Serum Cytokines Usefulness for Understanding the Pathology in Allergic Bronchopulmonary Aspergillosis and Chronic Pulmonary Aspergillosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurements
2.3. Cytokine Measurements
2.4. Statistical Analyses
2.5. Ethics Statement
3. Results
3.1. Characteristics of Study Participants
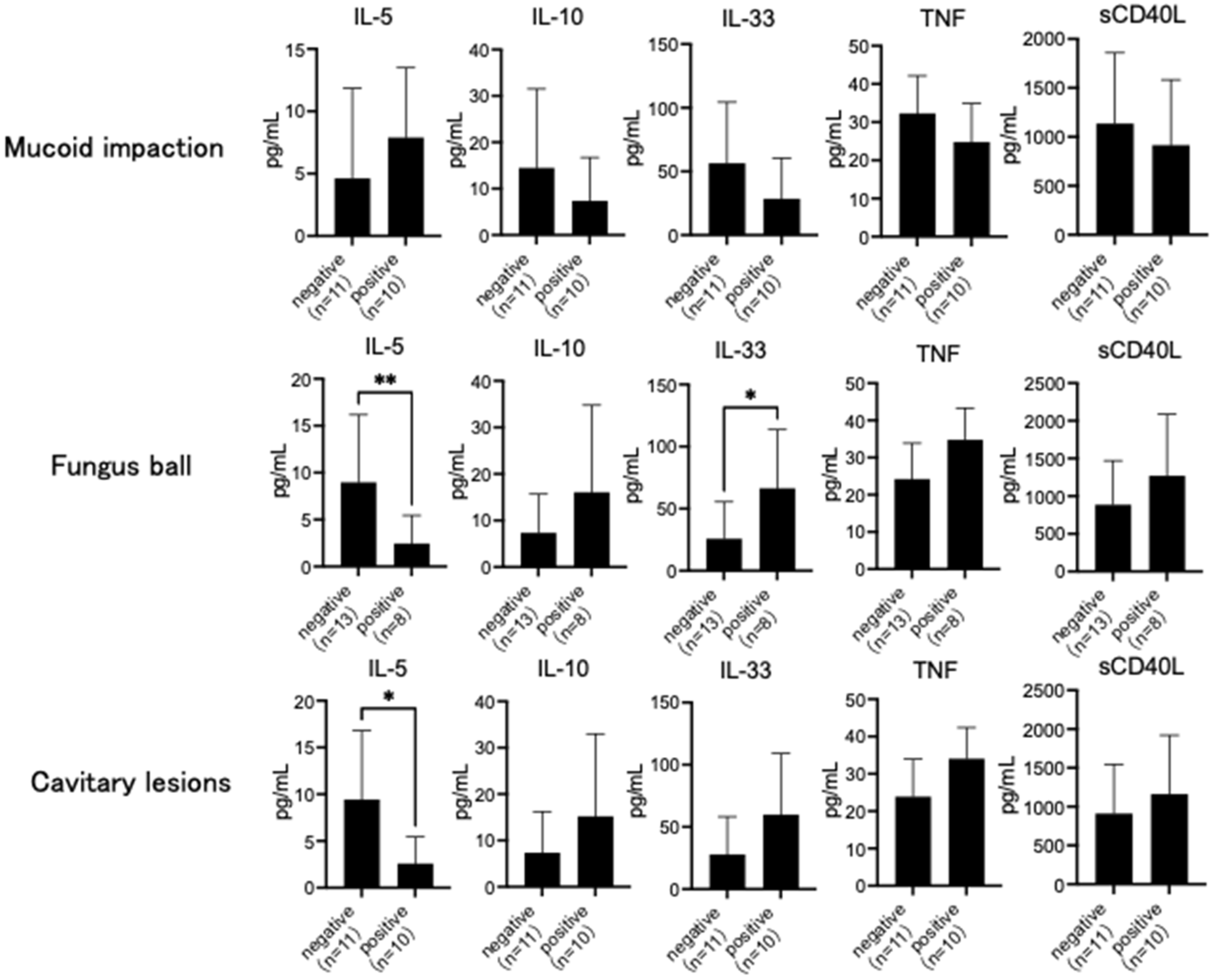
3.2. Comparison of Cytokines
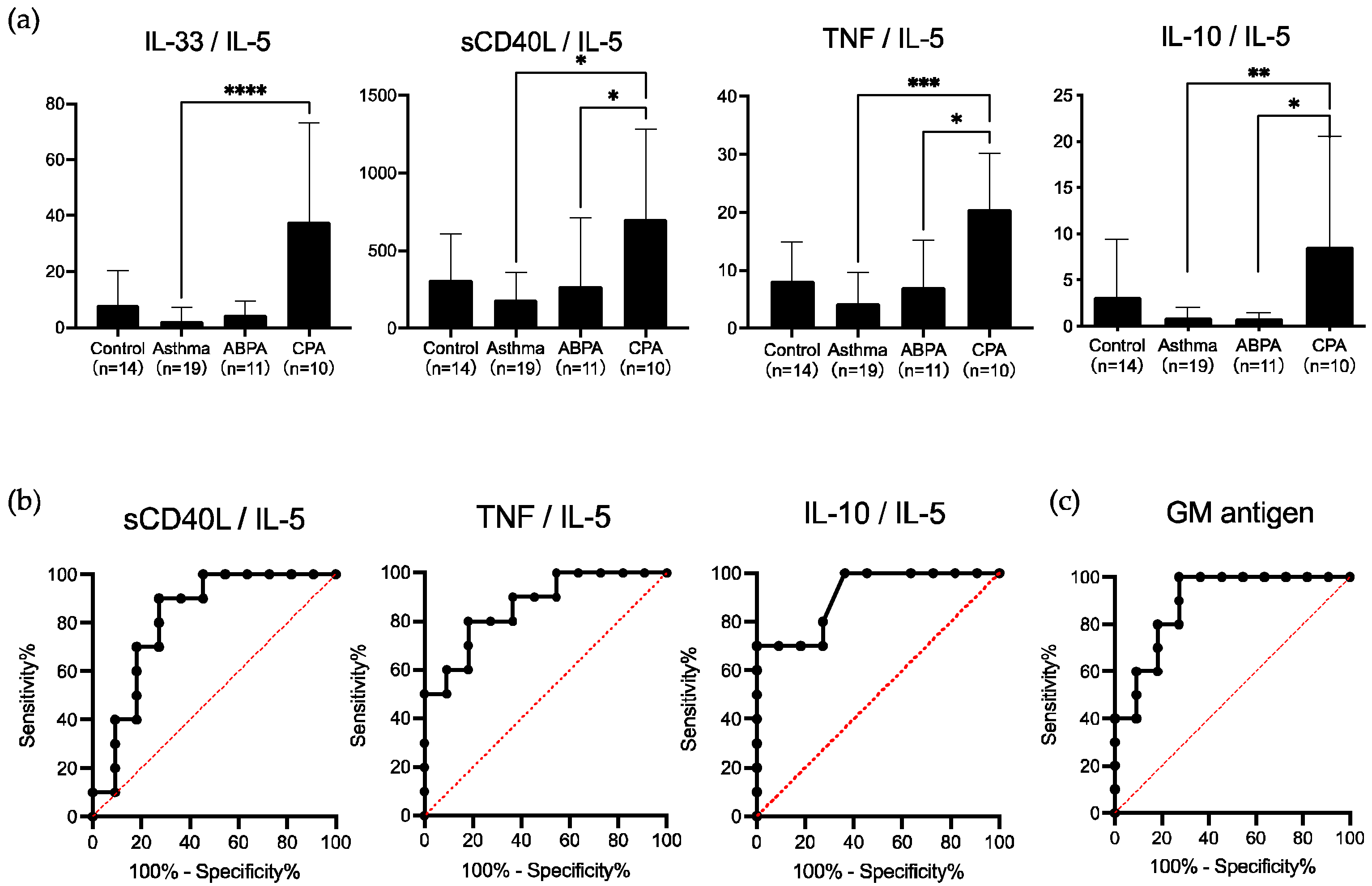
3.3. Cytokine Ratios
3.4. Usefulness of Cytokine Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asano, K.; Ueki, S.; Tamari, M.; Imoto, Y.; Fujieda, S.; Taniguchi, M. Adult-onset eosinophilic airway diseases. Allergy 2020, 75, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Sehgal, I.S.; Dhooria, S.; Muthu, V.; Prasad, K.T.; Bal, A.; Aggarwal, A.N.; Chakrabarti, A. Allergic bronchopulmonary aspergillosis. Indian J. Med. Res. 2020, 151, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Takazono, T.; Sheppard, D.C. Aspergillus in chronic lung disease: Modeling what goes on in the airways. Med. Mycol. 2017, 55, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, G.; Greenberger, P.A. Allergic bronchopulmonary aspergillosis. Allergy Asthma Proc. 2019, 40, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Cadranel, J.; Beigelman-Aubry, C.; Ader, F.; Chakrabarti, A.; Blot, S.; Ullmann, A.J.; Dimopoulos, G.; Lange, C.; European Society for Clinical, M.; et al. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur. Respir. J. 2016, 47, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Barac, A.; Kosmidis, C.; Alastruey-Izquierdo, A.; Salzer, H.J.F.; Cpanet. Chronic pulmonary aspergillosis update: A year in review. Med. Mycol. 2019, 57, S104–S109. [Google Scholar] [CrossRef] [PubMed]
- Lowes, D.; Chishimba, L.; Greaves, M.; Denning, D.W. Development of chronic pulmonary aspergillosis in adult asthmatics with ABPA. Respir. Med. 2015, 109, 1509–1515. [Google Scholar] [CrossRef]
- Kanj, A.; Abdallah, N.; Soubani, A.O. The spectrum of pulmonary aspergillosis. Respir. Med. 2018, 141, 121–131. [Google Scholar] [CrossRef]
- Sehgal, I.S.; Choudhary, H.; Dhooria, S.; Aggarwal, A.N.; Garg, M.; Chakrabarti, A.; Agarwal, R. Is There an Overlap in Immune Response Between Allergic Bronchopulmonary and Chronic Pulmonary Aspergillosis? J. Allergy Clin. Immunol. Pract. 2019, 7, 969–974. [Google Scholar] [CrossRef]
- Maiz, L.; Nieto, R.; Canton, R.; de la Pedrosa, E.G.G.; Martinez-Garcia, M.A. Fungi in Bronchiectasis: A Concise Review. Int. J. Mol. Sci. 2018, 19, 142. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.L.; Denning, D.W. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur. Respir. J. 2010, 37, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, R.; Aggarwal, A.N.; Garg, M.; Saikia, B.; Gupta, D.; Chakrabarti, A. Allergic bronchopulmonary aspergillosis with aspergilloma: An immunologically severe disease with poor outcome. Mycopathologia 2012, 174, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fayemiwo, S.; Moore, C.B.; Foden, P.; Denning, D.W.; Richardson, M.D. Comparative performance of Aspergillus galactomannan ELISA and PCR in sputum from patients with ABPA and CPA. J. Microbiol. Methods 2017, 140, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Hebisawa, A.; Ishiguro, T.; Takayanagi, N.; Nakamura, Y.; Suzuki, J.; Okada, N.; Tanaka, J.; Fukutomi, Y.; Ueki, S.; et al. New clinical diagnostic criteria for allergic bronchopulmonary aspergillosis/mycosis and its validation. J. Allergy Clin. Immunol. 2021, 147, 1261–1268.e5. [Google Scholar] [CrossRef] [PubMed]
- Rodland, E.K.; Ueland, T.; Bjornsen, S.; Sagen, E.L.; Dahl, C.P.; Naalsund, A.; Mollnes, T.E.; Brosstad, F.R.; Muller, F.; Aukrust, P.; et al. Systemic biomarkers of inflammation and haemostasis in patients with chronic necrotizing pulmonary aspergillosis. BMC Infect Dis. 2012, 12, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.L.; Gresnigt, M.S.; Smeekens, S.P.; Jacobs, C.W.; Magis-Escurra, C.; Jaeger, M.; Wang, X.; Lubbers, R.; Oosting, M.; Joosten, L.A.; et al. Pattern recognition pathways leading to a Th2 cytokine bias in allergic bronchopulmonary aspergillosis patients. Clin. Exp. Allergy 2015, 45, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Zhan, M.; Xu, B.; Zhao, L.; Li, B.; Xu, L.; Sun, Q.; Zhang, J.; Zhang, Z.; Chu, H. The Serum Level of IL-1B Correlates with the Activity of Chronic Pulmonary Aspergillosis. Can. Respir. J. 2018, 2018, 8740491. [Google Scholar] [CrossRef]
- Global Initiative for Asthma (GINA). Grobal Strategy for Asthma Management and Prevention, 2021. Available online: https://ginasthma.org/wp-content/uploads/2021/05/GINA-Main-Report-2021-V2-WMS.pdf (accessed on 20 October 2021).
- Agarwal, R.; Chakrabarti, A.; Shah, A.; Gupta, D.; Meis, J.F.; Guleria, R.; Moss, R.; Denning, D.W.; ABPA Complicating Asthma ISHAM Working Group. Allergic bronchopulmonary aspergillosis: Review of literature and proposal of new diagnostic and classification criteria. Clin. Exp. Allergy 2013, 43, 850–873. [Google Scholar] [CrossRef]
- Rodland, E.K.; Ueland, T.; Pedersen, T.M.; Halvorsen, B.; Muller, F.; Aukrust, P.; Froland, S.S. Activation of platelets by Aspergillus fumigatus and potential role of platelets in the immunopathogenesis of Aspergillosis. Infect Immun. 2010, 78, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Tracy, M.C.; Okorie, C.U.A.; Foley, E.A.; Moss, R.B. Allergic Bronchopulmonary Aspergillosis. J. Fungi 2016, 2, 17. [Google Scholar] [CrossRef]
- Fukushima, C.; Matsuse, H.; Obase, Y.; Fukahori, S.; Tsuchida, T.; Kawano, T.; Kohno, S.; Mukae, H. Liposomal Amphotericin B Fosters the Corticosteroids’ Anti-inflammatory Effect on Murine Allergic Bronchopulmonary Aspergillosis Model Airways. Inflammation 2019, 42, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Dimitriadou-Georgiadou, A.; Sein, T.; Kadiltsoglou, I.; Walsh, T.J. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun. 1998, 66, 5999–6003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filler, S.G.; Yeaman, M.R.; Sheppard, D.C. Tumor necrosis factor inhibition and invasive fungal infections. Clin. Infect Dis. 2005, 41 (Suppl. 3), S208–S212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cenci, E.; Mencacci, A.; Fe d’Ostiani, C.; Del Sero, G.; Mosci, P.; Montagnoli, C.; Bacci, A.; Romani, L. Cytokine- and T helper-dependent lung mucosal immunity in mice with invasive pulmonary aspergillosis. J. Infect Dis. 1998, 178, 1750–1760. [Google Scholar] [CrossRef] [Green Version]
- Mehrad, B.; Strieter, R.M.; Standiford, T.J. Role of TNF-alpha in pulmonary host defense in murine invasive aspergillosis. J. Immunol. 1999, 162, 1633–1640. [Google Scholar] [PubMed]
- Brieland, J.K.; Jackson, C.; Menzel, F.; Loebenberg, D.; Cacciapuoti, A.; Halpern, J.; Hurst, S.; Muchamuel, T.; Debets, R.; Kastelein, R.; et al. Cytokine networking in lungs of immunocompetent mice in response to inhaled Aspergillus fumigatus. Infect Immun. 2001, 69, 1554–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanagihara, Y.; Kajiwara, K.; Koshio, T.; Basaki, Y.; Ikizawa, K.; Mori, M.; Akiyama, K.; Kawamura, N.; Sakiyama, Y. Production of IL-4 and expression of CD40 ligand by human CD8 T cells. J. Allergy Clin. Immunol. 1999, 103, S405–S411. [Google Scholar] [CrossRef]
- Mion, F.; D’Inca, F.; Danelli, L.; Toffoletto, B.; Guarnotta, C.; Frossi, B.; Burocchi, A.; Rigoni, A.; Gerdes, N.; Lutgens, E.; et al. Mast cells control the expansion and differentiation of IL-10-competent B cells. J. Immunol. 2014, 193, 4568–4579. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, A.R.; Shokri, H.; Hassan Al-Heidary, S.; Ghafarifar, F. Evaluation of murine lung epithelial cells (TC-1 JHU-1) line to develop Th2-promoting cytokines IL-25/IL-33/TSLP and genes Tlr2/Tlr4 in response to Aspergillus fumigatus. J. Mycol. Med. 2018, 28, 349–354. [Google Scholar] [CrossRef]
- Garth, J.M.; Reeder, K.M.; Godwin, M.S.; Mackel, J.J.; Dunaway, C.W.; Blackburn, J.P.; Steele, C. IL-33 Signaling Regulates Innate IL-17A and IL-22 Production via Suppression of Prostaglandin E2 during Lung Fungal Infection. J. Immunol. 2017, 199, 2140–2148. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, T.; Tatematsu, M.; Fuchimukai, A.; Yamada, T.; Yamagata, K.; Takasuga, S.; Yamada, T. Trained innate lymphoid cells in allergic diseases. Allergol. Int. 2021, 70, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Kato, A. Group 2 Innate Lymphoid Cells in Airway Diseases. Chest 2019, 156, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Kaislasuo, J.; Guller, S.; Pal, L. Thermal stability of cytokines: A review. Cytokine 2020, 125, 154829. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 14) | Asthma (n = 19) | ABPA (n = 11) | CPA (n = 10) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 37 ± 7.626 | 56.7 ± 15.9 * | 47.1 ± 13.3 | 66.5 ± 10.6 *,*** | p < 0.0001 |
| Male, n (%) | 5 (35.7) | 5 (26.3) | 4 (36.3) | 8 (80.0) | p = 0.0410 |
| Body mass index (kg/m2) | 20.8 ± 3.26 | 23.8 ± 4.63 | 21.4 ± 3.42 | 18.0 ± 2.67 **,*** | p = 0.0007 |
| Smoker, n (%) | 6 (42.8) | 3 (15.7) | 5 (45.4) | 8 (80) | p = 0.0097 |
| IgE (IU/mL) | 122.0 ± 263.2 | 491.2 ± 944.0 | 3694 ± 3451 **** | 105.1 ± 184.8 | p < 0.0001 |
| Eosinophil count (/μL) | - | 275.2 ± 238.4 | 1198 ± 597.5 **** | 158.5 ± 103.4 | p < 0.0001 |
| Galactomannnan (C.O.I) | - | - | 0.33 ± 0.43 | 2.22 ± 3.21 | p = 0.0015 |
| Aspergillus precipitating antibody, n (%) | - | - | 4 (36.3) | 8 (80.0) | p = 0.0805 |
| Complications, n (%) | |||||
| COPD | - | 2 (10.5) | 0 | 3 (30) | p = 0.1870 |
| Bronchiectasis | - | 0 | 7 (63.6) | 4 (40) | p = 0.0005 |
| Tuberculosis sequelae | - | 0 | 0 | 1 (10) | p = 0.4762 |
| Post-thoracic surgery | - | 0 | 1 (9) | 2 (20) | p = 0.5865 |
| Lung cyst | - | 0 | 0 | 1 (10) | p = 0.4762 |
| Diabetes mellitus | - | 2 (10.5) | 2 (18.2) | 0 | p = 0.6111 |
| Chest CT findings | |||||
| mucoid impaction, n (%) | - | - | 10 (91) | 0 | p < 0.0001 |
| Consolidation, n (%) | - | - | 7 (63.6) | 5 (50) | p = 0.0003 |
| Bronchiectasis, n (%) | - | - | 7 (63.6) | 5 (50) | p = 0.0003 |
| Cavitary lesions, n (%) | - | - | 0 | 10 (100) | p < 0.0001 |
| Fungus balls, n (%) | - | - | 0 | 8 (80) | p = 0.0002 |
| Control (n = 14) | Asthma (n = 19) | ABPA (n = 11) | CPA (n = 10) | p-Value | |
|---|---|---|---|---|---|
| IL-1β | N.D. | N.D. | N.D. | N.D. | - |
| IL-2 | N.D. | 1.80 ± 0.73 | N.D. | 1.73 ± 0.54 | p = 0.4413 |
| IL-4 | N.D. | N.D. | N.D. | N.D. | - |
| IL-5 | 4.08 ± 2.92 | 11.46 ± 16.65 *** | 9.44 ± 7.37 *** | 2.60 ± 2.84 | p = 0.0099 |
| IL-6 | N.D. | 0.81 ± 0.53 | 90.35 ± 295.9 | 6.26 ± 4.23 ** | p < 0.0001 |
| IL-8 | 16.36 ± 7.40 | 42.01 ± 37.61 | 709.2 ± 1479 * | 61.25 ± 53.11 * | p = 0.0065 |
| IL-10 | 10.85 ± 24.13 | 3.12 ± 2.02 | 7.35 ± 8.86 | 15.23 ± 17.75 | p = 0.2573 |
| IL-13 | 0.40 ± 0.19 | 2.13 ± 2.37 *,*** | 0.63 ± 0.56 | 0.42 ± 0.22 | p = 0.0032 |
| IL-17A | N.D. | N.D. | N.D. | N.D. | - |
| IL-17F | N.D. | N.D. | N.D. | N.D. | - |
| IL-23 | 29.69 ± 13.18 | N.D. | 50.49 ± 46.47 | 155.9 ± 358.5 | p = 0.1022 |
| IL-25 | 0.51 ± 0.28 | 0.46 ± 0.51 | 0.78 ± 0.69 | 1.16 ± 1.00 | p = 0.0348 |
| IL-33 | 18.19 ± 19.16 | 6.07 ± 9.73 | 27.97 ± 30.23 | 59.91 ± 49.12 ** | p = 0.0002 |
| IFN-γ | N.D. | N.D. | N.D. | 5.26 ± 6.05 | p = 0.0297 |
| TNF | 19.19 ± 8.27 | 20.42 ± 11.7 | 23.85 ± 10.19 | 34.05 ± 8.36 *,** | p = 0.0021 |
| sCD40L | 735.6 ± 368.5 | 694.1 ± 202.7 | 912.4 ± 632.5 | 1162 ± 757.1 | p = 0.2740 |
| Diagnostic Markers | Value (%) | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
| ABPA | ||||
| IgE (cutoff, 602.1 IU/mL) | 100 | 100 | 100 | 100 |
| Eosinophil count (cutoff, 441.0/μL) | 100 | 100 | 100 | 100 |
| Chest CT findings | ||||
| mucoid impaction | 100 | 100 | 100 | 100 |
| CPA | ||||
| Age (cutoff, 61 years) | 70 | 81.8 | 77.8 | 75 |
| Body mass index (cutoff, 19.41 kg/m2) | 90 | 72.7 | 75.0 | 88.9 |
| Galactomannnan (cutoff index, 0.267) | 90 | 72.7 | 75.0 | 88.9 |
| Chest CT findings | ||||
| Cavitary lesions | 100 | 100 | 100 | 100 |
| Fungus balls | 80 | 100 | 100 | 84.6 |
| Cytokine ratios | ||||
| sCD40L/IL-5 (cutoff, 176.9) | 90 | 72.7 | 75.0 | 88.9 |
| TNF/IL-5 (cutoff, 14.68) | 80 | 81.8 | 81.8 | 75 |
| IL-10/IL-5 (cutoff, 2.47) | 70 | 100 | 100 | 78.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, Y.; Takazono, T.; Obase, Y.; Fukahori, S.; Ashizawa, N.; Hirayama, T.; Tashiro, M.; Yamamoto, K.; Imamura, Y.; Hosogaya, N.; et al. Serum Cytokines Usefulness for Understanding the Pathology in Allergic Bronchopulmonary Aspergillosis and Chronic Pulmonary Aspergillosis. J. Fungi 2022, 8, 436. https://doi.org/10.3390/jof8050436
Ito Y, Takazono T, Obase Y, Fukahori S, Ashizawa N, Hirayama T, Tashiro M, Yamamoto K, Imamura Y, Hosogaya N, et al. Serum Cytokines Usefulness for Understanding the Pathology in Allergic Bronchopulmonary Aspergillosis and Chronic Pulmonary Aspergillosis. Journal of Fungi. 2022; 8(5):436. https://doi.org/10.3390/jof8050436
Chicago/Turabian StyleIto, Yuya, Takahiro Takazono, Yasushi Obase, Susumu Fukahori, Nobuyuki Ashizawa, Tatsuro Hirayama, Masato Tashiro, Kazuko Yamamoto, Yoshifumi Imamura, Naoki Hosogaya, and et al. 2022. "Serum Cytokines Usefulness for Understanding the Pathology in Allergic Bronchopulmonary Aspergillosis and Chronic Pulmonary Aspergillosis" Journal of Fungi 8, no. 5: 436. https://doi.org/10.3390/jof8050436
APA StyleIto, Y., Takazono, T., Obase, Y., Fukahori, S., Ashizawa, N., Hirayama, T., Tashiro, M., Yamamoto, K., Imamura, Y., Hosogaya, N., Fukushima, C., Morinaga, Y., Yanagihara, K., Izumikawa, K., & Mukae, H. (2022). Serum Cytokines Usefulness for Understanding the Pathology in Allergic Bronchopulmonary Aspergillosis and Chronic Pulmonary Aspergillosis. Journal of Fungi, 8(5), 436. https://doi.org/10.3390/jof8050436






