Four New Highly Oxygenated Eremophilane Sesquiterpenes from an Endophytic Fungus Boeremia exigua Isolated from Fritillaria hupehensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Culture and Fermentation of Fungal Material
2.3. Extraction and Isolation
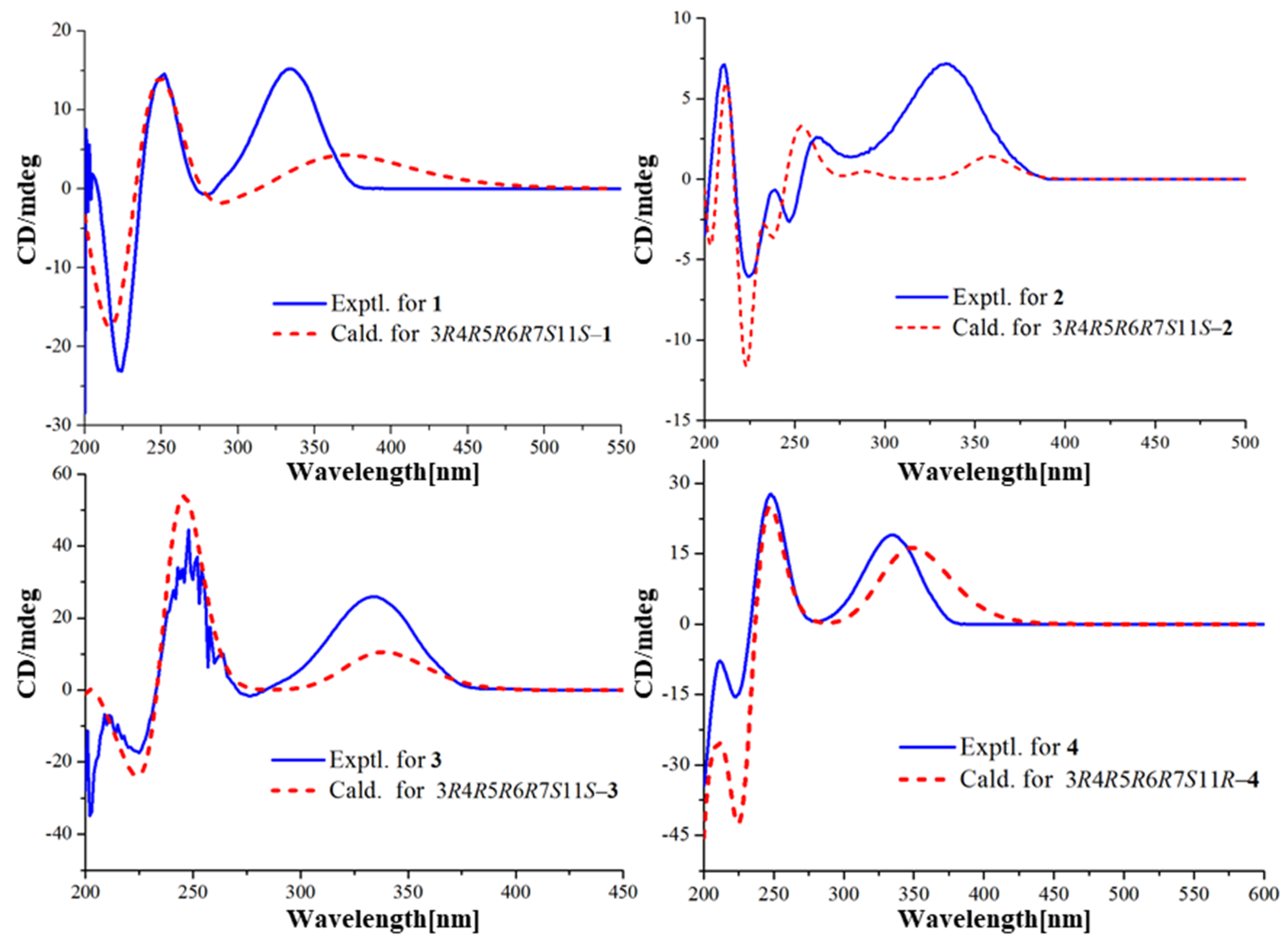
2.4. Quantum Chemical Calculations
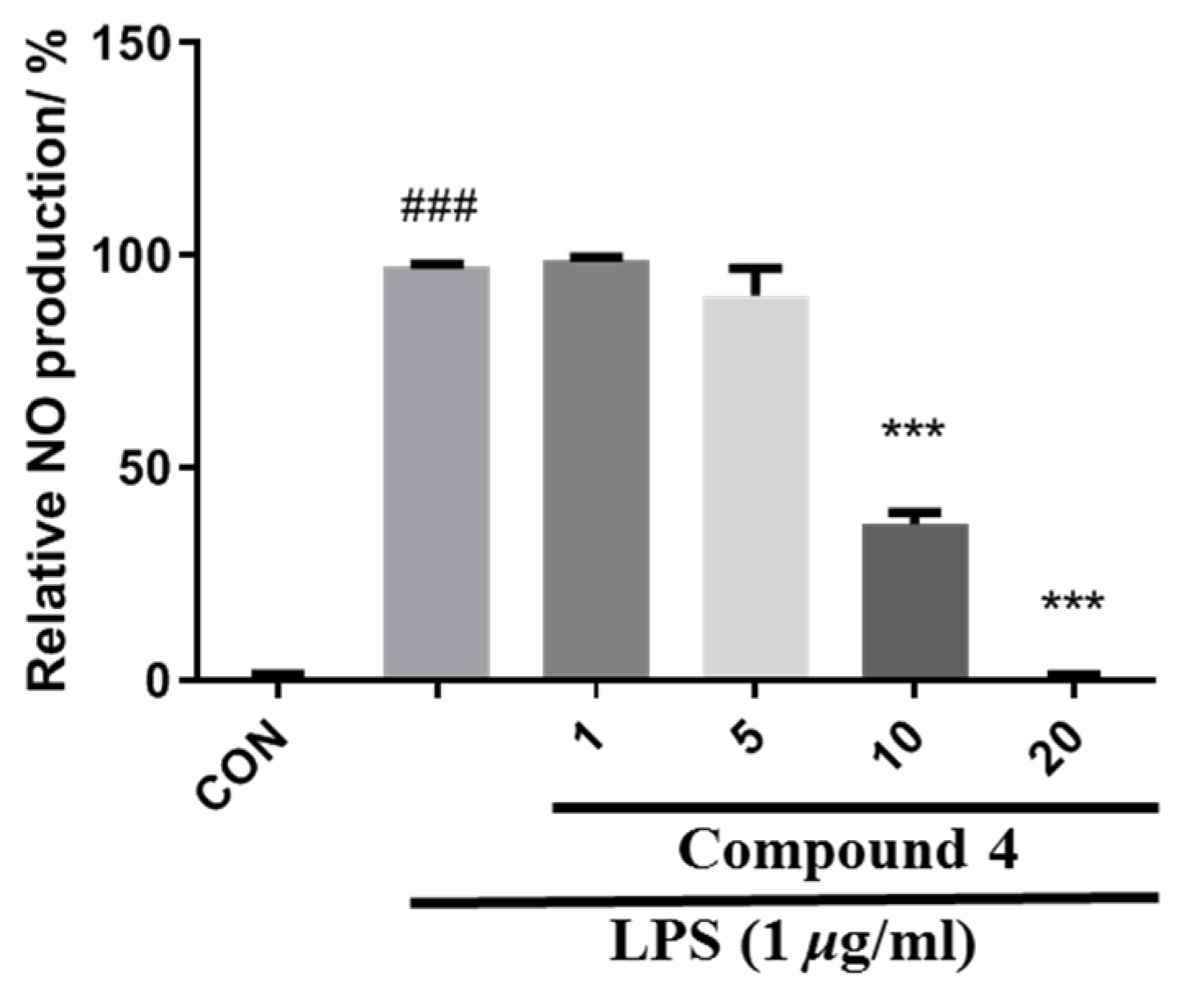
2.5. Nitric Oxide Production Inhibitory Assay
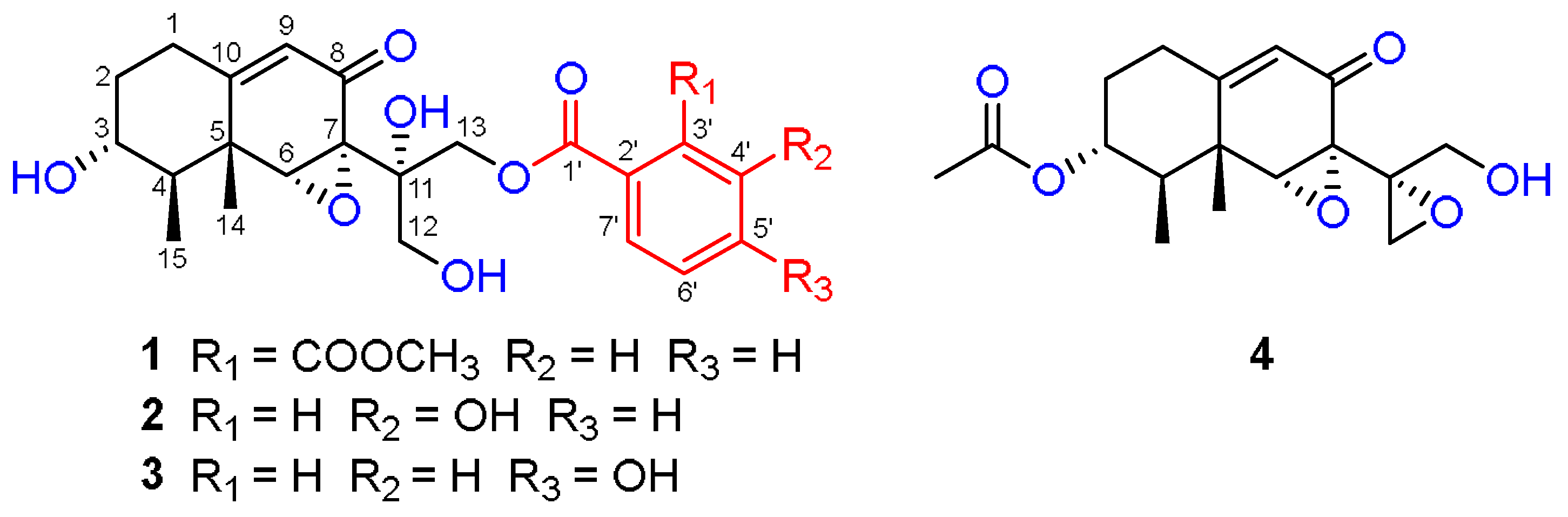
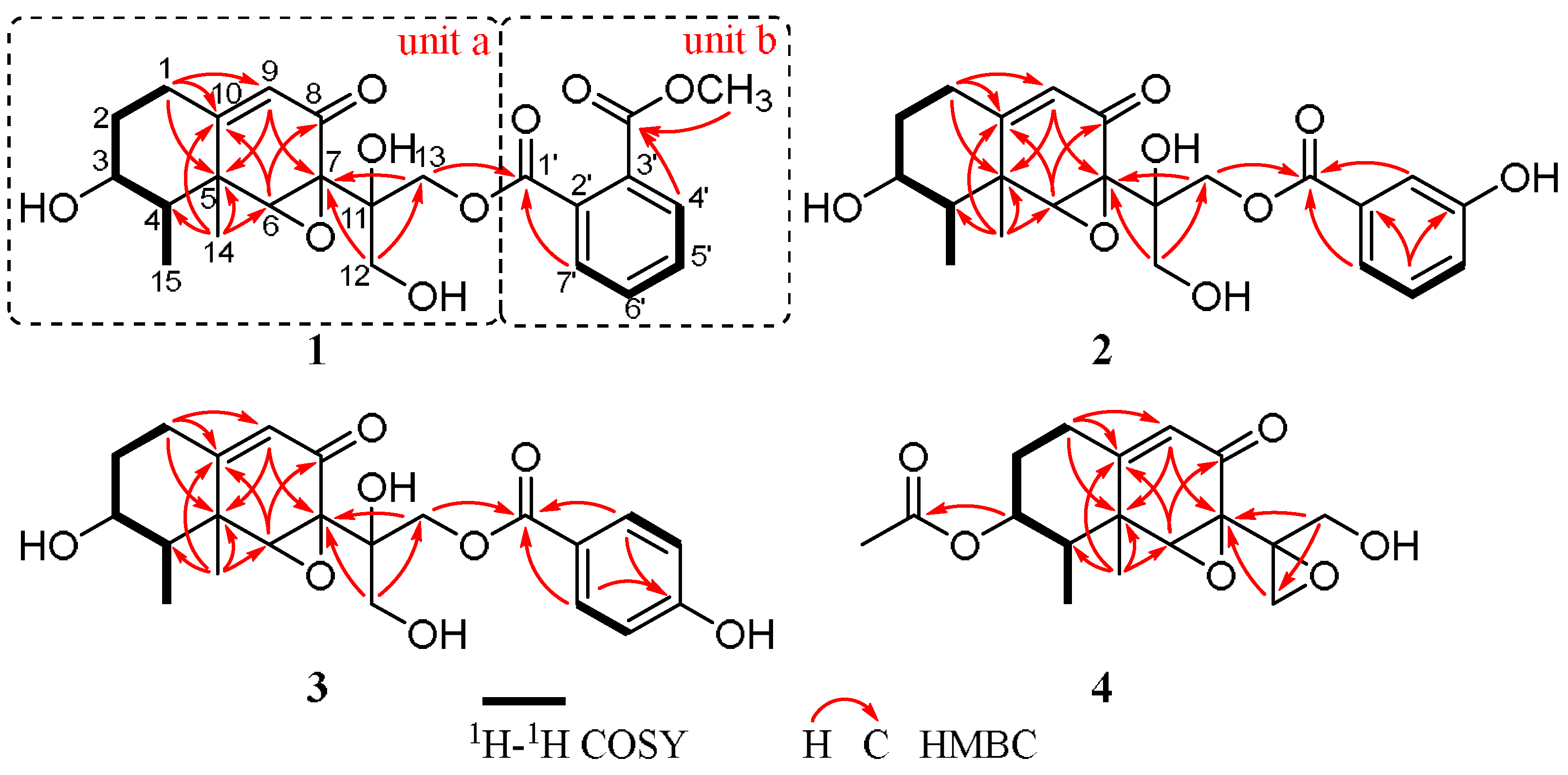
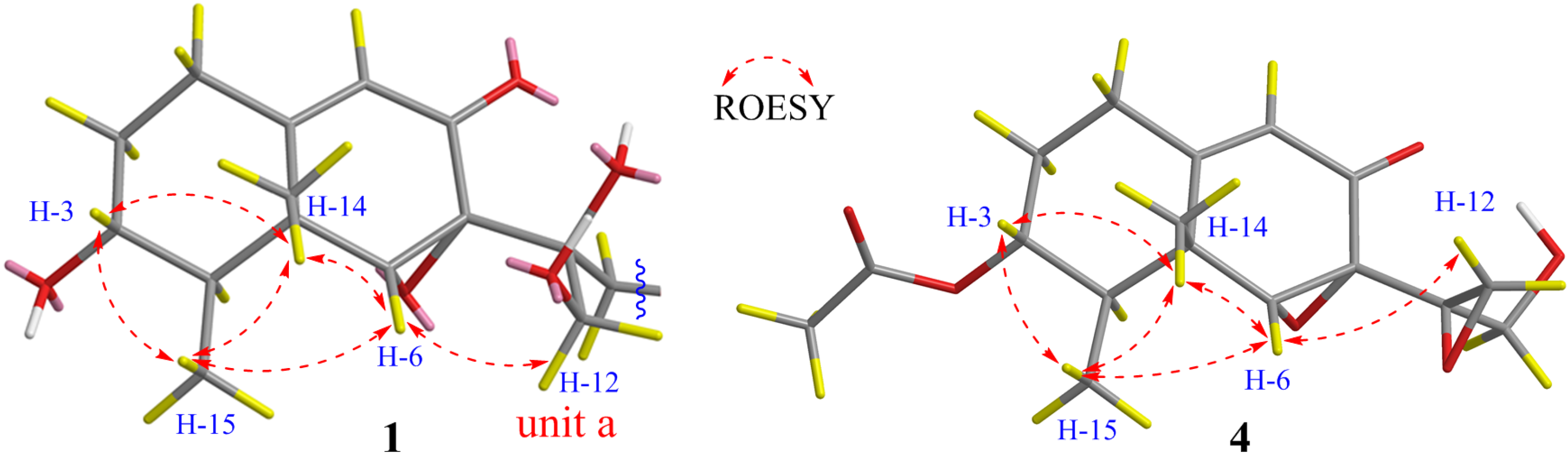
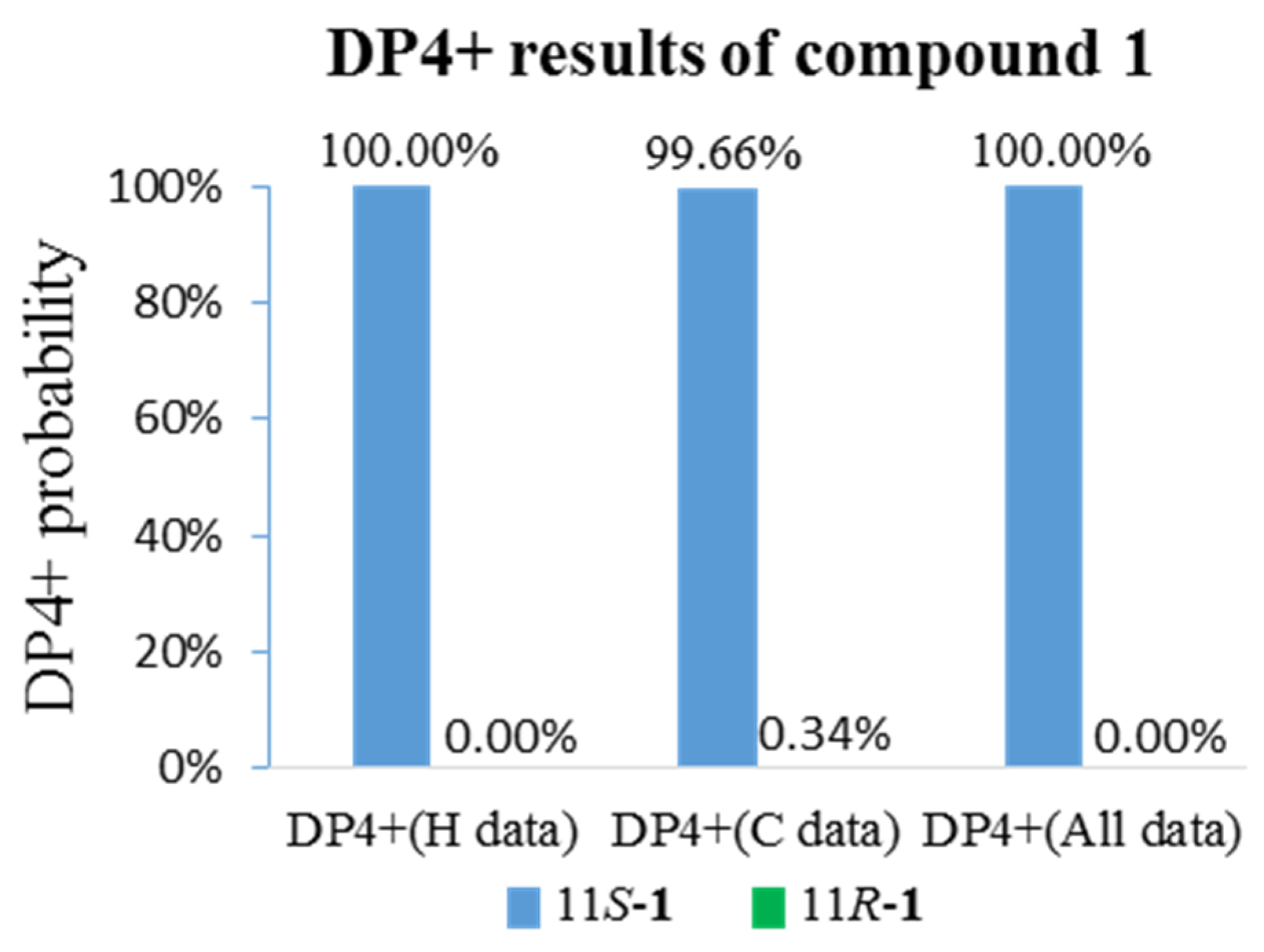
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2009, 26, 1125–1155. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liao, Z.X.; Liu, C.; Jia, H.Y.; Sun, J.Y. Eremophilane Sesquiterpenes from the Genus Ligularia. Chem. Biodivers. 2016, 13, 645–671. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.J.; Starks, C.M.; Manna, K.R.; Chappell, J.; Noel, J.P.; Coates, R.M. Stereochemistry and deuterium isotope effects associated with the cyclization-rearrangements catalyzed by tobacco epiaristolochene and hyoscyamus premnaspirodiene synthases, and the chimeric CH4 hybrid cyclase. Arch. Biochem. Biophys. 2006, 448, 31–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, C.J.; Kulka, M.; Zhang, J.Z.; Li, Y.M.; Guo, F.J. Occurrence and biological activities of eremophilane-type sesquiterpenes. Mini Rev. Med. Chem. 2014, 14, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Wongkanoun, S.; Wessel, A.C.; Bills, G.F.; Stadler, M.; Luangsa-ard, J.J. Phylogenetic and chemotaxonomic studies confirm the affinities of Stromatoneurospora phoenix to the coprophilous xylariaceae. J. Fungi 2020, 6, 144. [Google Scholar] [CrossRef]
- Li, C.S.; Ding, Y.Q.; Yang, B.J.; Hoffman, N.; Yin, H.Q.; Mahmud, T.; Turkson, J.; Cao, S.G. Eremophilane sesquiterpenes from Hawaiian endophytic fungus Chaetoconis sp. FT087. Phytochemistry 2016, 126, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Khan, B.; Zhao, S.S.; Wang, Z.Y.; Ye, Y.H.; Rajput, N.A.; Yan, W. Eremophilane sesquiterpenes and benzene derivatives from the endophyte microdiplodia sp. WGHS5. Chem. Biodivers. 2021, 18, e2000949. [Google Scholar] [CrossRef]
- Cheng, Z.B.; Zhao, J.J.; Liu, D.; Proksch, P.; Zhao, Z.M.; Lin, W.H. Eremophilane-type sesquiterpenoids from an Acremonium sp. fungus isolated from deep-sea sediments. J. Nat. Prod. 2016, 79, 1035–1047. [Google Scholar] [CrossRef]
- Wu, Q.X.; Shi, Y.P.; Yang, L. Unusual sesquiterpene lactones from Ligularia virgaurea spp. oligocephala. Org. Lett. 2004, 6, 2313–2316. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhang, D.W.; Zhang, M.; Zhao, J.L.; Chen, R.D.; Wang, N.; Zhang, D.; Dais, J.G. Eremophilane Sesquiterpenes from an Endophytic Fungus Periconia Species. J. Nat. Prod. 2016, 79, 2229–2235. [Google Scholar] [CrossRef]
- Kato, T.; Hirota, H.; Kuroda, C.; Gong, X.; Ohsaki, A. New eremophilane-type sesquiterpenes from Ligularia cymbulifera. Nat. Prod. Commun. 2017, 12, 1165–1167. [Google Scholar] [CrossRef] [Green Version]
- Silchenko, A.S.; Kalinovsky, A.I.; Ponomarenko, L.P.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Gorovoy, P.G.; Kim, N.Y.; Stonik, V.A. Structures of eremophilane-type sesquiterpene glucosides, alticolosides A-G, from the Far Eastern endemic Ligularia Alticola Worosch. Phytochemistry 2015, 111, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Bradfield, A.E.; Penfold, A.R.; Simonsen, J.L. The constitution of eremophilone and of two related hydroxy-ketones from the wood oil of Eremophila mitchelli. J. Chem. Soc. 1932, 2744–2759. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Fortkamp, D.; Abraham, W.R. Eremophilane-type sesquiterpenes from fungi and their medicinal potential. Biol. Chem. 2018, 399, 13–28. [Google Scholar] [CrossRef]
- Zhou, M.; Duan, F.F.; Gao, Y.; Peng, X.G.; Meng, X.G.; Ruan, H.L. Eremophilane sesquiterpenoids from the whole plant of Parasenecio albus with immunosuppressive activity. Bioorg. Chem. 2021, 115, 105247. [Google Scholar] [CrossRef]
- Chen, J.; Chen, C.; Yao, X.; Jin, X.; Gao, K. Eremophilane-type sesquiterpenoids with diverse skeletons from Ligularia sagittal. J. Nat. Prod. 2014, 77, 1329–1335. [Google Scholar] [CrossRef]
- Liu, M.Y.; Li, P.L.; Tang, X.L.; Luo, X.C.; Liu, K.C.; Zhang, Y.; Wang, Q.; Li, G.Q. Lemnardosinanes A-I: New bioactive sesquiterpenoids from soft coral Lemnalia sp. J. Org. Chem. 2021, 86, 970–979. [Google Scholar] [CrossRef]
- Wu, G.W.; Lin, A.Q.; Gu, Q.Q.; Zhu, T.J.; Li, D.H. Four new chloro-eremophilane sesquiterpenes from an antarctic deep-sea derived fungus, penicillium sp. PR19N-1. Mar. Drugs. 2013, 4, 1399–1408. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.B.; Jian, X.; Qiang, Z.; Rui, H.; Xu, B.; Yang, S.X.; Han, W.B.; Tang, J.J.; Gao, J.M. Eremophilane sesquiterpenoids with antibacterial and anti-inflammatory activities from the endophytic fungus Septoria rudbeckiae. J. Agric. Food Chem. 2021, 69, 11878–11889. [Google Scholar]
- Wang, A.; Yin, R.Y.; Zhou, Z.Y.; Gu, G.; Dai, J.G.; Lai, D.W.; Zhou, L.G. Eremophilane-type sesquiterpenoids from the endophytic fungus Rhizopycnis vagum and their antibacterial, cytotoxic, and phytotoxic activities. Front. Chem. 2020, 8, 596889. [Google Scholar] [CrossRef]
- Amaral, L.S.; Rodrigues, E. Two novel eremophilane sesquiterpenes from an endophytic xylariaceous fungus isolated from leaves of Cupressus lusitanica. J. Braz. Chem. Soc. 2010, 21, 1446–1450. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.J.; Nan, Z.D.; Li, W.H.; Huang, H.L.; Yuan, C.S. New eremophilanolides from Ligularia hodgsonii. Helv. Chim. Acta 2009, 92, 209–216. [Google Scholar] [CrossRef]
- Liu, Q.; Shen, L.; Wang, T.T.; Chen, C.J.; Qi, W.Y.; Gao, K. Novel modified furanoeremophilane-type sesquiterpenes and benzofuran derivatives from Ligularia veitchiana. Food Chem. 2010, 122, 55–59. [Google Scholar] [CrossRef]
- Ye, K.; Lv, X.; Zhang, X.; Wei, P.P.; Li, Z.H.; Ai, H.L.; Zhao, D.K.; Liu, J.K. Immunosuppressive Isopimarane Diterpenes from Cultures of the Endophytic Fungus Ilyonectria robusta. Front. Pharmacol. 2022, 12, 766441. [Google Scholar] [PubMed]
- Yang, H.X.; Wu, X.; Chi, M.J.; Li, Z.H.; Feng, T.; Ai, H.L.; Liu, J.K. Structure and cytotoxicity of trichothecenes produced by the potato-associated fungus Trichothecium crotocinigenum. Bioorg. Chem. 2021, 111, 104874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.X.; Ye, K.; Wei, P.P.; Lv, X.; Fan, Y.Z.; Yang, Y.L.; Ai, H.L.; Liu, J.K. Oblongolides from endophytic fungus Phoma bellidis Neerg. harbored in Tricyrtis maculata (D. Don) J.F.Macbr. Phytochemstry 2022, 198, 113126. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.T.; Yang, H.X.; Li, Z.H.; Liu, J.K.; Ai, H.L.; Wang, G.K.; Feng, T. Depsidones and diaryl ethers from potato endophytic fungus Boeremia exigua. Fitoterapia 2020, 141, 104483. [Google Scholar]
- Wavefunction Inc. Spartan 14; Wavefunction Inc.: Irvine, CA, USA, 2014. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the comparison of calculated and experimental electronic circular dichroism spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Jain, R.J.; Bally, T.; Rablen, P.R. Calculating accurate proton chemical shifts of organic molecules with density functional methods and modest basis sets. J. Org. Chem. 2009, 74, 4017–4023. [Google Scholar] [CrossRef] [Green Version]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef]
- Yu, W.W.; Ma, J.T.; He, J.; Li, Z.H.; Liu, J.K.; Feng, T. Cadinane sesquiterpenoids from the fungus Antrodiella albocinnamomea and their inhibitory activity against nitric oxide production. Phytochemistry 2022, 196, 113081. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, G.; Dhar, T.K.; Bhattacharyya, F.K.; Siddiqui, K.A. Mutagenic action of phaseolinone, a mycotoxin isolated from Macrophomina phaseolina. Aust. J. Biol. Sci. 1987, 40, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, T.K.; Siddiqui, K.A.I.; Ali, E. Structure of phaseolinone, a novel phytotoxin from Macrophomina phaseolina. Tetrahedron Lett. 1982, 23, 5459–5462. [Google Scholar]
| No. | δH (1) a | δC (1) b | δH (2) a | δC (2) b |
|---|---|---|---|---|
| 1 | 2.41 (tdd, 14.4, 5.0, 1.9) | 31.6, CH2 | 2.51 (tdd, 14.4, 5.0, 1.9) | 31.6, CH2 |
| 2.24 (dt, 14.4, 4.1) | 2.28 (dt, 14.4, 3.5) | |||
| 2 | 2.03 (dd, 12.3, 4.4) | 36.2, CH2 | 2.07 (dd, 12.2, 4.4) | 36.3, CH2 |
| 1.27 (ddd, 12.3, 5.0, 4.1) | 1.29 (ddd, 12.2, 5.0, 3.5) | |||
| 3 | 3.44 (td, 10.5, 4.4) | 71.1, CH | 3.53 (td, 10.5, 4.4) | 71.1, CH |
| 4 | 1.63 (dq, 10.5, 6.7) | 45.9, CH | 1.70 (dq, 10.5, 6.7) | 46.0, CH |
| 5 | 42.2, C | 42.3, C | ||
| 6 | 3.80 (s) | 63.9, CH | 3.91 (s) | 64.1, CH |
| 7 | 62.9, C | 62.8, C | ||
| 8 | 195.5, C | 195.4, C | ||
| 9 | 5.61 (d, 1.9) | 121.8, CH | 5.66 (d, 1.9) | 121.8, CH |
| 10 | 166.5, C | 166.5, C | ||
| 11 | 73.9, C | 74.2, C | ||
| 12 | 4.19 (d, 11.6) | 65.6, CH2 | 4.17 (d, 11.6) | 65.5, CH2 |
| 3.76 (d, 11.6) | 3.81 (d, 11.6) | |||
| 13 | 4.64 (d, 11.5) | 69.3, CH2 | 4.83 (d, 11.7) | 67.5, CH2 |
| 4.59 (d, 11.5) | 4.44 (d, 11.7) | |||
| 14 | 0.64 (s) | 18.3, CH3 | 1.03 (s) | 19.0, CH3 |
| 15 | 1.16 (d, 6.7) | 11.6, CH3 | 1.23 (d, 6.7) | 11.6, CH3 |
| 1’ | 169.6, C | 168.1, C | ||
| 2’ | 134.3, C | 132.4, C | ||
| 3’ | 132.8, C | 7.37 (dd, 2.6, 1.3) | 117.3, CH | |
| 4’ | 7.77 (dd, 6.4, 2.1) | 130.3, CH | 158.8, C | |
| 5’ | 7.62 (td, 6.4, 2.7) | 129.7, CH | 7.00 (dd, 7.9, 2.6) | 121.3, CH |
| 6’ | 7.61 (td, 6.4, 2.1) | 132.9, CH | 7.25 (t, 7.9) | 130.5, CH |
| 7’ | 7.59 (dd, 6.4, 2.7) | 132.3, CH | 7.44 (dd, 7.9, 1.3) | 121.8, CH |
| COOCH3 | 3.85 (s) | 53.5, CH3 | ||
| COOCH3 | 168.9, C |
| No. | δH (3) a | δC (3) b | δH (4) a | δC (4) b |
|---|---|---|---|---|
| 1 | 2.50 (tdd, 14.4, 4.8, 1.8) | 31.6, CH2 | 2.41 (tdd, 14.6, 5.0, 1.8) | 31.2, CH2 |
| 2.28 (dt, 14.4, 3.5) | 2.39 (dt, 14.6, 4.0) | |||
| 2 | 2.07 (dd, 12.5, 4.4) | 36.3, CH2 | 2.15 (dd, 12.3, 4.4) | 32.5, CH2 |
| 1.29 ddd, 12.5, 4.8,3.5 | 1.40 (ddd,12.3, 5.0, 4.0) | |||
| 3 | 3.53 (td, 10.6, 4.4) | 71.1, CH | 4.91 (td, 10.5, 4.4) | 74.2, CH |
| 4 | 1.69 (dq, 10.6, 6.8) | 45.9, CH | 1.95 (dq, 10.5, 6.8) | 43.1, CH |
| 5 | 42.2, C | 42.4, C | ||
| 6 | 3.89 (s) | 64.1, CH | 3.63 (s) | 65.5, CH |
| 7 | 62.8, C | 62.1, C | ||
| 8 | 195.3, C | 194.2, C | ||
| 9 | 5.65 (d, 1.8) | 121.8, CH | 5.75 (d, 1.8) | 121.7, CH |
| 10 | 166.4, C | 165.4, C | ||
| 11 | 74.3, C | 59.0, C | ||
| 12 | 4.17 (d, 11.6) | 65.5, CH2 | 2.87 (d, 5.1) | 48.3, CH2 |
| 3.81 (d, 11.6) | 2.66 (d, 5.1) | |||
| 13 | 4.82 (d, 11.7) | 67.2, CH2 | 4.07 (d, 12.3) | 62.0, CH2 |
| 4.39 (d, 11.7) | 3.72 (d, 12.3) | |||
| 14 | 1.02 (s) | 19.1, CH3 | 1.26 (s) | 18.6, CH3 |
| 15 | 1.22 (d, 6.8) | 11.6, CH3 | 1.14 (d, 6.8) | 11.4, CH3 |
| 1’ | 168.4, C | |||
| 2’ | 120.5, C | |||
| 3’ | 7.81 (d, 8.8) | 133.1, CH | ||
| 4’ | 6.74 (d, 8.8) | 116.8, CH | ||
| 5’ | 165.9, C | |||
| 6’ | 6.74 (d, 8.8) | 116.8, CH | ||
| 7’ | 7.81 (d, 8.8) | 133.1, CH | ||
| CH3CO | 2.06 (s) | 21.0, CH3 | ||
| CH3CO | 172.4, C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, H.-L.; Lv, X.; Ye, K.; Wang, M.-X.; Huang, R.; Shi, B.-B.; Li, Z.-H. Four New Highly Oxygenated Eremophilane Sesquiterpenes from an Endophytic Fungus Boeremia exigua Isolated from Fritillaria hupehensis. J. Fungi 2022, 8, 492. https://doi.org/10.3390/jof8050492
Ai H-L, Lv X, Ye K, Wang M-X, Huang R, Shi B-B, Li Z-H. Four New Highly Oxygenated Eremophilane Sesquiterpenes from an Endophytic Fungus Boeremia exigua Isolated from Fritillaria hupehensis. Journal of Fungi. 2022; 8(5):492. https://doi.org/10.3390/jof8050492
Chicago/Turabian StyleAi, Hong-Lian, Xiao Lv, Ke Ye, Meng-Xi Wang, Rong Huang, Bao-Bao Shi, and Zheng-Hui Li. 2022. "Four New Highly Oxygenated Eremophilane Sesquiterpenes from an Endophytic Fungus Boeremia exigua Isolated from Fritillaria hupehensis" Journal of Fungi 8, no. 5: 492. https://doi.org/10.3390/jof8050492
APA StyleAi, H.-L., Lv, X., Ye, K., Wang, M.-X., Huang, R., Shi, B.-B., & Li, Z.-H. (2022). Four New Highly Oxygenated Eremophilane Sesquiterpenes from an Endophytic Fungus Boeremia exigua Isolated from Fritillaria hupehensis. Journal of Fungi, 8(5), 492. https://doi.org/10.3390/jof8050492





