Grazing Effects of Soil Fauna on White-Rot Fungi: Biomass, Enzyme Production and Litter Decomposition Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of Litter
2.2. The Isolation and Culture of Decomposing Fungi
2.3. The Culturing of Grazers
2.4. Construction of Artificial Microcosm
2.5. Determination of Fungal Biomass in Decomposition Area
2.6. Enzyme Activity Determination in Decomposition Area
2.7. Zymography in the Grazing Area
2.8. Physical and Chemical Properties of Litter
2.9. Statistical Analysis
3. Results
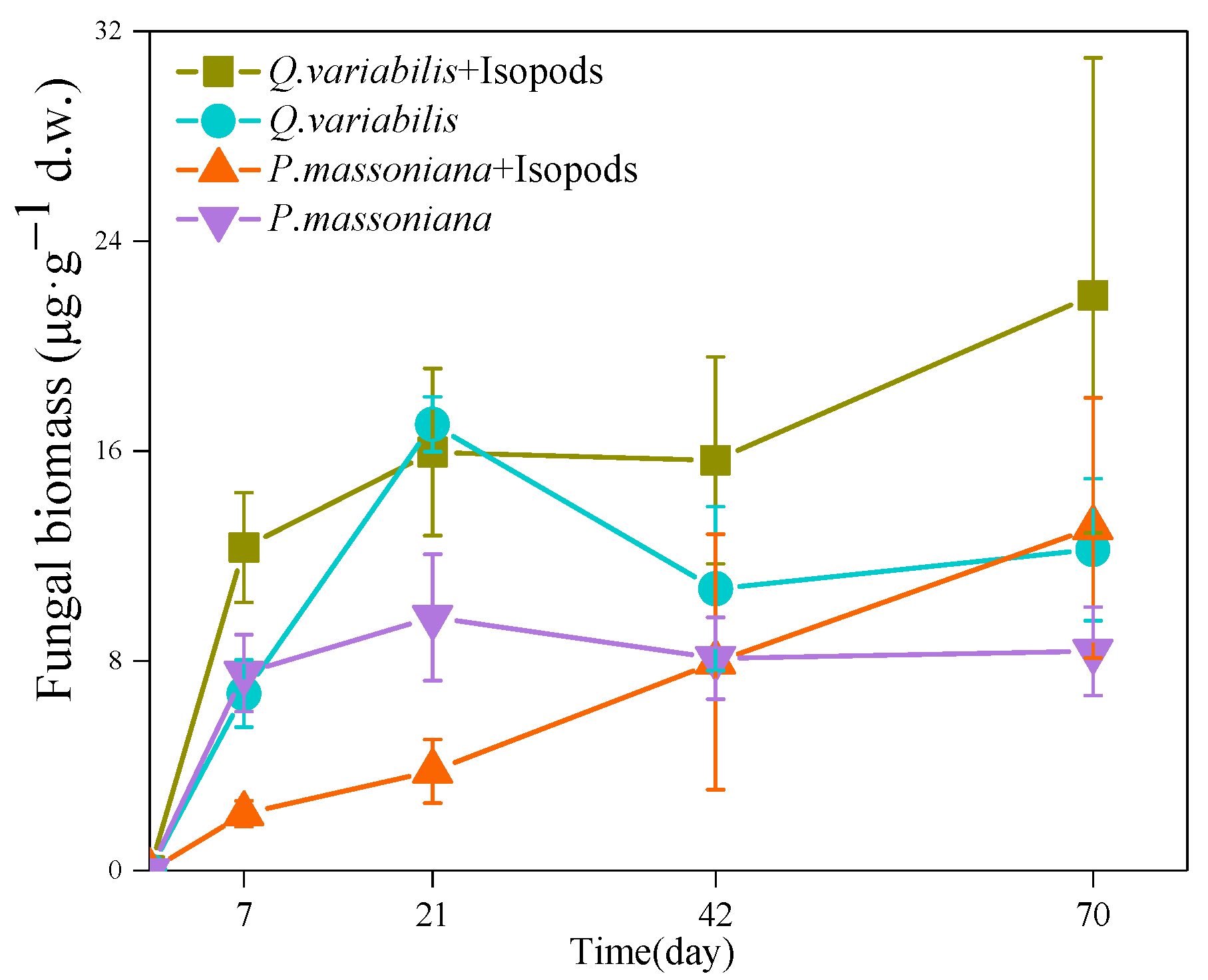
3.1. Fungal Biomass in the Decomposition Area
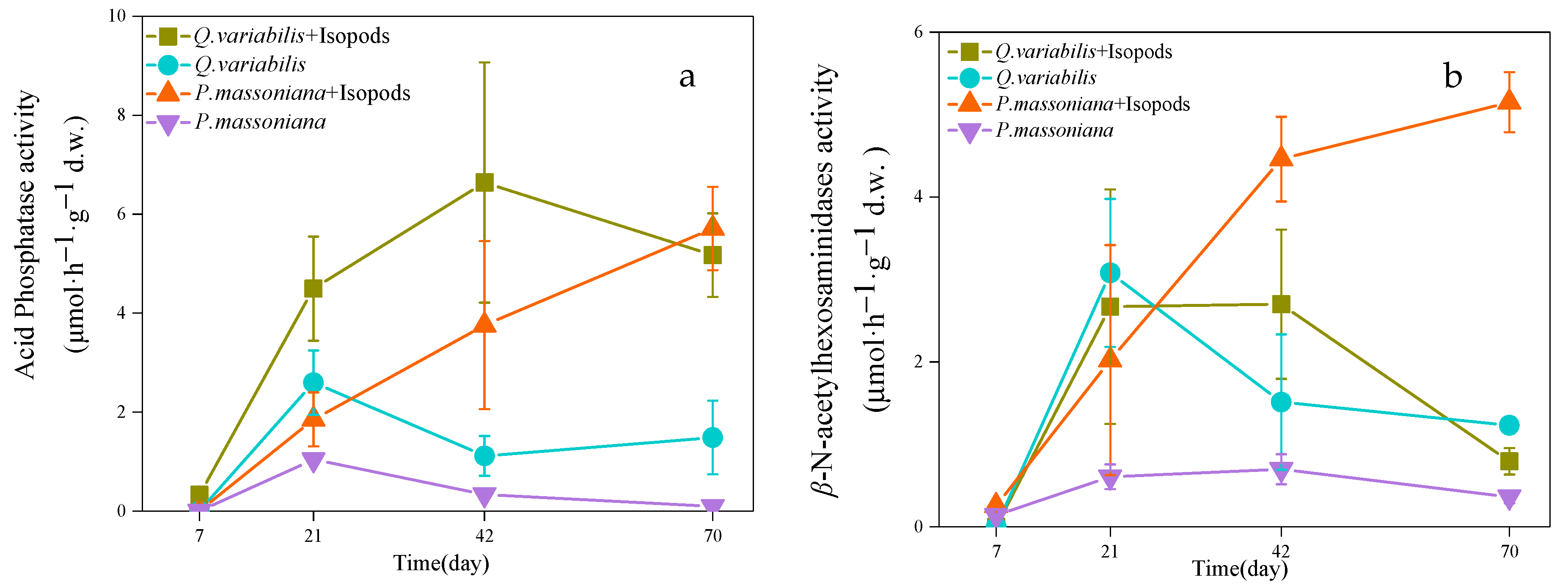
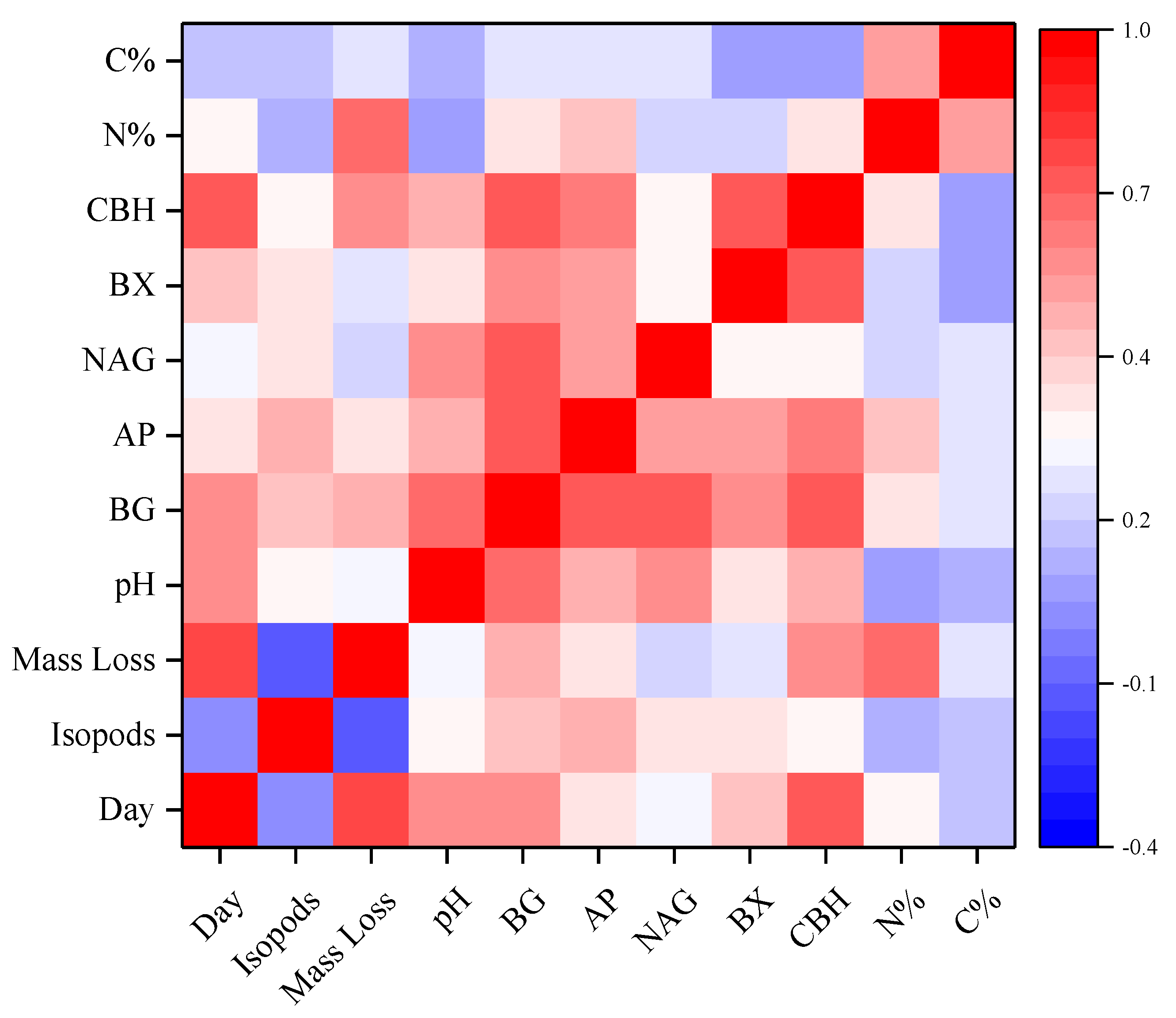
3.2. Enzyme Activity in Decomposition Area
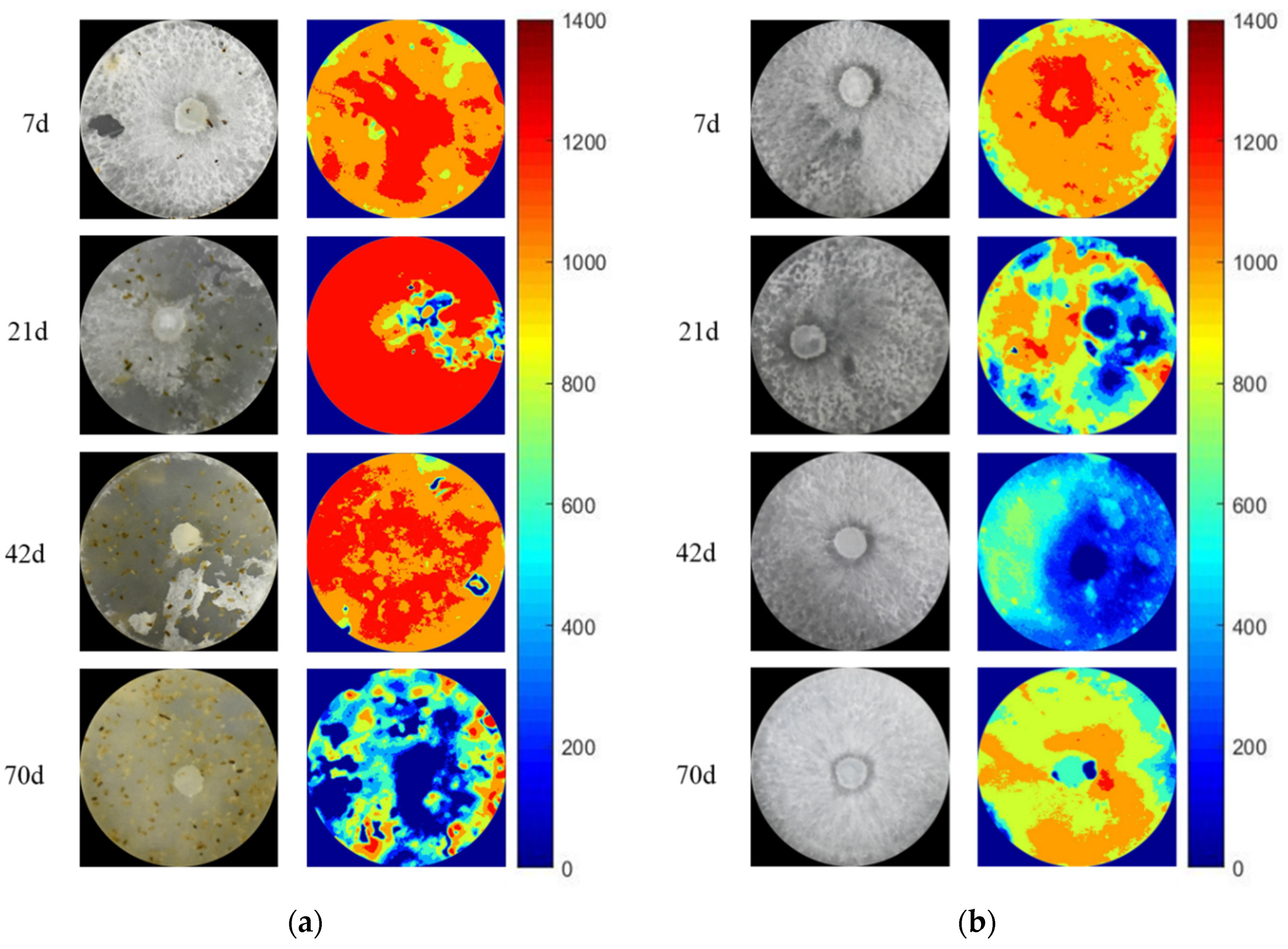
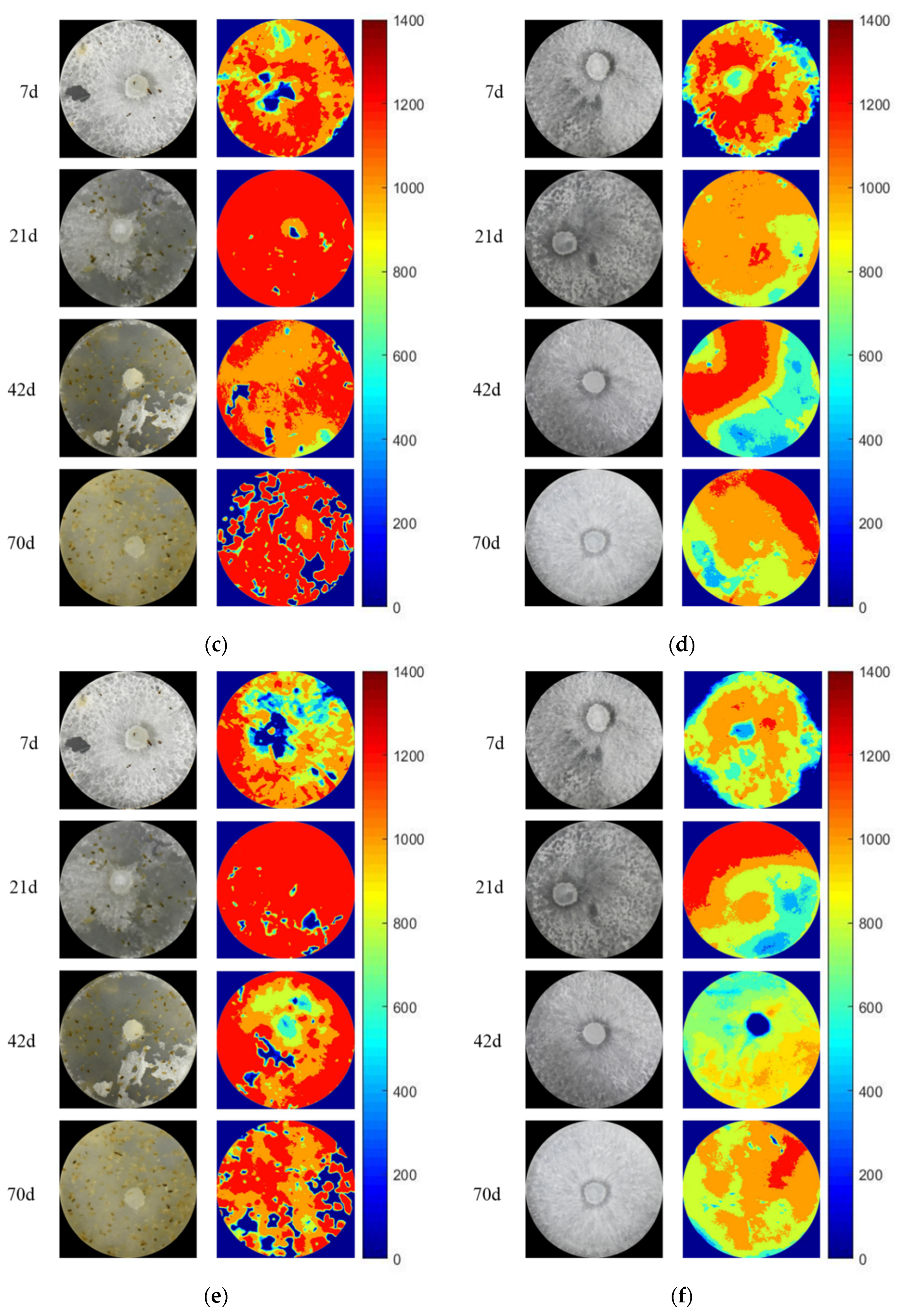
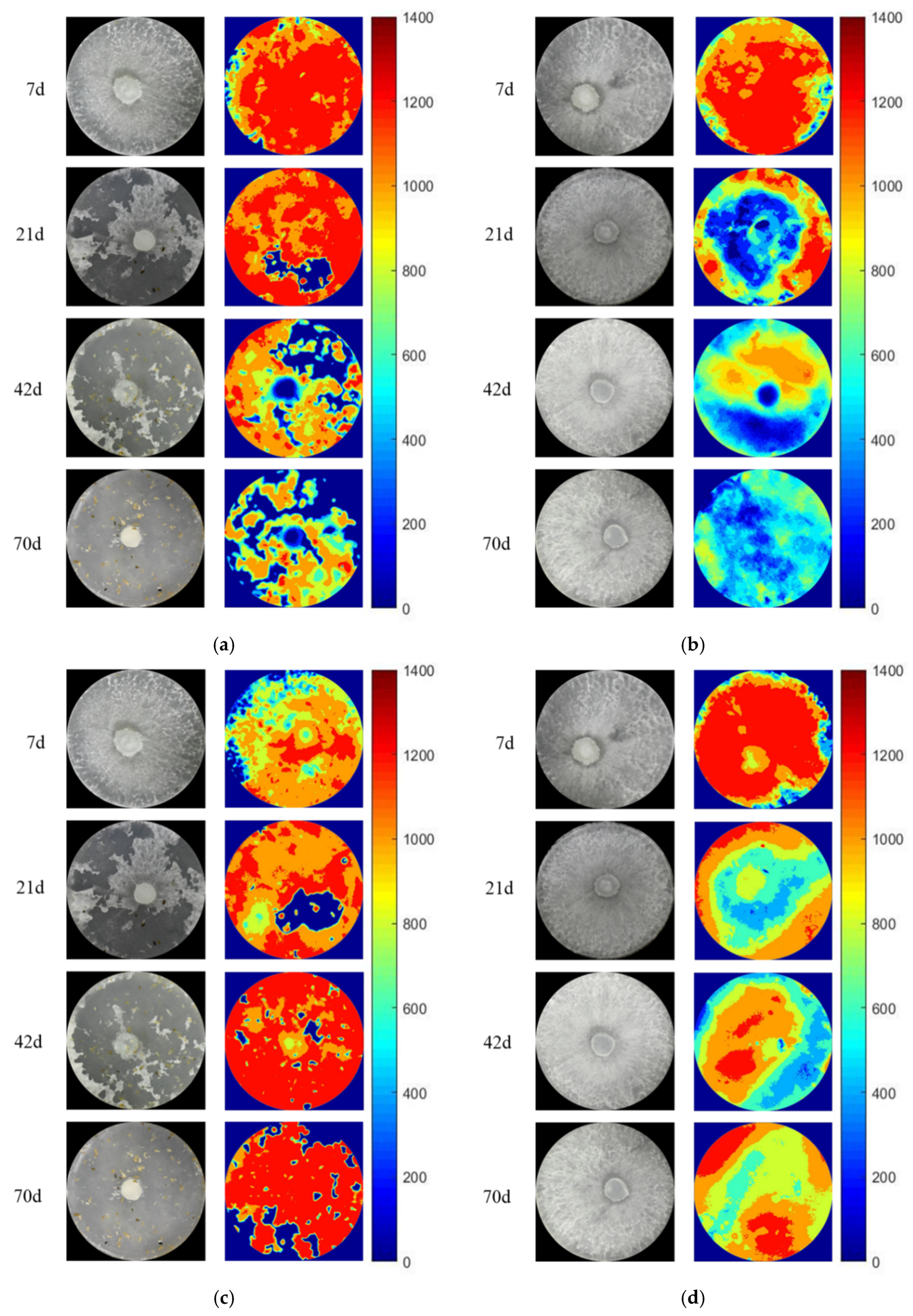
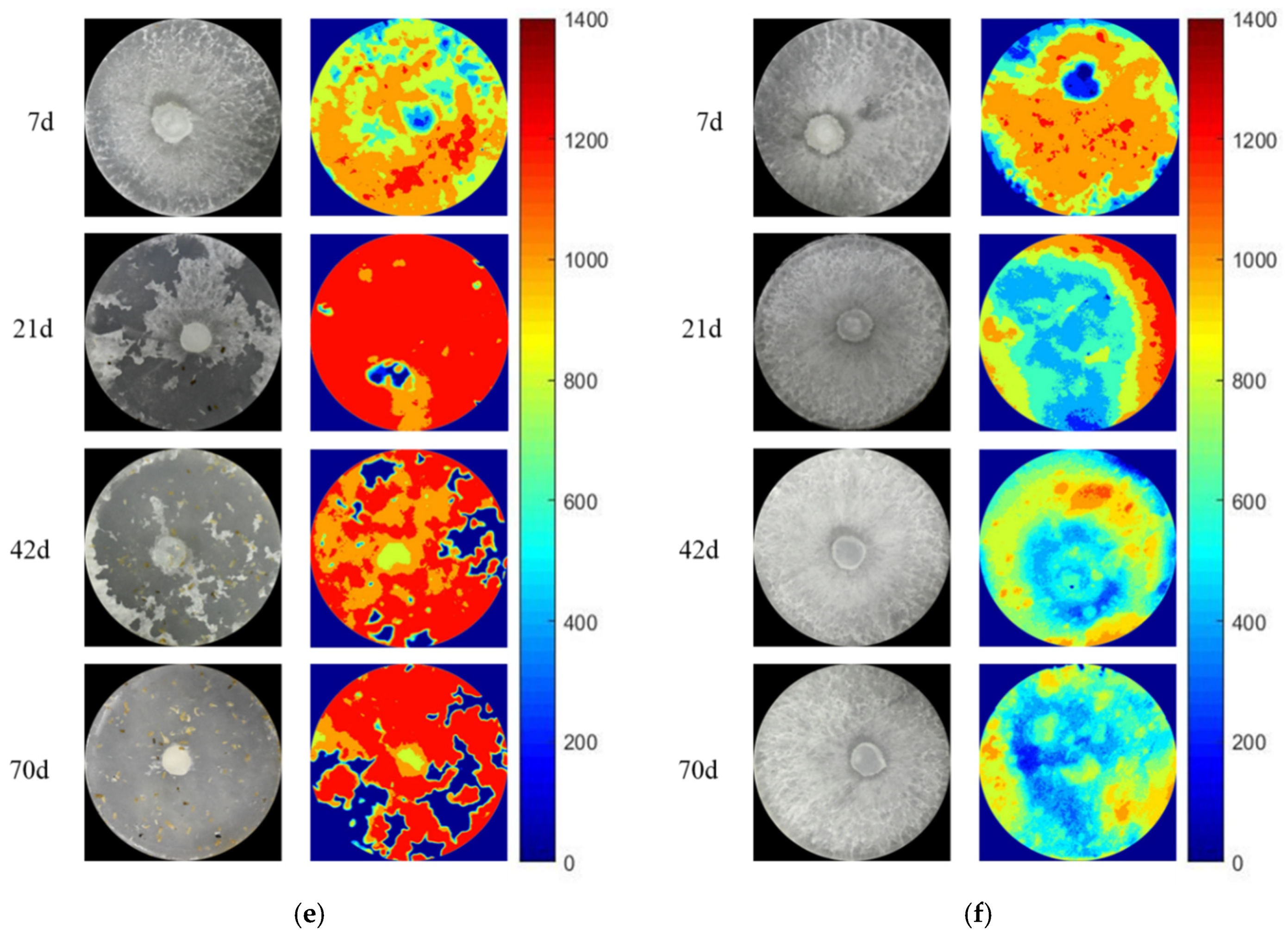
3.3. The Activity and Hotspot of Enzyme in Grazing Area
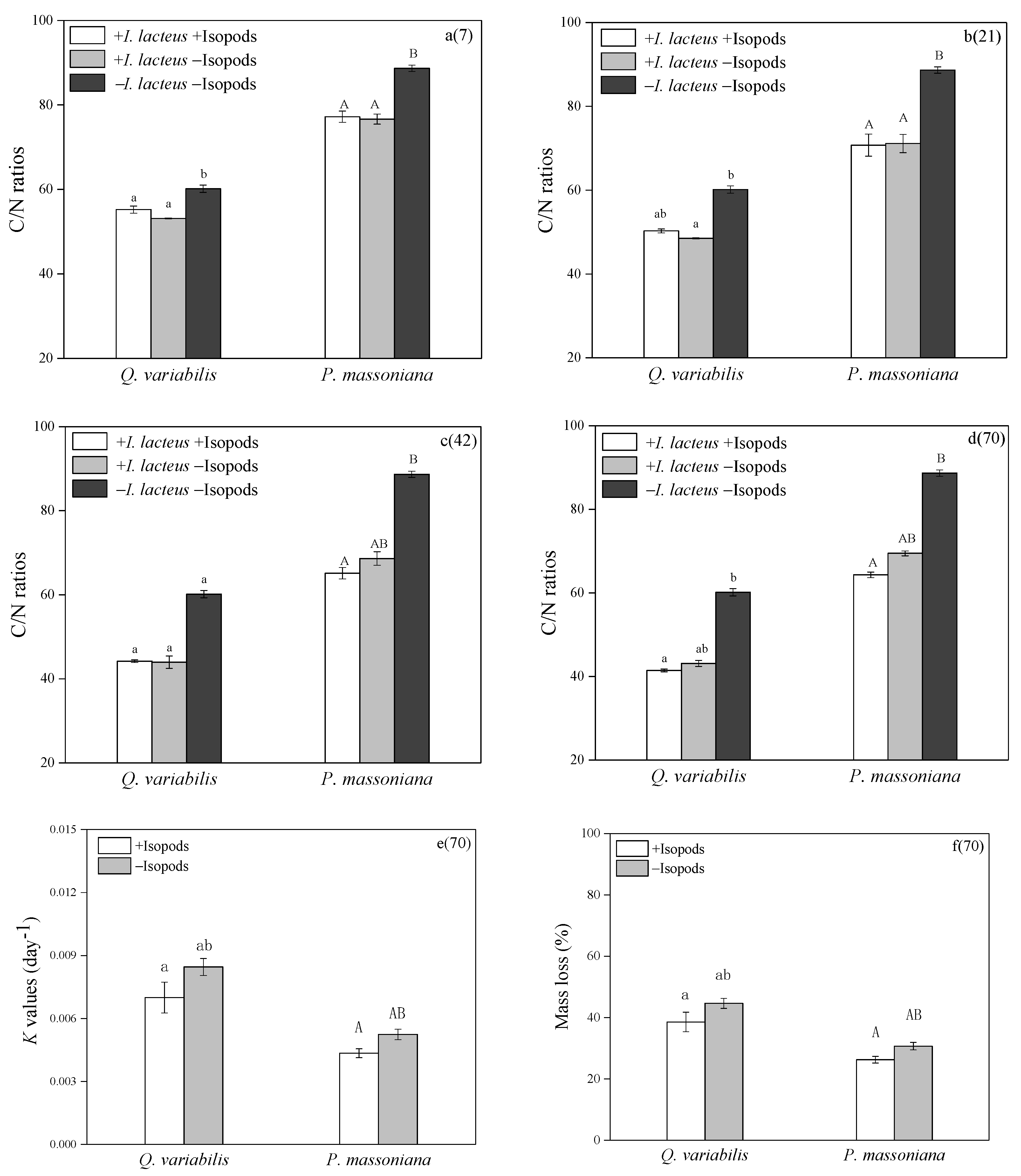
3.4. Litter Decomposition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chomel, M.; Guittonny-Larchevêque, M.; DesRochers, A.; Baldy, V. Home Field Advantage of Litter Decomposition in Pure and Mixed Plantations Under Boreal Climate. Ecosystems 2015, 18, 1014–1028. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.L.; Skowronski, N.; Hom, J. Invasive insects impact forest carbon dynamics. Glob. Change Biol. 2010, 16, 88–101. [Google Scholar] [CrossRef] [Green Version]
- Madritch, M.D.; Donaldson, J.R.; Lindroth, R.L. Canopy herbivory can mediate the influence of plant genotype on soil processes through frass deposition. Soil Biol. Biochem. 2007, 39, 1192–1201. [Google Scholar] [CrossRef]
- Allison, S.D.; Lu, Y.; Weihe, C.; Goulden, M.L.; Martiny, A.C.; Treseder, K.K.; Martiny, J.B. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef] [Green Version]
- Tordoff, G.M.; Chamberlain, P.M.; Crowther, T.W.; Black, H.I.J.; Jones, T.H.; Stott, A.; Boddy, L. Invertebrate grazing affects nitrogen partitioning in the saprotrophic fungus Phanerochaete velutina. Soil Biol. Biochem. 2011, 43, 2338–2346. [Google Scholar] [CrossRef]
- Voříšková, J.; Dobiášová, P.; Šnajdr, J.; Vaněk, D.; Cajthaml, T.; Šantrůčková, H.; Baldrian, P. Chemical composition of litter affects the growth and enzyme production by the saprotrophic basidiomycete Hypholoma fasciculare. Fungal Ecol. 2011, 4, 417–426. [Google Scholar] [CrossRef]
- Baldrian, P. Ectomycorrhizal fungi and their enzymes in soils: Is there enough evidence for their role as facultative soil saprotrophs? Oecologia 2009, 161, 657–660. [Google Scholar] [CrossRef]
- Baldrian, P.; Voříšková, J.; Dobiášová, P.; Merhautová, V.; Lisá, L.; Valášková, V. Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil 2011, 338, 111–125. [Google Scholar] [CrossRef]
- Jia, Y.; Lv, Y.; Kong, X.; Jia, X.; Tian, K.; Du, J.; Tian, X. Insight into the indirect function of isopods in litter decomposition in mixed subtropical forests in China. Appl. Soil Ecol. 2015, 86, 174–181. [Google Scholar] [CrossRef]
- Kourtev, P.S.; Ehrenfeld, J.G.; Huang, W.Z. Enzyme activities during litter decomposition of two exotic and two native plant species in hardwood forests of New Jersey. Soil Biol. Biochem. 2002, 34, 1207–1218. [Google Scholar] [CrossRef]
- Foudyl-Bey, S.; Brais, S.; Drouin, P. Litter heterogeneity modulates fungal activity, C mineralization and N retention in the boreal forest floor. Soil Biol. Biochem. 2016, 100, 264–275. [Google Scholar] [CrossRef]
- Lummer, D.; Scheu, S.; Butenschoen, O. Connecting litter quality, microbial community and nitrogen transfer mechanisms in decomposing litter mixtures. Oikos 2012, 121, 1649–1655. [Google Scholar] [CrossRef]
- Ji, Y.; Li, Q.; Tian, K.; Yang, J.; Hu, H.; Yuan, L.; Lu, W.; Yao, B.; Tian, X. Effect of sodium amendments on the home-field advantage of litter decomposition in a subtropical forest of China. Forest Ecol. Manag. 2020, 468, 118148. [Google Scholar] [CrossRef]
- Meehan, T.D.; Couture, J.J.; Bennett, A.E.; Lindroth, R.L. Herbivore-mediated material fluxes in a northern deciduous forest under elevated carbon dioxide and ozone concentrations. New Phytol. 2014, 204, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Bruns, T.D.; Lilleskov, E. A Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia 2005, 97, 762–769. [Google Scholar]
- Wilding, N.E.; Collins, N.M.E.; Hammond, P.M.E.; Webber, J.F.E. Insect-Fungus Interactions. J. Anim. Ecol. 1990, 59, 382. [Google Scholar]
- Boddy, L.; Jones, T.H. Interactions between basidiomycota and invertebrates. In British Mycological Society Symposia Series; Elsevier: Amsterdam, The Netherlands, 2008; Volume 28, pp. 155–179. [Google Scholar]
- Teuben, A.; Roelofsma, T.A.P.J. Dynamic interactions between functional groups of soil arthropods and microorganisms during decomposition of coniferous litter in microcosm experiments. Biol. Fert. Soils 1990, 9, 145–151. [Google Scholar] [CrossRef]
- David, J.; Handa, I.T. The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol. Rev. 2010, 85, 881–895. [Google Scholar] [CrossRef]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Species-specific effects of soil fauna on fungal foraging and decomposition. Oecologia 2011, 167, 535–545. [Google Scholar] [CrossRef]
- Crowther, T.W.; A’Bear, A.D. Impacts of grazing soil fauna on decomposer fungi are species-specific and density-dependent. Fungal Ecol. 2012, 5, 277–281. [Google Scholar] [CrossRef]
- Harrop-Archibald, H.; Didham, R.K.; Standish, R.J.; Tibbett, M.; Hobbs, R.J. Mechanisms linking fungal conditioning of leaf litter to detritivore feeding activity. Soil Biol. Biochem. 2016, 93, 119–130. [Google Scholar] [CrossRef]
- Crowther, T.W.; Jones, T.H.; Boddy, L.; Baldrian, P. Invertebrate grazing determines enzyme production by basidiomycete fungi. Soil Biol. Biochem. 2011, 43, 2060–2068. [Google Scholar] [CrossRef]
- Li, N. Study on Screening of Efficient Lignin-Degrading Fungus and Their Ligninase Producing Conditions; Northwest A&F University: Xianyang, China, 2008. (In Chinese) [Google Scholar]
- Chen, G.X. Terrestrial isopoda fauna of typical zones in China. Acta Zool. Sin. 2000, 3, 255–264. (In Chinese) [Google Scholar]
- Jia, Y. Effects of Nitrogen and Sodium Addition on Soil Fauna-Litter Decomposition System in Subtropical Forests; Nanjing University: Nanjing, China, 2015. (In Chinese) [Google Scholar]
- Crowther, T.W.; Boddy, L.; Jones, T.H. Outcomes of fungal interactions are determined by soil invertebrate grazers. Ecol. Lett. 2011, 14, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, S.; Norman, M.; Harris, J. A rapid ultrasonication method to determine ergosterol concentration in soil. Soil Biol. Biochem. 1995, 27, 1215–1217. [Google Scholar] [CrossRef]
- Jaroslav, N.; Vendula, V.K.; Věra, M. Activity and spatial distribution of lignocellulose-degrading enzymes during forest soil colonization by saprotrophic basidiomycetes. Enzym. Microb. Technol. 2007, 43, 186–192. [Google Scholar]
- The Allison Lab. Colorimetric Enzyme Assays. 1994. Available online: http://allison.bio.uci.edu/protocols/ (accessed on 9 December 2019).
- Hoang, D.T.T.; Razavi, B.S.; Kuzyakov, Y.; Blagodatskaya, E. Earthworm burrows: Kinetics and spatial distribution of enzymes of C-, N- and P-cycles. Soil Biol. Biochem. 2016, 99, 94–103. [Google Scholar] [CrossRef]
- Razavi, B.S.; Zarebanadkouki, M.; Blagodatskaya, E.; Kuzyakov, Y. Rhizosphere shape of lentil and maize: Spatial distribution of enzyme activities. Soil Biol. Biochem. 2016, 96, 229–237. [Google Scholar] [CrossRef]
- Fioretto, A.; Papa, S.; Curcio, E.; Sorrentino, G.; Fuggi, A. Enzyme dynamics on decomposing leaf litter of Cistus incanus and Myrtus communis in a Mediterranean ecosystem. Soil Biol. Biochem. 2000, 32, 1847–1855. [Google Scholar] [CrossRef]
- Cao, T.; Fang, Y.; Chen, Y.; Kong, X.; Yang, J.; Alharbi, H.; Kuzyakov, Y.; Tian, X. Synergy of saprotrophs with mycorrhiza for litter decomposition and hotspot formation depends on nutrient availability in the rhizosphere. Geoderma 2022, 410, 115662. [Google Scholar] [CrossRef]
- Tordoff, G.M.; Boddy, L.; Jones, T.H. Grazing by Folsomia candida (Collembola) differentially affects mycelial morphology of the cord-forming basidiomycetes Hypholoma fasciculare, Phanerochaete velutina and Resinicium bicolor. Mycol. Res. 2006, 110, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Crowther, T.W.; Jones, T.H.; Boddy, L. Interactions between saprotrophic basidiomycete mycelia and mycophagous soil fauna. Mycology 2012, 3, 77–86. [Google Scholar]
- Hedlund, K.; Boddy, L.; Preston, C.M. Mycelial responses of the soil fungus, Mortierella isabellina, to grazing by Onychiurus armatus (collembola). Soil Biol. Biochem. 1991, 23, 361–366. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Tiunov, A.V.; Scheu, S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 191–218. [Google Scholar] [CrossRef]
- Coulis, M.; Hättenschwiler, S.; Fromin, N.; David, J.F. Macroarthropod-microorganism interactions during the decomposition of Mediterranean shrub litter at different moisture levels. Soil Biol. Biochem. 2013, 64, 114–121. [Google Scholar] [CrossRef]


| Enzyme | EC | Substrate |
|---|---|---|
| β-1,4-glucosidase | 3.2.1.21 | p-nitrophenyl-β-d-glucoside |
| β-N-acetylhexosaminidases | 3.2.1.52 | p-nitrophenyl-N-acetyl-β-d-glucosaminide |
| Acid phosphatase | 3.1.3.1 | p-nitrophenyl-phosphate |
| Cellobiohydrolases | 3.2.1.91 | p-nitrophenyl-β-d-cellobioside |
| 1,4-β-xylosidase | 3.2.1.37 | p-nitrophenyl-β-d-xyloside |
| Enzyme | Treatment | Day | ||||
|---|---|---|---|---|---|---|
| 7 | 21 | 42 | 70 | |||
| Acid phosphatase | Q. variabilis | +Isopods | 612.37 ± 5.00 b | 620.29 ± 86.08 c | 608.31 ± 17.69 b | — |
| −Isopods | 531.81 ± 23.47 a | 316.33 ± 65.16 a,b | — | 409.07 ± 41.06 b | ||
| P. massoniana | +Isopods | 635.99 ± 6.20 b | 566.18 ± 70.85 b,c | 345.64 ± 44.68 a | 195.40 ± 82.38 a,b | |
| −Isopods | 613.77 ± 2.04 b | 215.82 ± 86.03 a | 117.81 ± 63.20 a | 108.32 ± 18.19 a | ||
| β-N-acetylhexosaminidases | Q. variabilis | +Isopods | 488.86 ± 83.05 a | 656.25 ± 72.65 a | 604.36 ± 24.57 b | 556.51 ± 75.28 a |
| −Isopods | 416.01 ± 59.80 a | 520.60 ± 52.14 a | 455.76 ± 15.42 a | 537.68 ± 14.79 a | ||
| P. massoniana | +Isopods | 503.75 ± 62.00 a | 478.25 ± 47.24 a | 656.23 ± 16.41 b | 477.55 ± 41.14 a | |
| −Isopods | 521.59 ± 102.21 a | 322.94 ± 112.26 a | 394.83 ± 9.30 a | 472.68 ± 29.62 a | ||
| β-1,4-glucosidase | Q. variabilis | +Isopods | 434.28 ± 24.79 a | 648.54 ± 64.08 a | 581.17 ± 18.33 b | 510.62 ± 85.87 a |
| −Isopods | 403.62 ± 10.33 a | 534.39 ± 68.87 a | 341.12 ± 18.27 a | 460.75 ± 40.11 a | ||
| P. massoniana | +Isopods | 470.92 ± 7.33 a | 634.28 ± 70.47 a | 544.82 ± 56.65 b | 385.41 ± 75.26 a | |
| −Isopods | 417.36 ± 44.33 a | 414.96 ± 98.73 a | 237.66 ± 59.17 a | 253.48 ± 84.15 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cao, T.; Lv, M.; Fang, Y.; Liu, R.; Luo, Y.; Xu, C.; Tian, X. Grazing Effects of Soil Fauna on White-Rot Fungi: Biomass, Enzyme Production and Litter Decomposition Ability. J. Fungi 2022, 8, 348. https://doi.org/10.3390/jof8040348
Chen Y, Cao T, Lv M, Fang Y, Liu R, Luo Y, Xu C, Tian X. Grazing Effects of Soil Fauna on White-Rot Fungi: Biomass, Enzyme Production and Litter Decomposition Ability. Journal of Fungi. 2022; 8(4):348. https://doi.org/10.3390/jof8040348
Chicago/Turabian StyleChen, Yunru, Tingting Cao, Meiqi Lv, You Fang, Run Liu, Yunchao Luo, Chi Xu, and Xingjun Tian. 2022. "Grazing Effects of Soil Fauna on White-Rot Fungi: Biomass, Enzyme Production and Litter Decomposition Ability" Journal of Fungi 8, no. 4: 348. https://doi.org/10.3390/jof8040348
APA StyleChen, Y., Cao, T., Lv, M., Fang, Y., Liu, R., Luo, Y., Xu, C., & Tian, X. (2022). Grazing Effects of Soil Fauna on White-Rot Fungi: Biomass, Enzyme Production and Litter Decomposition Ability. Journal of Fungi, 8(4), 348. https://doi.org/10.3390/jof8040348







