Abstract
Cantharellus is a well-known genus of edible mushrooms, belonging to the family Hydnaceae in the class Agaricomycetes. In this study, a phylogenetic overview of Cantharellus subg. Cinnabarinus and C. subg. Parvocantharellus in China is carried out with the description of four new species. Species description are based on morphological characters of basidiomata and phylogenetic analyses of multi-locus dataset of 28S + tef1 + rpb2. Among the new species, two species, C. chrysanthus and C. sinocinnabarinus, belong to C. subg. Cinnabarinus and two new species, C. convexus and C. neopersicinus, belong to C. subg. Parvocantharellus. Species delimitation characters of the new taxa are compared with closely related species. In addition, three new records of Cantharellus are reported for China: C. albovenosus and C. citrinus of subg. Cinnabarinus and C. koreanus of subg. Parvocantharellus. A key to the species of subg. Cinnabarinus in China was provided.
1. Introduction
Cantharellus Fr. was firstly described by Fries [1] based on the type species Cantharellus cibarius Fr. Most Cantharellus species are popular edible mushrooms, especially beloved in Europe. Cantharellus is an ectomycorrhizal genus, forming symbiosis with various plants, such as the trees of Fagaceae, Pinaceae, Betulaceae, Salicaceae, Juglandaceae, Leguminosae, etc. [2,3,4,5,6,7,8]. Species in Cantharellus are widely distributed and are especially rich in subtropical to tropical zones [3,9,10]. Up to now, about 300 species of Cantharellus have been reported worldwide [7]. However, the species diversity is poorly known in Asia in the past decades, and many specimens were named after European or North American species [6,11,12,13]. In recent years, some new species were reported from Asia based on the combination of morphological characters and DNA phylogenetic analyses [6,7,11,12,13,14,15,16]. Recent phylogenetic studies demonstrated that Cantharellus is monophyletic and forms a sister relationship with Craterellus Pers. [3,4,7]. Species in Cantharellus were divided into seven subgenera based on multi-locus phylogenetic analyses in Buyck et al. [3], and a subsequent study in Cao et al. [7]. Cantharellus subg. Cinnabarinus Buyck & V. Hofst., typified by C. cinnabarinus (Schwein.) Schwein. was introduced for a monophyletic assemblage of mostly quite small, yellow, orange, pink or red species, sometimes mixed with lilac-purple or brownish tones in the pileus center, strongly veined in the lamellate hymenophore with principally thin-walled hyphal endings and abundant in clamp connections [3,17,18]. Species in subg. Cinnabarinus are widely distributed in Asia, Europe, North America, Australasia and Africa, and 16 species have been reported worldwide. In China, a large number of Cantharellus species have been reported, but only two species in the subg. Cinnabarinus were recorded, i.e., C. cinnabarinus and C. phloginus, by S.C. Shao & P.G. Liu. Cantharellus cinnabarinus, originally reported from North America, was recorded to be widely distributed in China [19,20,21]; C. phloginus was described as being from southwestern China [22].
In this study, a number of Cantharellus specimens were collected from China; further study proved that they represented eight distinct species, five of which belong to the subg. Cinnabarinus and three to the subg. Parvocantharellus. Four species are described below as new to science, which would make a contribution to understanding the species diversity of Cantharellus in China, and revealing the phylogenetic relationships of Cantharellus species.
2. Materials and Methods
2.1. Morphological Studies
Photographs of fresh basidiomata were taken in the field. Specimens were dried and deposited in the Fungarium of Guangdong Institute of Microbiology (GDGM). Descriptions of macro-morphological characters and habitats were obtained from photographs and field notes. The color codes followed Kornerup and Wanscher [23]. Microscopic observations were carried out on tissue sections stained with 5% aqueous KOH and 1% aqueous Congo red under a light microscope (Carl Zeiss Microscopy GmbH, Göttingen, Germany) with a magnification up to 1000×. For basidiospore descriptions, the notation (a–)b–c(–d) describes basidiospore dimensions, where the range b–c represented 90% or more of the measured values and ‘a’ and ‘d’ were the extreme values; Lm and Wm indicated the average length and width (±standard deviation) of the measured basidiospores, respectively; Q referred to the length/width ratio of an individual basidiospore and Qm referred to the average Q value of all basidiospores ± sample standard deviation. All line-drawings of microstructures were made based on rehydrated materials.
2.2. DNA Extraction, PCR Amplification and Sequencing
Genomic DNA was extracted from the voucher specimens using the Sangon Fungus Genomic DNA Extraction kit (Sangon Biotech Co., Ltd., Shanghai, China) according to the manufacturer’s instructions. Primer pairs LROR/LR7 [24], tef1F/tef1R and RPB2-5FCanth/RPB2-7cRCanth [3,25] were used to amplify the LSU, tef1 and rpb2 region, respectively. PCR reactions were performed in a total volume of 25 μL containing 0.5 μL template DNA, 11 μL sterile deionized water, 0.5 μL of each primer and 12.5 μL 2 × PCR mix [DreamTaqtm Green PCR Master Mix (2×), Fermentas, USA]. Amplification reactions were performed in a Tprofessional Standard thermocycler (Biometra, Göttingen, Germany) under the following conditions: 95 °C for 4 min; then, 35 cycles of denaturation at 94 °C for 60 s, annealing at 53 °C (LSU)/50 °C (tef1)/52 °C (rpb2) for 60 s and extension at 72 °C for 60 s; with a final extension at 72 °C for 8 min. The PCR products were electrophoresed on 1% agarose gels and then send for sequencing on an ABI Prism® 3730 Genetic Analyzer (PE Applied Biosystems, Foster, CA, USA) at the Beijing Genomic Institute (BGI) using the same PCR primers. The raw sequences were assembled and checked with SeqMan implemented in Lasergene v7.1 (DNASTAR Inc., Madison, WI, USA). The newly generated sequences in this study were submitted to GenBank.
2.3. Phylogenetic Analyses
Sequences generated in this study and those downloaded from GenBank were combined and used for phylogenetic reconstruction. Detailed information of specimens included in this study was given in Table 1. Three sequence matrices, i.e., nrLSU, tef1 and rpb2, were aligned separately with software MAFFT v6.853 using the E-INS-i strategy [26] and then manually adjusted in MEGA 6 [27]. The ambiguous aligned regions and introns of the two protein-coding genes of tef1 and rpb2 were retained in the final analyses.
Maximum Likelihood (ML) analyses were inferred using RAxML v7.2.6 [28], and all parameters were kept as defaults except for choosing GTRGAMMAI as the model; statistical supports were obtained using rapid non-parametric bootstrapping with 1000 replicates. Bayesian Inference (BI) phylogenies were inferred using MrBayes 3.2.6 [29]; the best models of the multi-locus datasets were searched via the PartitionFinder 2 [30] for each locus, i.e., K80 + I + G, SYM + I + G and SYM + I + G for 28S, tef1 and rpb2, respectively. BI analysis using 4 chains were conducted by setting generations to 20 million and stoprul command with the value of stopval set to 0.01; trees were sampled every 1000 generations, the first 25% generations were discarded as burn-in and posterior probabilities (PP) were then calculated from the posterior distribution of the retained Bayesian trees. Cantharellus cibarius Fr. was selected as the outgroup based on recent studies [3,13]. The phylogenetic trees were visualized using FigTree v1.4.23.

Table 1.
Specimen information used in this study. Sequences newly generated in this study are in bold; HT, NT and ET refer to holotype, neotype and epitype, respectively.
Table 1.
Specimen information used in this study. Sequences newly generated in this study are in bold; HT, NT and ET refer to holotype, neotype and epitype, respectively.
| Taxa | Voucher | Locality | GenBank Accession No. | Reference | ||
|---|---|---|---|---|---|---|
| LSU | tef1 | rpb2 | ||||
| Cantharellus afrocibarius | BB 96.236 | Zambia | KF294669 | JX192994 | KF294747 | [3] |
| C. afrocibarius | BB 96.235 (HT) | Zambia | KF294668 | JX192993 | KF294746 | [3] |
| C. albovenosus | 1690 (HT) | South Korean | – | KY271942 | – | [11] |
| C. albovenosus | 1713 | South Korean | – | MW124387 | – | [11] |
| C. albovenosus | GDGM85853 | China | OM978952 | ON119062 | ON119006 | Present study |
| C. albovenosus | GDGM85846 | China | OM978950 | ON119060 | ON119004 | Present study |
| C. albovenosus | GDGM85142 | China | OM978949 | ON119059 | ON229082 | Present study |
| C. albovenosus | HMAS279296 | China | OM978948 | ON119066 | ON119010 | Present study |
| C. albovenosus | HMAS279284 | China | ON212414 | ON119064 | ON119008 | Present study |
| C. albovenosus | HMAS279292 | China | ON212412 | ON119065 | ON119009 | Present study |
| C. albovenosus | HMAS279262 | China | OM978947 | ON119063 | ON119007 | Present study |
| C. albovenosus | GDGM85852 | China | OM978951 | ON119061 | ON119005 | Present study |
| C. albus | HKAS107045 (HT) | China | MT782540 | MT776015 | MT776012 | [12] |
| C. albus | GDGM81399 | China | MZ605074 | MZ613977 | MZ614022 | [13] |
| C. albus | GDGM81064 | China | MZ605073 | MZ613976 | MZ614021 | [13] |
| C. appalachiensis | GRSM77088 | USA | DQ898690 | – | DQ898748 | [31] |
| C. appalachiensis | BB 07.123 | USA | KF294635 | GQ914979 | KF294711 | [3] |
| C. aurantinus | GDGM46278 (HT) | China | MZ766517 | MZ766560 | [13] | |
| C. aurantinus | GDGM46279 | China | MZ766518 | MZ766561 | MZ766571 | [13] |
| C. aurantinus | GDGM81899 | China | MZ766520 | MZ766563 | MZ766573 | [13] |
| C. aurantinus | GDGM84974 | China | MZ766521 | MZ766564 | MZ766572 | [13] |
| C. austrosinensis | GDGM81303 | China | MZ605084 | MZ613986 | MZ614029 | [13] |
| C. austrosinensis | GDGM81249 (HT) | China | MZ605082 | MZ613983 | MZ614027 | [13] |
| C. austrosinensis | GDGM80616 | China | MZ605081 | MZ613982 | MZ614026 | [13] |
| C. austrosinensis | GDGM81381 | China | MZ605086 | MZ613988 | MZ614031 | [13] |
| C. austrosinensis | GDGM81379 | China | MZ605085 | MZ613987 | MZ614030 | [13] |
| C. austrosinensis | GDGM81985 | China | MZ605087 | MZ613989 | MZ614032 | [13] |
| C. chrysanthus | GDGM45166 | China | OM978959 | ON119074 | ON119011 | Present study |
| C. chrysanthus | GDGM45937 | China | OM978960 | ON119075 | ON119012 | Present study |
| C. chrysanthus | GDGM85298 | China | OM978975 | ON119089 | ON119025 | Present study |
| C. chrysanthus | GDGM85305 | China | OM978976 | ON119090 | ON119026 | Present study |
| C. chrysanthus | GDGM53485 | China | OM978962 | ON119077 | ON119014 | Present study |
| C. chrysanthus | GDGM80220 (HT) | China | OM978970 | ON119083 | ON119019 | Present study |
| C. chrysanthus | GDGM82511 | China | OM978973 | ON119087 | ON119023 | Present study |
| C. chrysanthus | GDGM82516 | China | OM978974 | ON119088 | ON119024 | Present study |
| C. chrysanthus | GDGM80436 | China | OM978971 | ON119084 | ON119020 | Present study |
| C. chrysanthus | GDGM80202 | China | OM978965 | ON119080 | ON119016 | Present study |
| C. chrysanthus | GDGM80204 | China | OM978966 | ON119081 | ON119017 | Present study |
| C. chrysanthus | HMAS279434 | China | ON212413 | ON119091 | ON229079 | Present study |
| C. chrysanthus | GDGM80438 | China | – | ON119085 | ON119021 | Present study |
| C. chrysanthus | GDGM82473 | China | OM978972 | ON119086 | ON119022 | Present study |
| C. chrysanthus | GDGM77035 | China | OM978964 | ON119079 | ON229081 | Present study |
| C. chrysanthus | GDGM60524 | China | OM978963 | ON119078 | ON119015 | Present study |
| C. chrysanthus | GDGM80217 | China | OM978969 | ON119082 | ON119018 | Present study |
| C. chrysanthus | GDGM49628 | China | OM978961 | ON119076 | ON119013 | Present study |
| C. chrysanthus | GDGM87950 | China | OM978968 | – | ON119027 | Present study |
| C. chrysanthus | GDGM87951 | China | OM978967 | – | ON119028 | Present study |
| C. cibarius | GE 07.025 | France | KF294658 | GQ914949 | KF294736 | [3] |
| C. cibarius | BB 07.300 | Slovakia | KF294641 | GQ914950 | KF294718 | [3] |
| C. cinnabarinus | BB 04.263 (NT) | USA | – | GQ914983 | – | [32] |
| C. cinnabarinus | BB 07.053 | USA | KF294630 | GQ914984 | KF294705 | [32] |
| C. cinnabarinus | BB 07.001 | USA | KF294624 | GQ914985 | KF294698 | [32] |
| C. citrinus | 1691 (HT) | South Korean | – | MW124385 | – | [16] |
| C. citrinus | 1715 | South Korean | – | MW124388 | – | [16] |
| C. citrinus | 1710 | South Korean | – | MW124386 | – | [16] |
| C. citrinus | 1711 | South Korean | – | MW124384 | – | [16] |
| C. citrinus | GDGM86140 | China | OM978955 | ON119070 | ON119032 | Present study |
| C. citrinus | GDGM86141 | China | OM978956 | ON119071 | ON119033 | Present study |
| C. citrinus | GDGM80825 | China | – | ON119069 | ON119031 | Present study |
| C. citrinus | GDGM86142 | China | OM978957 | ON119072 | ON119034 | Present study |
| C. citrinus | GDGM80724 | China | OM978954 | ON119068 | ON119030 | Present study |
| C. citrinus | GDGM86143 | China | OM978958 | ON119073 | ON119035 | Present study |
| C. citrinus | GDGM80723 | China | OM978953 | ON119067 | ON119029 | Present study |
| C. coccolobae | 1064_RC. 14_24 | Guadeloupe | KX857088 | KX857020 | KX856992 | [33] |
| C. coccolobae | 1065_RC. 11_25 (HT) | Guadeloupe | KX857089 | KX857021 | KX856993 | [33] |
| C. congolensis | 1645/BB16.044 | Saharan Africa | KX857102 | KX857075 | KX857006 | [33] |
| C. congolensis | 1676/BB16.123 | Saharan Africa | KX857106 | KX857078 | KX857010 | [33] |
| C. aff. congolensis | BB 06.176 | Madagascar | KF294606 | – | KF294680 | [3] |
| C. aff. congolensis | BB 06.197 | Madagascar | KF294608 | – | KF294683 | [3] |
| C. convexus | GDGM54841 | China | OM978940 | ON119052 | ON119036 | Present study |
| C. convexus | GDGM70307 (HT) | China | OM978941 | ON119053 | ON119037 | Present study |
| C. corallinus | 1083_JJ_MO_CANT_2 | USA | – | KX857031 | – | [34] |
| C. corallinus | 1086_JJ_MO_CANT_5 | USA | – | KX857034 | – | [34] |
| C. corallinus | FLAS_F_61106 | USA | – | MK045368 | – | [34] |
| C. curvatus | BRNM:825749 (HT) | South Korea | MW124390 | [16] | ||
| C. cyphelloides | TNS F-61721 (HT) | Japan | NG059027 | – | – | [35] |
| C. decolorans | BB 08.278 (HT) | Madagascar | KF294654 | GQ914968 | KF294731 | [3] |
| C. fistulosus | DT_43 | Tanzania | JQ976965 | JX192997 | – | [3] |
| C. friesii | AH44798 | Spain | KR677522 | KX828831 | KX828752 | [36] |
| C. friesii | VDKO 1165 | Africa | – | KX834408 | KX881922 | [5] |
| C. galbanus | GDGM86249 (HT) | China | ZM766516 | MZ766568 | MZ766577 | [13] |
| C. garnierii | BB 09.024 | New Caledonia | KX857085 | KX857017 | KX856989 | [34] |
| C. garnierii | BB 09.283 | New Caledonia | KX857087 | KX857019 | KX856991 | [34] |
| C. garnierii | BB 09.033 | New Caledonia | KX857086 | KX857018 | KX856990 | [34] |
| C. garnierii | RF33 | New Caledonia | AY392768 | – | [37] | |
| C. garnierii | RF32 | New Caledonia | AY392767 | – | [37] | |
| C. koreanus | 1697 | South Korea | – | KY271940 | – | [11] |
| C. koreanus | 1689 (HT) | South Korea | – | KY271941 | – | [11] |
| C. koreanus | GDGM85306 | China | OM978978 | ON119093 | ON229077 | Present study |
| C. koreanus | GDGM79233 | China | OM978977 | ON119092 | ON229078 | Present study |
| C. koreanus | 1693 | South Korea | – | – | Unpublished | |
| C. koreanus | 1694 | South Korea | – | – | Unpublished | |
| C. koreanus | 1696 | South Korea | – | – | Unpublished | |
| C. luteolus | GDGM60393 (HT) | China | ZM766515 | MZ766566 | MZ766575 | [13] |
| C. luteolus | GDGM86247 | China | MZ766513 | MZ766567 | MZ766576 | [13] |
| C. luteolus | GDGM44258 | China | ZM766514 | MZ766566 | MZ766570 | [13] |
| C. luteovirens | GDGM81079 | China | MZ605092 | MZ613994 | MZ614036 | [13] |
| C. luteovirens | GDGM80672 (HT) | China | MZ605090 | MZ613992 | MZ614035 | [13] |
| C. luteovirens | GDGM80680 | China | MZ605091 | MZ613993 | – | [13] |
| C. minioalbus | GDGM78910 | China | MZ605098 | MZ613999 | MZ614043 | [13] |
| C. minioalbus | GDGM78901 (HT) | China | MZ605097 | MZ613998 | MZ614042 | [13] |
| C. minioalbus | GDGM78916 | China | MZ605100 | MZ614001 | MZ614045 | [13] |
| C. minor | BB 07.057 | USA | KF294632 | JX192979 | KF294707 | [3] |
| C. minor | BB 07.002 | USA | KF294625 | JX192978 | KF294699 | [3] |
| C. neopersicinus | GDGM85145-1 | China | OM978942 | ON119054 | ON119039 | Present study |
| C. neopersicinus | GDGM85145-2 | China | OM978945 | ON119055 | ON119040 | Present study |
| C. neopersicinus | GDGM85145-3 | China | OM978946 | ON119056 | ON119041 | Present study |
| C. neopersicinus | GDGM87366-1 (HT) | China | OM978943 | ON119057 | ON119042 | Present study |
| C. neopersicinus | GDGM87366-2 | China | OM978944 | ON119058 | ON119043 | Present study |
| C. phloginus | GDGM79007-1 | China | OM978979 | ON119094 | ON119044 | Present study |
| C. phloginus | GDGM79007-2 | China | OM978980 | ON119095 | ON119045 | Present study |
| C. phloginus | SSC99 (HT) | China | – | KF801096 | – | [22] |
| C. phloginus | SSC98 | China | – | KF801095 | – | [22] |
| C. phloginus | Yuan14468 | China | – | MW999424. | – | [7] |
| C. phloginus | Yuan14490 | China | – | MW999425 | – | [7] |
| C. phloginus | GDGM82514 | China | – | ON119096 | – | Present study |
| C. pseudominimus | JV 00.663 | Portugal | KF294657 | JX192991 | KF294735 | [3,10] |
| C. romagnesianus | AH44218 | Spain | KX828807 | KX828836 | KX828757 | [36] |
| C. roseofagetorum | AH44789 | Georgia | KX828812 | KX828839 | KX828760 | [36] |
| C. sinocinnabarinus | GDGM83229 | China | OM978983 | ON119098 | ON119047 | Present study |
| C. sinocinnabarinus | GDGM83238 | China | OM978985 | ON119101 | ON119051 | Present study |
| C. sinocinnabarinus | GDGM83023 | China | OM978981 | ON119097 | ON119050 | Present study |
| C. sinocinnabarinus | GDGM83232 | China | – | ON119100 | ON119049 | Present study |
| C. sinocinnabarinus | GDGM83027 | China | OM978982 | – | ON119046 | Present study |
| C. sinocinnabarinus | GDGM83230 (HT) | China | OM978984 | ON119099 | ON119048 | Present study |
| C. sinocinnabarinus | HKAS58243 | China | JF906727 | – | – | [20] |
| C. sinominor | GDGM80788 | China | MZ605105 | MZ614004 | MZ614048 | [13] |
| C. sinominor | GDGM80842 (HT) | China | MZ605107 | MZ614006 | MZ614050 | [13] |
| C. sinominor | GDGM80885 | China | MZ605108 | MZ614007 | MZ614051 | [13] |
| C. aff. subcyanoxanthus | BB 98.014 | Tanzania | KF294615 | JX192973 | KF294689 | [3] |
| C. tabernensis | BB 07.119 | USA | KF294634 | GQ914976 | KF294709 | [3] |
| C. tabernensis | BB 07.056 (ET) | USA | KF294631 | GQ914974 | KF294706 | [3,38] |
| C. texensis | 341/O7.120 | USA | JN940601 | GQ914987 | KF294710 | [3] |
| C. texensis | BB 07.018 | USA | KF294626 | GQ914988 | KF294701 | [3] |
| C. xanthocyaneus | 1751 | Congo | MT006309 | MT002277 | – | [39] |
| C. xanthocyaneus | Congo | MT006310 | MT002278 | – | [39] | |
| C. zangii | GDGM82389 | China | MZ605110 | MZ614009 | MZ614053 | [13] |
| C. zangii | GDGM82393 | China | MZ605111 | MZ614010 | MZ614054 | [13] |
| C. zangii | GDGM82374 | China | MZ605109 | MZ614008 | MZ614052 | [13] |
3. Results
3.1. Molecular Phylogeny
For phylogenetic analyses, a total of 152 sequences were newly produced in this study, containing 49 nrLSU, 51 tef1 and 52 rpb2, and 185 reliable sequences were downloaded from the GenBank database based on previous studies [3,13]. The combined dataset (LSU + tef1 + rpb2) contained 2892 characters (1311, 707 and 874 for LSU, tef1 and rpb2, respectively), of which 2013 were conserved and 708 were parsimony-informative. ML and BI analyses of the concatenated data set resulted in almost identical topologies, and no strongly-supported conflicts between ML and BI analyses were discovered; thus, only the tree inferred from ML analysis was displayed (Figure 1). Our phylogenetic analyses indicated that members of C. subg. Cinnabarinus formed a highly support monophyletic group (MLB/BPP = 100%/1.0). Five well-supported clades in the subg. Cinnabarinus were identified based on samples newly collected from China, including two new species, two species newly recorded in China and a known species in China. Besides, three well-supported clades in the subg. Parvocantharellus were firstly discovered in China, containing two new species and a newly recorded species from China.
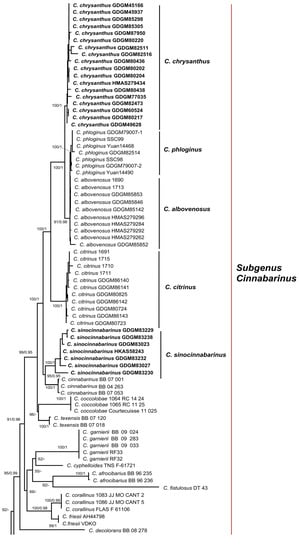
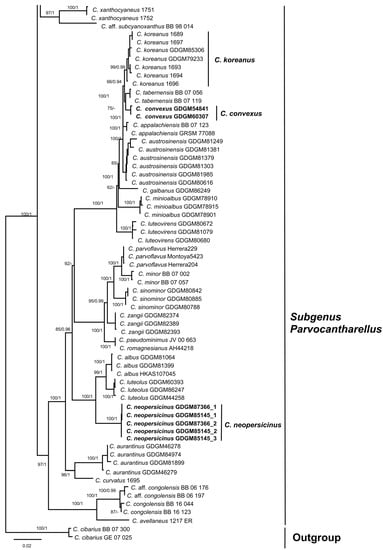
Figure 1.
Phylogenetic tree of representative species of Cantharellus inferred from LSU-tef1-rpb2 dataset by means of both ML and BI methods. Cantharellus cibarius Fr. served as outgroup. Bootstrap Supports (BS > 50%) and Bayesian Posterior Probabilities (BPP > 0.90) are shown on the supported branches. Bold names represent new species.
3.2. Taxonomy
3.2.1. Cantharellus subgen. Cinnabarinus Buyck & V. Hofst.

Figure 2.
Basidiomata of Cantharellus chrysanthus. (a,b) GDGM80220, holotype. (c) GDGM60524. (d) GDGM80438. (e) GDGM82516. (f) GDGM80217. (g) GDGM49628. (h) GDGM80436. (i) GDGM60334. (j) GDGM80202. (k) GDGM45937. (l) GDGM85298. (m) GDGM82473. Bars = 2 cm.
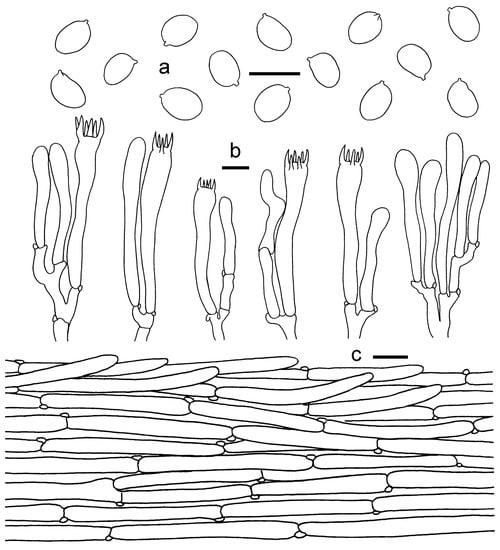
Figure 3.
Cantharelluschrysanthus (GDGM80220, Holotype). (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
MycoBank: MB843657.
GenBank: OM978970 for LSU, ON119083 for tef1 and ON119019 for rpb2.
Etymology—refers to the color of pileus similar to the yellow chrysanthemum flower.
Diagnosis—This species is characterized by its orange to orange-yellow pileus, pinkish white to orange white hymenophore, thin-walled pileipellis terminal hyphae, broadly ellipsoid basidiospores (7.5–9 × 5–6.5 μm) and long basidia up to 100 μm.
Type—CHINA. Guangdong Province, Shaoguan City, Ruyuan town, Nanling National Natural Reserve, alt. 500 m, 10 June 2020, Ming Zhang (GDGM80220).
Basidiomata small-sized. Pileus 20–60 mm broad, convex, with involute margin when young, then gradually to nearly applanate or broadly infundibuliform with depressed center and inflexed to straight, irregularly undulate or slightly cracked at maturity; surface dry or hygrophanous, glabrous or finely subtomentose, orange (5A7–6A7) to deep orange (5A8–6A8) when young, slightly fading to orange yellow to yellow (3A7–4A7) when mature. Context yellowish white to orange white (4A2–6A2), 1–2 mm thick in the center of the pileus, sharply attenuate towards margin, unchanging when exposed. Hymenophore decurrent, subdistant, composed of bifurcate, 2–3 mm high venose folds, particularly towards pileus margin, pinkish white (7A2–10A2), but in some specimens yellowish white to orange white, unchanging when bruised. Stipe 20–60 × 3–14 mm, central, cylindrical or slightly tapering towards base, solid, glabrous or finely pubescent, concolorous with pileus or paler, unchanging when handled. Odor fruity and pleasant. Taste mild.
Basidiospores 7.5–9 × 5–6.5 μm, Lm × Wm = 8.45(±0.47) × 5.98(±0.42) μm, Q = (1.25)1.28–1.6(1.64), Qm = 1.42 ± 0.1; broadly elliptical to subglobose, smooth, guttulate, thin-walled. Basidia 55–100 × 7–11 μm, 2–6-spored, narrowly clavate, colorless to hyaline in KOH; sterigmata 6–10 μm long. Pileipellis a cutis with long, repent and occasionally interwoven hyphae, subcylindrical cells that are 6–12 μm wide, thin-walled. Stipitipellis a cutis of cylindrical, parallel hyphae, 3–8 μm wide. Clamp connections abundant in all tissues.
Habitat and distribution—Solitary or scattered under Fagaceae trees mixed with other broadleaf trees in subtropical forests. Known from southern and southwestern China.
Additional specimen examined—China. Guangdong Province, Shaoguan City, Ruyuan town, Nanling National Natural Reserve, alt. 500 m, 7 June 2017, Ming Zhang (GDGM49628); same location, alt. 500 m, 21 July 2017, Ming Zhang (GDGM60524); same location, alt. 500 m, 9 June 2020, Ming Zhang (GDGM80436, GDGM80438); same location, alt. 500 m, 10 June 2020, Ming Zhang (GDGM80202, GDGM80204, GDGM80217, GDGM80220,); Huizhou city, Xiangtoushan National Natural Reserve, alt. 550 m, 17 May 2016, Ting Li (GDGM45937); Hunan Province, Rucheng town, Jiulongjiang National Forest Park, alt. 300 m, 4 September 2016, Ming Zhang (GDGM53485); Zhejiang province, Jinhua city, Wuyi Town, 23 August 2015, Tai-Hui Li (GDGM45166); Hangzhou City, Laohushan, 15 July 2021, Bao-Juan Ling (GDGM85298, GDGM85305); Qingyuan Town, Baishanzu National Natural Reserve, alt. 29 July 2020, Tai-Hui Li (GDGM82473); Longquan City, Fengyangshan National Natural Reserve, 25 August 2016, Rui-Lin Zhao (ZRL20161616, HMAS279434); Quzhou City, Kaihua County, He Tian township, Chi Keng village, 24 May 2021, Yi Li (GDGM87950); Quzhou City, Kaihua County, Shengtangou Scenic Spot, 30 May 2021, Yi Li (GDGM87951); Anhui Province, Huangshan City, Huangshan scenic spot, 11 August 2020, Ming Zhang (GDGM82511), same location, 13 August 2020, Ming Zhang (GDGM82516); Guizhou Province, Guiyang City, Longli County, Guanyin Village, bought from a wild mushroom market, 2 August 2019, alt. 1000 m, Yong He (GDGM77035).
Notes—Cantharellus chrysanthus is different from other Cantharellus species by the combined features of the orange to orange-yellow pileus, the pinkish white to orange white hymenophore, the thin-walled terminal hyphae of pileipellis, the broadly ellipsoid basidiospores (7.5–9 × 5–6.5 μm) and the long basidia up to 100 μm.
Phylogenetically, C. chrysanthus is related to C. albovenosus and C. phloginus in the analyses of the multi-locus datasets. However, C. albovenosus differs in its orange to reddish orange pileus with tomentoum or fibrilla, white to orange white and better-developed hymenophore, orange to reddish orange stipe, smaller basidiospores (7–8.5 × 5–6 μm) and shorter basidia (48–63 × 7–9 μm) [11]; Cantharellus phloginus, reported from southwest China, differs in its pastel red to pastel pink pileus and stipe, pale yellow to light yellow hymenophore, larger basidiospores [6.8–9.5 (–12) × 5–7 μm] and shorter basidia (60–95 × 8–10 μm) [22].

Figure 4.
Basidiomata of Cantharellus sinocinnabarinus. (a,c) GDGM83230. (b) GDGM83232. (d) GDGM83229. (e) GDGM832296. (f) GDGM83027. (g) GDGM83238. (h) HKAS58243. Bars = 2 cm.
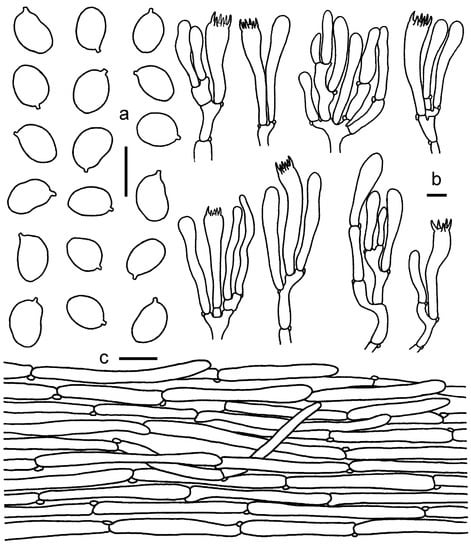
Figure 5.
Cantharellus sinocinnabarinus. (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
MycoBank: MB843658.
GenBank: OM978984 for LSU, ON119099 for tef1 and ON119048 for rpb2.
Etymology—Refers to the species distributed in China and is similar to C. cinnabarinus in morphology.
Diagnosis—This species is characterized by its small basidiomata, reddish orange to yellowish red pileus covered with white minute fibrils, yellowish orange to orange hymenophore and elongate elliptical basidiospores measuring (6.5–) 7–8 (9) × (4.5) 5–6 μm.
Type—China. Yunnan Province, Lijiang City, Yulong County, Jiuhe Village, 1 September 2020, alt. 2400 m, Ming Zhang (GDGM83230).
Basidiomata small-sized. Pileus 5–15 mm broad, applanate with a depressed center, not perforate; margin slightly incurved when young, applanate to reflexed with age; surface dry, orange, reddish orange to yellowish red (6A7–8A7), locally with white minute fibrils. Context thin, 0.5–1.5 mm thick, fleshy to fibrous, yellowish orange to reddish orange, unchanging when bruised. Hymenophore subdecurrent, with a clearly delimitation from stipe surface; lamellate ridges subdistant to close, well-developed, 1–2 mm high, appropriately bifurcate, with low interconnected low venose folds, particularly at pileus margin, yellowish orange to orange (4A7–6A7), unchanging when bruised. Stipe 10–15 mm long, 1–2.5 mm thick, subcylindrical, slightly tapering downward, glabrous or with obscure white minute fibrils, hollow, concolorous with pileus. Odor pleasant.
Basidiospores (100/4/4) (6.5)7–8(9) × (4.5)5–6 μm, Lm × Wm = 7.47(±0.5) × 5.21(±0.39) μm, Q = (1.25)1.27–1.6(1.67), Qm = 1.43 ± 0.09; elliptical to elongate elliptical. Basidia 50–75 × 10–12 μm, clavate, with 4–8 sterigmata. Pileipellis a cutis, composed of procumbent hyphae; hyphae 4–13 μm in diam., colorless, thin-walled. Hymenophoral trama composed of cylindrical hyphae 5–10 μm in diam. Stipitipellis a cutis, composed of procumbent, branched hyphae; hyphae 4–12 μm in diam., mostly 7 μm in diam. Cystidia absent. Clamp connections common.
Habitat and distribution—Gregarious on soil in subalpine mixed forest dominated by Cyclobalanopsis delavayi (Franch.) Schott. and Pinus yunnanensis Franch. Currently known from southwest China.
Additional specimens examined—China. Yunnan Province, Jianchuan County, Qianshi Mountain, 7 September 2009, alt. 2491 m, Yu23 (HKAS58243); Lijiang City, Yulong County, Jiuhe Village, 1 September 2020, alt. 2400 m, Ming Zhang (GDGM83229, GDGM83232, GDGM83027), Li-Qiang Wu (GDGM83238).
Notes—Cantharellus sinocinnabarinus can be easily recognized in the field by its small reddish orange basidiomata. Morphologically, C. sinocinnabarinus is similar to C. cinnabarinus, C. persicinus R.H. Petersen and C. texensis. However, the latter three species were all originally reported from North America; C. cinnabarinus and C. persicinus differ in their larger basidiomata (pileus up to 40 mm), thicker-walled hyphae of pileipellis terminal cells, and different sizes of basidiospores (6.7–7.57 × 3.82–4.68 μm for C. cinnabarinus, and 10.2–11.9 × 6.3–7.2 μm for C. persicinus) [32]; C. texensis differs in its robust basidiomata and longer but narrower basidiospores (8–8.95 × 3.7–4.3 μm), with a larger Q value (1.8–2.2) [32].
Shao et al. [20] has described a specimen (HKAS58243) under the name C. cinnabarinus on the basis of the LSU sequence, which is geographically close to C. sinocinnabarinus in southwest China. In this study, the specimen (HKAS58243) was re-examined; the morphological features and molecular phylogenetic analyses all demonstrated that it is actually C. sinocinnabarinus.
In the multi-locus phylogentic trees, specimens of C. sinocinnabarinus formed a well-supported independent terminal branch (BS = 100%, BPP = 1.0) in the subg. Cinnabarinus, and are closely related to C. cinnabarinus. However, they can be easily distinguished by the morphological features and large genetic distance.
Cantharellus albovenosus Buyck, Antonín & Ryoo, in Antonín, Hofstetter, Ryoo, Ka and Buyck, Mycol. Progr. 16(8): 757 (2017); Figure 6 and Figure 7.

Figure 6.
Basidiomata of Cantharellus albovenosus. (a,b) GDGM85852. (c,d) GDGM85846. (e) GDGM85142. Bars = 2 cm.
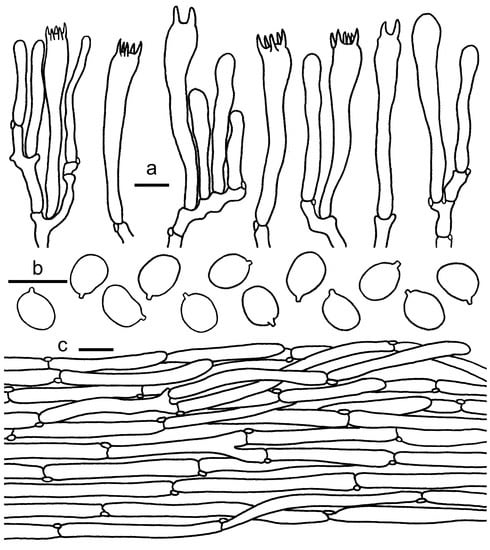
Figure 7.
Cantharellus albovenosus. (a) Basidia, basidiola and elements of the subhymenium. (b) Basidiospores. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
Basidiomata small-sized. Pileus 20–55 mm broad, convex at first, then broad applanate with a depressed centre, subinfundibuliform when mature or old; margin inflexed to straight when young, then undulate; surface tomentose when young, then glabrescent and radially (innately) fibrillose to finely striate and rugulose, orange, deep orange to reddish orange (5A6–7A6, 5A8–7A8), then pallescent to light orange at margin. Hymenophore decurrent, with a clearly delimitation from stipe surface; lamellate ridges, subdistant to distant, relatively well-developed, 1–1.5 mm high, appropriately bifurcate and interconnected with low veined folds, particularly towards pileus margin, white to orange white (5A2–6A2), unchanging when bruised. Stipe 25–50 × 2.5–9 mm, cylindrical and slightly clavate to bulbose at base, finely tomentose when young, then glabrous or with finely longitudinally fibrillose, concolorous with pileus, orange to reddish orange, sometimes paler to light orange in some specimens. Context white, orangish under pileipellis, solid, becoming hollow-fibrous in stipe. Odor spicy. Taste mild.
Basidiospores 7–8.5 × 5–6 μm, Lm × Wm = 7.9(±0.48) × 5.5(±0.34) μm, Q = (1.33)1.4–1.5(1.54), Qm = 1.44 ± 0.05; ellipsoid to subglobose, thin-walled, sometimes with granulose contents. Basidia 48–63 × 7–9 μm, 2–6-spored, clavate, sometimes subcapitate. Hymenial trama hyphae cylindrical to subinflated, sometimes irregular, thin-walled, 3–8 μm wide. Pileipellis a cutis composed of cylindrical, rarely subinflated, thin-walled, 4–10 μm wide hyphae; terminal cells 37–87 × 5–8 μm, adpressed, cylindrical, clavate or subfusoid. Stipitipellis a cutis of cylindrical, parallel, thin-walled, clamped, 3–7 μm wide hyphae.
Habitat and distribution—Scattered or gregarious on soil under mixed forest dominated by Fagaceae trees. Known to be from eastern China and Korea.
Specimens examined—China. Jiangsu Province, Nanjing City, Purple Mountain, 19 June 2021, alt. 150 m, Zi-Hang Zhang (GDGM85846); same location, 28 June 2021, Zi-Hang Zhang (GDGM85852, GDGM85853); Anhui Province, Huangshan National Scenic Area, 26 August 2021, alt. 1400 m, Chen-Jie Jiang (GDGM85142). Zhejiang Province, Lishui City, Jingning Town, Wangdongyang Alpine Wetland Nature Reserve 22 September 2016, Rui-Lin Zhao (HMAS279296, HMAS279292); same location, 23 September 2016, Rui-Lin Zhao (HMAS279262, HMAS279284).
Notes—Cantharellus albovenosus, recently reported from South Korea, is characterized by the combined features of the orange to reddish orange pileus, white to orange white and relatively well-developed lamellate hymenophore, the orange to reddish orange stipe, and the ellipsoid to nearly globose basidiospores (7–8.5 × 5–6 μm) [11]. Phylogenetically, C. albovenosus and C. phloginus clustered together in an almost similar phylogenetic position, and cannot be separated in our multi-locus phylogenetic tree (Figure 1). Morphologically, C. phloginus can be distinguished by its pastel red to pastel pink pileus and stipe, pale yellow to yellowish orange hymenophore and large basidiospores [6.8–9.5 (–12) × 5–7 μm] [22]. Ecologically, C. albovenosus is known from subtropical regions of South Korea and eastern China; meanwhile, C. phloginus is currently only known from tropical regions of southwest China. The distinguishable morphological features and different growth habits supported them as two distinct species, but some more effective molecular markers are needed to distinguish the two species.
Cantharellus citrinus Buyck, R. Ryoo & Antonín, in Buyck, Hofstetter, Ryoo, Ka and Antonín, MycoKeys 76: 35 (2020); Figure 8 and Figure 9.

Figure 8.
Basidiomata of Cantharellus citrinus. (a,b) GDGM86143. (c) GDGM86141. (d) GDGM80723. Bars = 2 cm.
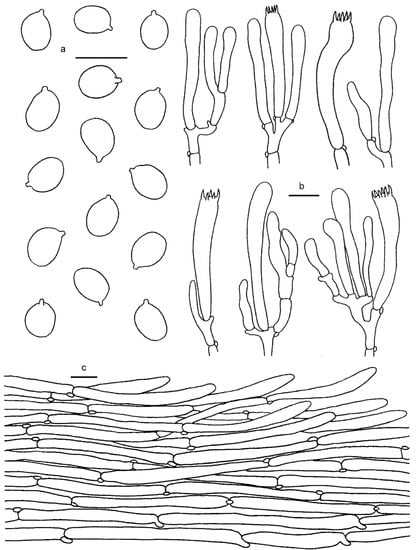
Figure 9.
Cantharellus citrinus. (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
Basidiomata small-sized. Pileus 15–45 mm broad, convex, with involute margin when young, then gradually to broadly infundibuliform with depressed center, irregularly undulate or slightly cracked margin when old; surface dry or hygrophanous, glabrous or finely subtomentose, greenish yellow, light yellow, yellow to yellowish orange (1A4–4A4, 1A7–4A7). Context yellowish white, 1 mm thick in the center of the pileus, sharply attenuate towards margin, unchanging when exposed. Hymenophore decurrent, subdistant, composed of bifurcate, less than 1 mm high veined folds, particularly towards pileus margin, white to yellowish white (1A2–3A2), unchanging when bruised. Stipe 15–30 × 3–5 mm, central, cylindrical or slightly tapering towards base, hollow, glabrous, concolorous with pileus or paler, unchanging when handled. Odor fruity and pleasant. Taste mild.
Basidiospores 7–9 × 5–6(6.5) μm, Lm × Wm = 7.77(±0.47) × 5.29(±0.40) μm, Q = (1.17)1.23–1.6(1.64), Qm = 1.47 ± 0.11; broadly elliptical to subglobose, smooth, guttulate, thin-walled. Basidia 55–65 × 7–8 μm, 4–6-spored, narrowly clavate, colorless to hyaline in KOH; sterigmata 5–10 μm long. Pileipellis a cutis with long, repent and occasionally interwoven hyphae, subcylindrical cells that are 5–15 μm wide, thin-walled. Stipitipellis a cutis of cylindrical, parallel hyphae, 5–10 μm wide. Clamp connections abundant in all tissues.
Habitat and distribution—Gregarious on soil under mixed forests in southwest China. Known from southwest China and Korea.
Specimen examined—China. Guizhou Province, Guiyang City, Longli County, Guanyin Village, bought from a wild mushroom market, 1 July 2020, alt. 1000 m, Ming Zhang (GDGM80825); Same location, 16 June 2020, Ting Li (GDGM80724, GDGM80723); 7 July 2021, Ming Zhang (GDGM86140, GDGM86141, GDGM86142, GDGM86143).
Notes—Cantharellus citrinus, recently reported from Korea [11], is characterized by its small basidiomata, greenish yellow to yellowish orange pileus, white to yellowish white hymenophore strongly bifurcate at pileus margin, glabrous and hollow stipe, and broadly elliptical to subglobose basidiospores [7–9 × 5–6 (6.5) μm]. In the multi-locus phylogentic tree, samples of C. citrinus formed a well-supported monophyletic terminal clade, and can be easily distinguished from other Cantharellus species.
Morphologically, C. citrinus might be easily identified as a species in the subg. Parvocantharellus by the small basidioma with a greenish yellow to yellowish orange pileus, and similar to C. galbanus Ming Zhang, C.Q. Wang & T.H. Li and C. luteovirens Ming Zhang, C.Q. Wang & T.H. Li. However, C. galbanus, recently reported from tropical China, differs in its smaller basidiomata, relatively well-developed hymenophore, and smaller basidiospores (6–7.5 × 4.8–5.5 µm) [13]; C. luteovirens, recently reported from subtropical China, differs in its yellow to yellowish-orange pileus, yellowish white to pale yellow hymenophore and smaller basidiospores (6–7.5 × 4.5–6 µm) [13].
Cantharellus phloginus S.C. Shao & P.G. Liu, in Shao, Buyck, Tian, Liu and Geng, Mycoscience 57(2): 146 (2016); Figure 10 and Figure 11.

Figure 10.
Basidiomata of Cantharellus phloginus (GDGM79007). Bar = 5 cm.
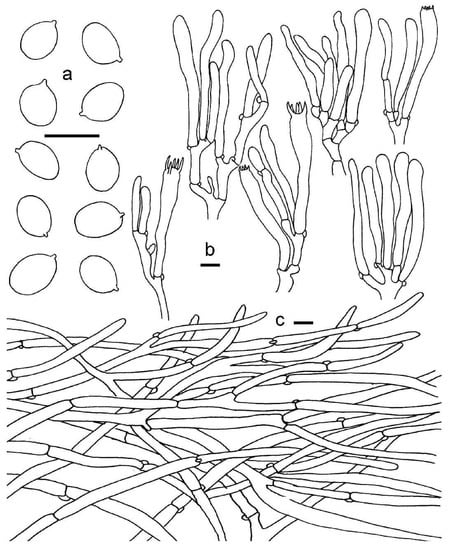
Figure 11.
Cantharellus phloginus. (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
Basidiomata small to medium-sized. Pileus 20–60 mm broad, applanate with a concave center, margin incurved at first, then becoming applanate or slightly reflexed with age, glabrous, pastel red to pastel pink (7A4–11A4); Context 2–3 mm thick, white, with pinkish hues under pileipellis, unchanging when bruised; Hymenophore decurrent, well-developed, lamellate ridges with anastomosing veins, forking towards pileus margin, pale yellow to light yellow (3A3–4A3), unchanging when touched. Stipe 20–40 × 4–8 mm, central, solid, subcylindrical, or slightly tapering towards base, glabrous, concolorous with pileus or paler to pinkish with yellowish hues, unchanging when handled. Odor fruity. Taste pleasant.
Basidiospores 6.8–9.5 (–12) × 5–7 μm, Lm × Wm = 8.49(±1.09) × 5.71(±0.69) μm, Q = (1.33)1.36–1.6(1.7), Qm = 1.49 ± 0.18; broadly ellipsoid to subglobose, smooth, guttulate. Basidia 60–95 × 8–10 μm, 2–6-spored, narrowly clavate, colorless to hyaline in KOH; sterigmata 3–7 μm long. Hymenophoral trama composed of cylindrical interwoven hyphae 3–13 μm in diam. Pileipellis a subcutis, composed of long, repent, branched, and slightly interwoven hyphae, with subcylindrical cells in 3–13 μm wide, thin-walled. Clamp connections abundant in all tissues.
Habitat and distribution—Gregarious or caespitose under mixed forests, dominated by Pinus sp. and Castanopsis in the tropical forest. Currently known to be southwest China.
Specimens examined—China. Yunnan Province, Puer City, alt. 1500 m, 26 August 2009, S.C. Shao 98 (HKAS58208, holotype); Puer City, bought from a mushroom market, alt. 1500 m, 28 September 2019, Ming Zhang (GDGM79007).
Notes—Cantharellus phloginus, recently reported from southwest China, is characterized by its pastel red to pastel pink pileus and stipe, pale yellow to yellowish orange, well-developed hymenophore, and ellipsoid basidiospores [6.8–9.5 (–12) × 5–7 μm] [22]. Morphologically, C. phloginus is similar to C. cinnabarinus and C. texensis Buyck & V. Hofst with the pinkish red pileus color. However, C. cinnabarinus differs in its small basidiomata, reddish pink pileus, small basidiospores [(6.4) 6.7–7.5 (8.1) × (3.7) 3.8–4.6 (5.2) μm] and thick-walled pileipellis [32]; C. texensis differs in its slender basidiomata, reddish pink pileus, relatively well developed hymenophore, small basidiospores [8–8.95 (9.4) × (3.3) 3.7–4.3 μm], and thinner-walled pileipellis that is faintly covered with zebroid incrustation [32]. Ecologically, C. phloginus occurs under trees of Pinus sp. and Castanopsis sp. in tropical regions of southwest China, while C. cinnabarinus and C. texensis occur on sandy loam in oak-pine forests in temperate regions of North America [32].
3.2.2. Cantharellus subgen. Parvocantharellus Eyssart. & Buyck

Figure 12.
Basidiomata of Cantharellus convexus. (a) GDGM70307. (b) GDGM54841. Bars = 2 cm.
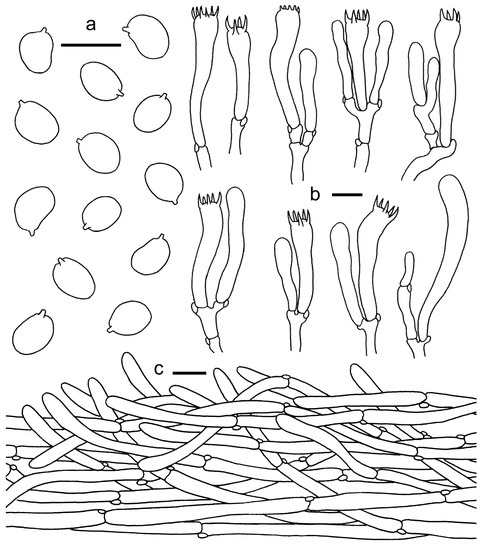
Figure 13.
Cantharellus convexus. (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
MycoBank: MB843659.
GenBank: OM978941 for LSU, ON119053 for tef1 and ON119037 for rpb2.
Etymology— “convexus” refers to the convex of the pileus center.
Diagnosis—This species can be easily distinguished from others in Cantharellus by its small basidiomata, yellowish white pileus, distant and well-developed lamellate hymenophore with or without bifurcate low veins and smaller basidiospores at 6–7 × 4.5–5 μm.
Type—China. Guangdong Province, Shaoguan City, Nanling National Nature Reserve, alt. 800 m, 29 July 2017, Ming Zhang (GDGM70307).
Basidiomata small-sized. Pileus 5–12 mm broad, convex when young, then gradually to nearly applanate with a central shallow depression at maturity; surface dry, tomentosus, mostly yellowish white, pale yellow to pale orange (2A2, 2A3–5A3), but in some specimens can be yellowish brown to brown, with a deeper center to olive brown to yellowish brown (4E5–5E5); margin wavy, incurved when young, decurved to slightly upturned at maturity, unchanging when handled. Context yellowish white, thin, unchanging when exposed. Hymenophore decurrent, lamellate ridges distant, relatively well developed, occasionally forking towards pileus margin, with or without bifurcate low veins between ridges, yellowish white to pale yellow (2A2–4A2, 2A3–4A3), unchanging when bruised. Stipe 10–20 × 1.5–3 mm, central, cylindrical or slightly tapering towards base, glabrous or faintly scaly, concolorous with pileus or paler, unchanging when handled. Odor not distinct.
Basidiospores (50/2/2) 6.0–7.0 × 4.5–5.0 μm, Lm × Wm = 5.71(±0.64) × 4.87(±0.49) μm, Q = (1)1.1–1.27(1.37), Qm = 1.17 ± 0.07, broadly ellipsoid to subglobose, smooth, guttulate. Basidia 32–50 × 7–9 μm, 4–6-spored, narrowly clavate, colorless to hyaline in KOH, sterigmata 3–7 μm long. Hymenophoral trama irregular, composed of colorless and branched hyphae, 5–22 μm wide, septate, thin-walled. Pileipellis a cutis with long, repent, branched, and usually interwoven hyphae consisting of subcylindrical cells in 3–15 μm wide, thin-walled; terminal cells appressed to suberect, mostly cylindrical, up to 110 μm long, 5–15 μm wide. Stipitipellis a cutis of cylindrical, parallel hyphae, 3–10 μm wide; terminal cells clavate or cylindrical. Clamp connections abundant in all tissues.
Habitat and distribution—Gregarious or scattered under broadleaf forests (dominated by Fagaceae trees) in subtropical China. Currently known from Guangdong and Hunan Province, Southern China.
Additional specimens examined—China. Hunan Province, Chenzhou City, Sanjiangkou Town, Jiulongjiang National Forest Park, under Castanopsis hystrix mixed with other broadleaf trees, alt. 200 m, 3 August 2017, Ming Zhang (GDGM54841).
Notes—Cantharellus convexus is characterized by its small basidiomata, convex pileus covered with fibrillose scales, distant and well-defined lamellate hymenophore without anastomosis between the folds, broad elliptic to subglobose basidiospores and thin-walled hyphae of the pileipellis. These traits taxonomically enable the placement of C. convexus into subg. Parvocantharellus.
Phylogenetically, two specimens of C. convexus formed an isolated lineage in subg. Parvocantharellus, and are closely related to C. tabernensis. A BLAST result of ITS sequence in the GenBank database also demonstrated that the similarity between C. convexus and C. tabernensis (JN944012, O7.064) is 93.7%. However, C. tabernensis, originally reported from North America, differs in its more robust basidiomata, dull orange-yellow to yellowish-brown pileus, vivid orange-yellow hymenophore and stipe and larger basidiospores (6–9 × 4.4–5.9 µm) [40]. Additionally, C. tabernensis, currently only known from Texas, Louisiana and Mississippi in North America, occurs in well-drained (sandy) soil in mixed woods, and near to Pinus elliottii Engelm. Meanwhile, C. convexus was found in broadleaf forests in southern China, close to Fagaceae trees. Another North America species, C. appalachiensis, also demonstrates a close relationship with C. convexus. However, C. appalachiensis differs in its larger and more robust basidiomata, with a drab yellow to dull brown pileus applanate with the center depressed, surface locally dull-grayish due to aggregate minute fibrils and with larger basidiospores (6.6–8.9 × 4.4–5.9 µm) [41,42].
Morphologically, C. convexus is similar to C. austrosinensis Ming Zhang, C.Q. Wang & T.H. Li, C. koreanus Buyck, Antonín & Ryoo and C. luteovirens. However, C. austrosinensis differs in its pastel yellow to greyish-yellow pileus, usually with a greyish-orange to brownish-orange center, broader basidiospores (6–8 × 4.8–6 µm) and strictly associated with coniferous trees (Pinus massoniana) [13]; C. koreanus, originally described from the temperate region of the Republic of Korea, differs in its dirty yellow-brown to pale brown pileus usually with a brown to dark brown center and larger basidiospores [6–8 (–9) × 4.2–5.5 (–6.5) μm] [11]; C. luteovirens differs in its yellow to orange pileus, greyish-yellow to greyish-orange hymenophores, broadly ellipsoid to subglobose basidiospores (7–8 × 5.2–6.5 μm) and is currently only found be associated with Acacia trees [13].

Figure 14.
Basidiomata of Cantharellus neopersicinus. (a–e) GDGM87366. (f,g) GDGM85145. Bars: (a,b,d–f) = 2 cm; (c,g) = 5 cm.
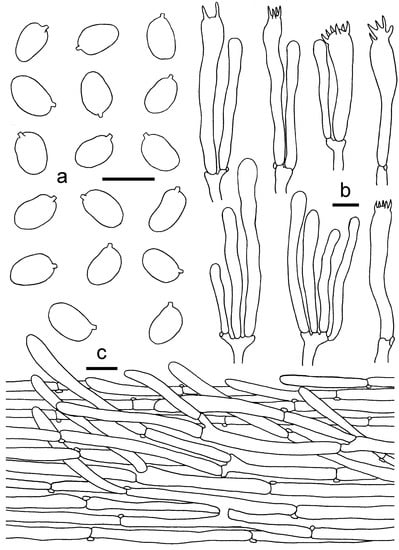
Figure 15.
Cantharellusneopersicinus. (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a,b) = 10 μm; (c) = 20 μm.
MycoBank: MB843660
GenBank: OM978943 for LSU, ON119057 for tef1 and ON119042 for rpb2
Etymology—refers to the color similar to Cantharellus persicinus.
Diagnosis—The pastel red to pink pileus, white to pinkish hymenophore with strongly bifurcate low veins and ellipsoid to subglobose [(6–)7–8.5(–9) × (4–)4.5–5.5(–6) μm], make C. neopersicinus easily distinguished from other species in the subg. Parvocantharellus.
Type—China. Guangdong Province, Leizhou City, Fangcha Village, under Eucalyptus robusta, alt. 105 m, 16 October 2021, Xiu-Yuan Chen (GDGM87366).
Basidiomata small-sized. Pileus 15–45 mm broad, convex when young, then gradually to nearly applanate with a central shallow depression at maturity; surface dry, glabrous, pastel red, pastel pink to pink (8A4–12A4); margin incurved when young, reflexed with age, wavy, sometimes irregularly split; unchanging when touched. Context thin, reddish white or pinkish (8A2–12A2), unchanging when exposed. Hymenophore decurrent, but clearly demarcated with stipe, lamellate ridges close to subdistant, poorly-developed, strongly forking towards pileus margin, with bifurcate low veins between ridges, white to pinkish, unchanging when bruised. Stipe 15–40 × 3–8 mm, central, cylindrical or slightly tapering towards base, hollow, glabrous, concolorous with pileus, unchanging when handled. Odor fruity. Taste mild.
Basidiospores (50/2/2) (6–)7–8.5(–9) × (4–)4.5–5.5(–6) μm, Lm × Wm = 7.78(±0.64) × 4.871(±0.46) μm, Q = (1.2)1.4–1.77(2), Qm = 1.6 ± 0.15, ellipsoid to subglobose, smooth, guttulate. Basidia 45–62 × 7–9 μm, 4–6-spored, narrowly clavate, colorless to hyaline in KOH, sterigmata 3–7 μm long. Hymenophoral trama irregular to subregular, composed of colorless and branched hyphae, 8–16 μm wide, septate, thin-walled. Pileipellis a cutis with long, repent to suberect, branched, and slightly interwoven hyphae, subcylindrical cells in 8–15 μm wide, thin-walled; terminal cells appressed, mostly cylindrical, up to 100 μm long, 5–15 μm wide. Stipitipellis a cutis of cylindrical, parallel hyphae, 3–8 μm wide, terminal cells cylindrical. Clamp connections abundant in all tissues.
Habitat and distribution—Gregarious or scattered under Eucalyptus robusta Smith in tropical China. Currently known from Guangdong Province, Southern China.
Additional specimens examined—China. Guangdong Province, Leizhou City, Fangcha Village, alt. 105 m, 25 October 2021, Xiu-Yuan Chen (GDGM85145).
Notes—Cantharellus neopersicinus is characterized by its small basidiomata, pastel red to pink pileus, poorly-developed lamellate hymenophore with strongly bifurcate low veins and ellipsoid to subglobose basidiospores [(6–) 7–8.5 (–9) × (4–) 4.5–5.5 (–6) μm]. Phylogenetic analyses based on multi-locus datasets demonstrated that C. neopersicinus was well nested into the subg. Parvocantharellus, formed a well-supported terminal clade, and was closely related to C. albus S.P. Jian & B. Feng and C. luteolus. However, C. albus, recently reported from China, can be easily distinguished by its white basidiomata slightly changing to yellowish when bruised, a spicy taste and smaller basidiospores (5.5–7.5 × 4.5–6 µm) [12,13]; C. luteolus differs in its small basidiomata, yellow to orange pileus, greyish-yellow to greyish-orange hymenophore and oval to subglobose basidiospores (7–8 × 5.2–6.5 μm) [13].
Morphologically, the pastel red to pink pileus color is easily reminiscent of the species C. cinnabarinus, C. coccolobae Buyck, P.-A. Moreau & Courtec., C. phloginus and C. persicinus. However, the former three species belong to the subg. Cinnabarinus, and can be easily distinguished from C. neopersicinus by the genetic distances. Besides, C. cinnabarinus differs in its cinnabar red to bright orange pileus, thick-walled hyphal terminal cells of pileipellis and smaller basidiospores (6.7–7.57 × 3.82–4.68 μm) [32]. Cantharellus coccolobae differs in its salmon orange hymenophore, white stipe context partly changing to yellowish when cut, large basidiospores [(7.9) 8.3–9.3 (9.8) × (4.8) 5.3–5.9 (6) µm], longer basidia up to 120 µm and the thick-walled hyphae of the pileipellis. Additionally, C. coccolobae was reported to be strictly associated with Coccoloba trees, while C. neopersicinus is under Eucalyptus trees [33]. Cantharellus phloginus is redescribed in this study and differs in its darker pileus color, pale yellow to light yellow hymenophore, white context and larger basidiospores [6.8–9.5 (–12) × 5–7 μm]. Cantharellus persicinus, originally reported from North America, differs in its more robust basidiomata, larger basidiospores (9.6–10.9 × 6.3–7.1 μm), and thick-walled cells of pileipellis. In addition, C. persicinus is reported to be associated with oaks or eastern hemlock [32,43,44].
Cantharellus koreanus Buyck, Antonín & Ryoo, in Antonín, Hofstetter, Ryoo, Ka and Buyck, Mycol. Progr. 16(8): 755 (2017); Figure 16 and Figure 17.

Figure 16.
Basidiomata of Cantharellus koreanus. (a,b) GDGM79233. (c,d) GDGM85306. Bars = 2 cm.
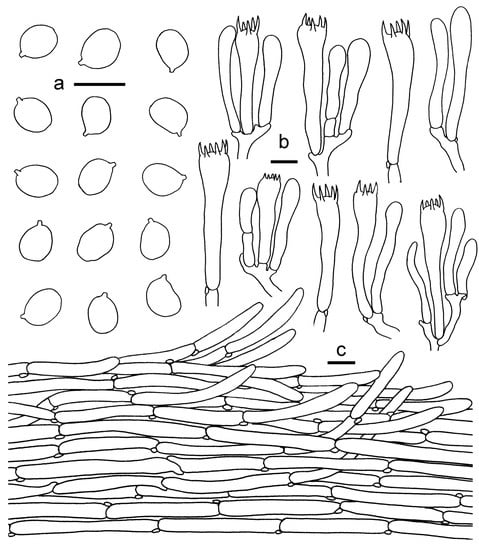
Figure 17.
Cantharellus koreanus. (a) Basidiospores. (b) Basidia, basidiola and elements of the subhymenium. (c) Pileipellis. Bars: (a, b) = 10 μm; (c) = 20 μm.
Basidiomata small-sized. Pileus 15–40 mm broad, convex at first, then gradually applanate with slightly an umbilicate centre; margin involute at first, undulate; surface dry, glabrous or finely tomentose-fibrillose at centre, mostly pale yellow to light yellow (1A3–4A3,1A4–4A4), olive brown to light brown (4D4–5D4) at centre, with obscurely sulcate at margin. Hymenophore with lamellate ridges; ridges broadly adnate to subdecurrent, with a clearly delimitation from the stipe surface, well-developed, bifurcate and with interconnected low veins, up to 1 mm high, yellowish white (2A2–4A2), unchanging when bruised. Stipe10–40 mm long, 2–5 mm thick, subcylindrical to cylindrical, slightly enlarged downward, but sometimes tapering towards base, glabrous or with faintly scaly, hollow, concolorous with pileus, darker and more somber than lamellae ridges. Odor fruity. Taste mild.
Basidiospores 5–8 × (4–) 4.5–6 μm, Lm × Wm = 7.05(±0.51) × 5.192(±0.34) μm, Q = (1.08)1.2–1.45(1.6), Qm = 1.36 ± 0.097, ellipsoid, broadly ellipsoid, thin-walled. Basidia 40–70 × 8–12 μm, 4–6-spored, narrowly clavate, sometimes subcapitate, thin-walled, clamped. Hymenophoral trama composed of clavate, subcylindrical, subregular, branched, thin-walled, clamped hyphae 5–12 μm wide. Pileipellis a cutis, composed of cylindrical, thin-walled hyphae, 5–15 μm wide; terminal cells clavate, fusoid to cylindrical, up to 100 μm long. Stipitipellis a cutis of cylindrical, parallel, branched, thin-walled hyphae 2–9 μm wide. Clamp connections abundant in all tissues.
Habitat and distribution—Gregarious or scattered under broadleaf forests (dominated by Fagaceae trees) in subtropical regions of China. Known from Hunan Province, China and Korea.
Specimens examined—China, Hunan Province, Zhangjiajie City, Zhangjiajie National Forest Park, alt. 1200 m, 17 July 2020, Wei-Qiang Qin (GDGM79233); same location, alt. 1100 m, 5 July 2021, Wei-Qiang Qin (GDGM85306).
Notes—Cantharellus koreanus, recently reported from Korea, is firstly reported from China in this study. It is characterized by the small basidiomata, the dirty yellow-brown to pale brown pileus with a brown to dark brown center, the well-development hymenophoral ridges with yellow tinge, and the ellipsoid to broadly ellipsoid basidiospores 6–8 (–9) × 4.2–5.5 (–6.5) μm in Antonín et al. [11] and 5–8 × (4–) 4.5–6 μm in this study.
Phylogenetically, C. koreanus is closely related to C. appalachiensis, C. austrosinensis and C. tabernensis. Indeed, C. koreanus is similar to C. appalachiensis, C. austrosinensis and C. tabernensis in morphology. However, C. appalachiensis differs in its larger and more robust basidiomata (pileus up to 50 mm broad), drab yellow to dull brown pileus, narrower basidia (5.5–9 μm in diam.), shorter and slightly thickened end cell of pileipellis, narrower hyphae of hymenophoral trama, and association with oaks and other hardwoods [41,45,46]; C. austrosinensis differs in its smaller basidiomata, pastel yellow to greyish-yellow pileus with a greyish-orange to brownish-orange center, shorter and narrower basidia (50–55 × 7–9 μm), interwoven hyphae of pileipellis, and symbiosis with coniferous trees [13]; C. tabernensis differs in its dull orange yellow to yellowish brown pileus, vivid orange yellow hymenophore and stipe, shorter and narrower basidia (35–55 × 5–8 μm), and distribution in North America [40,42,46].
In addition, several species were recently reported from China, and are also similar to C. koreanus in morphology, such as C. galbanus, C. luteolus Ming Zhang, C.Q. Wang & T.H. Li, C. luteovirens and C. sinominor Ming Zhang, C.Q. Wang & T.H. Li [13], but they can be easily separated from each other by the large genetic distances.
3.3. Key to Species of Subgenus Cinnabarinus in China
1 Basidiomata with pastel red or reddish orange tinge...................................................2
1’ Basidiomata without red tinge........................................................................................4
2 Pileus: small, always <20 mm broad..............................................C. sinocinnabarinus
2’ Pileus: relatively large, usually >20 mm wide...............................................................3
3 Basidiospores: 7–8.5 × 5–6 μm..................................................................C. albovenosus
3’ Basidiospores: 6.8–9.5 (–12) × 5–7 μm.........................................................C. phloginus
4 Pileus: greenish yellow to yellowish orange, hymenophore white to yellowish white; basidiospores: 7–9 × 5–6(6.5) μm.....................................................................C. citrinus
4’ Pileus: orange to orange-yellow, hymenophore pinkish white to orange white; basidiospores: 7.5–9 × 5–6.5 μm, basidia up to 100 μm....................................C. chrysanthus
4. Discussion
In this study, the species diversity of C. subg. Cinnabarinus from China were examined. Five species were identified based on morphological characters and multi-locus phylogenetic analyses, containing two new species C. chrysanthus and C. sinocinnabarinus, two newly recorded species C. albovenosus and C. citrinus to China, and a known species, C. phloginus. In addition, three species belonging to the subg. Parvocantharellus were firstly discovered from China, including two new species C. convexus and C. neopersicinus, and a new recorded species, C. koreanus.
In the past, the knowledge of species diversity of Cantharellus in China was poor and the specimens with large and yellow to orange basidiomata were mostly misidentified as the type species of the genus C. cibarius; meanwhile, specimens with small and yellow to orange red basidiomata were often inaccurately treated as C. minor Peck or C. cinnabarinus. However, a recent study proved that the distribution of C. cibarius is limited to northeast China, and the so-called “C. cibarius” reported from southwest China is actually C. yunnanensis W.F. Chiu [8]; meanwhile, the specimens labeled as “C. minor” in China were also proven to be misidentified, several new species with small basidiomata have been reported from China, and the distribution of C. minor with correctly identified specimens has not been found in China [13]. Cantharellus cinnabarinus was widely reported in China [19,21], but those photos of C. cinnabarinus used in the two literatures look like C. albovenosus; the correctly identified specimens of C. cinnabarinus in China have not been found in the present study. However, three morphologically similar species were discovered. The specimen HKAS58243 from southwest China, firstly identified as C. cinnabarinus in Shao et al. [20], was proven to be a native species of C. sinocinnabarinus in the present study. In addition, C. sinocinnabarinus seems to be restricted to subalpine habitats, and prefers symbiosis with Cyclobalanopsis delavayi and Pinus yunnanensis. The other two species, C. albovenosus and C. phloginus, are easily misidentified as C. cinnabarinus by their small basidiomata and reddish pileus color. However, C. albovenosus, recently reported from Korea, has been also found in eastern China, and C. phloginus seems to be restricted to tropical to subtropical regions in southwest China. Thus, we speculate that the specimens of “C. cinnabarinus” in Anhui, Guangdong, Jiangsu and Zhejiang provinces could be C. albovenosus, the distribution of “C. cinnabarinus” from tropical to subtropical regions of southwest China could be C. phloginus and the collections of “C. cinnabarinus” from subalpine regions of southwest China could be C. sinocinnabarinus.
Cantharellus neopersicinus, newly discovered in this study, is a remarkable species in Cantharellus. Morphologically, C. neopersicinus can be easily identified as a member of subg. Cinnabarinus or subg. Cantharellus, due to its pastel red to pink pileus and white to pinkish hymenophore; however, phylogenetic analyses demonstrated that it belongs to the subg. Parvocantharellus, which makes it the first species reported from China with pastel red to pink tinge in the subg. Parvocantharellus. Ecologically, C. neopersicinus is distributed in tropical areas of southern China, and currently, the only known symbiosis is with Eucalyptus robusta.
Cantharellus subg. Parvocantharellus, mainly composed of small-sized species, was suggested to be a monophyletic group, and closely related to the subg. Cinnabarinus [3]. However, in the present study, the subgenus was proven to be paraphyletic or polyphyletic; two species of C. cyanoxanthus R. Heim ex Heinem. and C. subcyanoxanthus Buyck, Randrianj. & Eyssart formed an isolated clade in the multi-locus phylogenetic tree, and could represent a separate generic clade. The result is similar to previous studies [13,16].
Species in the two subgenera are difficult to separate in morphology because most species share similar characteristics of small basidiomata, abundant clamps and thin-walled hyphal ends at the pileus surface. However, they formed two separate clades in the multi-locus phylogenetic trees, and can be easily distinguished by molecular phylogenetic evidence. In addition, the species in subg. Cinnabarinus mostly own distinct orange, pink or red tinge, and can be distinguished from subg. Parvocantharellus. In future work, more detailed morphological observations are needed to provide new evidences for distinguishing the two subgenera.
Author Contributions
Conceptualization, M.Z. and T.-H.L.; methodology, M.Z. and C.-Q.W.; performing the experiment, M.Z.; phylogenetic analysis, M.Z. and Y.L.; validation, M.Z., C.-Q.W., Y.L., M.-S.G., S.-C.S., W.-Q.Q., W.-Q.D. and T.-H.L.; writing—original draft preparation, M.Z.; writing—review and editing, C.-Q.W., Y.L., S.-C.S. and T.-H.L.; visualization, M.Z.; supervision, T.-H.L.; project administration, M.Z.; funding acquisition, M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 32070020 and 32170010), the Science and Technology Planning Project of Guizhou Province, China [No. Qian Ke He Fu Qi (2019) 4007], and Biodiversity Survey, Observation and Evaluation Project (2019–2023) of the Ministry of Ecology and Environment of China.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: (https://www.ncbi.nlm.nih.gov/; https://www.mycobank.org/page/Release%20names; accessed on 20 March 2022).
Acknowledgments
The authors sincerely thank the editors and anonymous reviewers for their efforts and contributions towards this manuscript. Sincere acknowledgments are expressed to the curators of the HMAS and HKAS for loan of the research specimens; to B. Bart (Muséum National d’Histoire Naturelle) for providing the sequences of Cantharellus koreanus; to W.Z. Ma (Kunming Institute of Botany, Chinese Academy of Sciences) and Z. Du (Institute of Microbiology, Chinese Academy of Sciences) for their assistance during the loan of specimens; to X.Y. Chen, Z.H. Zhang, and Y. He for the collaboration in the field.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fries, E.M. Systema Mycologicum, Sistens Fungorum Ordines, Genera et Species; Ex Offificina Berlingiana: Lundae, Sweden, 1821; Volume 1. [Google Scholar]
- Kumari, D.; Upadhyay, R.C.; Reddy, M.S. Cantharellus pseudoformosus, a new species associated with Cedrus deodara from India. Mycoscience 2011, 52, 147–151. [Google Scholar] [CrossRef]
- Buyck, B.; Kauff, F.; Eyssartier, G.; Couloux, A.; Hofstetter, V. A multilocus phylogeny for worldwide Cantharellus (Cantharellales, Agaricomycetidae). Fungal Divers. 2014, 64, 101–121. [Google Scholar] [CrossRef]
- Henkel, T.W.; Wilson, A.W.; Amie, M.C.; Dierks, J.; Uehling, J.K.; Roy, M.; Schimann, H.; Wartchow, F.; Mueller, G.M. Cantharellaceae of Guyana II: New species of Craterellus, new South American distribution records for Cantharellus guyanensis and Craterellus excelsus, and a key to the neotropical taxa. Mycologia 2014, 106, 307–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kesel, A.D.; Amalfi, M.; Kasongo Wa Ngoy, B.; Yorou, N.S.; Raspé, O.; Degreef, J.; Buyck, B. New and interesting Cantharellus from tropical Africa. Cryptog. Mycol. 2016, 37, 283–327. [Google Scholar] [CrossRef]
- Ogawa, W.; Endo, N.; Fukuda, M.; Yamada, A. Phylogenetic analyses of Japanese golden chanterelles and a new species description, Cantharellus anzutake sp. nov. Mycoscience 2018, 59, 153–165. [Google Scholar] [CrossRef]
- Cao, T.; Hu, Y.P.; Yu, J.R.; Wei, T.Z.; Yuan, H.S. A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud. Mycol. 2021, 99, 100121. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Liu, P.G.; Wei, T.Z.; Herrera, M. New insights into the taxonomy of the genus Cantharellus in China: Epityfication of C. yunnanensis W.F. Chiu and the first record of C. cibarius Fr. Cryptog. Mycol. 2021, 42, 25–37. [Google Scholar] [CrossRef]
- Corner, E.J.H. A Monograph of Cantharelloid Fungi; Oxford University Press: Oxford, UK, 1966. [Google Scholar]
- Buyck, B.; Kauff, F.; Cruaud, C.; Hofstetter, V. Molecular evidence for novel Cantharellus (Cantharellales, Basidiomycota) from tropical African miombo woodland and a key to all tropical African chanterelles. Fungal Divers. 2013, 58, 281–298. [Google Scholar] [CrossRef]
- Antonín, V.; Hofstetter, V.; Ryoo, R.; Ka, K.H.; Buyck, B. New Cantharellus species from the Republic of Korea. Mycol. Prog. 2017, 16, 75–759. [Google Scholar] [CrossRef]
- Jian, S.P.; Dai, R.; Gao, J.U.N.; Feng, B. Cantharellus albus, a striking new species from Southwest China. Phytotaxa 2020, 470, 133–144. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.Q.; Buyck, B.; Deng, W.Q.; Li, T.H. Multigene Phylogeny and Morphology Reveal Unexpectedly High Number of New Species of Cantharellus Subgenus Parvocantharellus (Hydnaceae, Cantharellales) in China. J. Fungi 2021, 7, 919. [Google Scholar] [CrossRef] [PubMed]
- An, D.Y.; Liang, Z.Q.; Jiang, S.; Su, M.S.; Zeng, N.K. Cantharellus hainanensis, a new species with a smooth hymenophore from tropical China. Mycoscience 2017, 58, 438–444. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Liang, Z.Q.; Xie, H.J.; Wu, L.L.; Xue, R.; Zeng, N.K. Cantharellus macrocarpus (Cantharellaceae, Cantharellales), a new species from tropical China. Phytotaxa 2021, 484, 170–180. [Google Scholar] [CrossRef]
- Buyck, B.; Hofstetter, V.; Ryoo, R.; Ka, K.-H.; Antonín, V. New Cantharellus species from South Korea. MycoKeys 2020, 76, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Eyssartier, G.; Buyck, B. Note nomenclaturale et systématique sur le genre Cantharellus. Doc. Mycol. 2001, 31, 55–56. [Google Scholar]
- Buyck, B.; Henkel, T.W.; Dentinger, B.T.M.; Séné, O.; Hofstetter, V. Multigene sequencing provides a suitable epitype, barcode sequences and a precise systematic position for the enigmatic, African Cantharellus miniatescens. Cryptog. Mycol. 2016, 37, 269–282. [Google Scholar] [CrossRef]
- Wu, X.L.; Dai, Y.C.; Li, T.H.; Yang, Z.L.; Song, B. Fungi of Tropical China; Science Press: Beijing, China, 2011. [Google Scholar]
- Shao, S.C.; Tian, X.F.; Liu, P.G. Two species with intercontinental disjunct distribution of the genus Cantharellus. J. Yunnan Agric. Univ. 2012, 27, 150–155. [Google Scholar]
- Yang, Z.L.; Wu, G.; Li, Y.C.; Wang, X.H.; Cai, Q. Common Edible and Poisonous Mushrooms of Southwestern China; Science Press: Beijing, China, 2021. [Google Scholar]
- Shao, S.C.; Buyck, B.; Tian, X.F.; Liu, P.G.; Geng, Y.H. Cantharellus phloginus, a new pink-colored species from southwestern China. Mycoscience 2016, 57, 144–149. [Google Scholar] [CrossRef]
- Kornerup, A.; Wanscher, J.H. Taschenlexikon der Farben, 3rd ed.; Muster-Schmidt Verlag: Göttingen, Germany, 1981. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [Green Version]
- Morehouse, E.A.; James, T.Y.; Ganley, A.R.D.; Vilgalys, R.; Berger, L.; Murphy, P.J.; Longcore, J.E. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol. Ecol. 2003, 12, 395–403. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Effificient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [Green Version]
- Moncalvo, J.M.; Nilsson, R.H.; Koster, B.; Dunham, S.M.; Bernauer, T.; Matheny, P.B.; McLenon, T.; Margaritescu, S.; Weiß, M.; Garnica, S.; et al. The cantharelloid clade: Dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 2006, 98, 937–948. [Google Scholar] [CrossRef]
- Buyck, B.; Cruaud, C.; Couloux, A.; Hofstetter, V. Cantharellus texensis sp. nov. from Texas, a Southern lookalike of C. cinnabarinus revealed by tef-1 sequence data. Mycologia 2011, 103, 1037–1046. [Google Scholar] [CrossRef]
- Buyck, B.; Moreau, P.-A.; Courtecuisse, R.; Kong, A.; Roy, M.; Hofstetter, V. Cantharellus coccolobae sp. nov. and Cantharellus garnieri two tropical members of Cantharellus subg. Cinnabarinus. Cryptog. Mycol. 2016, 37, 391–403. [Google Scholar] [CrossRef]
- Buyck, B. Special issue: Cantharellus. Cryptog. Mycol. 2016, 37, 255–258. [Google Scholar] [CrossRef]
- Suhara, H.; Kurogi, S. Cantharellus cyphelloides (Cantharellales), a new and unusual species from a Japanese evergreen broad-leaved forest. Mycol. Prog. 2015, 14, 55. [Google Scholar] [CrossRef]
- Olariaga, I.; Moreno, G.; Manjón, J.L.; Salcedo, I.; Hofstetter, V.; Rodríguez, D.; Buyck, B. Cantharellus (Cantharellales, Basidiomycota) revisited in Europe through a multigene phylogeny. Fungal Divers. 2017, 83, 263–292. [Google Scholar] [CrossRef]
- Ducousso, M.; Contesto, C.; Cossegal, M.; Prin, Y.; Rigault, F.; Eyssartier, G. Cantharellus garnierii sp. nov. from nickel mine maquis in New Caledonia. Cryptog. Mycol. 2004, 25, 115–125. [Google Scholar]
- Buyck, B.; Hofstetter, V. The contribution of tef-1 sequences to species delimitation in the Cantharellus cibarius complex in the southeastern USA. Fungal Divers. 2011, 49, 35–46. [Google Scholar] [CrossRef]
- Buyck, B.; Ndolo Ebika, S.T.; De Kesel, A.; Hofstetter, V. Tropical African Cantharellus Adans.: Fr. (Hydnaceae, Cantharellales) with lilac-purplish tinges revisited. Cryptog. Mycol. 2020, 41, 161–177. [Google Scholar] [CrossRef]
- Feibelman, T.P.; Bennett, J.W.; Cibula, W.G. Cantharellus tabernensis: A new species from the Southeastern United States. Mycologia 1996, 88, 295–301. [Google Scholar] [CrossRef]
- Ryvarden, L.; Petersen, R. Notes on cantharelloid fungi IV. Two new species of Cantharellus. Sven. Bot. Tidskr. 1971, 65, 399–405. [Google Scholar]
- Buyck, B.; Lewis, D.P.; Eyssartier, G.; Hofstetter, V. Cantharellus quercophilus sp. nov. and its comparison to other small, yellow or brown American chanterelles. Cryptog. Mycol. 2010, 31, 17–33. [Google Scholar]
- Petersen, R.H. Notes on Clavarioid Fungi. XIX. Colored illustrations of selected taxa, with comments on Cantharellus. Nova Hedwig. 1985, 42, 151–160. [Google Scholar]
- Kuo, M. Cantharellus persicinus. Retrieved from the MushroomExpert.Com. 2015. Available online: http://www.mushroomexpert.com/cantharellus_persicinus.html (accessed on 20 March 2022).
- Bigelow, H.E. The cantharelloid fungi of New England and adjacent areas. Mycologia 1978, 70, 707–756. [Google Scholar] [CrossRef]
- Montoya, L.; Herrera, M.; Bandala, V.M.; Ramos, A. Two new species and a new record of yellow Cantharellus from tropical Quercus forests in eastern Mexico with the proposal of a new name for the replacement of Craterellus confluens. MycoKeys 2021, 80, 91–114. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).