 2018, 21, 123–141. [Google Scholar] [CrossRef]
- Ódor, P.; Király, I.; Tinya, F.; Bortignon, F.; Nascimbene, J. Patterns and drivers of species composition of epiphytic bryophytes and lichens in managed temperate forests. For. Ecol. Manag. 2013, 306, 256–265. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y.H. Species dynamics of epiphytic macrolichens in relation to time since fire and host tree species in boreal forest. J. Veg. Sci. 2015, 26, 1124–1133. [Google Scholar] [CrossRef]
- Rosabal, D.; Burgaz, A.R.; Reyes, O.J. Substrate preferences and phorophyte specificity of corticolous lichens on five tree species of the montane rainforest of Gran Piedra, Santiago de Cuba. Bryologist 2013, 116, 113–121. [Google Scholar] [CrossRef]
- Benítez, A.; Aragón, G.; Prieto, M. Lichen diversity on tree trunks in tropical dry forests is highly influenced by host tree traits. Biodivers. Conserv. 2019, 28, 1909–2929. [Google Scholar] [CrossRef]
- González-Mancebo, J.M.; Losada-Lima, A.; McAlister, A. Host specificity of epiphytic bryophyte communities of a laurel forest on Tenerife (Canary Islands, Spain). Bryologist 2003, 106, 383–394. [Google Scholar] [CrossRef]
- Patiño, J.; González-Mancebo, J.M. Exploring the effect of host tree identity on epiphyte bryophyte communities in different Canarian subtropical cloud forests. Plant Ecol. 2011, 212, 433–449. [Google Scholar] [CrossRef]
- Gabriel, R.; Bates, J.W. Bryophyte community composition and habitat specificity in the natural forest of Terceira, Azores. Plant Ecol. 2005, 177, 125–144. [Google Scholar] [CrossRef]
- Sim-Sim, M.; Bergamini, A.; Luís, L.; Fontinha, S.; Martins, S.; Lobo, C.; Stech, M. Epiphytic bryophyte diversity on Madeira Island: Effects of tree species on bryophyte species richness and composition. Bryologist 2011, 114, 142–154. [Google Scholar] [CrossRef]
- González, M.N.; Rodrigo, J.D.; Suárez, C. Flora y Vegetación del Archipiélago Canario; Edirca Publisher: Las Palmas de Gran Canaria, Spain, 1986; 355p. [Google Scholar]
- Fernández, A. El Parque Nacional de Garajonay. La Gomera; de visita, G., Ed.; Organismo Autónomo de Parques Nacionales, Ministerio de Medio Ambiente: Madrid, Spain, 1998; 161p. [Google Scholar]
- Berthelot, S. Árboles y Bosques; Colección Territorio Canario; IDEA Publisher: Santa Cruz de Tenerife, Spain, 2005; 89p. [Google Scholar]
- Del Arco, M.J.; Wildpret, W.; Pérez, P.L.; Rodríguez, O.; Acebes, J.R.; García, A.; Martín, V.E.; Reyes, J.A.; Salas, M.; Díaz, M.A.; et al. Mapa de Vegetación de Canarias; GRAFCAN: Santa Cruz de Tenerife, Spain, 2006; 550p. [Google Scholar]
- Del Arco, M.J.; Rodríguez, O. Introducción. In Laurisilva Canari; Pérez, J.A., Ed.; Francisco Lemus Editor: Santa Cruz de Tenerife, Spain, 2005; pp. 1–14. [Google Scholar]
- Marzol, M.V.; Trujillo, J. El comportamiento del mar de nubes durante el verano en la isla de Tenerife a partir de su observación desde las torres de vigilancia. In Agua. Reflexiones para una Gestión Eficaz; Afonso-Carrillo, J., Ed.; Actas XIV Semana Científica Telesforo Bravo; Instituto de Estudios Hispánicos de Canarias: Puerto de la Cruz, Spain, 2019; pp. 21–44. [Google Scholar]
- Merwin, M.C.; Rentmeester, S.A.; Nadkarni, N.M. The influence of Host Tree species on the distribution of epiphytic Bromeliads in experimental monospecific plantations, La Selva, Costa Rica. Biotropica 2003, 35, 37–47. [Google Scholar] [CrossRef]
- Callaway, R.M.; Reinhart, K.O.; Moore, G.W.; Moore, D.J.; Pennings, S.C. Epiphyte host preferences and host traits: Mechanisms for species-specific interactions. Oecologia 2002, 132, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Laube, S.; Zotz, G. Neither Host-specific nor Random: Vascular epiphytes on three tree species in a Panamanian lowland forest. Ann. Bot. 2006, 97, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Lohmus, A.; Runnel, K. Ash dieback can rapidly eradicate isolated epiphyte populations in production forests: A case study. Biol. Conserv. 2014, 169, 185–188. [Google Scholar] [CrossRef]
- Asta, J.; Erhardt, W.; Ferretti, M.; Fornasier, F.; Kirschbaum, U.; Nimis, P.L.; Purvis, O.W.; Pirintsos, S.; Scheidegger, C.; Van Haluwyn, C.; et al. Mapping lichen diversity as an indicator of environmental quality. In Monitoring with Lichens-Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer Academic Publisher: Amsterdam, The Netherlands, 2002; pp. 273–279. [Google Scholar] [CrossRef]
- Asta, J.; Erhardt, W.; Ferretti, M.; Fornasier, F.; Kirschbaum, U.; Nimis, P.L.; Purvis, O.W.; Pirintsos, S.; Scheidegger, C.; Van Haluwyn, C.; et al. European Guideline for Mapping Lichen Diversity as an Indicator of Environmental Stress; British Lichen Society: London, UK, 2002; 20p. [Google Scholar]
- González-Montelongo, C.; Pérez-Vargas, I. Looking for a home: Exploring the potential of epiphytic lichens to colonize tree plantations in a Macaronesian laurel forest. For. Ecol. Manag. 2019, 453, 117541. [Google Scholar] [CrossRef]
- Robert, V.; Stegehuis, G.; Stalpers, J. The MycoBank Engine and Related Databases. 2005. Available online: http://www.mycobank.org (accessed on 10 January 2022).
- Nimis, P.L.; Martellos, S. ITALIC–The Information System on Italian Lichens, version 7.0; University of Trieste, Department of Biology: Trieste, Italy, 2021; Available online: http://dryades.units.it/italic (accessed on 10 January 2022).
- Llop, E.; Pinho, P.; Matos, P.; Pereira, M.J.; Branquinho, C. The use of lichen functional groups as indicators of air quality in a Mediterranean urban environment. Ecol. Ind. 2012, 13, 215–221. [Google Scholar] [CrossRef]
- Garrido-Benavent, I.; Llop, E.; Gómez-Bolea, A. The effect of agriculture management and fire on epiphytic lichens on holm oak trees in the eastern Iberian Peninsula. Lichenologist 2015, 47, 59–68. [Google Scholar] [CrossRef]
- Oliveros, J.C. (2007–2015) Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. Available online: https://bioinfogp.cnb.csic.es/tools/venny/index.html (accessed on 10 January 2022).
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Morales, E.A.; Lücking, R.; Anze, R. Una Introducción al Estudio de los Líquenes de Bolivia; Serie Ecología N°1; Universidad Católica Boliviana: San Pablo, Bolivia, 2009; 58p. [Google Scholar]
- Benítez, A.; Prieto, M.; Aragón, G. Large trees and dense canopies: Key factors for maintaining high epiphytic diversity on trunk bases (bryophytes and lichens) in tropical montane forests. Forestry 2015, 88, 521–527. [Google Scholar] [CrossRef]
- Kruskal, J.B. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, O. PAST. Paleontological Statistics. Version 3.25. 1999–2019. Available online: https://edisciplinas.usp.br/pluginfile.php/4407502/mod_resource/content/1/past3manual.pdf (accessed on 10 January 2022).
- Seaby, R.; Henderson, P.; Prendergast, J. Community Analysis Package, Versión 3.11. Searching for Structure in Community Data; PISCES Conservation Ltd.: Lymington, Reino Unido, 2004. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. Primer v5: User Manual/Tutorial; Primer-E. Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Cornejo, C.; Scheidegger, C. Lobaria macaronesica sp. nov., and the phylogeny of Lobaria sect. Lobaria (Lobariaceae) in Macaronesia. Bryologist 2010, 113, 590–604. [Google Scholar] [CrossRef]
- Tønsberg, T.; Blom, H.H.; Goffinet, B.; Holtan-Hartwig, J.; Lindblom, L. The cyanomorph of Ricasolia virens comb. nov. (Lobariacaeae, lichenized Ascomycetes). Opusc. Philolichenum 2016, 15, 12–21. [Google Scholar]
- GBIF (Global Biodiversity Information Facility). Available online: https://www.gbif.org/ (accessed on 15 June 2020).
- DiscoverLife. Available online: https://www.discoverlife.org/ (accessed on 10 January 2022).
- iNaturalist. Available online: https://www.inaturalist.org/ (accessed on 10 January 2022).
- Follmann, G. Lichen Flora and Lichen Vegetation of the Canary Islands. In Biogeography and Ecology in the Canary Islands; Kunkel, G., Ed.; Laboratorio de Botánica, Excmo, Cabildo Insular de Gran Canaria: Las Palmas, Spain, 1976; pp. 267–286. [Google Scholar]
- Mester, A. Laurisilva del Parque Nacional de Garajonay (Gomera), Incluyendo la Vegetación Epífita. Bachelor’s Thesis, Rheinisch-Westfälische Technische Hochschule Aachen, Aachen, Germany, 1986. [Google Scholar]
- Gil-González, M.L. Contribución al Estudio de la Flora y Vegetación Liquénica del Bosque de Madre del Agua (Agua García, Tenerife). Dpto; Biología Vegetal (Botánica), University of La Laguna: San Cristóbal de La Laguna, España, 1988; Unpublished Work. [Google Scholar]
- Li, S.; Liu, W.-Y.; Li, D.-W. Epiphytic lichen in subtropical forest ecosystems in southwest China: Species diversity and implications for conservation. Biol. Conserv. 2013, 159, 88–95. [Google Scholar] [CrossRef]
- Ellis, C.J.; Eaton, S.; Theodoropoulus, M.; Elliott, K. Epiphyte Communities and Indicator Species. An Ecological Guide for Scotland’s Woodlands; Royal Botanic Garden: Edinburgh, UK, 2015. [Google Scholar]
- Boch, S.; Martins, A.; Ruas, S.; Fontinha, S.; Carvalho, P.; Reis, F.; Bergamini, A.; Sim-Sim, M. Bryophyte and macrolichen diversity show contrasting elevation relationships and are negatively affected by disturbances in laurel forests of Madeira Island. J. Veg. Sci. 2019, 30, 1122–1133. [Google Scholar] [CrossRef]
- González-Mancebo, J.M.; Losada-Lima, A.; Patiño-Llorente, J. Forest floor bryophytes of laurel forest in Gomera (Canary Islands): Life strategies and influence of the tree species. Lindbergia 2004, 29, 5–16. [Google Scholar]
- Beltrán-Tejera, E. (Ed.) Hongos, Líquenes y Briófitos del Parque Nacional de Garajonay (La Gomera, Islas Canarias); O.A. Parques Nacionales, Serie Técnica; Ministerio de Medio Ambiente: Madrid, Spain, 2008; 853p. [Google Scholar]
- Heinrichs, S.; Ammer, C.; Mund, M.; Boch, S.; Budde, S.; Fischer, M.; Müller, J.; Schöning, I.; Schulze, E.D.; Schidt, W.; et al. Landscape-Scale mixtures of tree species are more effective than stand-scale mixtures for biodiversity of vascular plants, bryophytes and lichens. Forests 2019, 10, 73. [Google Scholar] [CrossRef]
- Lesica, P.; McCune, B.; Cooper, S.V.; Hong, W.S. Differences in lichen and bryophyte communities between old-growth and managed second-growth forests in the Swan Valley, Montana. Can. J. Bot. 1991, 69, 145–155. [Google Scholar] [CrossRef]
- Mezaka, A.; Kirillova, J. Epiphytic bryophytes and lichens and their functional trait relationships with host characteristics in the Lūznava Manor Park. Acta Biol. Univ. Daugavp. 2019, 19, 241–251. [Google Scholar]
- Staniaszek-Kik, M.; Chmura, D.; Zarnowiec, J. What factors influence colonization of lichens, liverworts, mosses and vascular plants on snags? Biologia 2019, 74, 375–384. [Google Scholar] [CrossRef]
- Tibell, L. Crustose lichens as indicators of forest continuity in boreal coniferous forests. Nord. J. Bot. 1992, 12, 427–450. [Google Scholar] [CrossRef]
- Ramírez-Morán, N.; León-Gómez, M.; Lücking, R. Use of lichen biotypes as bioindicators of perturbation in fragments of high Andean Forest (“Encenillo” Biological Reserve, Colombia). Caldasia 2016, 38, 31–52. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Plata, E.R.; Andrew, C.J.; Lücking, R.; Lumbsch, H.T. Phylogenetic diversity of Trentepohlialean algae associated with lichen-forming fungi. J. Phycol. 2011, 47, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Rivas Plata, E.; Lücking, R.; Lumbsch, H.T. When family matters: An analysis of Thelotremataceae (Lichenized Ascomycota: Ostropales) as bioindicators of ecological continuity in tropical forests. Biodivers. Conserv. 2008, 17, 1319–1351. [Google Scholar] [CrossRef]
- Barkmann, J.J. Phytosociology and Ecology of Cryptogamic Epiphytes. Including a Taxonomic Survey and Description of Their Vegetation Units in Europe; Van Gorcum Publisher: Assen, The Netherlands, 1969; 628p. [Google Scholar]
- Sipman, J.J.M. Corticolous lichens. In How to Sample the Epiphytic Diversity of Tropical Rain Forests. Ecotropica 1996, 2, 62–67. [Google Scholar]
- Wolseley, P.A.; Aguirre-Hudson, B. The ecology and distribution of lichens in tropical deciduous and evergreen forests of northern Thailand. J. Biogeogr. 1997, 24, 327–343. [Google Scholar] [CrossRef]
- Hauck, M.; Runge, M. Stemflow chemistry and epiphytic lichen diversity in dieback-affected spruce forest of the Harz Mountains, Germany. Flora 2002, 197, 250–261. [Google Scholar] [CrossRef]
- Hauck, M. Epiphytic Lichen Diversity and Forest Dieback: The Role of Chemical Site Factors. Bryologist 2003, 106, 257–269. [Google Scholar] [CrossRef]
- Lohmus, P.; Rosenvald, R.; Lomhus, A. Effectiveness of solitary retention trees for conserving epiphytes: Differential short-term responses of bryophytes and lichens. Can. J. For. Res. 2006, 36, 1319–1330. [Google Scholar] [CrossRef]
- Cáceres, M.E.S.; Lücking, R.; Rambold, G. Phorophyte specificity and environmental parameters versus stochasticity as determinants for species composition of corticolous crustose lichen communities in the Atlantic rain forest of northeastern Brazil. Mycol. Prog. 2007, 6, 117–136. [Google Scholar] [CrossRef]
- Pereira, I.; Müller, F.; Moya, M. Influence of Nothofagus bark pH on the lichen and bryophytes richness, Central Chile. Gayana Botánica 2014, 71, 120–130. [Google Scholar] [CrossRef]
- Buba, T.; Danmallam, B.A. Effects of tree size and bark roughness of Parkia biglobosa on Lichen colonization in Amurum forest reserve: Implication for conservation. J. Pure App. Sci. 2019, 17, 73–83. [Google Scholar] [CrossRef]
- Belinchón, R.; Martínez, I.; Aragón, G.; Escudero, A.; de la Cruz, M. Fine spatial pattern of an epiphytic lichen species is affected by habitat conditions in two forest types in the Iberian Mediterranean region. Fungal Biol. 2011, 115, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Merinero, S.; Rubio-Salcedo, M.; Aragón, G.; Martínez, I. Environmental factors that drive the distribution and abundance of a threatened cyanolichen in Southern Europe: A multi-scale aproach. Am. J. Bot. 2014, 101, 186–1885. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.; van Woudenberg, M.; Dorin, B.; Adcock, A.M.; McMullin, R.T.; Cottenie, K. The effects of bark quality on corticolous lichen community composition in urban parks of southern Ontario. Botany 2017, 95, 1141–1149. [Google Scholar] [CrossRef]
- Aboal, J.R. Los Flujos Netos Hidrológicos Y Químicos Asociados de un Bosque de Laurisilva en Tenerife. Ph.D. Thesis, Universtiy of La Laguna, Tenerife, Spain, 1998. Unpublished Work. [Google Scholar]
- Ohsawa, M.; Wildpret, W.; del Arco, M. (Eds.) Anaga Cloud Forest. A Comparative Study on Evergreen Broad-Leaved Forests and Trees of the Canary Islands and Japan; Laboratory of Ecology, Chiba University: Chiba, Japan, 1999; 315p. [Google Scholar]
- Terrón-Alfonso, A.; Burgaz, A.R.; Álvarez-Andrés, J. Líquenes de la provincia de Zamora (España). Bot. Complut. 2000, 24, 9–43. [Google Scholar]
- Casas-García, S.; Burgaz, A.R. Contribución al catálogo de la flora terrícola acidófila (líquenes y briófitos) de la provincia de Madrid (España). Bot. Complut. 2002, 26, 9–15. [Google Scholar]
- Kotelko, R.; Piercey-Normore, M.D. Cladonia pyxidata and C. pocillum; genetic evidence to regard them as conspecific. Mycologia 2010, 102, 534–545. [Google Scholar] [CrossRef]
- Barreno, E.; Pérez-Ortega, S. Líquenes de la Reserva Natural Integral de Muniellos, Asturias; KRK Ediciones; Consejería de Medio Ambiente, Ordenación del Territorio e Infraestructuras del Principado de Asturias: Asturias, Spain, 2003; 513p. [Google Scholar]
- Sicilia, D.C. Los Líquenes del Parque Nacional de Garajonay. Su Aplicación al Estudio de la Contaminación Ambiental. Ph.D. Thesis, University of La Laguna, Tenerife, Spain, 2007. Unpublished Work. 353p. [Google Scholar]
- Burgaz, A.R.; Ahti, T. Flora Liquenológica Ibérica. Vol. 4. Cladoniaceae; Sociedad Española de Liquenología, SEL: Madrid, Spain, 2009; 111p. [Google Scholar]
- Laundon, J.R. The species of Chrysothrix. Lichenologist 1981, 13, 101–121. [Google Scholar] [CrossRef]
- Matos, P.; Pinho, P.; Aragón, G.; Martínez, I.; Nunes, A.; Sorares, A.M.V.M.; Branquinho, C. Lichen traits responding to aridity. J. Ecol. 2015, 103, 451–458. [Google Scholar] [CrossRef]
- Lange, O.L.; Kilian, E.; Ziegler, H. Water vapor uptake and photosynthesis of lichens: Performance differences in species with green and blue-green algae as phycobionts. Oecologia 1986, 71, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Spribille, T.; Thor, G.; Brunnell, F.L.; Goward, T.; Björk, C.R. Lichens on dead wood: Species-substrate relationships in the epiphytic lichen floras of the Pacific Northwest and Fennoscandia. Ecography 2008, 31, 741–750. [Google Scholar] [CrossRef]
- Benítez, A.; Aragón, G.; González, Y.; Prieto, M. Functional traits of epiphytic lichens in response to forest disturbance and as predictors of total richness and diversity. Ecol. Indic. 2018, 86, 18–26. [Google Scholar] [CrossRef]
- Walser, J.-C. Molecular evidence for limited dispersal of vegetative propagules in the epiphytic lichen Lobaria pulmonaria. Am. J. Bot. 2004, 91, 1273–1276. [Google Scholar] [CrossRef]
- Armstrong, R.A. Dispersal, establishment and survival of soredia and fragments of the lichen, Hypogymnia physodes (L.) Nyl. New Phytol. 1990, 114, 239–245. [Google Scholar] [CrossRef]
- Snäll, T.; Hagström, A.; Rudolphi, J.; Rydin, H. Distribution pattern of the epiphyte Neckera pennata on three spation scales–importance of past landscape structure, connectivity and local conditions. Ecography 2004, 27, 757–766. [Google Scholar] [CrossRef]
- Wen-Zhang, M.; Wen-Yao, L.; Xing-Jiang, L. Species composition and life forms of epiphytic bryophytes in old-growth and secondary forests in Mt. Ailao, SW China. Cryptogam. Bryol. 2009, 30, 477–500. [Google Scholar]
- Mizuno, T.; Momohara, A.; Okitsu, S. The effects of bryophyte communities on the establishment and survival of an epiphytic fern. Folia Geobot. 2015, 50, 331–337. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Calderisi, M.; Giorgolo, F.; Bagella, S.; Bertini, G.; Chianucci, F.; Fratini, R.; Gottardini, E.; Cutini, A. Epiphytic lichen diversity and sustainable forest management criteria and indicators: A multivariate and modelling approach in coppice forests of Italy. Ecol. Indic. 2020, 115, 106358. [Google Scholar] [CrossRef]
- Miller, J.E.D.; Villella, J.; Stone, D.; Hardman, A. Using lichen communities as indicators of forest stand age and conservation value. For. Ecol. Manage. 2020, 475, 118436. [Google Scholar] [CrossRef]
- Giordani, P.; Malaspina, P. Do tree-related factors mediate the response of lichen functional groups to eutrophication? Plant Biosyst. 2016, 151, 1062–1072. [Google Scholar] [CrossRef]
- Koç, S.N.; Ataslar, E.; Türk, A.; Tufan-Cetin, O. Lichens of Barla Mountain in Isparta, Turkey: Diversity study and ecological assessment of the area. Plant Biosyst. 2016, 151, 985–995. [Google Scholar] [CrossRef]
- Munzi, S.; Cruz, C.; Maia, R.; Máguas, C.; Peretrello-Ramos, M.M.; Branquinho, C. Intra- and inter-specific variations in chitin in lichens along a N-deposition gradient. Environ. Sci. Pollut. Res. 2017, 24, 28065–28071. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Palacios, J.M.; Arévalo, J.R. Regeneration strategies of tree species in the laurel forest of Tenerife (The Canary Islands). Plant Ecol. 1998, 137, 21–29. [Google Scholar] [CrossRef]
- Arozena, M.E.; Panareda, J.M.; Figueiredo, A. El papel de Myrica faya como indicador de la dinámica del paisaje de la laurisilva en Canarias y Madeira. In Avances en Biogeografía. Áreas de Distribución: Entre Puentes y Barreras; Gómez, J., Arias, J., Olmedo, J.A., Serrano, J.L., Eds.; Universidad de Granada: Granada, Spain, 2016; pp. 601–610. [Google Scholar]
- Fernández-Palacios, J.M.; Arévalo, J.R.; Balguerías, E.; Barone, R.; De Nascimento, L.; Delgado, J.D.; Elias, R.B.; Fernández-Lugo, S.; Méndez, J.; Menezes de Sequira, M.; et al. La laurisilva. Canarias, Madeira y Azores; Macaronesia Editorial: Santa Cruz de Tenerfie, Spain, 2017; 435p. [Google Scholar]
- Hernández-Padrón, C.E.; Sicilia, D.; Pérez-Vargas, I.; Pérez de Paz, P.L. Líquenes y hongos liquenícolas. In Hongos, Líquenes y Briófitos del Parque Nacional de la Caldera de Taburiente; Beltrán, E., Ed.; O.A. de Parques Nacionales, Serie Técnica; Ministerio de Medio Ambiente: Madrid, Spain, 2004; pp. 233–350. [Google Scholar]
- Topham, P. Las Cañadas Del Teide. Lichenologist 1982, 14, 87–90. [Google Scholar] [CrossRef]
- Soto-Medina, E.; Lücking, R.; Bolaños, A. Especificidad de forófito y preferencias microambientales de los líquenes cortícolas en cinco forófitos del bosque premontano de finca Zíngara, Cali, Colombia. Rev. Biol. Trop. 2012, 60, 843–856. [Google Scholar] [CrossRef]
- Adams, D.B.; Risser, P.G. The effect of host specificity on the interspecific associations of bark lichens. Bryologist 1971, 74, 451–457. [Google Scholar] [CrossRef]
- Fritz, Ö.; Brunet, J.; Caldiz, M. Interacting effects of tree characteristics on the occurrence of rare epiphytes in a Swedish beech forest area. Bryologist 2009, 112, 488–505. [Google Scholar] [CrossRef]
- Sillet, S.C.; McCune, B.; Peck, J.E.; Rambo, T.R.; Ruchty, A. Dispersal limitations of epiphytic lichens result in species dependent on old-growth forests. Ecol. Appl. 2000, 10, 789–799. [Google Scholar] [CrossRef]
- Muñoz, J.; Felicísimo, Á.M.; Cabezas, F.; Burgaz, A.R.; Martínez, I. Wind as a longdistance dispersal vehicle in the southern hemisphere. Science 2004, 304, 1144–1147. [Google Scholar] [CrossRef]
- Ronnas, C.; Werth, S.; Ovaskainen, O.; Várkonyi, G.; Scheidegger, C.; Snäll, T. Discovery of long-distance gamete dispersal in a lichen-forming ascomycete. New Phytol. 2017, 216, 216–226. [Google Scholar] [CrossRef] [PubMed]
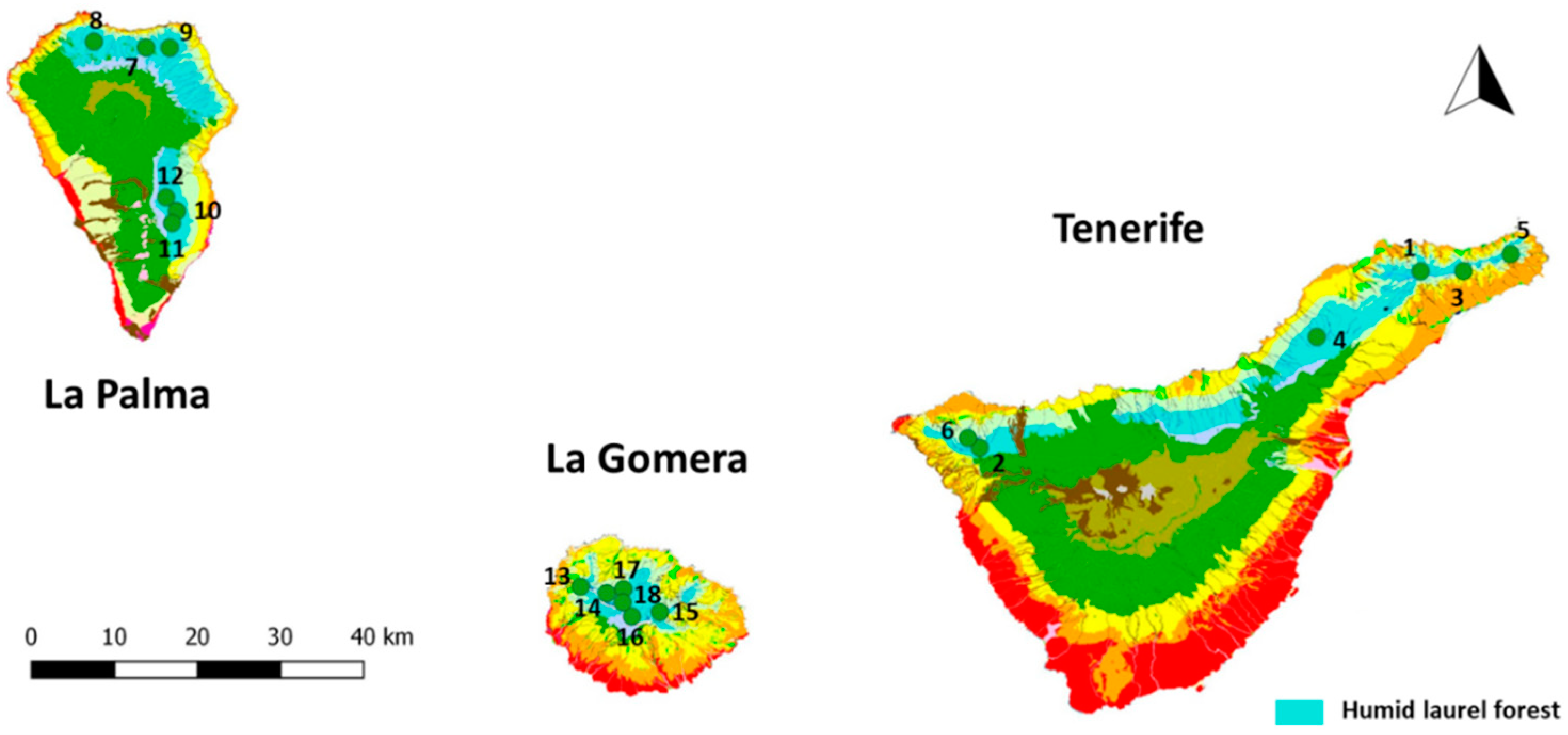
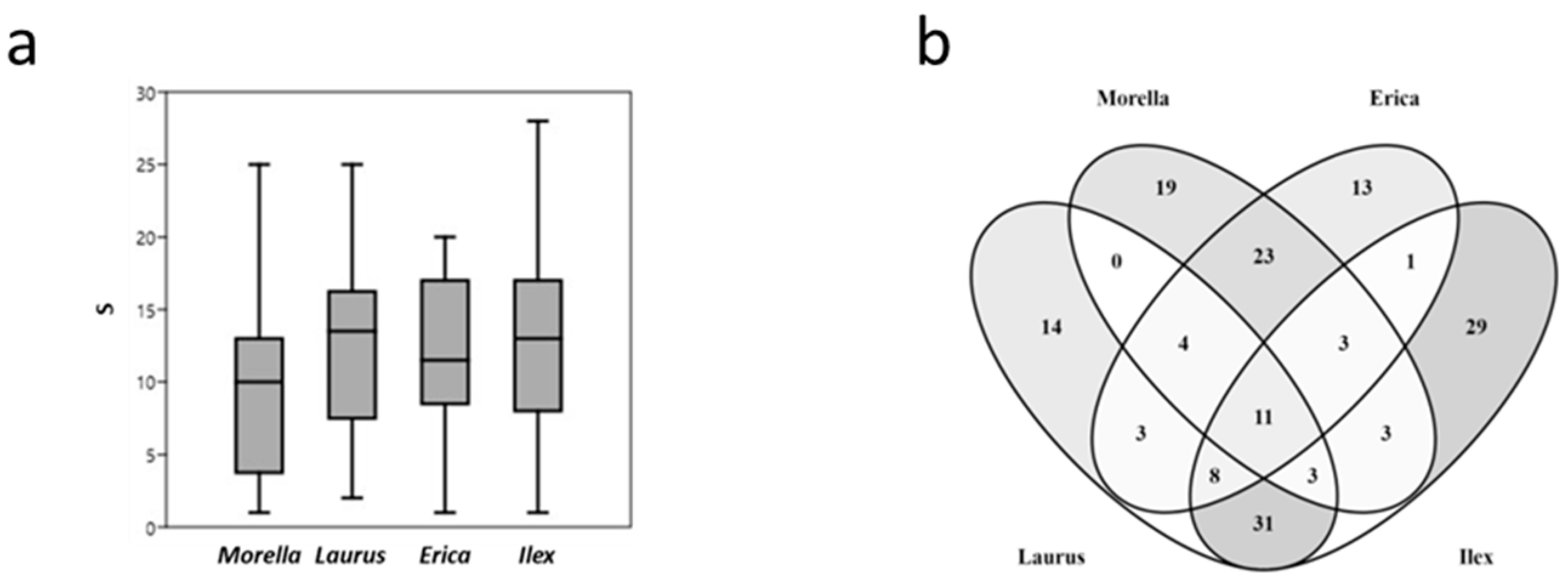
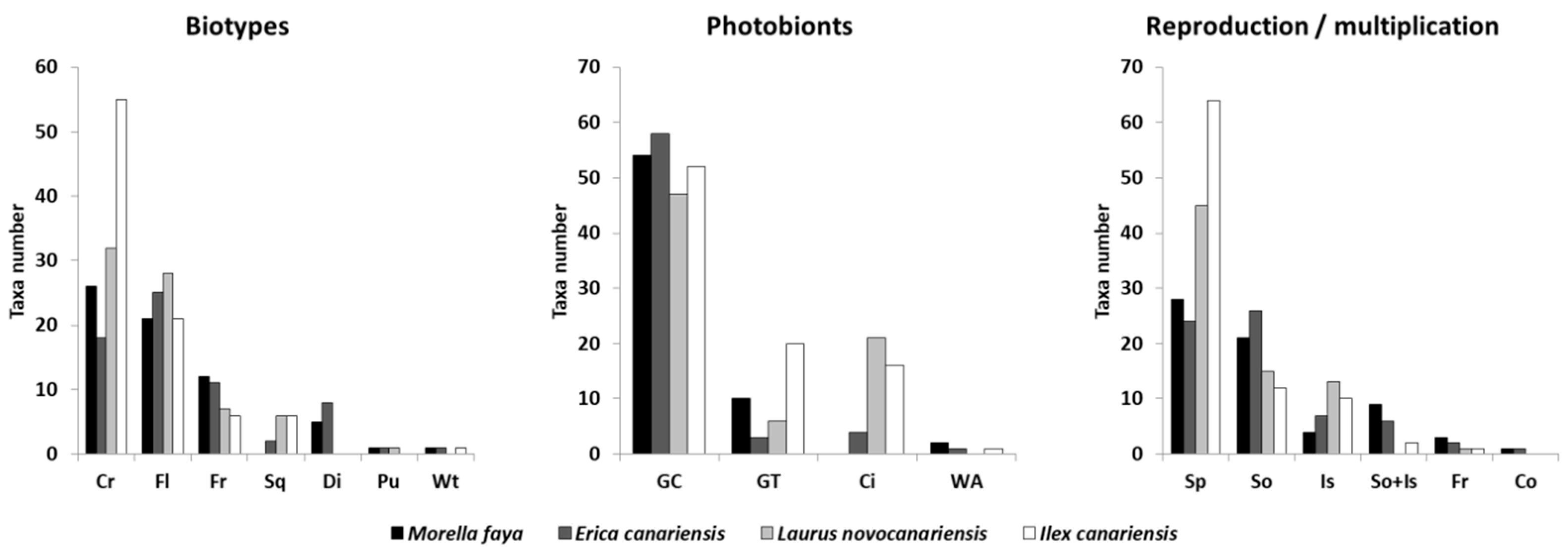
| Plot | Island | Locality | Universal Transverse Mercator (UTM) |
|---|---|---|---|
| 1 | Tenerife | Pista Las Hiedras (Anaga) | 375110/3157614 |
| 2 | Tenerife | Erjos (Teno) | 321907/3134428 |
| 3 | Tenerife | Cueva del Guanche (Anaga) | 380235/3157573 |
| 4 | Tenerife | Aguagarcía | 362558/3148881 |
| 5 | Tenerife | Hoya de Ijuana (Anaga) | 385900/3159815 |
| 6 | Tenerife | Las Portelas (Teno) | 320500/3135776 |
| 7 | La Palma | Mirador de Las Mimbreras | 223103/3190532 |
| 8 | La Palma | Don Pedro | 216864/3191489 |
| 9 | La Palma | Laguna de Barlovento | 225903/3190442 |
| 10 | La Palma | Hoyo del Infierno | 226243/3168304 |
| 11 | La Palma | Roque Niquiomo | 225640/3166681 |
| 12 | La Palma | La Pavona | 225015/3170197 |
| 13 | La Gomera | Lomito Ventoso | 273628/3116431 |
| 14 | La Gomera | Cordilleras de Vallehermoso | 276756/3115483 |
| 15 | La Gomera | El Bailadero | 283088/3112836 |
| 16 | La Gomera | El Contadero | 279720/3112282 |
| 17 | La Gomera | Fuensanta | 278779/3115994 |
| 18 | La Gomera | La Gollada Colorada | 278663/3114210 |
| Functional Traits | Types |
|---|---|
| Photobiont | Green algae |
| Trentepohliaceae | |
| Cyanobacteria | |
| Without thallus | |
| Growth form | Leprose/Pulverulent |
| Crustose | |
| Squamulose | |
| Foliose | |
| Fruticose | |
| Cladoniiform | |
| Reproductive strategy | Thallus fragmentation |
| Isidia | |
| Soralia | |
| Isidia + soralia | |
| Sexual (spores) | |
| Conidia |
| Functional Traits | Value | Signification |
|---|---|---|
| Tolerance to eutrophication | 1 | Lichens not resistant to eutrophication |
| 2 | Lichens resistant to a very weak eutrophication | |
| 3 | Lichen resistant to a weak eutrophication | |
| 4 | Lichen occurring in rather eutrophicated situations | |
| 5 | Lichens occurring in highly eutrophicated situations | |
| Water requirements | 1 | Hydro and hygrophytic species, in sites with a very high frequency of fog |
| 2 | Rather hygrophytic species, intermediate between 1 and 3 | |
| 3 | Mesophytic species | |
| 4 | Xerophytic species, but absent from extremely arid stands | |
| 5 | Very xerophytic species | |
| Solar irradiation | 1 | Species growing in very shaded situations |
| 2 | Species growing in shaded situations | |
| 3 | Species growing in sites with plenty of diffuse light but scarce direct solar irradiation | |
| 4 | Species growing in sun-exposed sites, but avoiding extreme solar irradiation | |
| 5 | Species growing in sites with very high direct solar irradiation | |
| Poleotolerance | 0 | Lichens that occur exclusively on old trees in ancient, undisturbed forests |
| 1 | Lichens mostly occurring in natural or semi-natural habitats | |
| 2 | Lichens also occur in moderately disturbed areas (agricultural areas, small settlements, etc.) | |
| 3 | Lichens also occur in heavily disturbed areas, incl. large towns | |
| Substrata pH | 1 | Species, which occur on very acid substrata |
| 2 | Species, which occur on acid substrata | |
| 3 | Species, which occur on subacid to subneutral substrata | |
| 4 | Species, which occur on slightly basic substrata | |
| 5 | Species, which occur on basic substrata |
| Morella | Erica | Laurus | Ilex | |
|---|---|---|---|---|
| Morella | 8.9 | 0.084 | 0.35 * | 0.38 * |
| Erica | 87% | 22 | 0.4 * | 0.59 * |
| Laurus | 96% | 92% | 14 | 0.033 |
| Ilex | 98% | 98% | 88% | 11 |
| Ilex canariensis | Laurus novocanariensis |
| Phlyctis agelaea (14%) Ricasolia virens (10%) Bacidia absistens (8.4%) Bacidia herbarum (8.1%) cf. Phyllopsora (6.4%) Athallia holocarpa (6.3%) Lecanora pulicaris (4.9%) Lobaria macaronesica (4.3%) Sticta canariensis cyanomorph. (4%) Thelotrema lepadinum (3.6%) Lecidella elaeochroma (3.6%) Lecanora rubicunda (3%) | Parmotrema perlatum (22%) Ricasolia virens (13%) Phlyctis agelaea (11%) Leucodermia leucomela (9.3%) Lobaria macaronesica (6.8%) Crocordia aurata (5.7%) Bacidia absistens (3.9%) Lobaria immixta (3%) |
| Erica canariensis | Morella faya |
| Parmotrema perlatum (32%) Chrysothrix candelaris (28%) Parmotrema reticulatum (6.1%) Leucodermia leucomela (6%) Platismatia glauca (5.9%) | Parmotrema perlatum (20%) Thelotrema lepadinum (17%) Parmotrema crinitum (9.3%) Parmelinopsis horrescens (8.8%) Chrysothrix candelaris (7.4%) Platismatia glauca (5.8%) Lecanora albella (4.1%) Leucodermia leucomela (2.7%) |
| Taxa | Presences | Worldwide Distribution * |
|---|---|---|
| Parmotrema perlatum | 202 | Cosmopolitan (except Africa) |
| Chrysothrix candelaris | 142 | Cosmopolitan |
| Leucodermia leucomelos | 117 | Cosmopolitan |
| Phlyctis agelaea | 84 | Holarctic (mainly Europe) |
| Bacidia absistens | 73 | Holarctic (mainly Europe) |
| Ricasolia virens | 73 | Holarctic (mainly Europe) |
| Platismatia glauca | 62 | Cosmopolitan (except Australia) |
| Lobaria macaronesica | 56 | Macaronesia |
| Lepra slesvicensis | 52 | Cosmopolitan |
| Parmotrema crinitum | 48 | Cosmopolitan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Montelongo, C.; Pérez-Vargas, I. Together Apart: Evaluating Lichen-Phorophyte Specificity in the Canarian Laurel Forest. J. Fungi 2022, 8, 1031. https://doi.org/10.3390/jof8101031
González-Montelongo C, Pérez-Vargas I. Together Apart: Evaluating Lichen-Phorophyte Specificity in the Canarian Laurel Forest. Journal of Fungi. 2022; 8(10):1031. https://doi.org/10.3390/jof8101031
Chicago/Turabian StyleGonzález-Montelongo, Cristina, and Israel Pérez-Vargas. 2022. "Together Apart: Evaluating Lichen-Phorophyte Specificity in the Canarian Laurel Forest" Journal of Fungi 8, no. 10: 1031. https://doi.org/10.3390/jof8101031
APA StyleGonzález-Montelongo, C., & Pérez-Vargas, I. (2022). Together Apart: Evaluating Lichen-Phorophyte Specificity in the Canarian Laurel Forest. Journal of Fungi, 8(10), 1031. https://doi.org/10.3390/jof8101031








