Biosynthesis of Xylariolide D in Penicillium crustosum Implies a Chain Branching Reaction Catalyzed by a Highly Reducing Polyketide Synthase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Strains
2.2. Transformation of Fungal Strains and Confirmation of Integration
2.3. Cultivation of Fungal Strains, Extraction and Isolation of Secondary Metabolites
2.4. Feeding with Prexylariolide D (2)
2.5. Incorporation of 13C-Labeled Precursors
3. Results
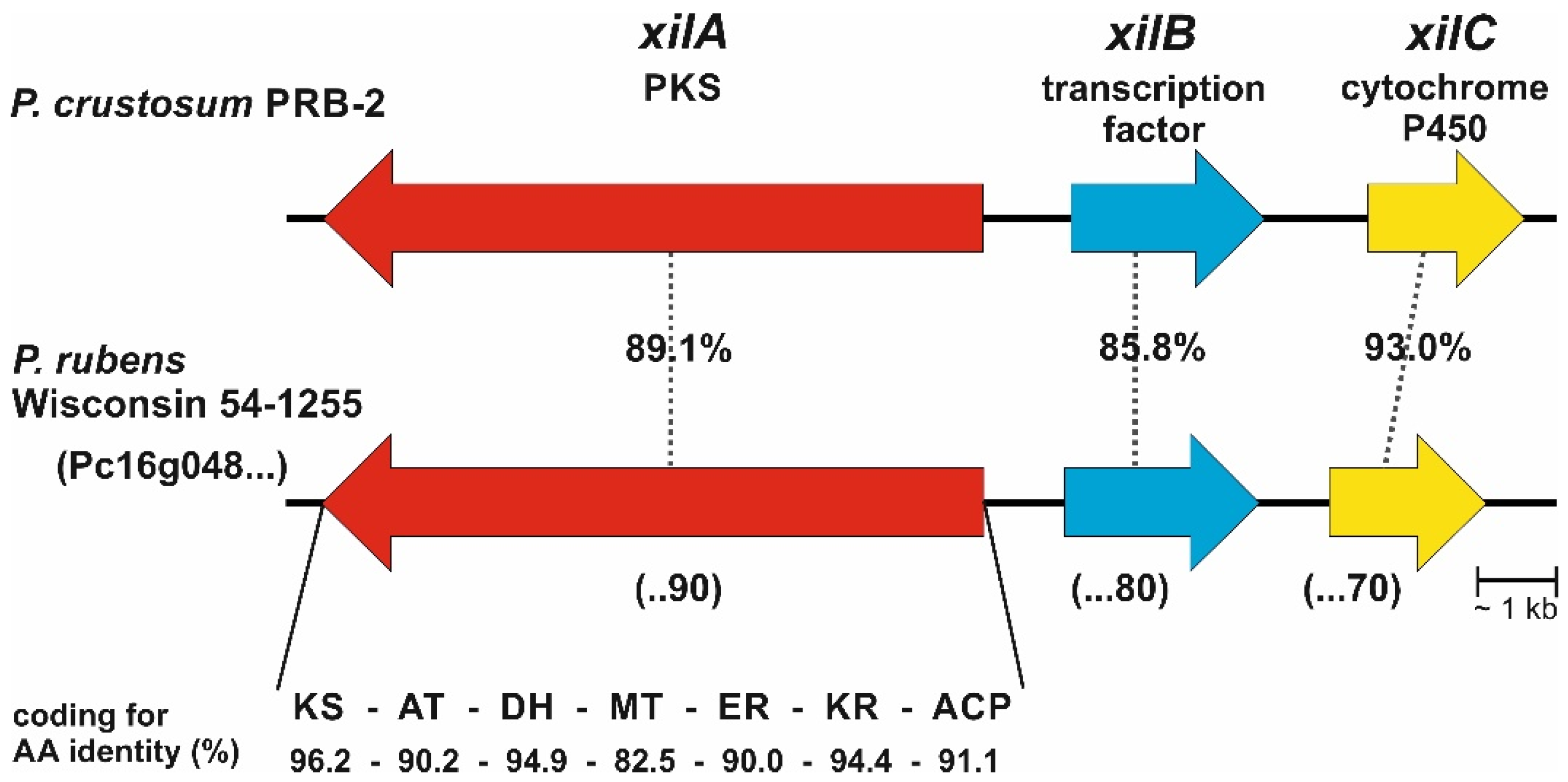
3.1. Bioinformatic Analysis
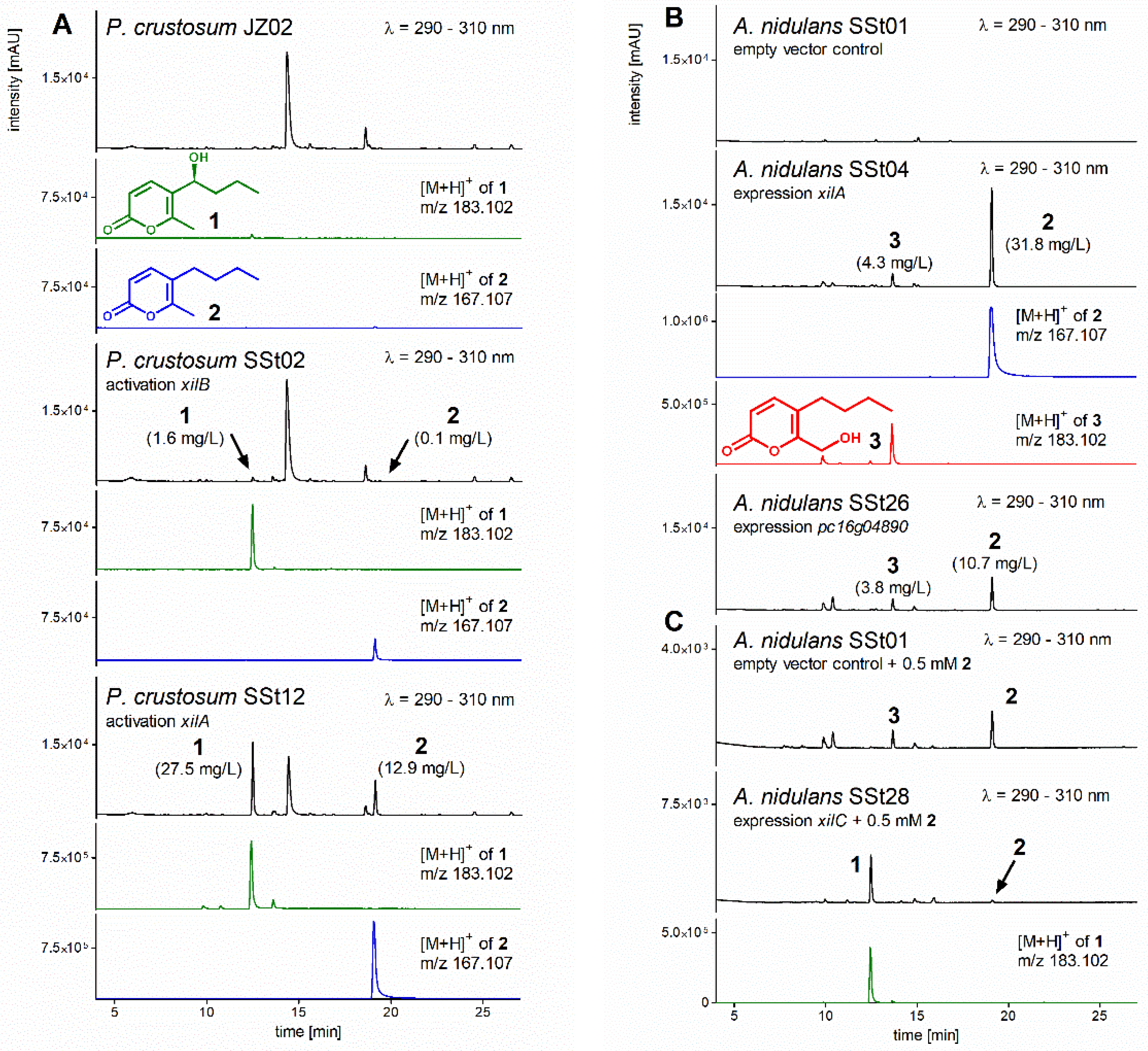
3.2. Activation of Transcription Factor xilB and PKS xilA in P. crustosum
3.3. Heterologous Expression of xilA and pc16g04890
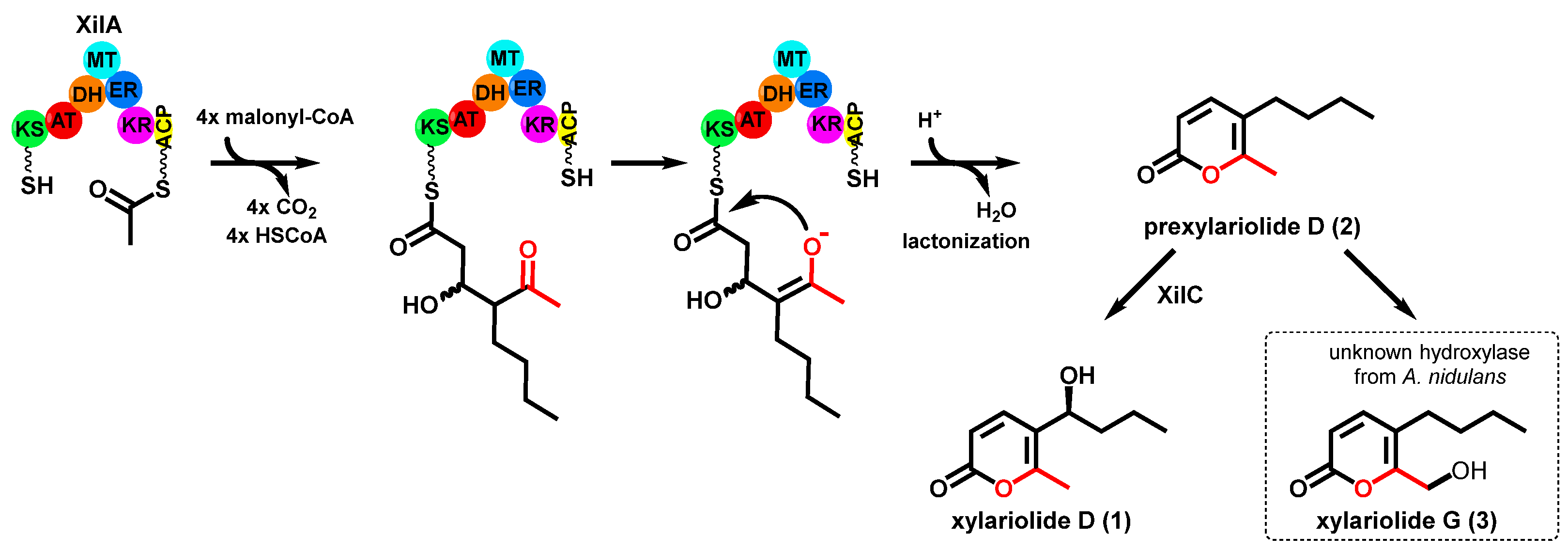
3.4. Structure Elucidation
3.5. Heterologous Expression of xilC and Feeding of Prexylariolide D
3.6. Feeding with 13C-Labeled Precursors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmeister, D.; Keller, N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.J. Polyketides, proteins and genes in fungi: Programmed nano-machines begin to reveal their secrets. Org. Biomol. Chem. 2007, 5, 2010. [Google Scholar] [CrossRef]
- Cox, R.J.; Simpson, T.J. Fungal type I polyketide synthases. Methods Enzymol. 2009, 459, 49. [Google Scholar]
- Yang, X.L.; Friedrich, S.; YIn, S.; Piech, O.; Williams, K.; Simpson, T.J.; Cox, R.J. Molecular basis of methylation and chain-length programming in a fungal iterative highly reducing polyketide synthase. Chem. Sci. 2019, 10, 8478. [Google Scholar] [CrossRef]
- Walker, P.D.; Weir, A.N.M.; Willis, C.L.; Crump, M.P. Polyketide β-branching: Diversity, mechanism and selectivity. Nat. Prod. Rep. 2021, 38, 723–756. [Google Scholar] [CrossRef]
- Bretschneider, T.; Heim, J.B.; Heine, D.; Winkler, R.; Busch, B.; Kusebauch, B.; Stehle, T.; Zocher, G.; Hertweck, C. Vinylogous chain branching catalysed by a dedicated polyketide synthase module. Nature 2013, 502, 124. [Google Scholar] [CrossRef]
- Wu, G.; Ma, H.; Zhu, T.; Li, J.; Gu, Q.; Li, D. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 2012, 68, 9745. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29. [Google Scholar] [CrossRef]
- Fan, J.; Liao, G.; Ludwig-Radtke, L.; Yin, W.-B.; Li, S.-M. Formation of terrestric acid in Penicillium crustosum requires redox-assisted decarboxylation and stereoisomerization. Org. Lett. 2020, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liao, G.; Kindinger, F.; Ludwig-Radtke, L.; Yin, W.-B.; Li, S.-M. Peniphenone and penilactone formation in Penicillium crustosum via 1,4-Michael additions of ortho-quinone methide from hydroxyclavatol to γ-butyrolactones from crustosic acid. J. Am. Chem. Soc. 2019, 141, 4225. [Google Scholar] [CrossRef] [PubMed]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef] [PubMed]
- Medema, M.H.; de Rond, T.; Moore, B.S. Mining genomes to illuminate the specialized chemistry of life. Nat. Rev. Genet. 2021, 22, 553. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Li, S.-M. Formation of 3-orsellinoxypropanoic acid in Penicillum crustosum is catalyzed by a bifunctional nonreducing polyketide synthase. Org. Lett. 2022, 24, 462. [Google Scholar] [CrossRef] [PubMed]
- Kindinger, F.; Nies, J.; Becker, A.; Zhu, T.; Li, S.-M. Genomic locus of a Penicillium crustosum pigment as an integration site for secondary metabolite gene expression. ACS Chem. Biol. 2019, 14, 1227. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.-M. Conversion of viridicatic acid to crustosic acid by cytochrome P450 enzyme-catalysed hydroxylation and spontaneous cyclisation. App. Microbol. Biotechnol. 2021, 105, 9181. [Google Scholar] [CrossRef]
- Mojardín, L.; Vega, M.; Moreno, F.; Schmitz, H.P.; Heinisch, J.J.; Rodicio, R. Lack of the NAD+-dependent glycerol 3-phosphate dehydrogenase impairs the function of transcription factors Sip4 and Cat8 required for ethanol utilization in Kluyveromyces lactis. Fungal. Genet. Biol. 2018, 111, 16. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Ahuja, M.; Oakley, C.E.; Entwistle, R.; Asokan, A.; Zutz, C.; Wang, C.C.; Oakley, B.R. Development of genetic dereplication strains in Aspergillus nidulans results in the discovery of aspercryptin. Angew. Chem. Int. Ed. Eng. 2016, 55, 1662. [Google Scholar] [CrossRef] [Green Version]
- Nies, J.; Li, S.-M. Prenylation and dehydrogenation of a C2-reversely prenylated diketopiperazine as a branching point in the biosynthesis of echinulin family alkaloids in Aspergillus ruber. ACS Chem. Biol. 2021, 16, 185. [Google Scholar] [CrossRef]
- Yin, W.B.; Chooi, Y.H.; Smith, A.R.; Cacho, R.A.; Hu, Y.; White, T.C.; Tang, Y. Discovery of cryptic polyketide metabolites from dermatophytes using heterologous expression in Aspergillus nidulans. ACS Synth. Biol. 2013, 2, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, P.; Ludwig-Radtke, L.; Yin, W.-B.; Li, S.-M. Isocoumarin formation by heterologous gene expression and modification by host enzymes. Org. Biomol. Chem. 2020, 18, 4946. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Chavez, F.; Zwahlen, R.D.; Bovenberg, R.A.L.; Driessen, A.J.M. Engineering of the filamentous fungus Penicillium chrysogenum as cell factory for natural products. Front. Microbiol. 2018, 9, 2768. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Kobayashi, T.; Oikawa, H.; Honma, M.; Ichihara, A. Enzymatic activity and partial purification of solanapyrone synthase: First enzyme catalyzing Diels-Alder reaction. Biochim. Biophys. Acta 1998, 1384, 387. [Google Scholar] [CrossRef]
- Katayama, K.; Kobayashi, T.; Chijimatsu, M.; Ichihara, A.; Oikawa, H. Purification and N-terminal amino acid sequence of solanapyrone synthase, a natural Diels-Alderase from Alternaria solani. Biosci. Biotechnol. Biochem. 2008, 72, 604. [Google Scholar] [CrossRef]
- Kasahara, K.; Miyamoto, T.; Fujimoto, T.; Oguri, H.; Tokiwano, T.; Oikawa, H.; Ebizuka, Y.; Fujii, I. Solanapyrone synthase, a possible Diels-Alderase and iterative type I polyketide synthase encoded in a biosynthetic gene cluster from Alternaria solani. Chembiochem 2010, 11, 1245. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhang, Z.Z.; Feng, Y.Y.; Gu, Q.Q.; Li, D.H.; Zhu, T.J. Secondary metabolites from Antarctic marine-derived fungus Penicillium crustosum HDN153086. Nat. Prod. Res. 2019, 33, 414. [Google Scholar] [CrossRef]
- Schlingmann, G.; Milne, L.; Carter, G.T. New α-pyrones produced by fungal culture LL-11G219 function as androgen receptor ligands. Tetrahedron 1998, 54, 13013. [Google Scholar] [CrossRef]
- Hu, Z.-Y.; Li, Y.-Y.; Lu, C.-H.; Lin, T.; Hu, P.; Shen, Y.-M. Seven novel linear polyketides from Xylaria sp. NCY2. Helv. Chim. Acta 2010, 93, 925. [Google Scholar] [CrossRef]
- Cadelis, M.M.; Gordon, H.; Grey, A.; Geese, S.; Mulholland, D.R.; Weir, B.S.; Copp, B.R.; Wiles, S. Isolation of a novel polyketide from Neodidymelliopsis sp. Molecules 2021, 26, 3235. [Google Scholar] [CrossRef]
- Tran, T.D.; Wilson, B.A.P.; Henrich, C.J.; Wendt, K.L.; King, J.; Cichewicz, R.H.; Stchigel, A.M.; Miller, A.N.; O’Keefe, B.R.; Gustafson, K.R. Structure elucidation and absolute configuration of metabolites from the soil-derived fungus Dictyosporium digitatum using spectroscopic and computational methods. Phytochemistry 2020, 173, 112278. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Fujii, I.; Sankawa, U.; Mayorga, M.E.; Timberlake, W.E.; Ebizuka, Y. Reidentification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tetrahedron Lett. 1999, 40, 91. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stierle, S.A.; Li, S.-M. Biosynthesis of Xylariolide D in Penicillium crustosum Implies a Chain Branching Reaction Catalyzed by a Highly Reducing Polyketide Synthase. J. Fungi 2022, 8, 493. https://doi.org/10.3390/jof8050493
Stierle SA, Li S-M. Biosynthesis of Xylariolide D in Penicillium crustosum Implies a Chain Branching Reaction Catalyzed by a Highly Reducing Polyketide Synthase. Journal of Fungi. 2022; 8(5):493. https://doi.org/10.3390/jof8050493
Chicago/Turabian StyleStierle, Sina A., and Shu-Ming Li. 2022. "Biosynthesis of Xylariolide D in Penicillium crustosum Implies a Chain Branching Reaction Catalyzed by a Highly Reducing Polyketide Synthase" Journal of Fungi 8, no. 5: 493. https://doi.org/10.3390/jof8050493
APA StyleStierle, S. A., & Li, S.-M. (2022). Biosynthesis of Xylariolide D in Penicillium crustosum Implies a Chain Branching Reaction Catalyzed by a Highly Reducing Polyketide Synthase. Journal of Fungi, 8(5), 493. https://doi.org/10.3390/jof8050493







